Abstract
Background
Plasminogen activator inhibitor-1 (PAI-1) promotes cellular aging both in vitro and in vivo. Telomere length is a marker of biological aging.
Objectives
To examine the cross-sectional and longitudinal associations between plasma PAI-1 and leukocyte telomere length in a large-scale epidemiological study of American Indians.
Methods
We measured leukocyte telomere length (LTL) and plasma PAI-1 in 2,560 American Indians who were free of overt CVD and participated in the Strong Heart Family Study (SHFS) clinical exam in 2001–2003. LTL and PAI-1 were repeatedly measured in 475 participants who attended SHFS clinical visits in both 2001–2003 and 1998–1999. Generalized estimating equation model was used to examine the cross-sectional and longitudinal associations between PAI-1 and LTL, adjusting for known risk factors.
Results
A higher level of plasma PAI-1 was negatively associated with shorter age-adjusted LTL (β=−0.023, 95%CI: −0.034 ~ −0.013). This association was attenuated (β=−0.015, 95%CI: −0.029 ~ −0.002) after adjustments for demographics, study site, lifestyle (smoking, drinking, physical activity), and metabolic factors (obesity, blood pressure, fasting glucose, insulin, lipids, kidney function). Further adjustment for hsCRP did not change this association (β=−0.015, 95%CI: −0.029 ~ −0.001). Longitudinal analysis revealed that change in plasma PAI-1 was also inversely associated with change in LTL after adjusting for demographics, follow-up years, lifestyle factors, changes in metabolic factors, baseline levels of PAI-1 and LTL (β=−0.0005, 95%CI: −0.0009 ~ −0.0001).
Conclusions
A higher level of plasma PAI-1 was associated with shorter LTL in American Indians. This finding may suggest a potential role of PAI-1 in biological aging among American Indians.
Keywords: Aging, Fibrinolysis, Indians, South American, Plasminogen Activator Inhibitor 1, Thrombosis, Telomere
INTRODUCTION
Telomeres are repetitive DNA sequences and their associated proteins at the end of chromosomes.[1] Telomere length shortens during each round of cell division, and when it reaches a critical value, cells stop division and enter senescence.[2] Thus, telomere length has been considered a cellular marker of biological aging. Shorter telomere length has been associated with various age-related diseases including cardiovascular disease (CVD),[3, 4] obesity,[5] diabetes,[4] and cancer.[6] Among American Indians participating in the Strong Heart Study (SHS), our group has recently reported associations of leukocyte telomere length with incident diabetes [7], onset and progression of carotid atherosclerosis [8], obesity [9], arterial aging [10], and life simple 7 factors [11].
Plasminogen activator inhibitor-1 (PAI-1), the primary physiological inhibitor of plasminogen activator,[12] plays a critical role in regulating fibrinolysis [13], fibrosis [14], and thrombosis [15]. Experimental studies have demonstrated a critical role of PAI-1 in cellular senescence both in vitro and in vivo.[16, 17] Human studies showed that plasma PAI-1 increased with age [13] and an elevated level of plasma PAI-1 was associated with multiple age-related conditions, including CVD,[18] obesity,[19, 20] insulin resistance,[20, 21] and vascular remodeling,[22] all of which have been related to shorter telomere length.[23] While existing evidence clearly suggests a potential role of PAI-1 in cellular aging and age-related conditions, little is known about the relationship between plasma PAI-1 and telomere length, a marker of biological aging, in large-scale epidemiological studies. To date, we are aware of two cross-sectional studies examining the association between plasma PAI-1 and leukocyte telomere length (LTL) in Africans and Europeans,[24, 25] but the sample size of these studies was rather small and the associations were substantially attenuated after adjustments for metabolic factors in both studies. A large-scale epidemiological study with extensive control of potential confounding variables is needed to confirm or refute the relationship between plasma PAI-1 and LTL. Moreover, previous research indicated that elevated plasma PAI-1 was almost always accompanied by inflammation,[26] a condition associated with shorter telomere length as well.[27–29] Thus, it is possible that the previously reported association between plasma PAI-1 and LTL could be modulated or confounded by inflammation. However, this has not been examined in previous studies. Further, no study has investigated the relationship between plasma PAI-1 and LTL in American Indians, a minority group suffering high rates of age-related diseases, e.g., diabetes, obesity, and CVD.[30, 31]
The goals of this study are twofold: (1) to investigate the cross-sectional and longitudinal associations between plasma PAI-1 and LTL in American Indians in the Strong Heart Family Study (SHFS); and (2) to examine whether the relationship between PAI-1 and LTL is modulated by inflammation assessed by high sensitive C-reactive protein (hsCRP).
MATERIALS AND METHODS
Study population
The Strong Heart Study (SHS) is a multicenter, population-based longitudinal study of CVD, diabetes and their risk factors in American Indians in tribes and communities residing in Arizona, North/South Dakota, and Oklahoma. The study design, survey methods, and laboratory techniques of the SHS have been described previously.[32] The SHFS, a component of the SHS, was initiated in 1997 by enrolling 967 participants from 30 families of the original SHS cohort members. The study was expanded in 2001–2003 by enrolling a total of 3,665 participants from 94 families including re-examination of most of the 967 members enrolled in 1998–1999. All participants provided written informed consent. The SHFS protocol has been described elsewhere [33] and was approved by the Institutional Reviews Boards from the Indian Health Service and the participating institutions. In the current study, 874 participants from one community were removed due to withdraw of consent. Our cross-sectional analysis finally included 2,560 participants who attended the clinical visit in 2001–2003, with exclusion of participants with overt CVD (N=135) or missing data on either LTL or plasma PAI-1 (N=96). The longitudinal analysis included 475 participants with repeated measurements of both LTL and plasma PAI-1 in samples collected at two visits (1998–1999, 2001–2003). Selection of study participants is shown in Figure 1.
Figure 1.
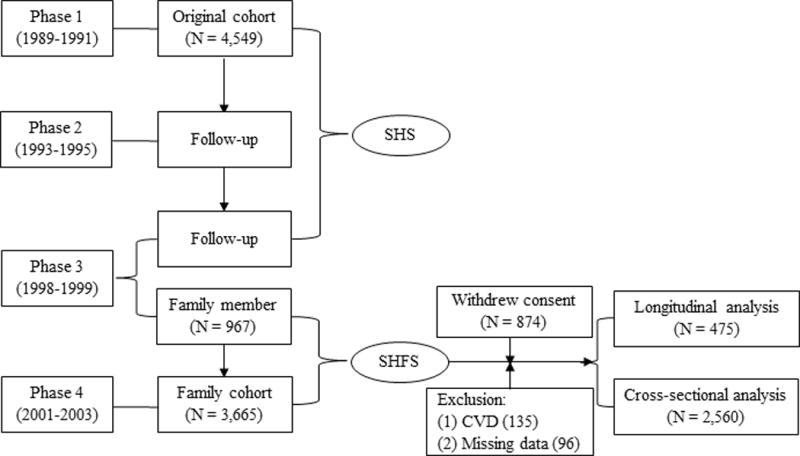
Flowchart illustrating the study design of SHFS and the selection of study participants.
The Strong Heart Family Study (SHFS) was initiated in 1997 by enrolling 967 family members of the original cohort, and then expanded in 2001–2003 by enrolling a total of 3,665 participants from 94 families including the re-examination of most of the 967 family members. The current analysis excluded 874 participants because one community withdrew consent for continuing participating in the study. After further excluding participants with overt CVD and missing data on either PAI-1 or LTL, a total of 2,560 participants were included in the cross-sectional analysis and 475 participants were included in the longitudinal analysis.
Measurement of LTL
Detailed methods for the measurement of LTL in the SHFS have been described previously.[7] Briefly, genomic DNA from peripheral blood was isolated according to standard protocols. LTL was quantified by quantitative PCR at the University of California, San Francisco using a high-throughput telomere length assay system. The telomere length assay determines the ratio of telomeric product/single copy gene (T/S) obtained using quantitative PCR according to protocols reported previously.[34] T/S ratio was calculated by taking the ratio between the mean of two T values and two S values attained for each of the three replicates. The three T/S ratios were averaged, and standard deviation and coefficient of variation (%CV, standard deviation/mean) were calculated. The T/S ratios were normalized to the mean of all samples and reported. For quality control, we included seven control DNA samples from various cancer cell lines in each assay plate. These control samples allowed us to create 8 standard curves, which were then integrated into a composite standard curve, used for T and S concentration calculations. For the purpose of quality control, about 20% of the samples selected randomly were measured twice. Intra- and inter-assay %CV was 4.6% and 6.9%, respectively.
Measurement of plasma PAI-1 and hsCRP
Plasma PAI-1 was measured using an enzyme-linked immunosorbent assay (ELISA) according to standard protocols described previously.[35] All antibodies and reagents were obtained from the Center for Thrombosis and Vascular Research, University of Leuven, Belgium. The assay %CV of this method was <5%. Serum hsCRP was measured by an ELISA assay developed in-house using purified CRP and anti-CRP antibodies from Calbiochem at the University of Vermont laboratory.[36] The assay %CV was 8%.
Assessment of other factors
Demographic information (age, sex, education level) was collected by standard questionnaires. Cigarette smoking was classified as current smoking, past smoking and never smoking. Current smoking was defined as having smoked at least 100 cigarettes in the entire life, having smoked cigarettes regularly, and smoking currently. Past smoking was defined as having smoked at least 100 cigarettes in the entire life, having smoked cigarettes regularly in the past, but not smoking currently. Never smoking was defined as never smoked or having smoked fewer than 100 cigarettes in the entire life. Alcohol drinking was classified as current drinkers, former drinkers and never drinkers. Current drinkers were those who had consumed any alcohol during the past year, former drinkers had stopped consuming alcohol for ≥12 months, and never drinkers were those who reported never drinking alcohol in their life time. Each participant was asked to wear a pedometer for seven consecutive days and to record the number of the steps taken daily in an activity diary. Physical activity was assessed by the mean number of steps per day calculated by averaging the total number of steps recorded in 7 consecutive days. Body weight (kg) and height (cm) were measured when participants wore light clothes and no shoes by trained staff. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). Waist circumference was measured at the level of the umbilicus while the participant was in a supine position. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times by trained staff using a standard mercury sphygmomanometer after the participants had been resting for at least 5 minutes and the mean of the last two measurements was used in statistical analysis. Fasting glucose, insulin, serum creatinine, and blood lipids including total cholesterol, triglycerides, low density lipoprotein cholesterol (LDL-C), and high density lipoprotein cholesterol (HDL-C), were measured by standard laboratory methods.[32] Estimated glomerular filtration rate (eGFR) was estimated based on serum creatinine by using the Modification of Diet in Renal Disease Study equation.[37]
Statistical analysis
Cross-sectional analysis
Given the strong correlation between age and LTL, we first regressed LTL on age and the residual from regression (i.e. age-adjusted LTL) was used in subsequent statistical analyses. PAI-1 and hsCRP were log-transformed (i.e. log-PAI-1, log-hsCRP) to improve normality.
To examine the cross-sectional association between plasma PAI-1 and LTL, we constructed a generalized estimating equation (GEE) model in which age-adjusted LTL was the dependent variable and plasma PAI-1 (continuous log-PAI-1 or categorized into quartiles) was the independent variable, adjusting for demographics (sex, education level), study site, lifestyle (smoking, drinking, physical activity), and metabolic factors (waist circumference, SBP, fasting glucose, insulin, LDL-C, HDL-C, eGFR). GEE regression model was used here to account for the family relatedness among study participants by including “family ID” as a cluster variable in the model. To examine whether and to what extent inflammation may potentially modulate the association between plasma PAI-1 and LTL, we additionally adjusted for log-hsCRP in the regression model.
Longitudinal analysis
Changes in LTL, plasma PAI-1, and metabolic factors were calculated by subtracting the follow-up values from their corresponding baseline measurements. To examine the longitudinal association between plasma PAI-1 and LTL, we constructed multivariate GEE model by regressing change in LTL (dependent variable) on change in plasma PAI-1 (independent variable), adjusting for demographics (age, sex, education level), study site, lifestyle (smoking, drinking) at baseline, and changes in metabolic factors (waist circumference, SBP, fasting glucose, insulin, LDL-C, HDL-C, eGFR) during the same time period, as well as follow-up years, plasma PAI-1 and LTL at baseline. Baseline plasma PAI-1 and LTL were adjusted to account for the longitudinal effect of their baseline values on telomere changes.
Sensitivity analysis
To examine whether diabetes influences our results, we performed separate analysis by excluding participants with overt diabetes at baseline. To examine whether antihypertensive medications affect our results, we excluded participants under antihypertensive medications. To examine whether smoking influences the association between plasma PAI-1 and LTL, we conducted stratification analysis by smoking status (smokers vs. non-smokers). A two-tailed P value less than 0.05 was considered statistically significant. All statistical analysis was conducted using SAS statistical software (version 9.4, Cary, North Carolina).
RESULTS
Characteristics of study participants
Characteristics of study participants are presented in Table 1. Mean age of the study participants was 38.9 ± 16.4 years. Women accounted for 60% of the study participants and had significantly longer age-adjusted LTL than men (P=0.009). Participants with higher plasma PAI-1 were more likely to be current smokers, current drinkers, and had higher BMI, waist circumference, fasting glucose, insulin, lipids, and hsCRP and shorter LTL than those with lower plasma PAI-1 (all P<0.05). No significant difference was detected for other variables listed in Table 1. Baseline characteristics of the 475 participants (39.9 ± 14.6 years) included in the longitudinal analysis was shown in Table 2.
Table 1.
Characteristics of SHFS participants examined in 2001–2003 according to quartiles of plasma PAI-1
| Quartiles of plasma PAI-1 (ng/mL) | |||||
|---|---|---|---|---|---|
| Characteristics | Q1 (≤ 28) |
Q2 (29 – 47) |
Q3 (48 – 73) |
Q4 (≥ 74) |
Trend P* |
| N | 675 | 634 | 623 | 628 | – |
| Age, year | 39.1±17.8 | 40.2±17.7 | 41.3±15.9 | 38.3±14.6 | 0.784 |
| Males, n (%) | 241 (35.70) | 262 (41.32) | 263 (42.22) | 251 (39.37) | 0.129 |
| Current smoking, n (%) | 210 (31.11) | 235 (37.07) | 235 (37.72) | 266 (42.36) | <0.001 |
| Current drinking, n (%) | 365 (54.07) | 372 (58.68) | 366 (58.75) | 409 (65.13) | 0.001 |
| Education, years | 12.1±2.4 | 12.2±2.3 | 12.4±2.3 | 12.1±2.2 | 0.718 |
| Physical activity, steps/day | 6796±4390 | 6244±4053 | 5725±3852 | 4978±3044 | <0.001 |
| Body mass index, kg/m2 | 27.1±5.8 | 30.2±7.0 | 32.4±7.4 | 35.4±7.5 | <0.001 |
| Waist circumference, cm | 91.6±14.9 | 99.8±17.7 | 105.7±17.4 | 111.9±17.2 | <0.001 |
| SBP, mmHg | 119.9±18.0 | 122.0±16.5 | 124.7±16.6 | 124.0±15.0 | <0.001 |
| DBP, mmHg | 73.8±10.8 | 75.4±10.6 | 77.6±11.1 | 78.5±10.9 | <0.001 |
| FPG, mg/dL | 100.8±40.8 | 103.3±36.4 | 110.7±46.5 | 119.9±54.2 | <0.001 |
| Insulin, uU/mL | 11.7±11.8 | 15.5±17.6 | 19.7±23.8 | 23.6±19.5 | <0.001 |
| Total cholesterol, mg/dL | 176.1±33.1 | 180.5±35.5 | 185.8±35.7 | 189.2±44.4 | <0.001 |
| Triglycerides, mg/dL | 125.2±71.3 | 140.7±82.1 | 171.0±105.2 | 224.3±326.7 | <0.001 |
| LDL-C, mg/dL | 94.1±28.2 | 101.1±30.1 | 102.3±29.4 | 102.5±32.3 | <0.001 |
| HDL-C, mg/dL | 57.2±15.9 | 51.9±13.6 | 50.2±13.5 | 47.1±13.2 | <0.001 |
| eGFR, mL/min/1.73 m2 | 95.9±26.6 | 95.9±23.6 | 97.5±25.8 | 101.2±25.0 | 0.001 |
| Log-hsCRP, mg/L | 0.8±1.3 | 1.0±1.3 | 1.4±1.1 | 1.6±1.1 | <0.001 |
| LTL, T/S ratio | 1.01±0.25 | 0.99±0.23 | 0.98±0.22 | 0.97±0.22 | <0.001 |
Sex-adjusted using GEEs to account for family relatedness of participants.
SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate; log-hsCRP: log-transformed high sensitivity C-reactive protein; LTL: leukocyte telomere length.
Table 2.
Characteristics of SHFS participants with available PAI-1 and LTL in both examinations conducted in 1998–1999 and 2001–2003 (N = 475)
| Characteristics | Mean/percentage |
|---|---|
| Age, year | 39.9±14.6 |
| Males, n (%) | 179 (37.68) |
| Current smoking, n (%) | 184 (38.98) |
| Current drinking, n (%) | 293 (62.08) |
| Education, years | 12.4±2.0 |
| Body mass index, kg/m2 | 31.3±7.4 |
| Waist circumference, cm | 102.4±18.6 |
| SBP, mmHg | 123.2±15.4 |
| DBP, mmHg | 76.7±9.3 |
| FPG, mg/dL | 112.3±55.4 |
| Insulin, uU/mL | 23.1±25.7 |
| Total cholesterol, mg/dL | 183.6±34.2 |
| Triglycerides, mg/dL | 132.8±125.3 |
| LDL-C, mg/dL | 117.6±28.8 |
| HDL-C, mg/dL | 44.4±12.8 |
| eGFR, mL/min/1.73 m2 | 94.6±23.6 |
| LTL, T/S ratio | 1.19±0.54 |
| Log-PAI-1 | 3.64±0.90 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; LDL-C: low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate; Log-PAI-1: log-transformed plasminogen activator inhibitor 1; LTL: leukocyte telomere length.
Cross-sectional association between plasma PAI-1 and telomere length
Table 3 shows the cross-sectional association between plasma PAI-1 and age-adjusted LTL. Regression using plasma PAI-1 as a continuous variable demonstrated that a higher log-PAI-1 was significantly associated with a shorter age-adjusted LTL (β=−0.023, P<0.001). This association was substantially attenuated but remained statistically significant after adjusting for demographics, study site, lifestyle and metabolic factors (β=−0.015, P=0.029). Additional adjustment for log-hsCRP did not change this association (β=−0.015, P=0.032).
Table 3.
Cross-sectional association between plasma PAI-1 and age-adjusted LTL among SHFS participants
| PAI-1 level | Unadjusted
|
Model 1*
|
Model 2†
|
|||
|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | |
| Continuous | ||||||
|
| ||||||
| Log-PAI-1 | −0.023 (0.006) | <0.001 | −0.015 (0.007) | 0.029 | −0.015 (0.007) | 0.032 |
| Quartiles | ||||||
| Quartile 1 | reference | – | reference | – | reference | – |
| Quartile 2 | −0.011 (0.011) | 0.300 | −0.015 (0.011) | 0.160 | −0.016 (0.011) | 0.145 |
| Quartile 3 | −0.023 (0.010) | 0.020 | −0.024 (0.011) | 0.033 | −0.025 (0.011) | 0.027 |
| Quartile 4 | −0.041 (0.011) | <0.001 | −0.027 (0.013) | 0.040 | −0.028 (0.013) | 0.035 |
| P for trend | <0.001 | 0.035 | 0.030 | |||
LTL: leukocyte telomere length; PAI-1: plasminogen activator inhibitor-1; Log-PAI-1: log-transformed plasminogen activator inhibitor-1.
Model 1 adjusted for demographics (sex, education level), study site, lifestyle factors (current smoking, current drinking, physical activity), and metabolic factors (waist circumference, systolic blood pressure, fasting glucose, insulin, low- and high- density lipoprotein cholesterol, estimated glomerular filtration rate).
Model 2 adjusted for covariates in model 1 plus log-transformed high sensitivity C-reactive protein.
Regression analysis using categorical plasma PAI-1 (in quartiles) also detected an association between PAI-1 level and age-adjusted LTL in the same direction. Compared with participants in the lowest quartile of plasma PAI-1, those in the highest quartile had significantly shorter age-adjusted LTL (β=−0.041, P<0.001). This association was attenuated after adjusting for demographics, study site, lifestyle and metabolic factors (β=−0.027, P=0.040). Additional adjustment for log-hsCRP did not attenuate this association (β=−0.028, P=0.035).
Longitudinal association between plasma PAI-1 and telomere length
Of the 475 participants with repeated measurements for both LTL and plasma PAI-1, LTL was shortened in 383 participants (81%) during an average 5.3 years of follow-up. The other 92 participants gained LTL. Our statistical analysis included all of the 475 participants as telomere lengthening might be associated with PAI-1 change during the follow-up period. Table 4 presents the longitudinal association between change in plasma PAI-1 and change in LTL. It shows that change in plasma PAI-1 was significantly and inversely associated with change in telomere length after adjusting for demographics, study site, lifestyle, changes in metabolic factors during the same time period, follow-up years, and baseline plasma PAI-1 and LTL (β=−0.0005, P=0.017). We did not find an association between baseline plasma PAI-1 and change in LTL (β=0.0003, P=0.254).
Table 4.
Longitudinal association between change in plasma PAI-1 and change in LTL among SHFS participants
| Sub-populations | N | Multivariate adjusted*
|
|
|---|---|---|---|
| β† (SE) | P | ||
| Total participants | 475 | −0.0005 (0.0002) | 0.017 |
| Excluding diabetic participants | 392 | −0.0005 (0.0002) | 0.014 |
| Excluding participants under antihypertensive treatment | 424 | −0.0005 (0.0002) | 0.017 |
LTL: leukocyte telomere length; PAI-1: plasminogen activator inhibitor-1.
Adjusted for age, sex, education level, study site, lifestyle (smoking, drinking), changes in metabolic factors (waist circumference, systolic blood pressure, fasting glucose, insulin, low- and high- density lipoprotein cholesterol, estimated glomerular filtration rate), follow-up years, and baseline plasma PAI-1 and LTL.
Change in LTL (T/S ratio) associated with per unit of change in plasma PAI-1.
Results of sensitivity analysis
After excluding participants with prevalent diabetes or those under antihypertensive medications, the cross-sectional association between plasma PAI-1 and age-adjusted LTL (Supplementary Tables S1 and S2) and the longitudinal association between change in plasma PAI-1 and change in LTL (Table 4) remained statistically significant. These results indicate that the observed associations between plasma PAI-1 and telomere length were unlikely to be attributed to prevalent diabetes and antihypertensive medications. The cross-sectional association between plasma PAI-1 and age-adjusted LTL (Supplementary Tables S3) and the longitudinal association between change in plasma PAI-1 and change in LTL (Supplementary Tables S4) appeared to be stronger in smokers than that in non-smokers.
DISCUSSION
In a large cohort of American Indians free of overt CVD, we demonstrated for the first time that plasma PAI-1 was significantly and inversely associated with leukocyte telomere length in both cross-sectional and longitudinal analyses, independent of many known risk factors and inflammation assessed by hsCRP. Our findings suggest that plasma PAI-1 may play a critical role in biological aging, probably through pathways beyond known risk factors, though the precise molecular mechanisms through which PAI-1 influences telomeric aging remain to be determined.
The observed inverse association between plasma PAI-1 and biological aging is in line with previous experimental studies showing that PAI-1 advanced cellular senescence both in vitro and in vivo.[16, 17] This association was also supported by previous experimental research which showed that PAI-1 deficiency (PAI-1−/− mice) attenuated cellular senescence and prolonged survival,[38] and pharmacologic inhibition of PAI-1 promoted telomere maintenance in mice.[17] Further, PAI-1 expression and plasma PAI-1 concentration were increased in the klotho mouse model (a premature aging model widely used in aging research).[39] Although the relationship between PAI-1 and biological aging has been evidenced by experimental [16, 17] and animal [17, 38, 39] studies, no large-scale epidemiological studies have investigated this association in any ethnic groups including American Indians. So far, we are aware of two human studies with rather small sample sizes examining the association between plasma PAI-1 and leukocyte telomere length.[24, 25] One examined several hemostatic factors including PAI-1, fibrinogen, von Willebrand factor, and D-dimer in 171 South Africans and 182 European descendants but no association was identified between plasma PAI-1 and telomere length after adjusting for metabolic factors.[24] Another study comprising of 272 elderly Europeans with diabetes and myocardial infarction examined the relationship of LTL to fibrinogen, hsCRP, and PAI-1 but found that telomere length was not correlated to any of these biomarkers.[25] Herein we identified a significant association between plasma PAI-1 and leukocyte telomere length in a large sample of American Indians (N=2,560) in the SHFS. Of note, our analysis showed that, the association between PAI-1 and LTL was largely attenuated (~50%) after adjusting for metabolic factors, indicating that the observed association could be modulated by these metabolic factors. This emphasizes the importance and necessity of adjustment for metabolic factors in examining the relationship between PAI-1 and LTL.
In a subsample including 475 participants with repeated measurements of both PAI-1 and LTL (average follow-up of 5.3 years), we examined the longitudinal association between PAI-1 and LTL. Results showed that a larger increase in plasma PAI-1 was associated with a larger reduction in telomere length. Although this observational association does not establish the causal role of PAI-1 in biological aging, this finding provides a hypothesis that is worth of further investigation in future research.
PAI-1 is secreted in response to inflammatory reactions.[40] As such, an elevated level of plasma PAI-1 was almost always accompanied by increased inflammatory responses.[26] Because inflammation has also been associated with shorter telomere length,[27–29] it may modulate the association between plasma PAI-1 and telomere length. To test this hypothesis, we conducted additional analysis by further adjusting for hsCRP in the multivariate GEE model. It shows that additional adjustment for hsCRP did not materially alter the observed association between plasma PAI-1 and telomere length. This suggests that plasma PAI-1 may play a role in biological aging through pathways beyond inflammation.
Some anti-hypertensive drugs e.g., angiotensin-converting enzyme inhibitors, can reduce plasma level of PAI-1 [41] and thus may influence our results. As such, we conducted sensitivity analysis by excluding participants who self-reported receiving anti-hypertensive medications. It shows that the observed associations, both cross-sectional and longitudinal, between plasma PAI-1 and telomere length persisted. Similarly, excluding participants with prevalent diabetes did not change the association between plasma PAI-1 and telomere length either. These results suggested that the observed association between plasma PAI-1 and biological aging may not be attributed to anti-hypertensive or hypoglycemic drugs. Both cross-sectional and longitudinal associations between plasma PAI-1 and telomere length appeared to be stronger in smokers than that in non-smokers.
Strengths of this study included the high quality data of telomere length, the longitudinal follow-up, the large sample size, and the extensive adjustments for known risk factors. However, several limitations should also be noted. First, similar to all other observational studies, we cannot establish the causal relationship between plasma PAI-1 and telomere length. A prospective study is needed to assess the temporal sequence between PAI-1 and biological aging. Second, our findings were derived from American Indians whose metabolic health profiles could be different from other ethnic groups. Thus, the generalizability of our results to other populations is unknown. However, our study may serve as a model for analysis of other ethnic groups with high rates of diabetes and obesity. Third, as hsCRP was not available in the clinical exam in 1998–1999, we were unable to assess whether the longitudinal relationship between PAI-1 and telomere length was independent of inflammation in our analysis.
In conclusion, a higher level of plasma PAI-1 was significantly associated with shorter telomere length in American Indians, independent of many known covariates including metabolic factors and inflammation. This finding suggests that PAI-1 may play a critical role in cellular aging and aging-related disorders, although its causal impact on biological aging warranted further investigation.
Supplementary Material
ESSENTIALS.
Plasminogen activator inhibitor-1 (PAI-1) advanced cellular senescence in experiment studies.
No population study exists on the association between PAI-1 and biological aging in American Indians.
We found cross-sectional and longitudinal associations between higher PAI-1 and shorter telomere length.
Our findings suggest a pathway linking PAI-1 with biological aging beyond of metabolic factors.
Acknowledgments
The authors would like to thank the Strong Heart Study participants, Indian Health Service facilities, and participating tribal communities for their extraordinary cooperation and involvement, which has contributed to the success of the Strong Heart Study. The views expressed in this article are those of the authors and do not necessarily reflect those of the Indian Health Service.
This study was supported by NIH grants R01DK091369, K01AG034259, R21HL092363 and cooperative agreement grants U01HL65520, U01HL41642, U01HL41652, U01HL41654, and U01HL65521.
Footnotes
Addendum
F. Yeh, J. Lin, L. G. Best, S. A. Cole, E. T. Lee, B. V. Howard, and J. Zhao designed and performed the study. H. Peng analyzed the data and wrote the paper. H. Peng and J. Zhao reviewed, edited, and finalized the paper. All authors gave final approval for the submitted paper.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Pare G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8:82–90. doi: 10.1161/CIRCGENETICS.113.000485. [DOI] [PubMed] [Google Scholar]
- 5.Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- 6.Weischer M, Nordestgaard BG, Cawthon RM, Freiberg JJ, Tybjaerg-Hansen A, Bojesen SE. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105:459–68. doi: 10.1093/jnci/djt016. [DOI] [PubMed] [Google Scholar]
- 7.Zhao J, Zhu Y, Lin J, Matsuguchi T, Blackburn E, Zhang Y, Cole SA, Best LG, Lee ET, Howard BV. Short leukocyte telomere length predicts risk of diabetes in american indians: the strong heart family study. Diabetes. 2014;63:354–62. doi: 10.2337/db13-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Lin J, Matsuguchi T, Blackburn E, Yeh F, Best LG, Devereux RB, Lee ET, Howard BV, Roman MJ, Zhao J. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: the Strong Heart Family Study. Aging (Albany NY) 2014;6:414–27. doi: 10.18632/aging.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Yeh F, Lin J, Matsuguchi T, Blackburn E, Lee ET, Howard BV, Zhao J. Short leukocyte telomere length is associated with obesity in American Indians: the Strong Heart Family study. Aging (Albany NY) 2014;6:380–9. doi: 10.18632/aging.100664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng H, Zhu Y, Yeh F, Cole SA, Best LG, Lin J, Blackburn E, Devereux RB, Roman MJ, Lee ET, Howard BV, Zhao J. Impact of biological aging on arterial aging in American Indians: findings from the Strong Heart Family Study. Aging (Albany NY) 2016;8:1583–92. doi: 10.18632/aging.101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng H, Mete M, Desale S, Fretts AM, Cole SA, Best LG, Lin J, Blackburn E, Lee ET, Howard BV, Zhao J. Leukocyte telomere length and ideal cardiovascular health in American Indians: the Strong Heart Family Study. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood. 1987;69:381–7. [PubMed] [Google Scholar]
- 13.Bonfigli AR, Sirolla C, Cenerelli S, Marra M, Boemi M, Franceschi C, Testa I, Mari D, Sacchi E, Testa R. Plasminogen activator inhibitor-1 plasma level increases with age in subjects with the 4G allele at position -675 in the promoter region. Thromb Haemost. 2004;92:1164–5. [PubMed] [Google Scholar]
- 14.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eren M, Painter CA, Atkinson JB, Declerck PJ, Vaughan DE. Age-dependent spontaneous coronary arterial thrombosis in transgenic mice that express a stable form of human plasminogen activator inhibitor-1. Circulation. 2002;106:491–6. doi: 10.1161/01.cir.0000023186.60090.fb. [DOI] [PubMed] [Google Scholar]
- 16.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–84. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, McAnally D, Smith LH, Miyata T, Vaughan DE. Plasminogen activator inhibitor-1 antagonist TM5441 attenuates Nomega-nitro-L-arginine methyl ester-induced hypertension and vascular senescence. Circulation. 2013;128:2318–24. doi: 10.1161/CIRCULATIONAHA.113.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635–41. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 19.Eksteen P, Pieters M, de Lange Z, Kruger HS. The association of clot lysis time with total obesity is partly independent from the association of PAI-1 with central obesity in African adults. Thromb Res. 2015;136:415–21. doi: 10.1016/j.thromres.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Lalic K, Jotic A, Rajkovic N, Singh S, Stosic L, Popovic L, Lukic L, Milicic T, Seferovic JP, Macesic M, Stanarcic J, Civcic M, Kadic I, Lalic NM. Altered Daytime Fluctuation Pattern of Plasminogen Activator Inhibitor 1 in Type 2 Diabetes Patients with Coronary Artery Disease: A Strong Association with Persistently Elevated Plasma Insulin, Increased Insulin Resistance, and Abdominal Obesity. Int J Endocrinol. 2015;2015:390185. doi: 10.1155/2015/390185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meigs JB, Mittleman MA, Nathan DM, Tofler GH, Singer DE, Murphy-Sheehy PM, Lipinska I, D’Agostino RB, Wilson PW. Hyperinsulinemia, hyperglycemia, and impaired hemostasis: the Framingham Offspring Study. JAMA. 2000;283:221–8. doi: 10.1001/jama.283.2.221. [DOI] [PubMed] [Google Scholar]
- 22.Diebold I, Kraicun D, Bonello S, Gorlach A. The ‘PAI-1 paradox’ in vascular remodeling. Thromb Haemost. 2008;100:984–91. [PubMed] [Google Scholar]
- 23.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–83. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 24.von Kanel R, Malan NT, Hamer M, van der Westhuizen FH, Malan L. Leukocyte telomere length and hemostatic factors in a South African cohort: the SABPA Study. J Thromb Haemost. 2014;12:1975–85. doi: 10.1111/jth.12733. [DOI] [PubMed] [Google Scholar]
- 25.Olivieri F, Lorenzi M, Antonicelli R, Testa R, Sirolla C, Cardelli M, Mariotti S, Marchegiani F, Marra M, Spazzafumo L, Bonfigli AR, Procopio A. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis. 2009;206:588–93. doi: 10.1016/j.atherosclerosis.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Kruithof EK. Regulation of plasminogen activator inhibitor type 1 gene expression by inflammatory mediators and statins. Thromb Haemost. 2008;100:969–75. [PubMed] [Google Scholar]
- 27.Masi S, Nightingale CM, Day IN, Guthrie P, Rumley A, Lowe GD, von Zglinicki T, D’Aiuto F, Taddei S, Klein N, Salpea K, Cook DG, Humphries SE, Whincup PH, Deanfield JE. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13- to 16-year-old adolescents. Arterioscler Thromb Vasc Biol. 2012;32:2029–34. doi: 10.1161/ATVBAHA.112.250589. [DOI] [PubMed] [Google Scholar]
- 28.Pedroso DC, Miranda-Furtado CL, Kogure GS, Meola J, Okuka M, Silva C, Calado RT, Ferriani RA, Keefe DL, dos Reis RM. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil Steril. 2015;103:542–7 e2. doi: 10.1016/j.fertnstert.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 29.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diagnosed diabetes among American Indians and Alaska Natives aged <35 years—United States, 1994–2004. MMWR Morb Mortal Wkly Rep. 2006;55:1201–3. [PubMed] [Google Scholar]
- 31.Fretts AM, Howard B, Kriska AM, Smith NL, Lumley T, Lee ET, Russell M, Siscovick D. Physical activity and incident diabetes in American Indians: the Strong Heart Study. Am J Epidemiol. 2009;170:632–9. doi: 10.1093/aje/kwp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132:1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 33.North KE, Howard BV, Welty TK, Best LG, Lee ET, Yeh JL, Fabsitz RR, Roman MJ, MacCluer JW. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157:303–14. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 34.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Declerck PJ, Alessi MC, Verstreken M, Kruithof EK, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood. 1988;71:220–5. [PubMed] [Google Scholar]
- 36.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–8. [PubMed] [Google Scholar]
- 37.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 38.Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GR, Mutlu GM, Miyata T, Vaughan DE. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci U S A. 2014;111:7090–5. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshita K, Yamamoto K, Ito M, Kondo T, Matsushita T, Hirai M, Kojima T, Nishimura M, Nabeshima Y, Loskutoff DJ, Saito H, Murohara T. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–54. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 40.Margetic S. Inflammation and haemostasis. Biochem Med (Zagreb) 2012;22:49–62. [PMC free article] [PubMed] [Google Scholar]
- 41.Sakata K, Shirotani M, Yoshida H, Urano T, Takada Y, Takada A. Differential effects of enalapril and nitrendipine on the fibrinolytic system in essential hypertension. Am Heart J. 1999;137:1094–9. doi: 10.1016/s0002-8703(99)70368-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


