Abstract
Objective
A systematic review on S-adenosylmethionine (SAMe) for treatment of neuropsychiatric conditions and co-morbid medical conditions.
Data Sources
Searches were conducted between 7/15/2015 and 9/28/2016 by combining search terms for SAMe (s-adenosyl methionine or s-adenosyl-l-methionine) with terms for relevant disease states including (major depressive disorder, MDD, depression, perinatal depression, human immunodeficiency virus, HIV, Parkinson's, Alzheimer's, dementia, anxiety, Schizophrenia, psychotic, 22q11.2, substance abuse, fibromyalgia, osteoarthritis, hepatitis, or cirrhosis). Additional studies were identified from prior literature. Ongoing clinical trials were identified through clinical trial registries.
Study Selection
Of the 174 records retrieved, 21 were excluded, as they were not original investigations. An additional 21 records were excluded, for falling outside of the scope of this review. Of the 132 studies included in this review, 115 were clinical trials and 17 were preclinical studies.
Data Extraction
A wide range of studies was included in this review in order to capture information that would be of interest to psychiatrists in clinical practice.
Results
This review of SAMe in the treatment of major depressive disorder found promising but limited evidence of efficacy and safety to support the use of SAMe as a monotherapy and as an augmentation for other antidepressants. Additionally, preliminary evidence suggests that SAMe may ameliorate symptoms in certain neurocognitive, substance use and psychotic disorders and co-morbid medical conditions.
Conclusions
SAMe holds promise as a treatment for multiple neuropsychiatric conditions, but the body of evidence has limitations. The encouraging findings support further study of SAMe in both psychiatric and co-morbid medical illnesses.
Introduction
Complementary, alternative, and integrative medicine (CAIM) includes a wide range of biological, psychological and mind-body treatments being used to enhance standard medical practices and improve patient outcomes. Integrative Psychiatry (IP), a form of CAIM, “seeks to enrich mainstream mental health care with valuable treatments from global healing traditions as well as from modern laboratories in related fields.” (1,2). CAIM interventions include nutraceuticals, classified by the US Food and Drug Administration [FDA] as “dietary supplements,” defined as products intended for ingestion that contain ingredients such as vitamins, minerals, amino acids, herbs or other botanicals and nutrient concentrates, metabolites, or constituents. Many patients with mental health disorders utilize these modalities, often without physician supervision (3,4). Understanding the growing evidence supporting the efficacy of certain CAIM therapies will prepare clinicians to better advise patients when discussing integrative treatments.
S-adenosylmethionine (SAMe) was discovered in 1952 by the late Italian scientist and former National Institutes of Health biochemistry director, Giulio Cantoni (5,6). It is an endogenous, intracellular amino acid metabolite and enzyme co-substrate involved in multiple crucial biochemical pathways, including biosynthesis of hormones and neurotransmitters (7-9). SAMe concentrations have been measured in blood and cerebrospinal fluid (CSF) with ranges established in normal (10,11) and disease states. SAMe deficiency in CSF has been reported in patients with rare inherited defects in folate and methionine metabolism (12,13) as well as in more common diseases such as depressive disorders, Alzheimer's Dementia, Parkinson's Disease and HIV infection (14,15). Deficiencies of folate and vitamin B12, necessary co-factors in the synthesis of SAMe, may account for decreased SAMe levels, especially in patients with depression and dementia. Studies have shown that with either oral or parenteral treatment, SAMe crosses the blood-brain barrier and increases CSF levels, including in patients with neuropsychiatric conditions (14,15). As a CAIM therapy, SAMe has been utilized for treatment of psychiatric and medical conditions in Europe for over 30 years. In the United States, it became better known after 1999 as an over-the-counter dietary supplement under the Dietary Supplement Health and Education Act (DSHEA).
This review summarizes clinical trials of SAMe for treatment of neuropsychiatric disorders and co-morbid conditions encountered by psychiatrists in practice. To provide information that will assist clinicians considering treatment options in a broad range of complex clinical situations, we discuss literature encompassing samples of patients with a wide variety of neuropsychological symptoms for whom decisions about psychiatric treatments may take into account co-existing medical conditions and medication interactions. In addition to preclinical research, we include results from controlled trials, open studies and case reports on SAMe monotherapy and augmentation therapy. SAMe safety, contraindications, and medication interactions are addressed. This review also highlights limitations of the current literature and suggests future potential areas for research.
Methods
A literature search conducted between 7/15/2015 and 9/28/2016 utilized electronic databases including PubMed, EMBASE, PsycINFO, Cochrane Library, CINAHL and Google Scholar by combining search terms for SAMe (s-adenosyl methionine or s-adenosyl-l-methionine) with terms for relevant disease states including (major depressive disorder, MDD, depression, perinatal depression, human immunodeficiency virus, HIV, Parkinson's, Alzheimer's, dementia, anxiety, Schizophrenia, psychotic, 22q11.2, substance abuse, fibromyalgia, osteoarthritis, hepatitis, or cirrhosis). Additional studies were identified using previous literature reviews, meta-analyses, books, and book chapters (Figure 1. PRISMA Flowchart). Ongoing clinical trials were identified through ClinicalTrials.gov and the WHO (World Health Organization) International Clinical Trials Registry Platform.
Figure 1. Flowchart of Studies Included in the Systematic Review.
Results
Preclinical Studies
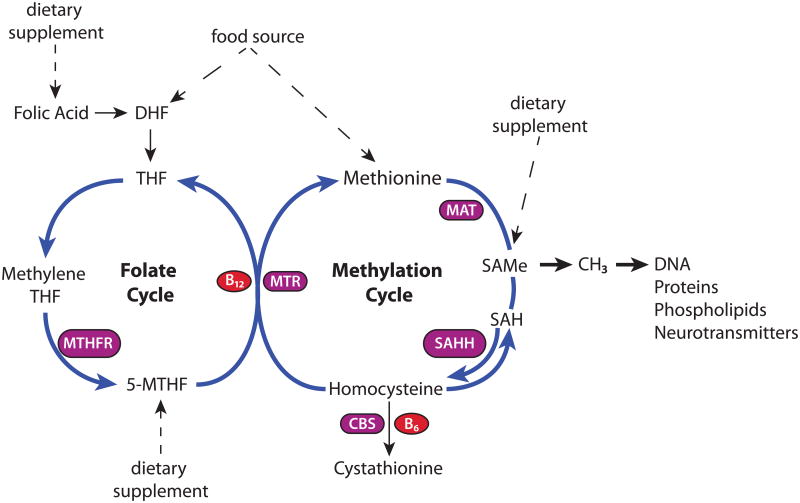
SAMe is the universal methyl donor in more than 100 methyltransferase reactions, which regulate essential metabolic pathways (see review, by 6,16). Methylation involves the transfer of a methyl group (CH3) to an acceptor molecule (Figure 2), including DNA bases, proteins, phospholipids, free amino acids and neurotransmitters. DNA methylation can turn gene transcription “on” or “off.” Similarly, methylation of proteins results in post-translational modifications that can regulate enzyme activity. Methylation of phospholipids is necessary for cell-membrane integrity and optimal function of receptors in the lipid membrane bilayer. Aberrant methylation has been implicated as a pathogenic mechanism in central nervous system (CNS) disorders, including depression and dementia (16,18). Methyl group donation is a target mechanism for disease prevention, to delay disease progression, and to enhance therapeutic outcomes (16,19).
Figure 2. S-adenosylmethionine in the Methylation Cycle.
DHF, dihydrofolate; THF, tetrahydrofolate; 5-MTHF, 5-methyltetrahydrofolate; SAMe, S-adenosylmethionine; SAH, S-adenosylhomocysteine; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine synthase; MAT, methionine adenosyltransferase; SAHH, S-adenosylhomocysteine hydrolase.
Reproduced with permission from Psychiatric Clinics of North America17
SAMe has been studied in animal models of depression (20,21). In rodents, SAMe dose-dependently decreases immobility time in the forced swimming test (22) and increases concentrations of CNS monoamine neurotransmitters, serotonin and norepinephrine (23). Animal studies show that chronic SAMe administration increases dopaminergic tone in brain regions, including rat striatum (24), and increases CNS beta-adrenergic receptor density and activity (25,26). Thus, studies of central monoaminergic neurotransmitters support proposed mechanisms for SAMe antidepressant effects. SAMe may also have modulatory effects on cell signaling pathways in the CNS. In rats, chronic treatment with SAMe resulted in a marked increase in calcium/calmodulin dependent protein kinase II (CaMKII) in synaptic vesicles from the hippocampus, as well as a marked increase of synapsin I in the synaptic cytosol of the hippocampus and frontal cortex (27). Typical antidepressants have been shown to activate CaMKII and synapsin I, which suggests that SAMe may share a similar modulatory action on neurotransmitter release.
A growing literature linking relative hypomethylation to disease pathophysiology in dementia includes reports of decreased SAMe concentrations in CSF in patients with Alzheimer's disease (28), hypomethylation of proteins that regulate levels of CNS phosphorylated-Tau, (29,30) and hypomethylation of genes that affect expression of beta-amyloid protein (31). SAMe affects site-specific methylation of DNA-promoter regions that regulate gene function, and carboxymethylation of proteins that can regulate b-amyloid and Tau proteins, neuropathological hallmarks of Alzheimer's disease (18).
Clinical Trials
Depressive Disorders
The antidepressant effects of SAMe were first described in 1970s (32). Early clinical studies used parenteral formulations, until an oral preparation became available in the 1980s (33). More than 50 clinical trials in the United States and Europe evaluated SAMe in the treatment of depressive disorders: 17 open-label trials with 708 patients (supplemental table, ST1); 19 double-blind, randomized placebo-controlled trials (RPCTs) of SAMe including 878 patients (Table 1); and 21 controlled trials comparing SAMe with other antidepressants with a total of 1591 patients (Table 2). Observations of SAMe-induced hypomania or mania in early studies (45,65,69,70,71) limited subsequent prospective clinical trials to unipolar major depressive episodes, though formal diagnostic criteria were not consistently used in early trials.
Table 1.
Controlled Trials of SAMe vs. Placebo for Depression
| Trial | Year | Patients enrolled (randomized) | Experimental Design | Study duration (days) | SAMe dose (mg/day) | Route | Primary Outcome Measure | Primary Outcome (SAMe vs. Placebo) P Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SAMe | Placebo | Positive | Negative | |||||||
| Fazio et al.34 | 1973 | 19 | 14 | 5 | double-blind | 8 | 45 | IV | HAM-D | < 0.01 | |
|
| |||||||||||
| Agnoli et al.35 | 1976 | 30 | 20 | 10 | double-blind | 15 | 45 | IM | HAM-D | P value for comparison not provided | |
|
| |||||||||||
| Barberi et al.36,a | 1978 | 40 | 20 | 20 | cross-over | 10 | 200 | IV | HAM-D | < 0.05 | |
|
| |||||||||||
| Muscettola et al.37 | 1982 | 20 | 10 | 10 | double-blind | 15 | 150 | IM | HAM-D | <0.01 | |
|
| |||||||||||
| Caruso et al.38 | 1984 | 49 | 25 | 24 | double-blind | 21 | 200 | IM | HAM-D | < 0.001 | |
|
| |||||||||||
| Carney et al.39 | 1986 | 32 | 15 | 17 | double-blind | 14 | 200 | IV | HAM-D | NSb | |
|
| |||||||||||
| Caruso et al.40 | 1987 | 59 | 30 | 29 | double-blind | 21 | 200 | IM | HAM-D | < 0.01 | |
|
| |||||||||||
| De Leo et al.41 | 1987 | 40 | 20 | 20 | double-blind | 30 | 200 | IM | ZSDS | < 0.05 | |
|
| |||||||||||
| Thomas et al.42 | 1987 | 20 | 9 | 11 | double-blind | 14 | 200 | IV | HAM-D | NSb | |
|
| |||||||||||
| Janicak et al.43 | 1988 | 15 | 7 | 5 | double-dummy | 15 | 400 | IV | HAM-D | < 0.02 | |
|
| |||||||||||
| Carrieri et al.44 | 1990 | 21 | 11 | 10 | cross-over | 15 | 1000 | PO | HAM-D | < 0.05 | |
|
| |||||||||||
| Kagan et al.45,a | 1990 | 18 | 9 | 9 | double-blind | 21 | 1600 | PO | HAM-D | < 0.05 | |
|
| |||||||||||
| Fava et al.46 | 1992 | 43 | 24 | 31 | double-blind | 42 | 1600 | PO | HAM-D | NSc,d,e | |
|
| |||||||||||
| Anacarani et al.47 | 1993 | 53 | 41 | 10 | double-blind | 21 | 400f | IV | IPAT-DS | NS g | |
|
| |||||||||||
| Salmaggi et al.48,a | 1993 | 80 | 40 | 40 | double-blind | 30 | 1600 | PO | HAM-D total | < 0.01 | |
|
| |||||||||||
| Cerutti et al.49,a,h | 1993 | 60 | 30 | 30 | double-blind | 30 | 1600 | PO | KSQ | NSi | |
|
| |||||||||||
| Delle Chiale et al.50 | 1997 | 75 | 40 | 35 | double-blind | 21 | 800 | IV | MADRS | < 0.05 | |
|
| |||||||||||
| Papakostas et al.51 | 2010 | 73 | 39 | 34 | double-blind/augmentation | 45 | 1600 | PO | HAM-D % response | < 0.05 | |
|
| |||||||||||
| Mischoulon et al.52 | 2014 | 189 | 64 | 60 | double-blind; escitalopram was third treatment group | 84 | 1600-3200 | PO | HAM-D total | NSc,d | |
SAMe-treated groups only. Significant (p<0.05) improvement by Day 10.
SAMe response rate greater than placebo, results not statistically significant
SAMe response rate equivalent to placebo
Considered failed trials (see text).
Describes a post-hoc analysis of TRH as a predictor of response to SAMe in n=32 from a placebo-controlled trial (n=43). Both the larger trial and this subset produced negative results.
SAMe does was 400mg IV given every other day at the end of a dialysis session.
p value for comparison not present in text, but graphed figure indicates no group difference.
Diagnosis was puerperal psychological distress
SAMe > placebo at 10 day assessment (p < 0.05), no significant differences at study endpoint.
Abbreviations.
HAM-D, Hamilton Rating Scale for Depression
IPAT-DS, Personality and Ability Testing – Depression Scale
KSQ, Kellner Symptom Questionnaire
NS, not statistically significant
ZSDS, Zung Self-Rating Depression Scale
Table 2.
Controlled Trials of SAMe vs. Other Antidepressants
| Trial | Year | Patients enrolled |
Experimental Design |
Duration (days) |
SAMe dose (mg/day) |
Route | Other Antidepressant |
Other dose (mg/day) |
Route | Primary Outcome Measure |
Relative Efficacy (SAMe vs. Other) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SAMe | Other | |||||||||||
| Mantero et al.53 | 1975 | 31 | 16 | 15 | double-blind | 21 | 75 | IM | Imipramine | 75 | IM | HAM-D | SAMe = Other |
|
| |||||||||||||
| Miccoli et al.54 | 1978 | 86 | 45 | 41 | double-blind | 21 | 200 | IV | Chlorimipramine or Amitryptyline | 100 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Barberi et al.36 | 1978 | 20 | 10 | 10 | double-blind | 20 | 200 | IV | Amitriptyline | 100 | IV | HAM-D | P value for comparison not provideda |
|
| |||||||||||||
| Del Vecchio et al.55 | 1978 | 28 | 14 | 14 | double-blind | 21 | 150 | IV | Chlorimipramine | 100 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Scarzella et al.56,b | 1978 | 20 | 10 | 10 | double-blind | 15 | 250 | IV | Chlorimipramine | 100 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Calandra et al.57 | 1979 | 24 | 12 | 12 | no blind | 15 | 150 | IV | Chlorimipramine | 100 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Monaco et al.58 | 1979 | 20 | 11 | 9 | double-blind | 15 | 200 | IV | Amitriptyline | 100 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Scaggion et al.59 | 1982 | 40 | 22 | 18 | double-dummy | 15 | 300 | IV | Nomifensine | 200 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Kufferle et al.60 | 1982 | 20 | 10 | 10 | double-blind | 18 | 150 | IV | Chlorimipramine | 50 | IV | HAM-D | SAMe = Other |
|
| |||||||||||||
| Ubago et al.61 | 1984 | 30 | 15 | 15 | double-blind | 30 | 100 | IV | Chlorimipramine | 50 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Bell et al.62,b | 1988 | 22 | 11 | 11 | double-dummy | 14 | 400 | IV | Imipramine | 150 | PO | HAM-D | SAMe > Otherc |
|
| |||||||||||||
| Janicak et al.43 | 1988 | 20 | 12 | 3 | double-dummy | 14 | 400 | IV | Imipramine | 150 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Cerutti et al.63 | 1989 | 20 | 20 | 20 | cross-over | 21 | 800 | PO | Minaprine | 200 | PO | HAM-D | SAMe > Otherc |
|
| |||||||||||||
| Bell et al.64 | 1990 | 28 | 14 | 14 | double-blind | 28 | 1600 | PO | Desipramine | 250 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| De Vanna et al.65,b | 1992 | 30 | 15 | 15 | double-blind | 42 | 1600 | PO | Imipramine | 140 | PO | MADRS | P value for comparison not provideda |
|
| |||||||||||||
| Bell et al.66 | 1994 | 17 | 11 | 6 | double-blind | 28 | 1600 | PO | Desipramine | 250 | PO | HAM-D | P value for comparison not provideda |
|
| |||||||||||||
| Delle Chiale et al.50 | 1997 | 122 | 57 | 65 | double-blind | 21 | 800 | IV | Chlorimipramine | 100 | IV | HAM-D | Other > SAMed |
|
| |||||||||||||
| Delle Chiale et al.67 | 2000 | 281 | 143 | 138 | double-blind | 42 | 1600 | PO | Imipramine | 150 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Delle Chiale et al.67 | 2000 | 295 | 147 | 148 | double-blind, dummy | 28 | 400 | IM | Imipramine | 150 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Pancheri et al.68 | 2002 | 293 | 146 | 147 | double-blind | 28 | 400 | IM | Imipramine | 150 | PO | HAM-D | SAMe = Other |
|
| |||||||||||||
| Mischoulon et al.52 | 2014 | 189 | 64 | 65 | double-blind, cross-over | 84 | 1600-3200 | PO | Escitalopram | 10-20 | PO | HAM-D | SAMe = Other |
Both groups demonstrated significant (p<0.05) improvements on primary outcome measure. P value for between-group comparison not provided.
SAMe-treated groups only. Significant (p<0.05) improvement by Day 10.
SAMe group demonstrated significant (p<0.05) improvement compared to other antidepressant group on primary outcome measure
Other antidepressant demonstrated significiant (p<0.05) improvement compared to SAMe on primary outcome measure
Abbreviations.
HAM-D, Hamilton Rating Scale for Depression
MADRS, Montgomery-Asberg's Rating Scale for Depression
SAMe Compared with Placebo
SAMe has been compared to placebo for depressive symptoms in 19 RCTs (Table 1). Five out of six controlled studies conducted between 1976 and 1988 reported that intravenous (200-400mg/day) or intramuscular (45-50mg/day) SAMe was more effective than placebo for depression (37,41,43,62,72). Starting in the 1990's, adequate oral doses (800-1600mg) of enteric-coated, stabilized SAMe could be utilized in clinical studies. Overall, twelve of the nineteen RPCTs showed the antidepressant effect of SAMe to be significantly greater than placebo for depressive syndromes (p<0.05, Table 1), though many of these used samples in which diagnostic criteria for MDD were not required or MDD was not a primary diagnosis. In one of two studies that failed to find a significant difference compared to placebo, an older, less stable oral form of SAMe was used, in which the tablets were degraded due to excess exposure to air (46). In the other study, both SAMe and escitalopram failed to outperform placebo (see below) (52).
A seminal 2002 meta-analysis by Hardy et al. (73), commissioned by the Agency for Healthcare Research and Quality (AHRQ), including 28 of 47 depression RCTs through the year 2000, remains the only SAMe meta-analysis published in the past fifteen years. This fairly exhaustive meta-analysis, excluded 2 potentially informative studies comparing SAMe against TCAs (50,67) due to insufficient statistical description, and one (64) because the authors were unable to obtain the paper. Regarding placebo comparisons, Cerutti et al. (49) was excluded because it covered postpartum depression. Fazio et al. (32) and Agnoli et al. (35) were excluded because their data were covered in other papers. Hardy and colleagues examined effect size and risk ratio of response in these studies. Only 3 studies were evaluable in the risk ratio analysis, which all favored SAMe. However, the authors could not draw definitive conclusions because overall power was modest due to small samples, differences between groups were nonsignificant, and studies had methodological limitations. The effect size analysis included 11 studies. The authors found no escalating dose-response effect, perhaps due to the mixture of studies using oral versus intramuscular SAMe. Nonetheless, the authors found that SAMe monotherapy was more effective than placebo in treating depression symptoms with an overall effect size of -0.65 (95% CI -1.05 to -0.25) (73). This corresponds to an improvement in the 17-item Hamilton Depression Rating Scale (HAM-D) of 5-6 points. While this is often considered clinically significant in a single trial, because the studies were based on different editions of the HAM-D with different numbers of items (e.g. 17 vs 21), the authors considered 10 points to represent clinically significant change. Thus, although SAMe demonstrated an advantage over placebo, the clinical significance is to be considered with caution.
SAMe Compared with Other Antidepressants
Several double-blind, randomized controlled trials (RCTs) compared SAMe to other antidepressants: tricyclic antidepressants (TCAs), nomifensine, minaprine and escitalopram (Table 2). Early RCTs showed parenteral SAMe (150-400mg/day) to be as effective or superior to TCAs (clomipramine, amitriptyline, imipramine) with fewer side effects (43,54,56,58,60). Subsequently, two larger studies (n=295, n=293) found intramuscular SAMe (400mg/day) to be as efficacious as oral imipramine (150mg/day) in treating MDD for 4 weeks (67,68). Additionally, two large studies by Di Padova and colleagues comparing SAMe against imipramine (74) suggested equivalency (effect size = 0.13, 95% CI -0.10 to 0.36), though these reports were not published in peer reviewed journals. Four RCTs, including one large study (n=281) (67) and three smaller studies (n≤30) (64,65,66) found oral SAMe (1600mg/day) to be as efficacious as oral desipramine (250mg/day) and oral imipramine (140-150mg/day). Overall, in 18 controlled trials, SAMe was as effective as chlorimipramine (CMI) (54,55,56,60,61), imipramine (43,53,62,67,68) and nomifensine (59).
One recent multi-center RCT (n=189) comparing SAMe, escitalopram and placebo failed to identify significant differences among the three arms at 12 weeks (52), perhaps due to an abnormally high placebo response rate. Re-analysis of the data from subjects enrolled at one of two sites (n=144) found that improvements in depression with SAMe were equivalent to improvements with escitalopram and significantly greater than with placebo (75). A second re-analysis of the same trial identified an inter-site difference in the proportion of women to men, and analyzed the outcomes separately for men and women (76) using the full sample from both sites. SAMe was found to be superior to placebo among males (n=51) but not females (n=62). Whether there is a significant gender-specific difference in antidepressant response requires validation by future studies.
Meta-analyses concluded that SAMe and TCAs were equally efficacious in treating depression (73,77). The Hardy et al. meta-analysis (73) examined studies of SAMe versus TCAs. Eleven studies included in the risk analysis for response, collectively produced risk ratios of approximately 1, which supported equality between SAMe and the comparison antidepressant drugs. The corresponding effect size analysis of 14 studies, also found a nonsignificant difference between SAMe and its comparators, suggesting equivalent efficacy. However, most of these studies were limited by the lack of an inactive placebo comparator arm.
SAMe in Combination with Other Antidepressants
A number of studies support the use of SAMe as an adjunctive treatment for MDD. An RCT of add-on parenteral SAMe (250mg/day) vs. placebo injections in patients receiving either CMI or mianserin showed improved clinical symptoms by day 10 in the group receiving parenteral SAMe compared to placebo (78). In an open-label trial, patients with MDD (n=30) who had not fully responded to a selective serotonin reuptake inhibitor (SSRI) or venlafaxine were treated with oral SAMe (800mg/day) for 2 weeks, followed by oral SAMe (1600mg/day) for an additional 4 weeks (79). At 6 weeks, 50% of patients achieved clinical response and 43% clinical remission. Reduction in depressive symptoms reached statistical significance at week 1 and remained significant through week 6 (p<0.001). In another open-label study, MDD patients (n=33) who failed to respond to at least 8 weeks of treatment with two adequate and stable doses of antidepressants were treated with a fixed dose of adjunctive SAMe (800mg/day) (80). At 8 weeks, clinical response was achieved in 60% of patients and remission in 36% based on HAM-D. Changes from baseline were significant by week 1 and remained significant by week 8 (p<0.001). In a RCT, outpatients with MDD (n=73) who were non-responders or partial responders to SSRI or serotonin-norepinephrine reuptake inhibitor (SNRI) antidepressants were randomized to receive adjunctive SAMe (up to 1600mg/day) or placebo for 6 weeks (51). Both response rates (SAMe: 36.1% vs. placebo: 17.6%) and remission rates (SAMe: 25.8% vs. placebo 11.7%) were significantly higher for patients receiving SAMe (p<0.05). A recent meta-analysis examining adjunctive nutraceuticals for depression demonstrated positive results for SAMe (81).
A multi-center, double-blind RPCT add-on study of 800mg SAMe (MSI-195, a novel SAMe formulation with improved bioavailability) for patients with MDD with inadequate responses to antidepressant treatment was completed in 2015 (82). Results are not yet published, but a press release from the sponsor stated that MSI-195 did not demonstrate efficacy over placebo, though post-hoc analysis identified a responsive sub-group of 143 subjects (74 on MSI-195 and 69 on placebo) after patients with obesity and/or unstable symptom profiles were excluded. In this subanalysis using last observation carried forward (LOCF), the MSI-195 produced a significant reduction in the Montgomery–Asberg Depression Rating Scale (MADRS) of -3.41 (p =0.031) relative to placebo, with an effect size of 0.36. (83).
SAMe in Depression with Co-morbid Medical Conditions
Depression and HIV
Relatively low concentrations of SAMe have been reported in the CSF from patients with depression or Human Immunodeficiency Virus (HIV) infection (14,15). An 8 week, open-label study assessed 20 HIV seropositive individuals with MDD treated with SAMe (800-1600mg/day) supplemented with vitamin B12 (1,000mcg/day) and folic acid (800mcg) (84). Intent-to-treat (ITT) analysis demonstrated significant reduction in Beck Depression Inventory (BDI) scores from baseline (Mean=33.5, SD=11.1) to week 8 (Mean= 6.6, SD= 6.1, p<0.001). Similarly, 17-item HAM-D scores significantly decreased from baseline (Mean=26.5, SD=6.8) to week 8 (Mean=7.7, SD=10.1, p<0.001). Between baseline and week 1, there was evidence for a rapid therapeutic effect on BDI and HAM-D (p< 0.01). At 8 weeks, the remission rate (17-item HAM-D of ≤ 7) was 79% for ITT analysis and 93% for the 15 subjects who completed the study. Two patients reported transient nausea and one reported transient diarrhea. No patients ended participation due to side effects. This encouraging result warrants further SAMe research in this population.
Depression and Parkinson's disease
Estimated rates of depression in patients with PD range from 30% to 50% (85). Significant side effects and potential interactions with selegiline, a monoamine oxidase inhibitor (MAOI) used to treat Parkinson's disease (PD), can limit use of prescription antidepressants (86). SAMe has been proposed to protect dopaminergic neurons from L-dopa induced neurotoxicity (87). In PD, chronic treatment with Levodopa (L-dopa) depletes blood levels of SAMe (88). L-dopa is methylated to 3-O-methyl-dopa by catechol-O-methyltransferase (COMT). Since SAMe is the methyl donor in this reaction, its levels become depleted with L-dopa treatment. Pre-clinical studies show that acute treatment with L-dopa markedly depletes SAMe levels in liver and brain tissue (25). In PD, L-dopa treatment is associated with increased levels of total homocysteine in plasma (89) and CSF (90), as a by-product of increased COMT methylation of L-dopa.
In three small trials in patients with PD and MDD (n=13, n=21, n=32), SAMe (1600 – 4000 mg/day) significantly reduced depression scores on HAM-D (p<0.05) (44, 91,92). A 10 week, open-label study involving patients with co-morbid treatment-resistant MDD and PD (n=13) showed significant improvement in HAM-D depression scores following administration of SAMe monotherapy (800-3600mg/daily) (91). Mean HAM-D dropped from a baseline of 27.09 (SD= 6.04) to 9.55 (SD= 7.29, p<0.002) after 10 weeks. Out of eleven completers, ten had 50% or more improvement on HAM-D. Two patients dropped out due to increased anxiety; other side effects included nausea (n=1) and diarrhea (n=2). In another 12 week double-blind, placebo-controlled RCT in patients with PD and depression (n=32), both SAMe and escitalopram groups had significantly improved depression scores compared to placebo (p<0.05) (92). In two out of three clinical trials, SAMe improved PD motor symptoms (91,92). Larger controlled studies are needed to follow-up these preliminary findings in PD (93).
Depression and Osteoarthritis or Fibromyalgia
SAMe has been reported to exert clinically significant anti-inflammatory and analgesic effects (73,94-98). While the mechanism remains to be elucidated, SAMe does not appear to alter the eicosanoid system in the same manner as NSAIDs, but may enhance proteoglycan synthesis and secretion (33,94). In several RCTs including more than 22,000 patients, SAMe was as effective as nonsteroidal anti-inflammatory drugs (NSAIDs) in relieving pain in osteoarthritis (99-106). An AHRQ meta-analysis of eight studies comparing SAMe to NSAIDs, found equivalent efficacy on the primary outcome measure of pain symptoms (visual analog scale, VAS; comparative effect size = 0.11; 95% CI [-0.56, 0.35]) (73). In three out of four small RCTs with fibromyalgia patients, SAMe (200-800mg) significantly reduced pain symptom primary outcomes including the VAS (p<0.05) (107-110) compared to placebo. Concurrently, SAMe improved symptoms of depression (HAM-D or BDI, p<0.05). These results warrant further investigation into SAMe treatment for patients with MDD and rheumatologic co-morbidities.
Sexual Dysfunction Secondary to Depression or Antidepressant Medication
Sexual dysfunction is commonly associated with MDD as well as with chronic use of most standard antidepressant treatment, leading to interest in agents that specifically treat or reduce the emergence of antidepressant-induced sexual dysfunction (111). One single-site RCT (described in detail above) of SAMe augmentation in SSRI/SNRI non-responders examined whether adjunctive SAMe was associated with greater improvement in sexual functioning than adjunctive placebo (112). Relative to those who got placebo, men treated with adjunctive SAMe demonstrated significantly lower arousal dysfunction (p=0.0012) and degree of erectile dysfunction (p=0.01) at study endpoint, independent of treatment-associated change in depression severity. Whether SAMe may benefit male arousal and erectile dysfunction in MDD, can be further assessed in prospective trials as well as in re-analyses of previously published studies.
Neurocognitive Disorders and Cognitive Function
MDD is commonly associated with cognitive impairment. Preclinical and early clinical trials provide some support for beneficial effects of SAMe alone or in combination with other nutraceuticals, on cognitive function (17, 113-115). A secondary analysis of data from a RCT on adjunctive SAMe for MDD (n=46) demonstrated that oral SAMe (1600mg/day), as compared to placebo, improved two memory-related cognitive functions (recall, p=0.04 and word-finding, p=0.09), but not five other cognitive domains (116). These preliminary findings suggest that SAMe may have beneficial effects on memory-related cognition in MDD. Further studies are needed to evaluate whether this effect is independent of improvement in depressive symptoms.
Linnebank and colleagues found that CSF levels of SAMe in patients with Alzheimer's Disease were significantly reduced compared to controls (28). A one year, open-label study that treated early-stage Alzheimer's Disease patients (n=14) with a nutriceutical formulation (NF) containing SAMe (400mg) in addition to other vitamins (folic acid, 400μg; vitamin B12, 6μg) and nutriceuticals (alpha-tocopherol, 30 IU; N-acetyl cysteine, 600mg; acetyl-L-carnitine, 500mg) demonstrated improvement in cognitive symptoms as assessed by the Dementia Rating Scale (DRS) and clock-drawing tests (117). Family caregivers also reported improvement in multiple domains of the Neuropsychiatric Inventory (NPI). In another pilot study in moderate-to-late stage Alzheimer's disease (n=12), treatment with a similar NF containing SAMe did not show statistical separation between active and placebo groups, but there were some suggestive signals in post-hoc data analyses, including greater delay in cognitive decline as measured by the DRS and clock-drawing tests among those who got active NF (118). Recently, a larger, multi-site, phase II RCT was conducted in AD patients (n=106) randomized to receive either a NF containing SAMe (400mg) or placebo for 3 to 6 months (119). Relative to the placebo group, within 3 months, the NF cohort demonstrated improved cognitive performance on the Clox-1 (p=0.0083, CI [0.4481, 2.9343]) and the age and education-adjusted Dementia Rating Scale (DRS; p=0.0266, CI [0.1722, 2.7171]). Notably, in the NF group, there was significant improvement in the DRS memory domain scores from baseline to 3 month endpoint (p<0.0001, CI [1.2348, 3.2283]). Across all studies evaluating the cognitive effects of NF containing SAMe, the treatment was well tolerated. Interpretation of the results of these trials with regard to effect of SAMe is limited because SAMe comprised one of multiple ingredients in the NF. Future studies in AD may include clinical trial designs that isolate the contribution of SAMe to cognitive improvement.
Psychotic Disorders
Aggression in schizophrenia (SCZ) has been linked to a genetic variant of the catechol-O-methyltransferase (COMT) gene associated with low activity of an enzyme critical for neurotransmission (120). As SAMe increases COMT enzymatic activity (121), researchers investigated its utility in managing aggression in a subset of SCZ patients. In one RCT, 18 patients with chronic SCZ and the low-activity, COMT polymorphism (codon 158 polymorphism) were randomly assigned to either SAMe (800mg/day) or placebo for 8 weeks (122). There was a significant decrease in the primary outcome measure of aggression (Overt Aggression Scale) from baseline to 8 week endpoint in only the SAMe group (p=0.016), resulting in a significant group by time interaction (p=0.032). While there were no significant group differences in side effects, the study was terminated because worsening irritability in two subjects who received SAMe.
The 22q11.2 deletion syndrome (22q11.2DS) is a genetic disorder associated with high rates of psychiatric co-morbidity including psychosis, depression and attention-deficit/hyperactivity disorder (ADHD) (123). Individuals with this syndrome are missing one copy of the COMT gene. The ability of SAMe to increase COMT enzymatic activity has been proposed as a potential therapeutic mechanism for alleviating psychiatric symptoms in this patient population. A 12-week randomized, double-blind, cross-over, placebo-control study assessed feasibility and safety of SAMe (1600mg/day) in 22q11.2DS patients (n=12) (124). There were no significant group differences found for the randomized population in the primary outcome measure (Clinical Global Impression Scale) (125). The subset with 22q11.2DS and comorbid depression (n=5) who received oral SAMe, demonstrated numerically greater improvement in Children's Depression Rating Scale-Revised (CDRS-R) scores compared to those who received placebo. Future studies may include larger samples of subjects with greater symptom severity.
Liver Disease Associated with Substance Use Disorders, Infections, Cholestasis
SAMe may have a role in treating depression in patients who develop hepatitis or cirrhosis due to comorbid alcohol dependence (126) or intravenous drug use (127) as it does not exacerbate hepatic dysfunction. Preclinical and clinical studies suggest that SAMe can improve liver function (e.g. decreased transaminase levels) or liver disease outcomes in hepatitis, alcoholic and viral liver cirrhosis and cholestasis (73,128-133). In the largest study in this group, Mato and colleagues conducted a RCT with n=123 patients with alcoholic liver cirrhosis. The primary outcome, measured as the overall all-cause mortality or liver transplantation at 2 year study endpoint was 30% in the placebo group and 16% in the SAMe group, although the difference was not statistically significant (p=0.077). As part of the post-hoc analysis, when patients in Child C class (least favorable prognostic group) were excluded (n=8), overall mortality or liver transplantation was significantly greater in the placebo group compared to the SAMe group (29% vs. 12%, p=0.025) (131).
Use in Pregnancy, Risks and Medication Interactions
The need for safer treatments for depression in women during pregnancy and post-partum is urgent, particularly in light of evidence that untreated maternal MDD may adversely affect fetal and neonatal development. The use of certain prescription antidepressants has been associated with increased risk of birth defects (134). SAMe has been evaluated in intrahepatic cholestasis of pregnancy in conjunction with the bile acid urosdeoxycholic acid (UDCA) (135,136). UDCA is a natural bile acid commonly used to reverse impaired bile formation (137). A systematic review and meta-analysis of ten RCTs (n= 727 pregnant women with intrahepatic cholestasis of pregnancy) found that a combination of ursodeoxycholic acid (UDCA) and SAMe significantly (p<0.05) reduced rates of Caesarian sections, preterm birth, and fetal asphyxia (138). The mean endogenous CSF concentration of SAMe in normal infants and youth may be greater than in adults, though rigorous studies of age-related changes in levels of SAMe are needed (139). Trials of SAMe during pregnancy and breast-feeding with long-term monitoring of child development would be worthwhile.
The most common side effect of SAMe is nausea and, less frequently, diarrhea, abdominal discomfort, or vomiting. Occasionally agitation, anxiety, or insomnia can occur in patients sensitive to activating effects of SAMe. As with other antidepressants, SAMe can trigger hypomanic or manic symptoms in patients with bipolar disorder. Overall, SAMe has a favorable side effect profile in that it does not cause sexual dysfunction or weight gain. Another advantage is that SAMe does not cause cognitive or memory dysfunction, particularly important in patients with dementia, age-related cognitive or memory decline, and traumatic brain injury. SAMe is well tolerated in geriatric patients as indicated in open trials showing improved recall and word finding scores (115).
SAMe has few known adverse interactions with other drugs. One case of serotonin syndrome in a 71 year old woman treated with escalating doses of clomipramine (CMI) while on SAMe was reported (140). Her symptoms developed 48-72 hours after the dose of CMI was increased three-fold (25mg/day to 75mg/day), while the dose of SAMe was kept constant. It is likely that her symptoms developed from the rapid dose escalation of CMI. No other cases of serotonin syndrome attributable to SAMe have been reported, including in trials where SAMe was used to augment SSRIs and TCAs. Furthermore, in an open trial that included 60 depressed patients taking MAOIs, augmentation with SAMe was beneficial and caused no adverse effects (141). In patients with medication-induced elevated LFTs, SAMe reduced or normalize liver functions (141). The theoretical possibility that SAMe could induce hyper-homocysteinemia has never been substantiated, nor has any confirmed case been reported. A small study of adults given a high dose of oral SAMe (1600mg for 5 days) showed no change in serum homocysteine levels (142).
Discussion
This review of the role of S-adenosylmethionine in the treatment of Major Depressive Disorder found encouraging evidence of efficacy and safety of SAMe as a monotherapy and as an augmentation for other antidepressants. Since the US FDA Agency for Healthcare Research and Quality (AHRQ) review (73), additional studies have generally supported SAMe as efficacious for treatment of MDD and comparable to several prescription antidepressants, though comparisons against newer generation antidepressants are needed. In addition to depression, this review found supportive early evidence for SAMe in certain neurocognitive, substance abuse and psychotic disorders. Studies of SAMe in primary anxiety disorders were not identified. Additional clinical studies are needed to further delineate the role of SAMe in neuropsychiatric conditions.
Clinical reviews often exclude studies in patients with co-morbid medical illnesses or concurrent prescription medications. Consequently, because of these exclusions, clinical opportunities for using SAMe are often overlooked. Depressed patients present with a broad array of co-morbid conditions, concurrent medications, and medication-related side effects. SAMe may ameliorate symptoms associated with medical conditions such as hepatic disease, osteoarthritis, fibromyalgia, and cognitive and memory decline. As is the case with many over the counter natural products, the evidence so far suggests that, compared to prescription medications, SAMe may cause fewer and less severe side effects and considerably fewer drug interactions. Moreover, SAMe may prevent or reverse side effects caused by other medications, such as liver or sexual dysfunction. Knowing that evidence generally supports the safety and efficacy of SAMe in both psychiatric and medical illnesses could impact clinical decision making.
Certain limitations in our review should be noted. First, the methodology of studies cited in this review varies due to the inclusion of diverse neuropsychiatric conditions, smaller studies, open trials and larger RCTs. This heterogeneity limits interpretation on the clinical significance of SAMe in neuropsychiatric disorders. Second, the relatively modest body of research during the past 15 years precluded our undertaking a new meta-analysis. The consensus of this Work Group was that the relatively limited new material, would not significantly change the overall conclusions of the Hardy meta-analysis, which, while generally positive, were cautious and acknowledged the limitations of the body of work, such as small samples, different doses and delivery systems, and concerns about publication biases (73).
Careful consideration of pursuing treatment with SAMe as opposed to a registered antidepressant is required on the part of the clinician and the patient. Clinicians who recommend SAMe need to inform their patients that this compound has not been tested as rigorously as its FDA-approved counterparts, and as such, its relative efficacy cannot be guaranteed. However, the risks of SAMe compare quite favorably with prescription antidepressants, particularly in that it does not cause sexual dysfunction or weight gain (two of the most common causes for antidepressant discontinuation) and it is less likely to be life-threatening in patients who are at risk for overdosing during suicide attempts. No cases of death by SAMe overdose have been reported. In a mouse study, lethal oral dose of SAMe was equivalent to over 400,000 mg in a 70 kg man (National Library of Medicine, 1999 RTECS (Registry of Toxic Effects of Chemical Substances), Bethesda, MD, Record Nos. 7176, 7177). Although the cost of SAMe is not covered by insurance companies, compared with the high co-payments on many prescriptions, it may be a reasonable expense (2). Patients should be discouraged from self-medicating their depression, and should be encouraged to seek professional evaluation before starting any treatment.
Conclusion
This review provides a broad perspective on the role of SAMe in the treatment of depression, neuropsychiatric disorders, and co-morbid medical conditions. Notably, encouraging evidence is found for the safety and efficacy of SAMe as a monotherapy and as an adjunctive agent for Major Depressive Disorder, though with several caveats in view of the heterogeneity of the studies and methodological concerns as discussed. Preliminary evidence suggests SAMe may hold promise in a number of neuropsychiatric conditions and co-morbid medical illnesses. Exploration of the full range of potential benefits of SAMe through controlled clinical studies is much needed and is advised.
Supplementary Material
Clinical Points.
- Clinical opportunities for using S-adenosylmethionine (SAMe) may include multiple neuropsychiatric disorders and co-morbid medical conditions.
- S-adenosylmethionine (SAMe) is a viable treatment in MDD, and early evidence suggests that it holds promise for a number of neuropsychiatric conditions.
- Additional research is needed to strengthen the body of evidence.
Acknowledgments
We appreciate the helpful review and input from American Psychiatric Association Council on Research.
Funding/Support: None
Footnotes
Disclosures: Anup Sharma: Dr. Sharma reports no competing interests. He is member of the American Psychiatric Association (APA) Council on Research and Quality.
Patricia Gerbarg: Dr. Gerbarg receives royalties from two books that include information about SAMe. She serves as a consultant for NCCAM Award #8T007483: The Treatment of Depression with Yoga and Walking.
Teodoro Bottiglieri: Dr. Bottiglieri reports having been the chairman of the advisory board for Methylation Sciences Inc., holding stock options in Methylation Sciences Inc., Scientific Advisor to Gnosis S.p.A. Nestle Health Sciences and Pamlab Inc. and having received research funding from Nestle Health Sciences, Pamlab Inc., distributor of B vitamins as a medical food.
Lila Massoumi: Dr. Massoumi reports no competing interests.
Linda L. Carpenter: Dr. Carpenter reports consulting income from Magstim Ltd. and research support from the National Institutes of Health and through clinical trial contracts between Butler Hospital and Neuronetics, Inc., NeoSync, and Cervel. She is member of the American Psychiatric Association (APA) Council on Research and Quality.
Helen Lavretsky: Dr. Lavretsky reports no competing interests. She reports grant support from the NIMH, NCCIH, Forest Research Institute, Alzheimer's Research and Prevention foundation (APRF)
Philip R. Muskin: Dr. Muskin reports no competing interests.
Richard P. Brown: Dr. Brown serves as a consultant to Humanetics and holds a patent on the use of 7-keto DHEA for PTSD. He receives royalties from two books that include information about SAMe and receives honoraria for lectures on CAIM that may include information on SAMe.
David Mischoulon: Dr Mischoulon has received research support from the FisherWallace, Nordic Naturals, Methylation Sciences, Inc. (MSI), and PharmoRx Therapeutics. He has received honoraria from the Massachusetts General Hospital Psychiatry Academy. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
As Work Group of the American Psychiatric Association Council on Research
References
- 1.Muskin PR, Gerbarg PL, Brown RP. Along roads less traveled: Complementary, alternative, and integrative treatments. Psychiatr Clin North Am. 2013;36:xiii–xv. doi: 10.1016/j.psc.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Brown RP, Gerbarg PL, Muskin PR. How to Use of Herbs, Nutrients and Yoga in Mental Health Care. New York: W. W. Norton; 2009. [Google Scholar]
- 3.Kessler RC, Soukup J, Davis RB, Foster DF, Wilkey SA, Van Rompay MI, Eisenberg DM. The use of complementary and alternative therapies to treat anxiety and depression in the united states. Am J Psychiatry. 2001;158:289–94. doi: 10.1176/appi.ajp.158.2.289. [DOI] [PubMed] [Google Scholar]
- 4.Elkins G, Rajab MH, Marcus J. Complementary and alternative medicine use by psychiatric inpatients. Psychol Rep. 2005;96:163–6. doi: 10.2466/pr0.96.1.163-166. [DOI] [PubMed] [Google Scholar]
- 5.Cantoni GL. The nature of the active methyl donor formed enzymatically from l-methionine and adenosinetriphosphate1, 2. Journal of the American Chemical Society. 1952;74:2942–3. [Google Scholar]
- 6.Cantoni GL. The role of s-adenosylhomocysteine in the biological utilization of s-adenosylmethionine. Prog Clin Biol Res. 1985;198:47–65. [PubMed] [Google Scholar]
- 7.Curcio M, Catto E, Stramentinoli G, Algeri S. Effect of s-adenosyl-l-methionine on serotonin metabolism in rat brain. Prog Neuropsychopharmacol. 1978;2:65–71. doi: 10.1016/0364-7722(78)90023-1. [DOI] [PubMed] [Google Scholar]
- 8.Bottiglieri T, Laundy M, Martin R, Carney MWP, Nissenbaum H, Toone BK, et al. S-adenosylmethionine influences monoamine metabolism. The Lancet. 1984;324:224. doi: 10.1016/s0140-6736(84)90507-5. [DOI] [PubMed] [Google Scholar]
- 9.Otero-Losada ME, Rubio MC. Acute changes in 5-HT metabolism after s-adenosyl-l-methionine administration. Gen Pharmacol. 1989;20:403–6. doi: 10.1016/0306-3623(89)90186-9. [DOI] [PubMed] [Google Scholar]
- 10.Strauss KA, Ferreira C, Bottiglieri T, Zhao X, Arning E, Zhang S, Soltys K. Liver transplantation for treatment of severe S-adenosylhomocysteine hydrolase deficiency. Molecular genetics and metabolism. 2015;116:44–52. doi: 10.1016/j.ymgme.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW. Reynolds EH Homocysteine, folate, methylation, and monoamine metabolism in depression. Journal of Neurology, Neurosurgery & Psychiatry. 2000;69:228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surtees R, Leonard J, Austin S. Association of demyelination with deficiency of cerebrospinal-fluid s-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991;338:1550–4. doi: 10.1016/0140-6736(91)92373-a. [DOI] [PubMed] [Google Scholar]
- 13.Hyland K, Smith I, Bottiglieri T, Perry J, Wendel U, Clayton PT, Leonard JV. Demyelination and decreased s-adenosylmethionine in 5,10-methylenetetrahydrofolate reductase deficiency. Neurology. 1988;38:459–62. doi: 10.1212/wnl.38.3.459. [DOI] [PubMed] [Google Scholar]
- 14.Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH. Cerebrospinal fluid s-adenosylmethionine in depression and dementia: Effects of treatment with parenteral and oral s-adenosylmethionine. J Neurol Neurosurg Psychiatry. 1990;53:1096–8. doi: 10.1136/jnnp.53.12.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castagna A, Le Grazie C, Accordini A, Giulidori P, Cavalli G, Bottiglieri T, Lazzarin A. Cerebrospinal fluid s-adenosylmethionine (same) and glutathione concentrations in HIV infection: Effect of parenteral treatment with same. Neurology. 1995;45:1678–83. doi: 10.1212/wnl.45.9.1678. [DOI] [PubMed] [Google Scholar]
- 16.Bottiglieri T. S-Adenosyl-L-methionine (same): From the bench to the bedside--molecular basis of a pleiotrophic molecule. Am J Clin Nutr. 2002;76:1151S–7S. doi: 10.1093/ajcn/76/5.1151S. [DOI] [PubMed] [Google Scholar]
- 17.Bottiglieri T. Folate, vitamin B12, and s-adenosylmethionine. Psychiatric Clinics of North America. 2013;36:1–13. doi: 10.1016/j.psc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Scarpa S, Cavallaro RA, D'Anselmi F, Fuso A. Gene silencing through methylation: An epigenetic intervention on alzheimer disease. J Alzheimers Dis. 2006;9:407–14. doi: 10.3233/jad-2006-9406. [DOI] [PubMed] [Google Scholar]
- 19.Mischoulon D, Fava M. Role of s-adenosyl-l-methionine in the treatment of depression: A review of the evidence. Am J Clin Nutr. 2002;76:1158S–61S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- 20.Benelli A, Filaferro M, Bertolini A, Genedani S. Influence of s-adenosyl-l-methionine on chronic mild stress-induced anhedonia in castrated rats. Br J Pharmacol. 1999;127:645–54. doi: 10.1038/sj.bjp.0702589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genedani S, Saltini S, Benelli A, Filaferro M, Bertolini A. Influence of same on the modifications of brain polyamine levels in an animal model of depression. Neuroreport. 2001;12:3939–42. doi: 10.1097/00001756-200112210-00017. [DOI] [PubMed] [Google Scholar]
- 22.Czyrak A, Rogoz Z, Skuza G, et al. Antidepressant activity of S-adenosyl-L-methionine in mice and rats. J Basic Clin Physiol Pharmacol. 1992;3:1–17. doi: 10.1515/jbcpp.1992.3.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Otero-Losada ME, Rubio MC. Acute effects of S-adenosyl-L-methionine on catecholaminergic central function. Eur J Pharmacol. 1989;163:353–56. doi: 10.1016/0014-2999(89)90205-7. [DOI] [PubMed] [Google Scholar]
- 24.Bottiglieri T, Hyland K. Effect of S-adenosylmethionine on dopamine metabolism in the rat striatum: an in-vivo microdialysis study. Soc Neurosci Abstracts. 1996;2:834. [Google Scholar]
- 25.Cohen B, Stramentinoli G, Sosa AL, et al. Effects of the novel antidepressant S-adensosylmethionine on alpha 1 and beta-adrenoreceptors in rat brain. Eur J Pharmacol. 1989;170:201–207. doi: 10.1016/0014-2999(89)90540-2. [DOI] [PubMed] [Google Scholar]
- 26.Cimino M, Vantini G, Aalgeri S. Age-related modification of dopaminergic and beta-Adrenergic receptor system: restoration to normal activity by modifying membrane fluidity with S-adenosylmethionine. Life Sci. 1984;34:2029–2039. doi: 10.1016/0024-3205(84)90367-9. [DOI] [PubMed] [Google Scholar]
- 27.Consogno E, Tiraboschi E, Iuliano E, Gennarelli M, Racagni G, Popoli M. Long-term treatment with S-adenosylmethionine induces changes in presynaptic CaM kinase II and synapsin I. Biological psychiatry. 2001;50:337–344. doi: 10.1016/s0006-3223(01)01176-3. [DOI] [PubMed] [Google Scholar]
- 28.Linnebank M, Popp J, Smulders Y, Smith D, Semmler A, Farkas M, Kulic L, Cvetanovska G, Blom H, Stoffel-Wagner B, Kölsch H, Weller M, Jessen F. S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer's disease. Neurodegener Dis. 2010;7:373–8. doi: 10.1159/000309657. [DOI] [PubMed] [Google Scholar]
- 29.Sontag JM, Nunbhakdi-Craig V, Montgomery L, Arning E, Bottiglieri T, Sontag E. Folate deficiency induces in vitro and mouse brain region-specific downregulation of leucine carboxyl methyltransferase-1 and protein phosphatase 2A B(alpha) subunit expression that correlate with enhanced tau phosphorylation. J Neurosci. 2008;28:11477–87. doi: 10.1523/JNEUROSCI.2816-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bottiglieri T, Arning E, Wasek B, Nunbhakdi-Craig V, Sontag JM, Sontag E. Acute administration of L-DOPA induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J Neurosci. 2012;32:9173–81. doi: 10.1523/JNEUROSCI.0125-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuso A, Nicolia V, Ricceri L, Cavallaro RA, Isopi E, Mangia F, Fiorenza MT, Scarpa S. S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol Aging. 2012;33:1482.e1–1. doi: 10.1016/j.neurobiolaging.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Fazio C, Andreoli V, Agnoli A, et al. Therapy of schizophrenia and depressive disorders with S-Adenosyl-L-Methionine. IRCS. 1974;2:1015. [Google Scholar]
- 33.Stramentinoli G. Pharmacologic aspects of s-adenosylmethionine: pharmacokinetics and pharmacodynamics. Am J Med. 1987;83:35–42. doi: 10.1016/0002-9343(87)90849-7. [DOI] [PubMed] [Google Scholar]
- 34.Fazio C, Andreoli V, Agnoli A, et al. Therapeutic effects and mechanism of action of S-adenosyl-L-methionine (SAM) in depressive syndromes [in Italian] Minerva Med. 1973;64:1515–1529. [PubMed] [Google Scholar]
- 35.Agnoli A, Andreoli V, Casacchia M, et al. Effect of s-adenosyl-l-methionine (SAMe) upon depressive symptoms. Psychiatr Res. 1976;13:43–54. doi: 10.1016/0022-3956(76)90008-x. [DOI] [PubMed] [Google Scholar]
- 36.Barberi A, Puscateri C. Sugli effetti clinici dell s-adenosil-I-metionina (SAMe) nelle sindromi depressive. Minerva Psichiatr. 1978;19:235–243. [Google Scholar]
- 37.Muscettola G, Galzenati M, Balbi A. SAMe versus placebo: A double blind comparison in major depressive disorders. Adv Biochem Psychopharmacol. 1982;32:151–6. [PubMed] [Google Scholar]
- 38.Caruso I, Fumagalli M, Boccassini L, Puttini P, Giniselli G, Cavallari G. Antidepressant activity of S-adenosylmethionine. The Lancet. 1984;323:904. doi: 10.1016/s0140-6736(84)91360-6. [DOI] [PubMed] [Google Scholar]
- 39.Carney MW, Edeh J, Bottiglieri T, et al. Affective illness and S-adenosyl methionine: a preliminary report. Clin Neuropharmacol. 1986;9:379–385. doi: 10.1097/00002826-198608000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Caruso I, Fumagali M, Boccazzini L, et al. Treatment of depression in rheumatoid arthritis patients: a comparison of S-adenosylmethionine (SAMe) and placebo in a double-blind study. Clin Trials. 1987;24:305–310. [Google Scholar]
- 41.De Leo D. S-adenosylmethionine as an antidepressant. Curr Ther Res. 1987;41:865–70. [Google Scholar]
- 42.Thomas CS, Bottiglieri T, Edeh J. The influence of S-adenosylmethionine (SAM) on prolactin in depressed patients. Int Clin Psychopharmacol. 1987;2:97–102. doi: 10.1097/00004850-198704000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Janicak PG, Lipinski J, Davis JM, Comaty JE, Waternaux C, Cohen B, et al. S-adenosylmethionine in depression. A literature review and preliminary report. Ala J Med Sci. 1988;25:306–13. [PubMed] [Google Scholar]
- 44.Carrieri PB, Indaco A, Gentile S, Troisi E. S-adenosylmethionine treatment of depression in patients with parkinson's disease: A double-blind, crossover study versus placebo. Current Therapeutic Research. 1990 [Google Scholar]
- 45.Kagan BL, Sultzer DL, Rosenlicht N, Gerner RH. Oral s-adenosylmethionine in depression: A randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 1990;147:591–5. doi: 10.1176/ajp.147.5.591. [DOI] [PubMed] [Google Scholar]
- 46.Fava M, Rosenbaum JF, Birnbaum R, Kelly K, Otto MW, MacLaughlin R. The thyrotropin response to thyrotropin-releasing hormone as a predictor of response to treatment in depressed outpatients. Acta Psychiatr Scand. 1992;86:42–5. doi: 10.1111/j.1600-0447.1992.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 47.Ancarani E, Biondi B, Bolletta A. Major depression complicating hemodialysis in patients with chronic renal failure: a multicenter, double-blind, controlled trial of S-adenosyl-L-methionine versus placebo. Curr Ther Res. 1993;54:680–686. [Google Scholar]
- 48.Salmaggi P, Bressa GM, Nicchia G, Coniglio M, La Greca P, Le Grazie C. Double-blind, placebo-controlled study of s-adenosyl-l-methionine in depressed postmenopausal women. Psychother Psychosom. 1993;59:34–40. doi: 10.1159/000288642. [DOI] [PubMed] [Google Scholar]
- 49.Cerutti R, Sichel MP, Perin M, Grussu P, Zulian O. Psychological distress during puerperium: A novel therapeutic approach using s-adenosylmethionine. Current Therapeutic Research. 1993;53:707–16. [Google Scholar]
- 50.Delle Chiale R, Boissard G. Paper presented at the World Biological Psychiatry Congress [abstract 90-56] Bioi Psych. 1997;42:245. [Google Scholar]
- 51.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–8. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 52.Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Baer L, et al. A double-blind, randomized, placebo-controlled clinical trial of s-adenosyl-l-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Psychiatry. 2014;75:370–6. doi: 10.4088/JCP.13m08591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mantero M, Pastorino P, Carolei A, Agnoli A. Controlled double-blind study (SAMe-imipramine) in depressive syndromes [in Italian] Minerva Med. 1975;66:4098–4101. [PubMed] [Google Scholar]
- 54.Miccoli L, Porro V, Bertolino A. Comparison between the antidepressant activity and of s-adenosylmethionine (SAMe) and that of some tricyclic drugs. Acta Neurol (Napoli) 1978;33:243–55. [PubMed] [Google Scholar]
- 55.Del Vecchio M, Iorio G, Cocorullo M, et al. Has SAMe (Ado-Met) an anti- depressant effect: a preliminary trial versus chlorimipramine. Riv Sper Freniatr. 1978;102:344–358. [Google Scholar]
- 56.Scarzella R, Appiotti A. Confronto clinico in doppio cieco della same versus clorimipramina nelle sindromi depressive. Rivista Sperimentale Freniatria. 1978;102:359–65. [Google Scholar]
- 57.Calandra C, Roxas M, Rapisarda V. Antidepressant action of SAM in comparison to chlorimipramine: hypotheses to interpret the mechanism of action [in Italian] Minerva Psichiatr. 1979;20:147–152. [PubMed] [Google Scholar]
- 58.Monaco P, Quattrocchi F. Study of the antidepressive effects of a biological transmethylating agent (s-adenosyl-methione or SAM) Riv Neurol. 1979;49:417–39. [PubMed] [Google Scholar]
- 59.Scaggion G, Baldan L, Domanin S, et al. Azione antidepressiva della SAMe a confronto con il nomifensine maleato. Minerva Psichiatr. 1982;23:93–97. [PubMed] [Google Scholar]
- 60.Küfferle B, Grünberger J. Early clinical double-blind study with s-adenosyl-l-methionine: A new potential antidepressant. Adv Biochem Psychopharmacol. 1982;32:175–80. [PubMed] [Google Scholar]
- 61.Ubago JG, Gonzales Infante JM, Blanco Picabea A. Valoracion clinicade la accion antidepresiva de la sulfoadenosil-L-mentionina comparada con la de la clorimiprimina. Actas Luso Esp Neurol Psiquiatr. 1984;2:73–80. [PubMed] [Google Scholar]
- 62.Bell KM, Plon L, Bunney WE, Potkin SG. S-adenosylmethionine treatment of depression: A controlled clinical trial. Am J Psychiatry. 1988;145:1110–4. doi: 10.1176/ajp.145.9.1110. [DOI] [PubMed] [Google Scholar]
- 63.Cerutti PG, Savoini G, D'Avola G, et al. S-adenosil-metbionina. Valuazione dell'efficacia della s-adenosil-metionina nel trattamento delle sindromi depressive: studio clinico controllato versus minaprina. Basi Razionali Ter. 1989;19:591–595. [Google Scholar]
- 64.Bell MB, Carreon D, Pion L, et al. Oral s-adenosylmethionine in the treatment of depression: a double-blind comparison with desipramine. Study Report BioResearch file. 1990 In. [Google Scholar]; Bressa GM. S-adenosyl-L-mehionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand Suppl. 1994;154:7–14. doi: 10.1111/j.1600-0404.1994.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 65.De Vanna M, Rigamonti R. Oral S-adenosyl-L-methionine in depression. Current Therapeutic Research. 1992;52:478–485. [Google Scholar]
- 66.Bell KM, Potkin SG, Carreon D, Pion L. S-adenosylmethionine blood levels in major depression: changes with drug treatment. Acta Neurol Scand Suppl. 1994;154:15–18. doi: 10.1111/j.1600-0404.1994.tb05404.x. [DOI] [PubMed] [Google Scholar]
- 67.Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular s-adenosyl-l-methionine 1,4-butanedisulfonate (same) in the treatment of major depression: Comparison with imipramine in 2 multicenter studies. Am J Clin Nutr. 2002;76:1172S–6S. doi: 10.1093/ajcn/76/5.1172S. [DOI] [PubMed] [Google Scholar]
- 68.Pancheri P, Scapicchio P, Chiaie RD. A double-blind, randomized parallel-group, efficacy and safety study of intramuscular s-adenosyl-l-methionine 1,4-butanedisulphonate (same) versus imipramine in patients with major depressive disorder. Int J Neuropsychopharmacol. 2002;5:287–94. doi: 10.1017/S1461145702003085. [DOI] [PubMed] [Google Scholar]
- 69.Carney MWP, Martin R, Bottiglieri T, et al. Switch mechanism in affective illness and S-adenosylmethionine [letter] Lancet. 1983;1:820–821. doi: 10.1016/s0140-6736(83)91876-7. [DOI] [PubMed] [Google Scholar]
- 70.Lipinski JF, Cohen BM, Frankenburg F, et al. Open trial of S-adenosylmethionine for treatment of depression. Am J Psychiatry. 1984;141:448–450. doi: 10.1176/ajp.141.3.448. [DOI] [PubMed] [Google Scholar]
- 71.Carney MWP, Chari TKN, Bottiglieri T, Reynolds EH, Toone BK. Switch mechanism in affective illness and oral S-adenosylmethionine (SAM) [letter] Br J Psychiatry. 1987;150:724–725. doi: 10.1192/bjp.150.5.724. [DOI] [PubMed] [Google Scholar]
- 72.Andreoli V, Campedelli A, Maffei F. La s-adenosil-l-metionina (same) in geropsichiatria: Uno studio clinico controllato “in aperto” nelle sindromi depressive. Minerva Psichiatr. 1978;25:172–80. [Google Scholar]
- 73.Hardy ML, Coulter ID, Favreau JT, Morton SC, Venuturupalli SR, Chiapelli F, et al. Evidence Reports/Technology Assessments. 64. Rockville (MD): Agency for Healthcare Research and Quality (US); 2002. Oct, S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease. Report No.: 02-E034. [Google Scholar]
- 74.Di Padova C, Giudici A, Boissard G. Ademetionine and depression. In: Mato JM, Caballero A, editors. V Workshop on Methionine Metabolism: Molecular mechanisms and clinical implications. Vol. 2000. Granada, Spain: Feb 20–24, 2000. pp. 295–9. [Google Scholar]
- 75.Sarris J, Papakostas GI, Vitolo O, Fava M, Mischoulon D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: Efficacy and effects of histamine and carnitine as moderators of response. J Affect Disord. 2014;164:76–81. doi: 10.1016/j.jad.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 76.Sarris J, Price LH, Carpenter LL, Tyrka AR, Ng CH, Papakostas GI, et al. Is S-adenosyl methionine (SAMe) for depression only effective in males? A re-analysis of data from a randomized clinical trial. Pharmacopsychiatry. 2015 doi: 10.1055/s-0035-1549928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bressa GM. S-adenosyl-l-methionine (SAMe) as antidepressant: Meta-analysis of clinical studies. Acta Neurol Scand Suppl. 1994;154:7–14. doi: 10.1111/j.1600-0404.1994.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 78.Alvarez E, Udina C, Guillamat R. Shortening of latency period in depressed patients treated with SAMe and other antidepressant drugs. Cell Biol Rev S. 1987;1:103–10. [Google Scholar]
- 79.Alpert JE, Papakostas G, Mischoulon D, Worthington JJ, Petersen T, Mahal Y, et al. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: An open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661–4. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
- 80.De Berardis D, Marini S, Serroni N, Rapini G, Iasevoli F, Valchera A, et al. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: An open label, fixed dose, single-blind study. ScientificWorldJournal. 2013;204649 doi: 10.1155/2013/204649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. American Journal of Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15091228. [DOI] [PubMed] [Google Scholar]
- 82.Add-On Study of MSI-195 (S-Adenosyl-L-Methionine, SAMe) for Patients With Major Depressive Disorder (MDD) 2013 Jul 26; www.clinicaltrials.gov. Web. 12 Oct. 2015.
- 83.Press Release: MSI Methylation Sciences Inc. [Accessed 23, 2016];(MSI) Announces Results From the Horizon Phase 2 Trial for its Novel Treatment, MSI-195, for Major Depressive Disorder (MDD) http://methylationsciences.com/index.php/media/
- 84.Shippy RA, Mendez D, Jones K, Cergnul I, Karpiak SE. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38. doi: 10.1186/1471-244X-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Movement Disorders. 2008;23:183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- 86.Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson's disease. The Journal of neuropsychiatry and clinical neurosciences. 2001;13:187–196. doi: 10.1176/jnp.13.2.187. [DOI] [PubMed] [Google Scholar]
- 87.Werner P, Di Rocco A, Prikhojan A, Rempel N, Bottiglieri T, Bressman S, Yahr MD. COMT-dependent protection of dopaminergic neurons by methionine, dimethionine and S-adenosylmethionine (SAM) against L-dopa toxicity in vitro. Brain research. 2001;893:278–281. doi: 10.1016/s0006-8993(00)03298-4. [DOI] [PubMed] [Google Scholar]
- 88.Cheng H, Gomes-Trolin C, Aquilonius SM, Steinberg A, Löfberg C, Ekblom J, Oreland L. Levels of l-methionine s-adenosyltranferase activity in erythrocytes and concentrations of s-adenosylmethionine and s-adenosylhomocysteine in whole blood of patients with parkinson's disease. Experimental Neurology. 1997;145:580–5. doi: 10.1006/exnr.1997.6466. [DOI] [PubMed] [Google Scholar]
- 89.Belcastro V, Pierguidi L, Castrioto A, Menichetti C, Gorgone G, Ientile R, Tambasco N. Hyperhomocysteinemia recurrence in levodopa- treated Parkinson's disease patients. European Journal of Neurology. 2010;17:661–665. doi: 10.1111/j.1468-1331.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 90.Isobe C, Abe T, Terayama Y. L-Dopa therapy increases homocysteine concentration in cerebrospinal fluid from patients with Parkinson's disease. Journal of Clinical Neuroscience. 2010;17:717–721. doi: 10.1016/j.jocn.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 91.Di Rocco A, Rogers JD, Brown R, Werner P, Bottiglieri T. S-Adenosyl-Methionine improves depression in patients with parkinson's disease in an open-label clinical trial. Mov Disord. 2000;15:1225–9. doi: 10.1002/1531-8257(200011)15:6<1225::aid-mds1025>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 92.Varanese S, Hirsh S, Howard J, et al. 7th International Congress on Mental Dysfunction & Other Non-Motor Features in Parkinson's Disease. Barcelona, Spain: Dec 9-12, 2010. Safety and preliminary efficacy evaluation of SAM-e and escitalopram in the treatment of depression associated with PD. [Google Scholar]
- 93.Varanese S, Birnbaum Z, Rossi R, Di Rocco A. Treatment of advanced Parkinson's disease. Parkinsons Dis. 2011 doi: 10.4061/2010/480260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.di Padova C. S-adenosylmethionine in the treatment of osteoarthritis. Review of the clinical studies. Am J Med. 1987;83:60–5. doi: 10.1016/0002-9343(87)90853-9. [DOI] [PubMed] [Google Scholar]
- 95.Zhang M, Borovikova LV, Wang H, Metz C, Tracey KJ. Spermine inhibition of monocyte activation and inflammation. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 96.Di Benedetto P, Iona LG, Zidarich V. Clinical evaluation of s-adenosyl-l-methionine versus transcutaneous electrical nerve stimulation in primary fibromyalgia. Current Therapeutic Research. 1993;53:222–9. [Google Scholar]
- 97.Ianniello A, Ostuni PA, Sfriso P, Menenghetti L, Zennaro A, Todesco S. S-Adenosyl-L-Methionine in sjögren's syndrome and fibromyalgia. Current Therapeutic Research. 1994;55:699–706. [Google Scholar]
- 98.Grassetto M, Varotto A. Primary fibromyalgia is responsive to s-adenosyl-l-methionine. Current Therapeutic Research. 1994;55:797–806. [Google Scholar]
- 99.Berger R, Nowak H. A new medical approach to the treatment of osteoarthritis: Report of an open phase IV study with ademetionine (gumbaral) Am J Med. 1987;83:84–8. doi: 10.1016/0002-9343(87)90858-8. [DOI] [PubMed] [Google Scholar]
- 100.Schumacher HR. Osteoarthritis: The clinical picture, pathogenesis, and management with studies on a new therapeutic agent, s-adenosylmethionine. Am J Med. 1987;83:1–4. [PubMed] [Google Scholar]
- 101.Bradley JD, Flusser D, Katz BP, Schumacher HR, Jr, Brandt KD, Chambers MA, Zonay LJ. A randomized, double blind, placebo controlled trial of intravenous loading with s-adenosylmethionine (SAM) followed by oral SAM therapy in patients with knee osteoarthritis. The Journal of Rheumatology. 1994;21:905–11. [PubMed] [Google Scholar]
- 102.Vetter G. Double-blind comparative clinical trial with S-adenosylmethionine and indomethacin in the treatment of osteoarthritis. The American journal of medicine. 1987;83:78–80. doi: 10.1016/0002-9343(87)90856-4. [DOI] [PubMed] [Google Scholar]
- 103.Caruso I, Pietrogrande V. Italian double-blind multicenter study comparing S-adenosylmethionine, naproxen, and placebo in the treatment of degenerative joint disease. The American journal of medicine. 1987;83:66–71. doi: 10.1016/0002-9343(87)90854-0. [DOI] [PubMed] [Google Scholar]
- 104.Maccagno A, Di Giorgio EE, Caston OL, Sagasta CL. Double-blind controlled clinical trial of oral S-adenosylmethionine versus piroxicam in knee osteoarthritis. The American journal of medicine. 1987;83:72–77. doi: 10.1016/0002-9343(87)90855-2. [DOI] [PubMed] [Google Scholar]
- 105.Glorioso S, Todesco S, Mazzi A, Marcolongo R, Giordano M, Colombo B, Passeri M. Double-blind multicentre study of the activity of S-adenosylmethionine in hip and knee osteoarthritis. International journal of clinical pharmacology research. 1984;5:39–49. [PubMed] [Google Scholar]
- 106.Müller-Fassbender H. Double-blind clinical trial of S-adenosylmethionine versus ibuprofen in the treatment of osteoarthritis. The American journal of medicine. 1987;83:81–83. doi: 10.1016/0002-9343(87)90857-6. [DOI] [PubMed] [Google Scholar]
- 107.Tavoni A, Vitali C, Bombardieri S, Pasero G. Evaluation of s-adenosylmethionine in primary fibromyalgia: A double-blind crossover study. Am J Med. 1987;83:107–10. doi: 10.1016/0002-9343(87)90862-x. [DOI] [PubMed] [Google Scholar]
- 108.Tavoni A, Jeracitano G, Cirigliano G. Evaluation of s-adenosylmethionine in secondary fibromyalgia: A double-blind study. Clin Exp Rheumatol. 1998;16:106. [PubMed] [Google Scholar]
- 109.Jacobsen S, Danneskiold-Samsøe B, Andersen RB. Oral s-adenosylmethionine in primary fibromyalgia. Double-blind clinical evaluation. Scand J Rheumatol. 1991;20:294–302. doi: 10.3109/03009749109096803. [DOI] [PubMed] [Google Scholar]
- 110.Volkmann H, Norregaard J, Jacobsen S, Danneskiold-Samsøe B, Knoke G, Nehrdich D. Double-blind, placebo-controlled cross-over study of intravenous S-adenosyl-L-methionine in patients with fibromyalgia. Scandinavian journal of rheumatology. 1997;26:206–211. doi: 10.3109/03009749709065682. [DOI] [PubMed] [Google Scholar]
- 111.Taylor MJ, Rudkin L, Bullemor-Day P, Lubin J, Chukwujekwu C, Hawton K. Strategies for managing sexual dysfunction induced by antidepressant medication. Cochrane Database Syst Rev. 2013;5:CD003382. doi: 10.1002/14651858.CD003382.pub3. [DOI] [PubMed] [Google Scholar]
- 112.Dording CM, Mischoulon D, Shyu I, Alpert JE, Papakostas GI. SAMe and sexual functioning. European Psychiatry. 2012;27:451–4. doi: 10.1016/j.eurpsy.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Chan A, Shea TB. Effects of dietary supplementation with n-acetyl cysteine, acetyl-l-carnitine and s-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human apoe4. Neuromolecular Med. 2007;9:264–9. doi: 10.1007/s12017-007-8005-y. [DOI] [PubMed] [Google Scholar]
- 114.Shea TB, Chan A. S-adenosyl methionine: A natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer's disease. J Alzheimers Dis. 2008;13:67–70. doi: 10.3233/jad-2008-13107. [DOI] [PubMed] [Google Scholar]
- 115.Fontanari D, Di Palma C, Giorgetti G, Violante F, Voltolina M. Effects of S-adenosyl-L-methionine on cognitive and vigilance functions in the elderly. Current therapeutic research. 1994;55:682–689. [Google Scholar]
- 116.Levkovitz Y, Alpert JE, Brintz CE, Mischoulon D, Papakostas GI. Effects of s-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. J Affect Disord. 2012;136:1174–8. doi: 10.1016/j.jad.2011.04.059. [DOI] [PubMed] [Google Scholar]
- 117.Chan A, Paskavitz J, Remington R, Rasmussen S, Shea TB. Efficacy of a vitamin/nutriceutical formulation for early-stage Alzheimer's disease: A 1-year, open-label pilot study with a 16-month caregiver extension. Am J Alzheimers Dis Other Demen. 2008;23:571–85. doi: 10.1177/1533317508325093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer's disease: A placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24:27–33. doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Remington R, Bechtel C, Larsen D, Samar A, Doshanjh L, Fishman P, et al. A phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer's disease. J Alzheimers Dis. 2015;45:395–405. doi: 10.3233/JAD-142499. [DOI] [PubMed] [Google Scholar]
- 120.Soyka M. Neurobiology of aggression and violence in schizophrenia. Schizophrenia Bulletin. 2011;37:913–20. doi: 10.1093/schbul/sbr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsao D, Diatchenko L, Dokholyan NV. Structural mechanism of s-adenosyl methionine binding to catechol o-methyltransferase. PLoS One. 2011;6:e24287. doi: 10.1371/journal.pone.0024287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Strous RD, Ritsner MS, Adler S, Ratner Y, Maayan R, Kotler M, et al. Improvement of aggressive behavior and quality of life impairment following s-adenosyl-methionine (sam-e) augmentation in schizophrenia. Eur Neuropsychopharmacol. 2009;19:14–22. doi: 10.1016/j.euroneuro.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 123.Tang KL, Antshel KM, Fremont WP, Kates WR. Behavioral and Psychiatric Phenotypes in 22q11. 2 Deletion Syndrome. Journal of Developmental & Behavioral Pediatrics. 2015;36:639–650. doi: 10.1097/DBP.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Green T, Steingart L, Frisch A, Zarchi O, Weizman A, Gothelf D. The feasibility and safety of s-adenosyl-l-methionine (same) for the treatment of neuropsychiatric symptoms in 22q11. 2 deletion syndrome: A double-blind placebo-controlled trial. Journal of Neural Transmission. 2012;119:1417–23. doi: 10.1007/s00702-012-0831-x. [DOI] [PubMed] [Google Scholar]
- 125.Hedges DW, Brown BL, Shwalb DA. A direct comparison of effect sizes from the clinical global impression- improvement scale to effect sizes from other rating scales in controlled trials of adult social anxiety disorder. Human Psychopharmacology: Clinical and Experimental. 2009;24:35–40. doi: 10.1002/hup.989. [DOI] [PubMed] [Google Scholar]
- 126.Agricola R, Dalla Verde G, Urani R, Di Palma C, Giorgetti V. S-adenosyl-L-methionine in the treatment of major depression complicating chronic alcoholism. Current therapeutic research. 1994;55:83–92. [Google Scholar]
- 127.Russo AL, Monaco M, Pani A, Fontanari D. Efficacy of s-adenosyl-l-methionine in relieving psychologic distress associated with detoxification in opiate abusers. Current therapeutic research. 1994;55:905–913. [Google Scholar]
- 128.Frezza M, Centini G, Cammareri G, Le Grazie C, Di Padova C. S-adenosylmethionine for the treatment of intrahepatic cholestasis of pregnancy. Results of a controlled clinical trial. Hepatogastroenterology. 1990;37:122–5. [PubMed] [Google Scholar]
- 129.Friedel HA, Goa KL, Benfield P. S-adenosyl-L-methionine. A review of its pharmacological properties and therapeutic potential in liver dysfunction and affective disorders in relation to its physiological role in cell metabolism. Drugs. 1989;38:389–416. doi: 10.2165/00003495-198938030-00004. [DOI] [PubMed] [Google Scholar]
- 130.Lieber CS. Role of S-adenosyl-L-methionine in the treatment of liver diseases. J Hepatol. 1999;30:1155–9. doi: 10.1016/s0168-8278(99)80274-8. [DOI] [PubMed] [Google Scholar]
- 131.Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, et al. S-adenosylmethionine in alcoholic liver cirrhosis: A randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081–9. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 132.Milkiewicz P, Mills CO, Roma MG, Ahmed-Choudhury J, Elias E, Coleman R. Tauroursodeoxycholate and s-adenosyl-l-methionine exert an additive ameliorating effect on taurolithocholate-induced cholestasis: A study in isolated rat hepatocyte couplets. Hepatology. 1999;29:471–6. doi: 10.1002/hep.510290215. [DOI] [PubMed] [Google Scholar]
- 133.Testino G, Leone S, Ansaldi F, Borro P. Silymarin and s-adenosyl-l-methionine (same): Two promising pharmacological agents in case of chronic alcoholic hepathopathy. A review and a point of view. Minerva Gastroenterol Dietol. 2013;59:341–56. [PubMed] [Google Scholar]
- 134.Reefhuis J, Devine O, Friedman JM, Louik C, Honein MA National Birth Defects Prevention Study. Specific ssris and birth defects: Bayesian analysis to interpret new data in the context of previous reports. BMJ. 2015;351:h3190. doi: 10.1136/bmj.h3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frezza M, Pozzato G, Chiesa L, Stramentinoli G, Padova CD. Reversal of Intrahepatic Cholestasis of Pregnancy in Women after High Dose S-Adenosyl-L-Methionine Administration. Hepatology. 1984;4:274–278. doi: 10.1002/hep.1840040217. [DOI] [PubMed] [Google Scholar]
- 136.Sun QF, Ding JG, Wang XF, Fu RQ, Yang JX, Hong L, et al. Efficacy and safety of intravenous stronger neo-minophagen C and s-adenosyl-l-methionine in treatment of pregnant woman with chronic hepatitis B: A pilot study. Med Sci Monit. 2010;16:PR9–14. [PubMed] [Google Scholar]
- 137.Beuers U, Trauner M, Jansen P, Poupon R. New paradigms in the treatment of hepatic cholestasis: From UDCA to FXR, PXR and beyond. Journal of hepatology. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 138.Zhou F, Gao B, Wang X, Li J. Meta-analysis of ursodeoxycholic acid and S-adenosylmethionine for improving the outcomes of intrahepatic cholestasis of pregnancy. [Article in Chinese]. [abstract only] Zhonghua Gan Zang Bing Za Zhi. 2014;22:299–304. doi: 10.3760/cma.j.issn.1007-3418.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 139.Surtees R, Hyland K. A method for the measurement of S-adenosylmethionine in small volume samples of cerebrospinal fluid or brain using high-performance liquid chromatography-electrochemistry. Anal Biochem. 1989;181:331–335. doi: 10.1016/0003-2697(89)90252-2. [DOI] [PubMed] [Google Scholar]
- 140.Iruela LM, Minguez L, Merino J, Monedero G. Toxic interaction of S-adeno-sylmethionine and clomipramine [letter] Am J Psychiatry. 1993;150:522. doi: 10.1176/ajp.150.3.522b. [DOI] [PubMed] [Google Scholar]
- 141.Torta R, Zanalda E, Rocca P, Ravizza L. Inhibitory activity of s-adenosyl-l-methionine on serum gamma-glutamyl-transpeptidase increase induced by psychodrugs and anticonvulsants. Current Therapeutic Research. 1988;44:144–59. [Google Scholar]
- 142.Gören JL, Stoll AL, Damico KE, Sarmiento IA, Cohen BM. Bioavailability and lack of toxicity of s-adenosyl-l-methionine (SAMe) in humans. Pharmacotherapy. 2004;24:1501–7. doi: 10.1592/phco.24.16.1501.50943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.