Abstract
Objective
Thyroid hormone influences lipoprotein metabolism. The role of menopausal status in this association has not been extensively studied. The aim of the present study is to evaluate the association between lipid parameters and mild elevations of thyrotropin (TSH), and whether menopause influences this relationship.
Study design
A cross-sectional study was conducted with a sample of 2,914 women (aged 14–102 years) from the SardiNIA study.
Main outcome measures
The association of TSH with blood lipid levels was examined using regression analyses, according to menopausal status.
Results
Postmenopausal women had lower serum TSH concentrations and higher levels of total cholesterol, low-density lipoprotein cholesterol (LDLc), high-density lipoprotein cholesterol (HDLc), and triglycerides than did premenopausal women (p = 0.001 or less for all). In premenopausal women, after adjusting for the confounders age, BMI, smoking, insulin and glycaemia, TSH showed a direct relation to the levels of total cholesterol (β= 0.046, p = 0.010), LDLc (β= 0.044, p = 0.016) and triglycerides (β= 0.085, p < 0.001), but no association with HDLc level. In the postmenopausal group, TSH was directly associated only with triglyceride levels (β= 0.103, p = 0.014).
Conclusions
The association between mild elevation of TSH and lipid levels is influenced by menopausal status. Further research is needed to clarify this finding.
Keywords: Cholesterol, Subclinical hypothyroidism, TSH, Menopause, Estrogen
1. Introduction
Thyroid hormones exert a wide range of effects in several systems including cardiovascular function. It has long been observed that overt hypothyroidism is associated with accelerated atherosclerosis but the role of subclinical hypothyroidism is not completely understood [1,2]. Thyroid hormone significantly influences lipoprotein metabolism, and overt hypothyroidism is a well-known cause of hyperlipidaemia [3]. Moreover, similar biochemical changes were also observed in subclinical hypothyroidism, in which high levels of thyrotropin (TSH) were associated with an increase of lipid abnormalities that were reversible by levothyroxine supplement therapy [4]. Recently, abnormalities in lipoprotein pattern have also been reported in subjects with TSH in the upper normal range [5], although with some gender difference [6]. Thus, serum lipids can change along with TSH levels even when thyroid hormone levels are normal.
Thus far, few large studies have investigated the association of TSH levels within the reference range with level of lipids in women in the general population. Menopause usually leads to changes in hormonal status, metabolism and lipid profile. Reduced oestrogen production from ovaries results in increased plasma cholesterol levels, both in total and low density lipoprotein cholesterol (LDLc), with a reduction in high density lipoprotein cholesterol (HDLc) [7]. The changes in lipid profile are thus similar to those observed in overt hypothyroidism, for which replacement therapy is mandatory. On the contrary, treatment of subclinical hypothyroidism is not universally accepted, particularly in the elderly for whom mild TSH elevation may be a normal manifestation of aging.
The objective of this study was to investigate whether menopause might influence the relation between TSH and lipid parameters in a sample with normal or slightly elevated TSH.
2. Methods
2.1. Study population
This analysis is based on data from the SardiNIA study, a population-based survey that investigates genetic and phenotypic traits associated with aging [8]. Features of this project have been described elsewhere [9]. Briefly, all residents from four towns (Lanusei, Arzana, Ilbono, and Elini) in a valley in Sardinia (Italy), aged 14 years and older, were invited to participate. Since November 2001, participants had visited and their blood samples analysed about every 3 years, generating three complete surveys.
2.2. Exposure assessment and outcome assessment
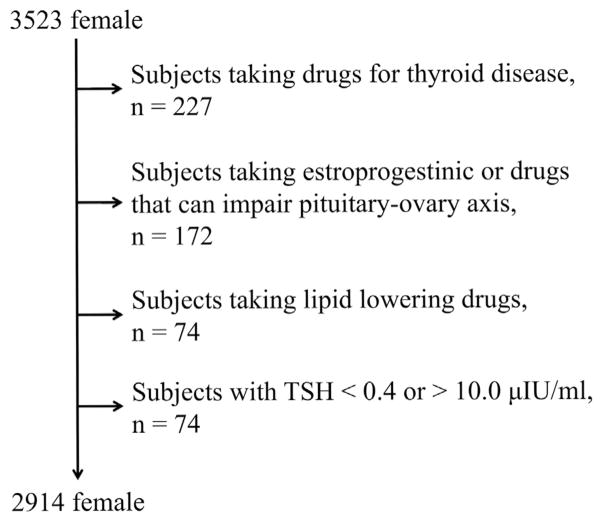
A detailed medical history, which included age at menopause, a physical examination, anthropometric and biochemical measurements, was assessed by computer-aided face-to-face interviews. Physical examinations were performed by trained medical staff following a standardized procedure. We analysed data from a first visit of participants, focusing on women with normal or mildly elevated TSH (range 0.4–10.0 μIU/ml) and free thyroxine within the reference range. From the original cohort of 6,148 subjects, those who reported taking thyroid medications (thyroid hormone replacement or thyrostatics), lipid lowering drugs, or hormonal replacement therapy were excluded, yielding a final population of 2,914 (age 14–102 years), as shown in Fig. 1. Each participant signed an Informed Consent. All study methods were approved by the local ethics committee, Azienda Sanitaria Locale 4 (ASL4).
Fig. 1.
Flowsheet of included cases.
2.3. Covariates assessment
Venous blood samples were drawn between 7 and 8 a.m. after an overnight fast. Plasma triglycerides and total cholesterol were determined by an enzymatic method (Abbott Laboratories ABA-200 ATC Biochromatic Analyser, Irving, TX, USA); HDLc by dextran sulphate–magnesium precipitation; and LDLc by the Friedewald formula. Serum TSH was assessed with the Siemens TSH assay (Immulite 2000) according to the manufacturer’s instructions. The method is a solid-phase, two-site chemiluminescent immunometric assay (normal range 0.4–4.0 μIU/ml). Fasting plasma glucose concentration was measured by the glucose oxidase method (Beckman Instruments Inc., Fullerton, CA, USA). Body mass index (BMI) was calculated as weight (kg)/height2 (m2). Smokers were defined as current consumers of at least one cigarette per day.
2.4. Statistical analysis
First, each parameter distribution gaussianity was assessed by the Shapiro-Wilk test. Because of skewed distributions, we calculated the sample descriptive statistics by using non-parametric tests, and reported results were expressed as median and 25th–75th percentiles. Distributions of categorical variables were expressed as absolute number and percentages. The Wilcoxon rank sum test was used to compare all continuous variables in women before and after menopause. Differences in frequencies were tested by the exact Fisher test. Because of the skewed distribution, lipid parameters were mathematically transformed (inverse normal transformation) for the regression analyses. Multiple linear regression tests were performed, separately, after stratifying the sample set by menopausal status, with each lipid parameter as a dependent variable and age, BMI, smoking, insulin, glycaemia as independent variables. Age at menopause was further included as independent variable only in models which analysed post-menopausal women. In a further analysis, we repeated the above analyses in women with serum TSH level within the normal range (TSH 0.4–4.0 μIU/ml). Collinearity was assessed by using the tolerance and variation-inflation factor (VIF). Collinearity was found if the tolerance was less than 0.1 and the VIF more than 10, respectively. Autocorrelation and heteroscedasticity were further tested with Durbin-Watson Test and Breusch-Pagan Test, respectively. Significance was set at p < 0.05 in Stata 12.0.
3. Results
Relevant features of the sample are in Table 1. Postmenopausal women had lower serum TSH, higher levels of total cholesterol, LDLc, HDLc, triglycerides, glycaemia and increased BMI compared to premenopausal subjects (p = 0.001 or less for all). To test the role of TSH on lipid parameters, multiple regression analyses were run (Table 2). In premenopausal women, after adjusting for the covariates age, BMI, smoking, insulin, glycaemia, TSH showed a direct relation with total cholesterol (β= 0.046, p = 0.010), LDLc (β= 0.044, p = 0.016), and triglycerides (β= 0.085, p < 0.001), but no association with HDLc (β= −0.007, p = 0.720).
Table 1.
Main characteristics of the sample.
| Variable | Before menopause (n = 2026) | After menopause (n = 888) | p value |
|---|---|---|---|
| Age (years) | 33.7 (24.8–41.7) | 61.8 (56.3–69.4) | <0.001 |
| Years since menopause | – | 12.8 (6.4–20.1) | – |
| BMI (kg/m2) | 22.1 (20.2–24.7) | 27.4 (24.2–30.8) | <0.001 |
| TSH (μIU/ml) | 1.87 (1.30–2.62) | 1.49 (0.92–2.23) | <0.001 |
| Total cholesterol (mg/dl) | 195 (172–220) | 229 (205–256) | <0.001 |
| LDL (mg/dl) | 113 (95–136) | 142 (119–163) | <0.001 |
| HDL (mg/dl) | 66 (58–76) | 68 (59–79) | 0.001 |
| Triglycerides (mg/dl) | 58 (43–82) | 79 (58–111) | <0.001 |
| Glycemia (mg/dl) | 81 (76–87) | 89 (82–98) | <0.001 |
| Insulin (μ/ml) | 6.3 (4.3–9.4) | 7.0 (4.3–10.8) | <0.001 |
| Smokers [n, (%)] | 385 (19.0%) | 50 (5.6%) | <0.001 |
Data are expressed as median and interquartile range or absolute number and percentage.
Abbreviations: BMI, body mass index; TSH, thyrotropin; LDL, low density lipoprotein cholesterol, HDL, high density lipoprotein cholesterol.
Table 2.
Multivariate linear regression models for lipid parameters.
| Total cholesterol | LDL | HDL | Triglycerides | ||
|---|---|---|---|---|---|
| Before menopausea TSH (0.4–10.0 μIU/ml) | β | 0.046** | 0.044** | −0.007 | 0.085*** |
| 95% CI | 0.111–0.081 | 0.008–0.079 | −0.044–0.030 | 0.049–0.121 | |
| After menopauseb TSH (0.4–10.0 μIU/ml) | β | 0.032 | 0.019 | 0.002 | 0.054* |
| 95% CI | −0.018–0.083 | 0.032–0.069 | −0.048–0.052 | 0.005–0.102 |
Data are expressed as β(Beta coefficient) and 95% CI (95% confidence interval).
Abbreviations: LDL, low density lipoprotein cholesterol, HDL, high density lipoprotein cholesterol, TSH, thyrotropin.
p < 0.05.
p < 0.01.
p < 0.001.
Adjusted for age, body mass index, glycemia, insulin, and smoking habits.
Adjusted for age, body mass index, glycemia, insulin, and smoking habits, and age of menopause.
In the postmenopausal group, TSH showed no relation with total cholesterol (β= 0.032, p = 0.210), LDLc (β= 0.019, p = 0.470), or HDLc (β= 0.002, p = 0.940). However, triglycerides were still positively associated with TSH (β= 0.054, p = 0.028).
In a separate analysis we tested the association between lipid parameters and TSH in women with TSH within the reference range (TSH 0.4–4.0 μIU/ml), stratified by menopausal status, as shown in Table 3. In premenopausal women TSH was directly related to total cholesterol (β= 0.069, p = 0.008), LDLc (β= 0.057, p = 0.029), and triglycerides (β= 0.099, p < 0.001). Again, HDLc showed no relation to TSH (β= 0.018, p = 0.525). In postmenopausal women TSH once more retained a positive relation with triglycerides (β= 0.103, p = 0.014), but no significant association with other lipid parameters (p = 0.345 or higher).
Table 3.
Multivariate linear regression models for lipid parameters in subjects with normal TSH value (TSH 0.4–4.0 μUI/ml).
| Before menopause | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|
|
| ||||
| β(95% CI) | β(95% CI) | β(95% CI) | β(95% CI) | |
| Age | 0.036 (0.031;0.040)** | 0.030 (0.025;0.034)** | 0.020 (0.015;0.025)** | 0.018 (0.013;0.022)** |
| BMI | 0.011 (−0.001;0.022) | 0.029 (0.018;0.041)** | −0.058(−0.071;−0.046)** | 0.039 (0.027;0.051)** |
| Smoke | −0.028 (−0.132;0.077) | −0.007 (−0.113;0.099) | −0.190(−0.302;−0.078)** | 0.253 (0.144;0.362)** |
| Insulin | 0.008 (0.001;0.014)* | 0.004 (−0.003;0.01) | 0.003 (−0.004;0.009) | 0.017 (0.011;0.024)** |
| Glycaemia | 0.003 (0.001;0.006) | 0.003 (0.001;0.006) | 0.001 (−0.002;0.004) | −0.001(−0.005;0.002) |
| TSH | 0.069 (0.018;0.121)** | 0.057 (0.006;0.109)* | 0.018 (−0.037;0.072) | 0.099 (0.046;0.153)** |
| After menopause | Total cholesterol | LDL | HDL | Triglycerides |
| β(95% CI) | β(95%CI) | β(95%CI) | β(95% CI) | |
| Age | −0.012 (−0.019;−0.004)** | −0.013(−0.021;−0.006)** | −0.005 (−0.012;0.002) | 0.007 (0.001;0.015)* |
| Age at menopause | 0.010 (−0.005;0.024) | 0.011 (−0.003;0.025) | 0.004 (−0.010;0.018) | 0.003 (−0.011;0.016) |
| BMI | 0.010 (−0.004;0.025) | 0.014 (−0.001;0.028) | −0.026(−0.040;−0.011)** | 0.041 (0.027;0.055)** |
| Smoke | −0.008 (−0.316;0.300) | 0.001 (−0.308;0.311) | −0.149 (−0.451;0.152) | 0.182 (−0.113;0.477) |
| Insulin | −0.003 (−0.009;0.004) | −0.003 (−0.010;0.004) | −0.004 (−0.010;0.003) | 0.006 (0.001;0.013)* |
| Glycaemia | −0.001 (−0.004;0.001) | −0.002 (−0.004;0.001) | −0.003 (−0.005;−0.001)* | 0.004 (0.002;0.007)** |
| TSH | 0.041 (−0.045;0.127) | 0.012 (−0.075;0.099) | 0.011 (−0.073;0.095) | 0.103 (0.021;0.185)* |
Data are expressed as beta coefficient (β) and 95% confidence interval (95% CI).
Abbreviations: LDL, low density lipoprotein cholesterol, HDL, high density lipoprotein cholesterol, TSH, thyrotropin.
p < 0.05.
p < 0.01.
4. Discussion
Aging is usually associated with a worsening of lipid metabolism, both in men and in women [10]. LDLc progressively increases for a reduction of its catabolism, due to lower activity of the hepatic LDLc receptor [11]. However, other factors, such as oestrogen deficiency, changes in BMI, body fat distribution, and insulin sensitivity could contribute to these metabolic changes [12]. The importance of oestrogen for lipid metabolism is well documented. Before menopause women generally show a more favourable lipid profile compared to men. After menopause, there is a shift to worst lipid status, with increased LDLc levels [11].
We found that menopausal status significantly modifies the association between TSH and lipid parameters. In particular, TSH level was significantly positively associated with total cholesterol, LDLc, and triglycerides in premenopausal women and only with triglycerides in postmenopausal women.
The mechanism by which TSH levels, even within the normal range, might affect the lipid levels is not clear. Nevertheless, studies have shown that TSH acts directly on various tissues. TSH receptors are expressed in a variety of extrathyroidal cells and are involved in adipogenesis [13] and lipolysis [14]. In particular, TSH receptors are present in hepatocytes [15], where, independently of thyroid hormones, TSH can upregulate the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase and thereby increase cholesterol content in liver and serum in thyroidectomised rats [16]. Only recently it has been emphasized the role of sex hormone in the association between TSH and lipids [6]. Menopause and increased TSH level shared the mechanism by which interact with LDLc metabolism. Indeed, oestrogen deficiency as well as hypothyroidism cause a decrease of LDLc receptors which, in turns, lead to a reduced clearance of LDLc from the serum [12]. The reason why in premenopausal women a mild increase of TSH concentration can increase LDLc concentration but not in postmenopausal women is unclear and will require more studies. However, it is reasonable to hypothesize that other factors, such as different intestinal cholesterol absorption and hampered activity of thyroid hormone on LDLc receptor could explain the different effect.
Our study also showed that mild elevation of TSH was positively associated with triglycerides, regardless the gonadal status. Lipoprotein lipase (LPL) is a key enzyme that hydrolyses the triglycerides in lipoprotein. LPL is increased by thyroid hormone and LPL deficiency leads to hypertriglyceridemia [17]. Women with normal ovarian steroid production have a higher LPL activity in the gluteal and femoral region, whereas with menopause LPL activity decreases in the gluteofemoral region [18]. On the contrary, omental adipocytes (but not subcutaneous adipocytes) from postmenopausal women are larger and have higher LPL activity compared with premenopausal women [19], reflecting a shift toward visceral fat storage in states of oestrogen deficiency. In women, visceral obesity is associated with elevated levels of androgens [20]. Since oestrogen down-regulates the density of androgen receptors, when oestrogen levels become low, visceral fat accumulation may occur. Excess visceral adiposity is strongly associated with insulin resistance and atherogenic dyslipidaemia, and represents a major cardiovascular disease (CVD) risk factor. These changes in lipid metabolism induced by oestrogen deficiency and visceral fat accumulation might partly explain the more quick acceleration in CVD morbidity rates observed in women after the age of 45 years [21]. Thus, it could be speculated that these factors might be jointly responsible for our findings in postmenopausal women. Further studies are needed to elucidate the mechanisms that are responsible for the different activity that TSH has on triglycerides in women before and after menopause.
We also found that HDLc is not associated to TSH, as in premenopausal as in postmenopausal women. Cholesteryl ester transfer protein transfers cholesterol from HDLc to LDLc and very low density lipoprotein cholesterol and its concentrations are decreased in hypothyroidism but it is accepted that there is no consistent effect of subclinical hypothyroidism on HDLc concentration [22].
There are some limitations of the present study that should be considered when interpreting its findings. First, the cross sectional nature of the study precludes causal inferences. Further, all the subjects had only one assessment of TSH, thus potentially including individuals with non thyroidal illness or transient abnormality. Finally, we did not included in the analysis dietary lipid intake and the family history of dyslipidaemia which might give a misleading interpretation of lipid profile.
Many literature reports have previously analysed the effect of thyroid hormone on lipid metabolism. However, results of cross-sectional studies of lipids levels in patients with subclinical hypothyroidism have been inconsistent [23,24] and conflicting results have been reported on the effect of levothyroxine treatment on lipid parameters [25–27]. The effect of menopause in the association between lipid parameters and TSH is less studied. Park et al. found an association between TSH and lipids (LDLc and triglycerides) in a cohort of 949 euthyroid postmenopausal women [28] and similar results have been obtained in another study [29]. Although many variables in methodological approaches used might in part explain these contrasting results, age, sex hormones, degree of duration of hypothyroidism, smoking habits, genetic traits, and the baseline level of cholesterol are likely clinical variables involved in the disparate results obtained in these studies.
Overall, thyroid disorders are highly prevalent, occurring more frequently in women [30]. Aging together with decreased oestrogen levels and adipose tissue redistribution might partially explain the increased CVD risk seen after menopause even in the presence of somewhat high TSH levels. The current guidelines recommend to prescribe levothyroxine supplement therapy in subclinical hypothyroidism with TSH values >10 μIU/ml [31], but to be more conservative when TSH values are between 4 and 10 μIU/ml, in particular in older subjects. Our findings add further support for a conservative attitude in prescribing hormonal treatment in post-menopausal women with mildly elevation of TSH level.
Acknowledgments
Funding
This work was supported in part by Contract NO1-AG-1-2109 from the NIH, National Institute on Aging.
Footnotes
Contributors
APD wrote the manuscript and performed the data collection.
MS did the statistical analysis and edited the manuscript.
MGP edited the manuscript.
MD edited the manuscript and performed the data collection.
SL edited the manuscript and performed the data collection.
GD edited the manuscript.
DS edited the manuscript.
FC edited the manuscript.
Ethical approval
This work has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Each participant gave written informed consent, and the protocol was approved. All study methods were approved by the local ethics committee, Azienda Sanitaria Locale 4 (ASL4).
Provenance and peer review
This article has undergone peer review.
Conflict of interest
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Gao N, Zhang W, Zhang YZ, Yang Q, Chen SH. Carotid intima-media thickness inpatients with subclinical hypothyroidism: a meta-analysis. Atherosclerosis. 2013;227:18–25. doi: 10.1016/j.atherosclerosis.2012.10.070. [DOI] [PubMed] [Google Scholar]
- 2.Delitala AP, Filigheddu F, Orrù M, AlGhatrif M, Steri M, Pilia MG, Scuteri A, Lobina M, Piras MG, Delitala G, Lakatta EG, Schlessinger D, Cucca F. No evidence of association between subclinical thyroid disorders and common carotid intima medial thickness or atherosclerotic plaque. Nutr Metab Cardiovasc Dis. 2015;25:1104–1110. doi: 10.1016/j.numecd.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24:1–13. doi: 10.1385/ENDO:24:1:001. [DOI] [PubMed] [Google Scholar]
- 4.Danese MD, Ladenson PW, Meinert CL, Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 5.Asvold BO, Vatten LJ, Nilsen TI, Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 6.Meisinger C, Ittermann T, Tiller D, Agger C, Nauck M, Schipf S, Wallaschofski H, Jørgensen T, Linneberg A, Thiery J, Kluttig A, Greiser KH, Werdan K, Burkhardt K, Völzke H. Sex-specific associations between thyrotropin and serum lipid profiles. Thyroid. 2014;24:424–432. doi: 10.1089/thy.2013.0259. [DOI] [PubMed] [Google Scholar]
- 7.Jenner JL, Ordovas JM, Lamon-Fava S, Schaefer MM, Wilson PW, Castelli WP, Schaefer EJ. Effects of age sex, and menopausal status on plasma lipoprotein(a) levels. The Framingham Offspring Study. Circulation. 1993;87:1135–1141. doi: 10.1161/01.cir.87.4.1135. [DOI] [PubMed] [Google Scholar]
- 8.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LA, Loi F, Pilia MG, Delitala A, Spurgeon H, Najjar SS, AlGhatrif M, Lakatta EG. Longitudinal perspective on the conundrum of central arterial stiffness blood pressure, and aging. Hypertension. 2014;64:1219–1227. doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delitala AP, Orrù M, Filigheddu F, Pilia MG, Delitala G, Ganau A, Saba PS, Decandia F, Scuteri A, Marongiu M, Lakatta EG, Strait J, Cucca F. Serum free thyroxine levels are positively associated with arterial stiffness in the SardiNIA study. Clin Endocrinol (Oxf) 2015;82:592–597. doi: 10.1111/cen.12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton M. Cholesterol and atherosclerosis: modulation by oestrogen. Curr Opin Lipidol. 2013;24:214–220. doi: 10.1097/MOL.0b013e3283613a94. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. doi: 10.1016/0021-9150(93)90225-j. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer EJ, Lamon-Fava S, Cohn SD, Schaefer MM, Ordovas JM, Castelli WP, Wilson PW. Effects of age gender, and menopausal status on plasma low density lipoprotein cholesterol and apolipoprotein B levels in the Framingham Offspring Study. J Lipid Res. 1994;35:779–792. [PubMed] [Google Scholar]
- 13.Lu M, Lin RY. TSH stimulates adipogenesis in mouse embryonic stem cells. J Endocrinol. 2008;196:159–169. doi: 10.1677/JOE-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA, Sorisky A. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism. 2010;59:547–553. doi: 10.1016/j.metabol.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, Gao L, Zhao JJ. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med. 2009;13:4636–4642. doi: 10.1111/j.1582-4934.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, Yu C, Qin C, Liu J, Tian X, Sun X, Fu R, Zhang L, Zhang X, Lu Y, Zou J, Wang L, Guan Q, Gao L, Zhao J. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52:1401–1409. doi: 10.1002/hep.23800. [DOI] [PubMed] [Google Scholar]
- 17.Lam KS, Chan MK, Yeung RT. High-density lipoprotein cholesterol, hepatic lipase and lipoprotein lipase activities in thyroid dysfunction—effects oft reatment. Q J Med. 1986;59:513–521. [PubMed] [Google Scholar]
- 18.Björntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- 19.Tchernof A, Desmeules A, Richard C, Laberge P, Daris M, Mailloux J, Rhéaume C, Dupont P. Ovarian hormone status and abdominal visceral adipose tissue metabolism. J Clin Endocrinol Metab. 2004;89:3425–3430. doi: 10.1210/jc.2003-031561. [DOI] [PubMed] [Google Scholar]
- 20.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 22.Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97:326–333. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 23.Vierhapper H, Nardi A, Grösser P, Raber W, Gessl A. Low-density lipoprotein cholesterol in subclinical hypothyroidism. Thyroid. 2000;10:981–984. doi: 10.1089/thy.2000.10.981. [DOI] [PubMed] [Google Scholar]
- 24.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 25.Tzotzas T, Krassas GE, Konstantinidis T, Bougoulia M. Changes in lipoprotein(a) levels in overt and subclinical hypothyroidism before and during treatment. Thyroid. 2000;10:803–808. doi: 10.1089/thy.2000.10.803. [DOI] [PubMed] [Google Scholar]
- 26.Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Müller B. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: a double blind, placebo-controlled trial (Basel Thyroid Study) J Clin Endocrinol Metab. 2001;86:4860–4866. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- 27.Brenta G, Berg G, Arias P, Zago V, Schnitman M, Muzzio ML, Sinay I, Schreier L. Lipoprotein alterations hepatic lipase activity, and insulin sensitivity in subclinical hypothyroidism: response to L-T(4) treatment. Thyroid. 2007;17:453–460. doi: 10.1089/thy.2006.0302. [DOI] [PubMed] [Google Scholar]
- 28.Park HT, Cho GJ, Ahn KH, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Lee KW, Kim SH. Thyroid stimulating hormone is associated with metabolic syndrome in euthyroid postmenopausal women. Maturitas. 2009;62:301–305. doi: 10.1016/j.maturitas.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Geng H, Zhang X, Wang C, Zhao M, Yu C, Zhang B, Wang Y, Ban B, Zhao J. Even mildly elevated TSH is associated with an atherogenic lipid profile in postmenopausal women with subclinical hypothyroidism. Endocr Res. 2015;40:1–7. doi: 10.3109/07435800.2013.879166. [DOI] [PubMed] [Google Scholar]
- 30.Delitala AP, Pilia MG, Ferreli L, Loi F, Curreli N, Balaci L, Schlessinger D, Cucca F. Prevalence of unknown thyroid disorders in a Sardinian cohort. Eur J Endocrinol. 2014;171:143–149. doi: 10.1530/EJE-14-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce SH, Brabant G, Duntas LH, Monzani F, Peeters RP, Razvi S, Wemeau JL, Guideline ETA. Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215–228. doi: 10.1159/000356507. [DOI] [PMC free article] [PubMed] [Google Scholar]