Abstract
Diabetes mellitus represents a group of metabolic diseases that are characterized by hyperglycemia caused by either lack of insulin production or a reduced ability to respond to insulin. It is estimated that there were 347 million people worldwide who suffered from diabetes in 2008 and incidence is predicted to double by 2050. Neuropathy is the most common complication of long-term diabetes and approximately 30% of these subjects develop chronic neuropathic pain. A distinct acute, severe form of neuropathic pain, called insulin neuritis or treatment-induced painful neuropathy of diabetes (TIND), may also occur shortly after initiation of intensive glycemic control, with an incidence rate of up to 10.9%.
The pathological mechanisms leading to TIND, which is mostly unresponsive to analgesics, are not yet understood, impeding the development of therapies. Studies to date have been clinical and with limited cohorts of patients. In the current study, we developed chronic and acute insulin-induced neuropathic pain in mice with type 2 insulin-resistant diabetes. Furthermore, we determined that insulin-induced acute allodynia is independent of glycemia levels, can also be induced with Insulin-like Growth Factor 1 (IGF1) and be prevented by inhibition of AKT, providing evidence of an insulin/IGF1 signaling pathway-based mechanism for TIND. This mouse model is useful for the elucidation of mechanisms contributing to TIND and for the testing of new therapeutic approaches to treat TIND.
Keywords: Diabetes, neuropathy, insulin neuritis, insulin, TIND
INTRODUCTION
Diabetes mellitus comprises a group of metabolic diseases that are characterized by hyperglycemia caused by either lack of insulin production or a reduced ability to respond to insulin. About 347 million people worldwide were estimated to suffer from diabetes in 2008 and incidence is predicted to double by 2050 (Danaei et al., 2011). The majority of cases represent type 2 diabetes, whereas 5–10% of diabetic patients suffer from type 1 diabetes. In both forms, neuropathy is the most common complication of long-term diabetes and approximately 30% of such subjects develop chronic neuropathic pain (Abbott et al., 2011).
A distinct, acute, severe form of neuropathic pain may also occur shortly after initiation of intensive glycemic control. First described by Caravati in 1933, the condition was considered a rare iatrogenic neuropathy (Caravati, 1933) and its mechanisms are still unknown. This neuropathy is sometimes referred to as insulin neuritis or acute painful diabetic neuropathy of rapid glycemic control, but has most recently been classified as treatment-induced painful neuropathy of diabetes (TIND) (Tesfaye et al., 1996, Knopp et al., 2013, Gibbons and Freeman, 2015). Both type 1 and type 2 diabetic patients experience TIND, which develops following onset of treatment with insulin (Gibbons and Freeman, 2015). While TIND occurred following treatment with oral hypoglycemic agents, it is reported with less frequency (Leow and Wyckoff, 2005, Knopp et al., 2013). Previous studies originally reported incidences to be around 1%, however, a more recent, extensive clinical study has found that up to 10.9% of study patients met the criteria for TIND (Gibbons and Freeman, 2015). The number of patients suffering from TIND is prone to increase with the predicted doubling of the diabetic population (Danaei et al., 2011, Gibbons and Freeman, 2015) and the recommendation of rigorous glycemic control implemented following the Diabetes Control and Complications Trial (DCCT) and Epidemiology of Diabetes Interventions and Complications (EDIC) studies (DCCT research group, 1988, Martin et al., 2006, Albers et al., 2010).
TIND is characterized by allodynia and/or burning painful paraesthesias and may occur distally in a length-dependent fashion or be more generalized and involved proximal sites including the trunk (Archer et al., 1983, Tesfaye et al., 1996, Dabby et al., 2009, Gibbons and Freeman, 2010). Patients start to experience excruciating neuropathic pain within 2–4 weeks of treatment initiation (Dabby et al., 2009). This pain is frequently described as severe and, in one study, is rated 10 on a scale of 1–10 by all subjects (Gibbons and Freeman, 2010), despite being treated with multiple medications. The pain phenotype can vary and it is particularly notable that allodynia and burning pain are experienced after insulin therapy, while allodynia is not experienced after instituting glycemic control using oral hypoglycemic agents (Dabby et al., 2009). TIND is refractory to currently available therapeutic interventions (Dabby et al., 2009, Gibbons and Freeman, 2010), including all of the first line treatments for “classic” chronic painful diabetic neuropathy such as tricyclic antidepressants, anticonvulsants or serotonin reuptake inhibitors. In some case reports, pain persisted, and even increased, despite use of analgesics and sedatives, with cessation of insulin treatment being the only approach to alleviating pain – but leading to severe hyperglycemia (Caravati, 1933). While the disease is resistant to treatment with multiple analgesics (Knopp et al., 2013), the condition subsides within weeks to months for most patients, but can last years (Caravati, 1933, Gibbons and Freeman, 2010).
The pathological mechanisms leading to TIND are not understood, which impedes the development of targeted therapies. The currently proposed theories are endoneurial ischaemia, hypoglycemic microvascular damage and regenerating nerve firing (Llewelyn et al., 1986, Tesfaye et al., 1996, Gibbons and Freeman, 2010). It has been suggested that institution of good glucose control, or perhaps insulin per se, may promote axonal regeneration that causes pain sensations. Prominent regenerative activity in the absence of active degeneration was observed in patients suffering from TIND and pain may possibly result from ectopic generation of impulses in the regenerating axon sprouts (Archer et al., 1983, Llewelyn et al., 1986). More recently, peripheral nerve degeneration described as diffuse damage to unmyelinated and lightly myelinated nerve fibers and a decrease in intra-epidermal nerve fibers that was temporally related to the rapid improvement in glucose control were observed in patients suffering from TIND (Gibbons and Freeman, 2010). TIND could also result from changes in nerve vasculature. Proliferating epineurial blood vessels, similar to those found in retinopathy, have been described in nerve of patients suffering from TIND. Some patients showed severe abnormalities, such as arterial attenuation and arterial-venous shunting that could lead to endoneurial ischemia (Tesfaye et al., 1996). Similarly, insulin was shown to reduce nerve nutritive blood flow and increase arterio-venous shunt flow when administered by intravenous infusion at doses from 0.04U/kg up to 32U/kg (Kihara et al., 1994). However, normal vasa nervosum was observed in other patients suffering from TIND (Archer et al., 1983, Vital et al., 1997).
Studies to date are clinical with limited cohorts of patients, in part because of the lack of awareness about the disorder. Establishing an animal model becomes necessary to study the mechanism of TIND and potential therapeutics. In this study, we developed a protocol for acute and chronic insulin-induced neuropathic pain in mice with type 2 insulin-resistant diabetes. Furthermore, we determined that insulin-induced acute allodynia is independent of glycemia levels, can also be induced through Insulin-like Growth Factor 1 (IGF-1) and can be alleviated by an AKT inhibitor, providing evidence of an insulin/IGF1 signaling pathway-based mechanism for TIND.
Experimental procedures
Animals and diet
Adult female C57Bl6 mice were purchased from Harlan Industries/Envigo (Placentia, CA, USA). Inducible insulin receptor knockdown (IR-KD) mice were purchased from Taconic (USA) and male db/db (5 months old, BKS.Cg-Dockm7m +/+ Leprdb/J) mice were purchased from Jackson Laboratory (USA). A total of 74 animals were housed 3–4 per cage with free access to food and water and maintained in a vivarium approved by the American Association for the Accreditation of Laboratory Animal Care. All animal studies were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of California San Diego. Six to 8 mice were used per group. C57Bl6 mice were fed a low fat diet (LFD, 10% kcal from fat, Research Diet Inc., New Brunswick, NJ, USA) or a high fat diet (HFD, 60% kcal from fat, Research Diet Inc., New Brunswick, NJ, USA) up to 33 weeks. IR-KD mice were fed a regular diet but were maintained on doxycycline water to induce a reduction of insulin receptor expression (Seibler et al., 2007). db/db mice were fed a regular diet.
Chronic insulin-induced painful neuropathy
After 14 weeks of HFD regimen, once insulin resistance was confirmed by glucose tolerance test, a group of HFD mice was treated with 1U insulin (Humulin R) subcutaneous injections five times a week between 9 and 11 am. HFD regimen and insulin injection continued for 8 weeks. Tactile allodynia and responses to thermal stimuli were measured 5–6h and 6–7h, respectively, after insulin injection (unless otherwise specified) once a week.
Acute insulin-induced painful neuropathy
After 15 weeks of HFD regimen, once insulin resistance was confirmed by glucose tolerance test, other groups of HFD mice received a single dose of saline, insulin (0.5 or 2.0U) or IGF1 (2 mg/kg, Prospec Inc., NJ, USA) sc injection and tactile allodynia was assessed every 30 min for 210 min.
Glucose tolerance test
To assess insulin resistance, glucose was administered at 1.5g/kg ip after on overnight fast. Glucose levels were measured using a strip-operated reflectance meter in a blood sample obtained by tail prick every 30 min for 2 h.
Tactile Allodynia
Impaired sensation to touch is one of the most common pain reported by patients suffering from TIND. Therefore, a modified testing regime was performed to measure response to light touch or tactile allodynia using von Frey filaments (Chaplan et al., 1994) for mice suffering from insulin-induced painful neuropathy. The mice were acclimated for 10–15 minutes on the wire mesh platform. Von Frey filaments (Stoelting, Wood Dale, IL, USA) were used to determine the 50% paw withdrawal threshold (PWT), using the up-down method developed by Dixon (Dixon, 1980). In brief, the filaments were applied in sequence upon the plantar surface of the hindpaw and pulsed for 5 seconds. The response was positive if the mouse lifted its paw, in which case the next lightest filament was chosen. A lack of response resulted in the filament of increasing weight for the following measurement. This process was repeated until four measurements had been made after an initial change in the behavior or until the lightest filament received a positive response. The resulting sequence was used to interpolate the 50% response threshold as described previously (Chaplan et al., 1994). Initial measurements at time 0 established the baseline for PWT and blood glucose. Following baseline measurements, HFD, db/db or IR-KD mice were injected subcutaneously with insulin (Humilin R). Mice were placed back in their cages for approximately 10 minutes in order to remain hydrated and then allowed to acclimate for 10 minutes on the wire mesh platform before the next time point. This process of measuring, returning to the cages and acclimating was repeated every 30 minutes from time 0 to time 210 minutes. Blood glucose measurements were taken at time 0, 30, 60, 150 and 210 minutes with a OneTouch UltraMini© blood glucose meter and OneTouch Ultra© blood glucose strips. A standardized testing regime was performed weekly, once a week, to measure tactile allodynia for the chronic model of insulin neuritis.
Thermal responses latency
Another common pain sensation reported by patients suffering from TIND is impaired temperature sensation. We have therefore performed thermal evoked pain using the thermal testing apparatus (UARD, San Diego, CA, USA). Mice were placed in an observation chamber on top of testing apparatus and allowed to acclimate to the warmed glass surface (30oC) and surroundings for 30 minutes. The mobile heat source was placed below the center of the hind paw and turned on, activating a timer and locally warming the glass surface. Paw withdrawal triggered movement sensors that stopped the timer and turned off the heat source. Both paws were measured and the mean used as a composite score for each mouse. To avoid tissue damage, the upper cut-off limit was set at 20 seconds. The response latency was converted to the response temperature using a withdrawal time to floor temperature calibration curve (Calcutt, 2004).
Nerve conduction velocity
Motor nerve conduction velocity (MNCV) was measured in the sciatic nerve: interosseus muscle system of isoflurane-anesthetized animals as described (Jolivalt et al., 2015). Core and nerve temperatures were monitored using rectal and near-nerve thermistor probes and maintained at 37°C using a heated platform and heating lamp. The sciatic nerve was stimulated (1-5V, 0.05ms square wave) via needle electrodes placed at the sciatic notch or ankle to deliver a single supramaximal stimulus. Evoked M responses were recorded from interosseous muscles of the ipsilateral foot using fine needle electrodes, amplified and processed through a MacLab data acquisition system for display on a computer running LabChart software (AD Instruments, Dunedin, New Zealand). The difference in M wave peak latency after stimulation at the sciatic notch and ankle was recorded as the time required for the motor nerve conduction between those two sites. The distance between stimulation sites was measured using digital calipers and divided by the M-wave latency difference to calculate sciatic motor nerve conduction velocity (MNCV). Three measurements were made per mouse and the median used to represent MNCV for that animal.
Drug administration
Gabapentin (200 mg/kg, ip) or saline was injected in a single dose given on day 52 of the chronic insulin-induced allodynia, 1 hour after the daily injection of 1U insulin. Tactile responses were assessed for the following 4 hours. Gabapentin (200 mg/kg, ip) or saline was also injected 90 min before a single bolus injection of 2U insulin in another group of HFD mice. Similarly, an AKT inhibitor (AKT inhibitor IV, EMD) was injected ip at 1 or 2.5 mg/kg 30 min prior to a single bolus injection of 2U insulin in another group of HFD mice. Tactile responses were assessed for the following 4 hours after AKT inhibitor or gabapentin injections.
Statistical analysis
Mice were assigned numbers for injections to prevent observer bias. Data are presented as group mean ± SEM. After verifying normality of distribution and equality of variance, statistical analyses were performed by one-way ANOVA followed by Dunnett’s post-hoc test or two-way repeated measures ANNOVA followed by Tukey or Sidak’s post-hoc test.
RESULTS
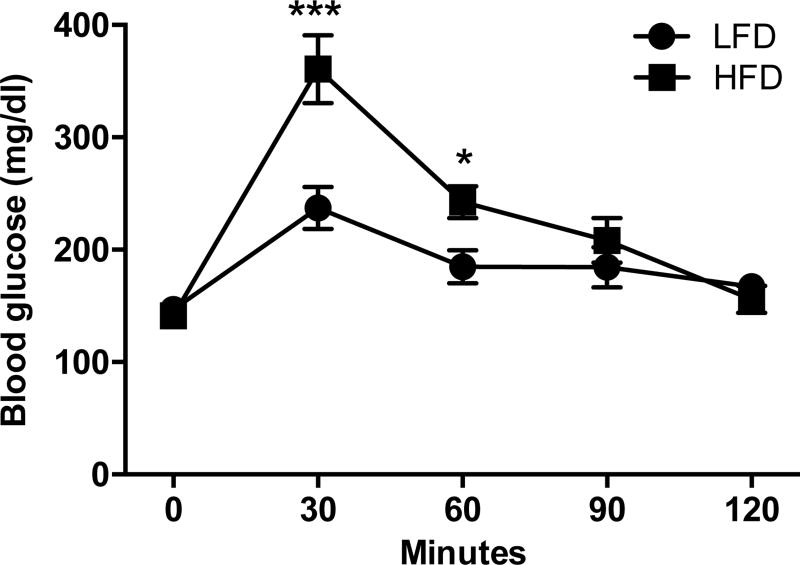
C57BL6 mice were fed a HFD or LFD for 14 weeks before to undergo a glucose tolerance test. Fourteen weeks of HFD induced insulin resistance in C57Bl6 mice compared to LFD fed C57BL6 mice (Fig. 1).
Figure 1. Glucose tolerance test demonstrating insulin resistance in HFD mice.
Blood glucose levels in C57Bl6 mice fed a low fat diet (LFD) or a high fat diet (HFD) following an ip injection of glucose at 1.5g/kg after an overnight fast. Two-way repeated measures ANOVA followed by Sidak’s post-hoc test, **p<0.01, ***p<0.001, F(4,28)=9.008, n=6. Mean±s.e.m.
Chronic model
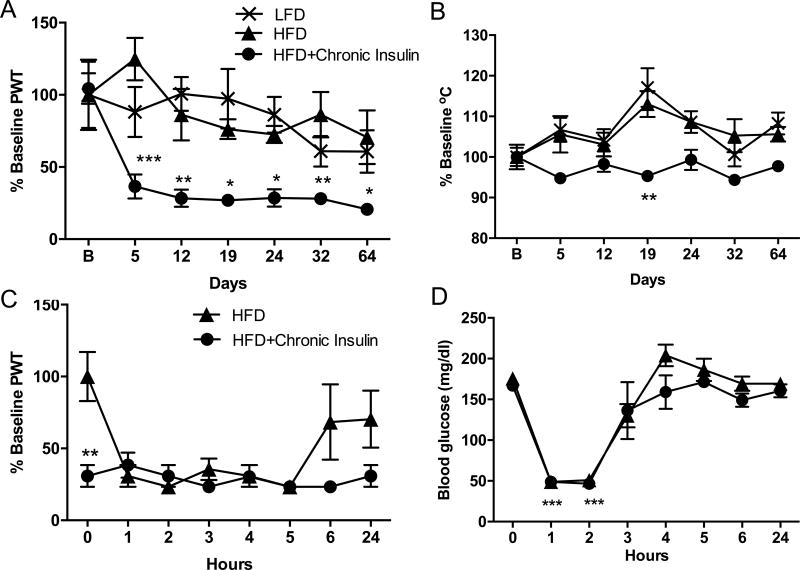
After 14 weeks on HFD, mice were injected daily, 5 days a week, with 1U insulin subcutaneously (sc). Tactile and thermal responses were assessed 5–7 hours after the last injection, once a week for 64 days, along with blood glucose levels. Thermal responses did not varied significantly (Fig. 2B) over the tested period while a significant (F(6,18)=5.164, p=0.0030, p<0.01) and persistent pain response or allodynia to von Frey filaments stimulation was detected by day 5 after the initial insulin injection, and up to 64 days (Fig. 2A). Allodynia was detected 72h after the last insulin injection on day 64 (Fig. 2A), further demonstrating a chronicity of the effect rather than an acute effect of insulin injections. Weekly testing of the mice did not induce allodynia as demonstrated by non-allodynic responses of LFD and HFD mice that were tested weekly in parallel with the HFD mice receiving insulin (Fig. 2A). This data show that chronic injection of 1U insulin induces a persistent allodynia, but not themal hyperalgesia, in HFD mice.
Figure 2. Daily insulin injection induces persistent allodynia.
(A) Tactile responses to von Frey filaments 5–6 hrs after the last injection for mice fed a low fat diet (LFD), a high fat diet (HFD) or a HFD and receiving daily 1U insulin injection sc (HFD+Chronic Insulin). Two-way repeated measures ANOVA followed by Sidak’s post-hoc test versus HFD group, *p<0.05, **p<0.01***p<0.001, n=4–6. (B) Responses to thermal stimulation 6–7 hrs after the last injection for mice fed a low fat diet (LFD), a high fat diet (HFD) or a HFD and receiving daily 1U insulin injection sc (HFD+Chronic Insulin). Two-way repeated measures ANOVA followed by Sidak’s post-hoc test versus HFD group, *p<0.05, **p<0.01***p<0.001, n=4–6. B: Baseline, Day 5 to Day 32, measurements were performed 5–7hrs after insulin injection while Day 64, tactile and thermal responses were assessed 72 hrs after the last insulin injection. (C) Time course of tactile responses on day 60 after injection of 1U insulin to previously untreated HFD mice (HFD) and to HFD receiving daily insulin injection for the past 59 days (HFD+Chronic Insulin). Twoway repeated measures ANOVA followed by Sidak’s post-hoc test, ** p<0.01 chronic vs HFD, n=6 (D) Blood glucose levels on day 60 after injection of 1U insulin to previously untreated HFD mice (HFD) and to HFD receiving daily insulin injection for the past 59 days (HFD+Chronic Insulin). Two-way repeated measures ANOVA followed by Tukey’s post-hoc test, ***p<0.001 vs baseline, n=6. Mean±s.e.m.
On day 60, responses to von Frey filaments were assessed hourly after insulin injection (Fig. 2C) along with blood glucose levels (Fig. 2D). Twenty-four hours after the last insulin injection, allodynia was present in HFD mice receiving daily injection of insulin (chronic group) while the responses from previously untreated HFD mice were similar to baseline values (0.43±0.10 vs 0.51±0.12, respectively). Following insulin injection, both groups of mice were allodynic between 1 and 5 hours post-injection. Responses of HFD mice receiving daily insulin (chronic group) did not decrease further and remained allodynic up to 24 hours (Fig. 2C). In contrast, HFD mice that received only a single insulin injection (HFD group) developed allodynia that persisted for 5 hours before to recover to values similar to baseline by 6 hours and up to 24 hours (Fig. 2C). In the meantime, blood glucose levels followed the same pattern for both groups of mice (Fig. 2D). Blood glucose levels were similar for both groups of mice in the morning of day 60, 24 hours after the last injection administered to the chronic group. Blood glucose levels were significantly (F(7,21)=72.71, p<0.001) decreased for 2 hours before to rise back to initial value by 3–4 hours post-insulin injection (Fig. 2D). These results demonstrate that a single insulin injection induces a reversible allodynia within 6 hours while daily injection of insulin induces a chronic allodynia independent of glucose excursions.
On day 65, motor nerve conduction velocity (MNCV) was measured on the left paw 24 hours after the last insulin injection. Both, HFD and HFD mice receiving daily injection of insulin (chronic group) had significantly (F(2,16)=33.51, p<0.001) slower MNCV when compared to LFD mice (LFD: 60.1±1.8 vs. HFD: 42.2±1.9 vs. HFD+ chronic insulin; 43.3±1.0 m/s).
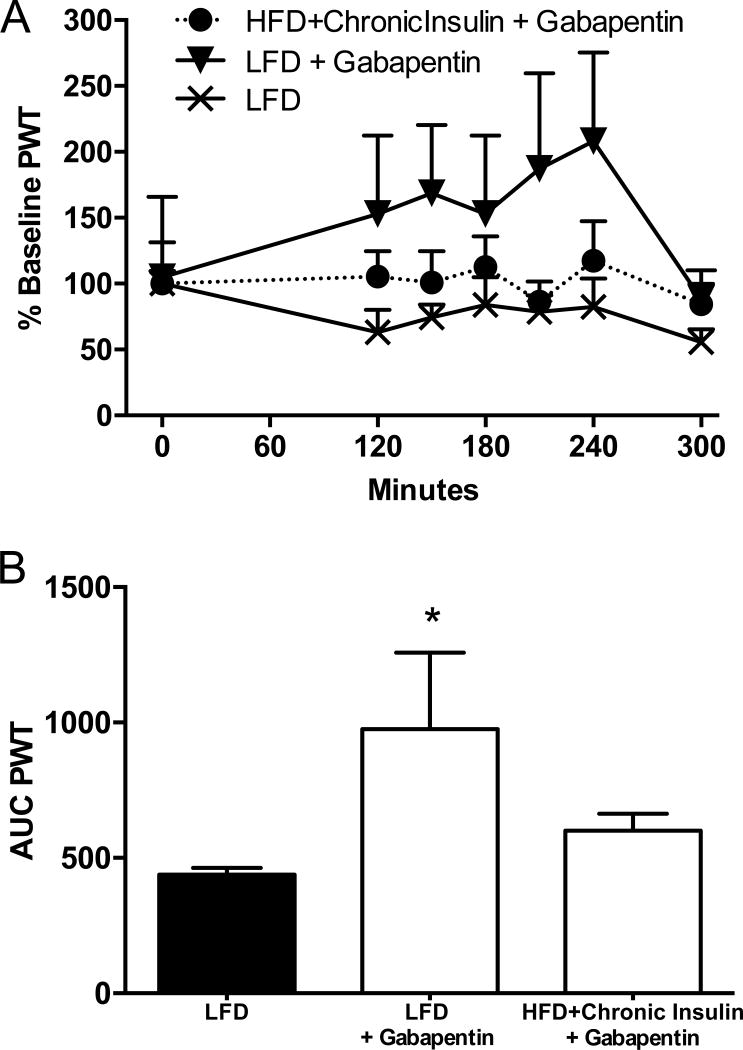
Gabapentin is one of the first line of treatment used against TIND in diabetic patients, however with little efficacy (Dabby et al., 2009). In order to test if our mouse model of TIND was representative of the human condition, we evaluated the effect of gabapentin on tactile allodynia in mice receiving daily insulin injections. On day 52, 24 hours after the last insulin injection, paw withdrawal thresholds (PWT), expressed as percent of baseline values, were 36±11% (PWT: 0.29±0.06) and 84±15% (PWT: 0.43±0.08) for HFD mice receiving daily injection of insulin and LFD mice, respectively. Gabapentin given 90 min after the daily injection of insulin to mice of the chronic group did not alleviate allodynia for the 4 subsequent hours (Fig. 3A). In contrast, gabapentin administration to LFD mice increased their PWT (Fig. 3A). When reporting the area under the curve (AUC), only AUC for LFD mice receiving gabapentin was significantly (F(2,18)=3.788, p<0.05) increased (Fig. 3B) when compared to LFD receiving saline. This shows the lack of efficacy of gabapentin to alleviate allodynia in our murine model of TIND, mirroring the lack of efficacy in human TIND.
Figure 3. Effect of Gabapentin.
(A) Tactile responses to von Frey filaments were assessed in mice fed a low fat diet (LFD) ± gabapentin at 200mg/kg ip or a HFD and receiving daily 1U insulin injection sc (HFD+Chronic Insulin) + Gabapentin at 200mg/kg ip. (B) Area under the curve (AUC) of time courses in (A). One-way ANOVA followed by Dunnet’s post hoc test, *p<0.05 vs LFD, n=6. Mean+s.e.m.
Acute model
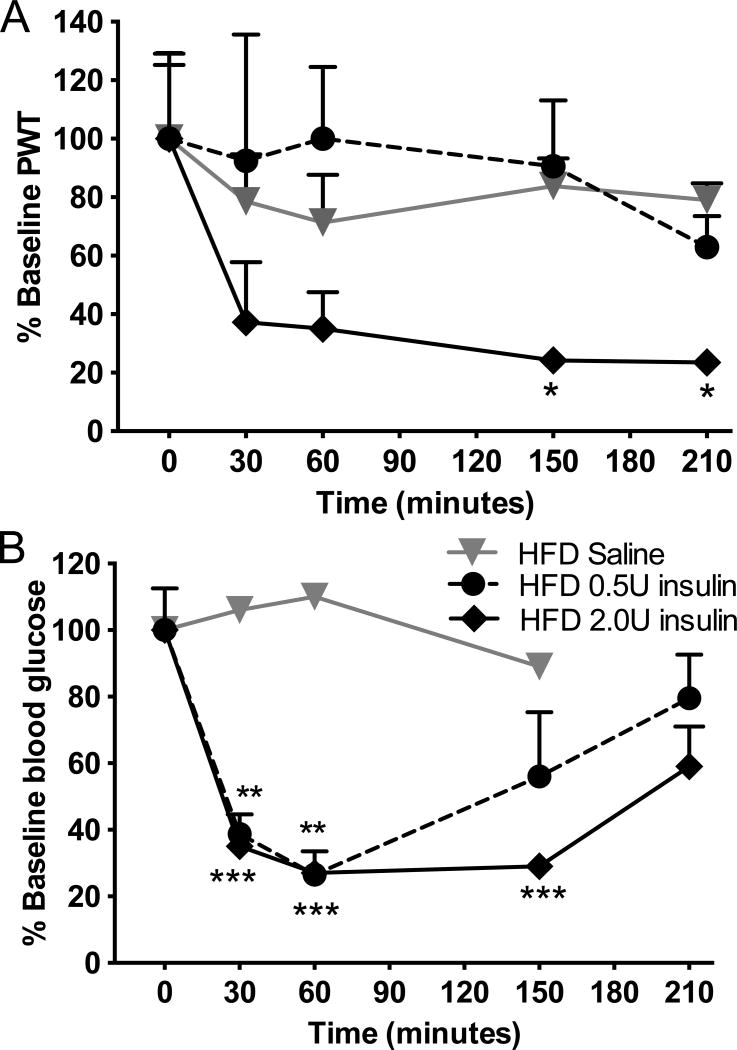
In order to further understand the role of glucose in the development of TIND, the acute effect of variable doses of insulin was tested in another group of mice fed a HFD diet with a similar insulin resistance as the HFD mice shown in Fig 1. From 15 weeks on HFD onwards, HFD mice were injected with a single dose of 2.0 or 0.5U insulin, after which tactile responses and blood glucose levels were assessed acutely for 3 hours. PWT to von Frey filaments were significantly (p<0.05) reduced in insulin-resistant mice receiving 2.0U of insulin sc, whereas 0.5U insulin, had no effect and values remained similar to mice receiving saline (Fig. 4A). In contrast and despite systemic insulin resistance, blood glucose levels were lowered to a similar level at 60 min post-insulin injection by both 0.5U and 2.0U insulin, with the later having a more prolonged effect (Fig. 4B). Notably, blood glucose levels remained above the hypoglycemic range, declining from 207±11 to 56±5 mg/dl by 60 min in HFD mice receiving 0.5 or 2.0U insulin. This acute injection paradigm demonstrates that insulin-induced tactile allodynia in mice is dependent on the insulin dose but not on glucose levels.
Figure 4. Insulin acutely induces tactile allodynia in HFD mice.
(A) Tactile responses to von Frey filaments for HFD mice receiving saline (HFD saline), insulin at 0.5U (HFD 0.5U insulin) or insulin at 2.0U (HFD 2.0U insulin). Two-way repeated measures ANOVA followed by Sidak’s post-hoc test, *p<0.05 vs baseline, n=8. (B) Blood glucose levels in HFD mice receiving saline (HFD saline), insulin at 0.5U (HFD 0.5U insulin) or insulin at 2.0U (HFD 2U insulin). Two-way ANOVA, **p<0.01, ***p<0.001 vs baseline followed by Tuley’s post hoc test, n=8. Mean+s.e.m.
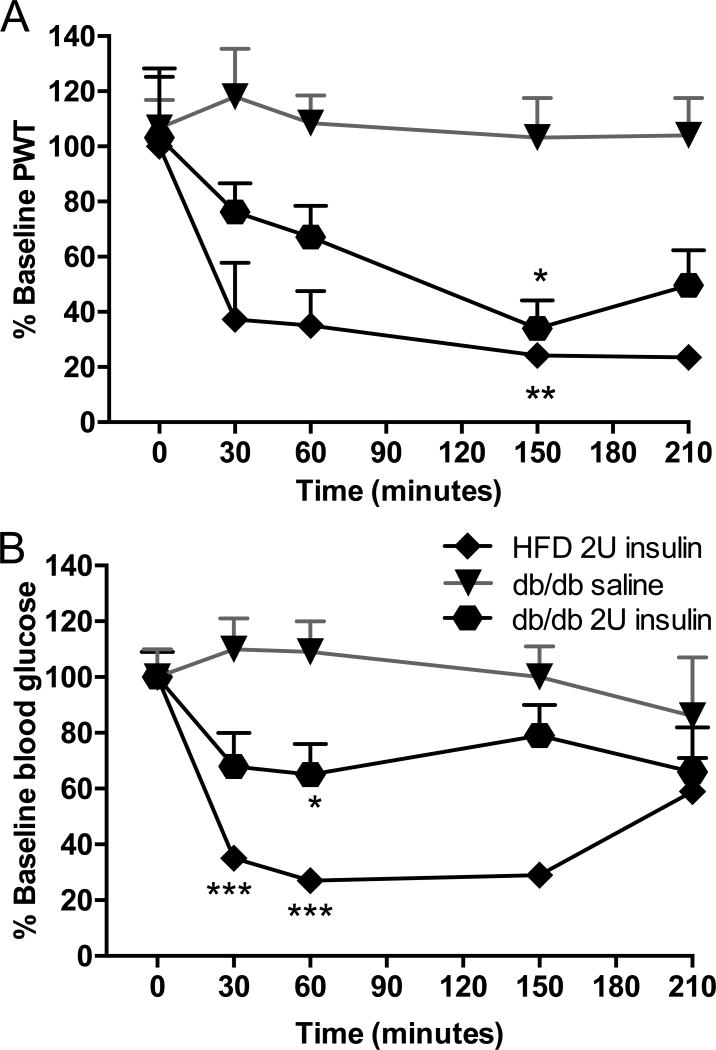
We compared the results obtained in HFD mice, a model of early type 2/obesity related diabetes to db/db mice, a model of established type 2 diabetes. Paw response threshold to von Frey filaments was also significantly reduced in db/db mice receiving 2.0U insulin sc (Fig 5A). A single injection of 2.0U insulin significantly (F(1,88)=8.853, p<0.05) lowered blood glucose levels from 478±43 to a minimum, still hyperglycemic, of 309±53 mg/dl by 60min (Fig 5B). A single injection of 2.0U insulin induces allodynia in a genetic mouse model of type 2 diabetes to levels similar to those observed in HFD mice, despite persistent hyperglycemia.
Figure 5. Insulin acutely induces tactile allodynia in db/db mice.
(A) Tactile responses to von Frey filaments for HFD mice receiving 2U insulin (HFD 2U insulin), db/db mice receiving 2U insulin (db/db 2U insulin) or saline (db/db Saline). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05 vs baseline, n=11. (B) Blood glucose levels in HFD mice receiving 2U insulin (HFD 2U insulin), db/db mice receiving 2U insulin (db/db 2U insulin) or saline (db/db Saline). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05, ***p<0.001 vs baseline, n=11. Mean+s.e.m.
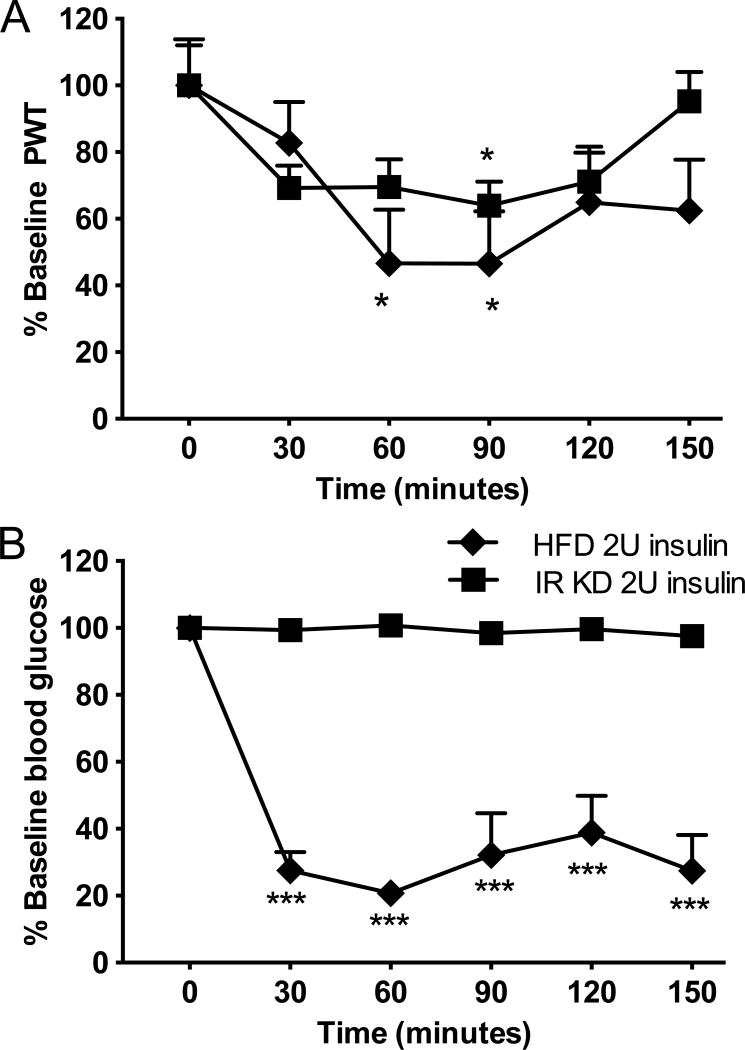
A conditional transgenic mouse model of type 2 diabetes with reduced insulin receptor expression when drinking water containing doxycycline (Seibler et al., 2007) was also used to assess paw responses to von Frey filament after insulin injection. These mice developed significant insulin resistance (Fig. 6B). Injection of 2.0U insulin induced tactile allodynia (Fig. 6A) similar to that induced in HFD mice, however blood glucose (BG) levels remain elevated (BG: 591±4 mg/dl, Fig. 6B).
Figure 6. Insulin acutely induces tactile allodynia in insulin receptor knockdown mice without affecting blood glucose levels.
(A) Tactile responses to von Frey filaments for HFD mice receiving 2U insulin (HFD 2U insulin) or insulin receptor knockdown mice receiving 2U insulin (IR KD 2U insulin). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05 vs baseline, n=8. (B) Blood glucose levels in HFD mice receiving 2U insulin (HFD 2U insulin) or insulin receptor knockdown mice receiving 2U insulin (IR KD 2U insulin). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, ***p<0.001 vs baseline, n=8. Mean+s.e.m.
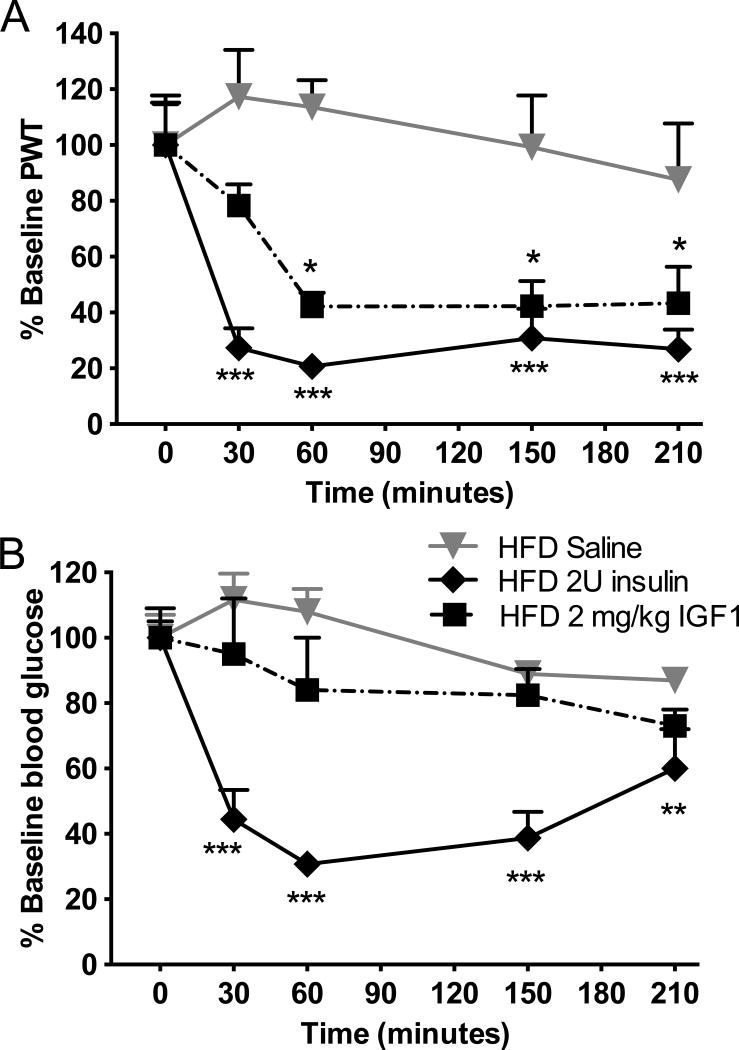
Insulin can activate IGF1 receptors at high doses and vice-versa (Kim and Accili, 2002). Additionally, as insulin and IGF1 share similar signaling pathways but IGF1 does not affect blood glucose levels (Morgado et al., 2011), IGF1 was injected sc at 2mg/kg, an equivalent dose to 2U insulin, to HFD mice, in order to further assess the role of insulin versus glucose in the pathophysiology of TIND. Similarly to insulin, IGF1 induced a significant (F(4,105)=6.901, p<0.05) allodynia (Fig. 7A) without affecting blood glucose levels (Fig. 7B).
Figure 7. IGF1 acutely induces tactile allodynia in HFD mice without affecting blood glucose levels.
(A) Tactile responses to von Frey filaments for HFD mice receiving saline (HFD saline), insulin at 2U (HFD 2U insulin) or IGF1 at 2mg/kg (HFD 2mg/kg IGF1). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05, ***p<0.001 vs baseline, n=8. (B) Blood glucose levels in HFD mice receiving saline (HFD saline), insulin at 2U (HFD 2U insulin) or IGF1 at 2mg/kg (HFD 2mg/kg IGF1). Two-way repeated measures ANOVA followed by Tukey’s post hoc test, **p<0.01, ***p<0.001 vs baseline, n=8. Mean+s.e.m.
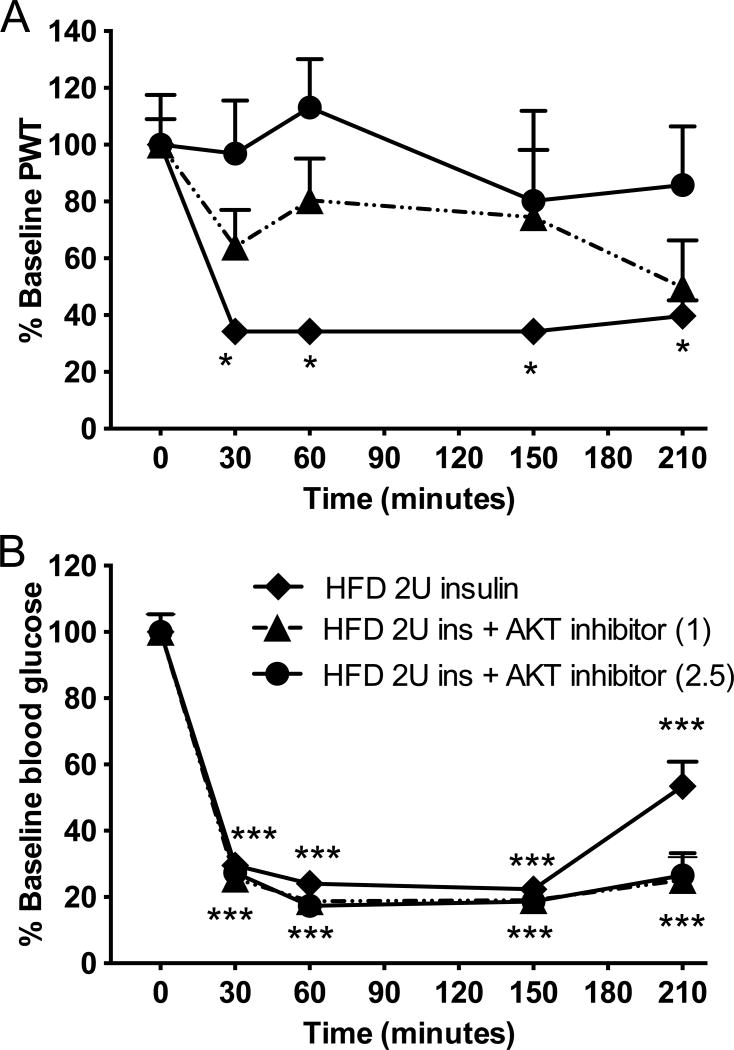
The role of insulin, rather than of glucose changes, in our murine model of TIND, demonstrated in Fig 4 to 7, was further investigated with an inhibitor of AKT, a downstream kinase of the insulin/IGF1 signaling pathway. Injection of AKT inhibitor IV at 1 or 2.5 mg/kg to HFD mice receiving 2.0U insulin prevented insulin-induced allodynia in a dose-dependent manner (Fig 8A), while neither doses affected blood glucose levels (Fig 8B).
Figure 8. Effect of AKT inhibitor IV on acute insulin-induced tactile allodynia.
(A) Tactile responses to von Frey filaments were assessed in HFD mice receiving 2U insulin and HFD receiving 2U insulin after pretreatment with AKT Inhibitor IV at 1 or 2.5 mg/kg ip. Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05 vs baseline, n=7–8. (B) Blood glucose levels in HFD mice receiving 2U insulin and HFD receiving 2U insulin after pretreatment with AKT Inhibitor IV at 1 or 2.5 mg/kg ip. Two-way repeated measures ANOVA followed by Tukey’s post hoc test, ***p<0.001 vs baseline, n=7–8. Mean+s.e.m.
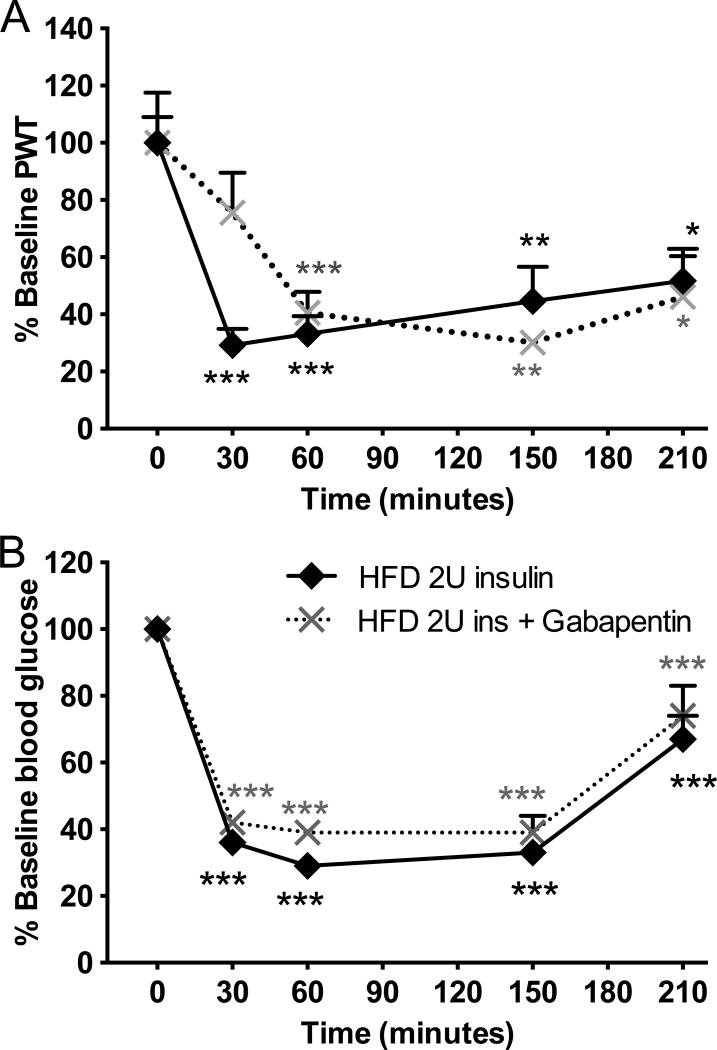
In order to test the validity of our acute TIND mouse model in relation to human TIND and our chronic TIND mouse model, HFD mice were pretreated with 200mg/kg ip gabapentin 90 min (peak efficacy in mice model of painful diabetic neuropathy) prior to receive 2.0U insulin sc. Similar to results from human and our chronic TIND model, gabapentin pretreatment did not prevent insulin-induced allodynia nor blood glucose changes in HFD mice receiving 2.0U insulin (Fig. 9A, B).
Figure 9. Effect of gabapentin on acute insulin-induced tactile allodynia.
(A) Tactile responses to von Frey filaments were assessed in HFD mice receiving 2U insulin and HFD receiving 2U insulin after pretreatment with gabapentin at 200mg/kg ip. Two-way repeated measures ANOVA followed by Tukey’s post hoc test, *p<0.05, **p<0.01, ***p<0.001 vs baseline, n=7–8. (B) Blood glucose levels in HFD mice receiving 2U insulin and HFD receiving 2U insulin after pretreatment with gabapentin at 200mg/kg ip. Two-way repeated measures ANOVA followed by Tukey’s post hoc test, ***p<0.001 vs baseline, n=7–8. Mean+s.e.m.
DISCUSSION
In this study, we have shown that a form of TIND in response to insulin can be modeled in mice. HFD fed mice that build up insulin resistance and mild hyperglycemia (Anderson et al., 2014), develop allodynia lasting 4–5 hours after an acute single insulin injection. HFD mice also developed a chronic persistent allodynia that lasted at least 72 hours after the last injection for HFD mice receiving 1U insulin daily. The protracted insulin-induced allodynia developed after a minimum of 5 days of daily insulin injections. Our model is restricted to development of allodynia as thermal hyperalgeisa was not detected in our HFD mice receiving daily injection of insulin, a divergence from the human TIND. However, allodynia is the common pain sensation described by patients suffering from TIND after treatment with insulin or hypoglycemic agent (Dabby et al., 2009). Despite this limitation, these acute (single insulin injection) and chronic (daily insulin injection for at least 5 days) models will allow us to understand mechanisms involved in TIND and develop new therapeutic approaches.
Independently of blood glucose levels, a single insulin injection induced an acute allodynia while daily injection of insulin induced a persistent allodynia in C57Bl6 mice fed a HFD. In reported human cases of TIND, the neuropathic pain severity correlated with the magnitude of decrease in HbA1c levels, with a 20% risk to develop TIND associated with 2–3% points decrease in HbA1c levels over 3 months compared to a 80% risk with more than 4% points change (Gibbons and Freeman, 2015). However, insulin exhibits a number of properties independent of glucose regulation such as survival, maintenance, repair and regeneration in the PNS (Apfel, 1999) and it is therefore difficult to separate the physiological and neuropathic effect of insulin whilst treating diabetes. Using insulin injections in HFD and in transgenic mouse models allows a separation of the effect of insulin from that of glycemic control that is not possible in human conditions without jeopardizing the health of the patients. The mouse model is therefore divergent from the human condition but may help to dissect specific mechanisms. Using our chronic model but more specifically with our acute model, we found that changes in glucose levels were not correlated with the neuropathic pain. Allodynia was detected with low dose insulin or with IGF1 that did not affect blood glucose levels. Allodynia was also detected in transgenic (IR-KD and db/db) mice displaying hyperglycemia and for which the blood glucose remained elevated even after insulin injection. This indicates that insulin induces a pain response in diabetic mice independent of blood glucose levels, glycemic excursion or hypoglycemia.
Some mechanistic hypotheses have been proposed based on descriptions of few TIND cases and their associated pathology. These hypotheses range from immunological or inflammatory response to insulin (Knorr-Held and Meier, 1990, Younger et al., 1996, Wilson et al., 2003), hemodynamic changes, similar to those found in retinopathy (Kihara et al., 1994, Tesfaye et al., 1996) or nerve regeneration leading to ectopic responses (Archer et al., 1983, Llewelyn et al., 1985, Llewelyn et al., 1991). However, each of these proposed mechanisms have been refuted by others (Archer et al., 1983, Britland et al., 1992, Vital et al., 1997). Our mouse model of TIND also suggests that large fibers structural changes are not participating in the etiology of TIND induced by insulin. Nerve conduction velocity in HFD mice receiving insulin was similar to nontreated HFD mice, not improving neither worsening, suggesting that large fibers structures changes are not contributing to TIND, as observed in the human condition (Gibbons 2017). The lack of effect of insulin on NCV was also shown in insulin-deficient diabetic mice receiving low levels of constant insulin not sufficient to fully correct blood glucose (Yorek et al., 2014) and in insulin-deficient diabetic rats with insulin implants (Calcutt et al., 1996). Nerve blood flow and nerve vasculature remain to be assess in order to fully disregard the contribution of vasculature defects. More recently, other mechanisms inferred from cell cultures or other neuropathies have been proposed such as insulin-induced NMDA receptors trafficking leading to increased excitability (Liu et al., 1995, Skeberdis et al., 2001, Chen and Roche, 2009) or activation of the insulin-signaling pathway, which may lead to neuropathic pain via PI3K/AKT or GSK3 activation, (Xu et al., 2007, Guedes et al., 2008, Choi et al., 2010, Mazzardo-Martins et al., 2012, Weng et al., 2014)
Our study favors the direct role of insulin and activation of its signaling pathway in the etiology of TIND. Insulin has direct physiological actions on primary sensory neurons in vitro (Fernyhough et al., 1993) and ex vivo (King et al., 2015) and peripheral nerves express insulin receptors (Sugimoto et al., 2000). Similarly, IGF1 increases neurite outgrowth in cultured (Recio-Pinto et al., 1986, Caroni and Grandes, 1990), its receptors are expressed in sciatic nerve (Wuarin et al., 1994) and more particularly in Schwann cells (Cheng et al., 1996), and their activation stimulates a similar PI3K/AKT signaling pathway as insulin (Belfiore et al., 2009). Activation of the insulin/IGF1-signaling pathway may play a major role in insulin-induced neuropathic pain. Indeed, there is some precedence for this, as elements of the insulin-signaling pathway have been implicated in other forms of neuropathic pain. Activation of AKT was associated with intraplantar carrageenan induced-allodynia, which was prevented by inhibitors of phopshoinositol-3 kinase (PI3K) and of AKT (Choi et al., 2010). Similar results implicating AKT activation were observed for neuropathic pain following spinal nerve ligation (Xu et al., 2007, Guedes et al., 2008), sciatic nerve transection (Guedes et al., 2008), capsaicin (Sun et al., 2007) or nerve growth factors injections (Bonnington and McNaughton, 2003). In our acute insulin injection model, AKT inhibitor prevented insulin-induced allodynia, further supporting the role of insulin rather than rapid glucose changes in the ethiology of TIND.
In ”classic” diabetic neuropathic pain, gabapentin efficacy is dependent upon its binding to α2 δ1 calcium channels (Luo et al., 2002). The lack of efficacy of gabapentin in our TIND models demonstrate that α2 δ1 calcium channels are not contributing to TIND, as supported by the similar paucity or lack of efficacy of gabapentin in human (Wilson et al., 2003, Dabby et al., 2009). The use of gabapentin demonstrates that our mouse model may be suitable to study the mechanisms involved in TIND that can be translated back to human. However, our models represent an incomplete picture of the causes of TIND and have limitations. TIND in human has been reportedly caused by rapid changes in glycemic control, which was not observed in our murine models. And the changes in glycemic levels were precipitated by a variety of approaches, including strict insulin regimes, insulinomas (hyperinsulinemia associated with hypoglycemia), a history of diabetic anorexia and oral hypoglycemic agents (Gibbons and Freeman, 2015). As most hypoglycemic agents are insulin sensitizers improving insulin availability and action (Mudaliar and Henry, 2001), the occurrence of TIND following their use does not negate our hypothesis that insulin signaling is central to the development of TIND. A number of experiments, including chronic administration of IGF1 and use of hypoglycemic agents, remain to be performed in order to definitely support a role of insulin/IGF1 signaling pathway in TIND. Nonetheless, it is the first attempt to model TIND that will allow us to decipher the mechanisms involved.
Despite limitations, we have demonstrated translatability of our murine model with the human disorder, that TIND is independent of glucose levels changes and that the insulin/IGF1 signaling pathway plays a major role in the ethiology of acute TIND in our murine model. This new mouse model of TIND will allow further testing of different recent hypothesis of TIND mechanisms and to assess new therapeutic approaches aimed at alleviating this debilitating insulin-induced pain, a phenomenon likely to increase in frequency as aggressive glycemic control is recommended to control diabetes.
Highlights.
-
-
Insulin induces neuropathic pain in High-Fat diet mice.
-
-
Insulin-induced pain is independent of glucose levels.
-
-
Our murine model of treatment-induced neuropathy of diabetes provides evidence for an insulin-IGF1 pathway-based mechanism.
Acknowledgments
The authors are thankful to Dr. Nigel Calcutt for fruitful discussions. This work was funded by a Juvenile Diabetes Research Foundation Career Development Award and National Institutes of Health grant AG039736 to CGJ.
Abbreviations
- HFD
high fat diet
- IGF1
Insulin-like Growth Factor 1
- IR-KD
Inducible insulin receptor knockdown
- LFD
Low fat diet
- MNCV
motor nerve conduction velocity
- PWT
50% paw withdrawal threshold
- TIND
treatment-induced painful neuropathy of diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes care. 2011;34:2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM, Diabetes C Complications Trial /Epidemiology of Diabetes I, Complications Research G. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NJ, King MR, Delbruck L, Jolivalt CG. Role of insulin signaling impairment, adiponectin and dyslipidemia in peripheral and central neuropathy in mice. Dis. Model Mech. 2014;7:625–633. doi: 10.1242/dmm.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel SC. Neurotrophic factors in the therapy of diabetic neuropathy. Am J Med. 1999;107:34S–42S. doi: 10.1016/s0002-9343(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Archer AG, Watkins PJ, Thomas PK, Sharma AK, Payan J. The natural history of acute painful neuropathy in diabetes mellitus. J. Neurol., Neurosurg, Psychiatry. 1983;46:491–499. doi: 10.1136/jnnp.46.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr. Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Bonnington JK, McNaughton PA. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britland ST, Young RJ, Sharma AK, Clarke BF. Acute and remitting painful diabetic polyneuropathy: a comparison of peripheral nerve fibre pathology. Pain. 1992;48:361–370. doi: 10.1016/0304-3959(92)90085-P. [DOI] [PubMed] [Google Scholar]
- Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med. 2004;99:55–65. doi: 10.1385/1-59259-770-x:225. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Jorge MC, Yaksh TL, Chaplan SR. Tactile allodynia and formalin hyperalgesia in streptozotocin-diabetic rats: effects of insulin, aldose reductase inhibition and lidocaine. Pain. 1996;68:293–299. doi: 10.1016/s0304-3959(96)03201-0. [DOI] [PubMed] [Google Scholar]
- Caravati C. Insulin Neuritis: a case report. Va Med Monthly. 1933;59:745–746. [Google Scholar]
- Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110:1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron. 2009;62:471–478. doi: 10.1016/j.neuron.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Randolph A, Yee D, Delafontaine P, Tennekoon G, Feldman EL. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J Neurochem. 1996;66:525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabby R, Sadeh M, Lampl Y, Gilad R, Watemberg N. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed Pharmacother. 2009;63:707–709. doi: 10.1016/j.biopha.2008.08.011. [DOI] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- DCCT research group d. Factors in development of diabetic neuropathy. Baseline analysis of neuropathy in feasibility phase of Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes. 1988;37:476–481. [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67:534–541. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH, Freeman R. Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain. 2015;138:43–52. doi: 10.1093/brain/awu307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons CH. Treatment induce neuropathyof diabetes-long term implications in Type 1 diabetes. J Diabetes Complication. 2017 doi: 10.1016/j.jdiacomp.2017.01.010. in press. [DOI] [PubMed] [Google Scholar]
- Guedes RP, Araujo AS, Janner D, Bello-Klein A, Ribeiro MF, Partata WA. Increase in reactive oxygen species and activation of Akt signaling pathway in neuropathic pain. Cell Mol Neurobiol. 2008;28:1049–1056. doi: 10.1007/s10571-008-9279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivalt CG, Rodriguez M, Wahren J, Calcutt NA. Efficacy Of A Long-Acting C-Peptide Analogue Against Peripheral Neuropathy In Streptozotocin-Diabetic Mice. Diabetes, Obes Metab. 2015;17:781–788. doi: 10.1111/dom.12477. [DOI] [PubMed] [Google Scholar]
- Kihara M, Zollman PJ, Smithson IL, Lagerlund TD, Low PA. Hypoxic effect of exogenous insulin on normal and diabetic peripheral nerve. Am J Physiol. 1994;266:E980–E985. doi: 10.1152/ajpendo.1994.266.6.E980. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Accili D. Signalling through IGF-I and insulin receptors: where is the specificity? Growth Horm & IGF Res. 2002;12:84–90. doi: 10.1054/ghir.2002.0265. [DOI] [PubMed] [Google Scholar]
- King MR, Anderson NJ, Liu C, Law E, Cundiff M, Mixcoatl-Zecuatl TM, Jolivalt CG. Activation of the insulin-signaling pathway in sciatic nerve and hippocampus of type 1 diabetic rats. Neuroscience. 2015;303:220–228. doi: 10.1016/j.neuroscience.2015.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp M, Srikantha M, Rajabally YA. Insulin neuritis and diabetic cachectic neuropathy: a review. Curr Diabetes Rev. 2013;9:267–274. doi: 10.2174/1573399811309030007. [DOI] [PubMed] [Google Scholar]
- Knorr-Held S, Meier C. Mast cells in human polyneuropathies: their density and regional distribution. Clin Neuropathol. 1990;9:121–124. [PubMed] [Google Scholar]
- Leow MK, Wyckoff J. Under-recognised paradox of neuropathy from rapid glycaemic control. Postgrad Med J. 2005;81:103–107. doi: 10.1136/pgmj.2004.021162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Brown JC, 3rd, Webster WW, Morrisett RA, Monaghan DT. Insulin potentiates N-methyl-D-aspartate receptor activity in Xenopus oocytes and rat hippocampus. Neurosci Lett. 1995;192:5–8. doi: 10.1016/0304-3940(95)11593-l. [DOI] [PubMed] [Google Scholar]
- Llewelyn JG, Gilbey SG, Thomas PK, King RH, Muddle JR, Watkins PJ. Sural nerve morphometry in diabetic autonomic and painful sensory neuropathy. A clinicopathological study. Brain. 1991;114(Pt 2):867–892. doi: 10.1093/brain/114.2.867. [DOI] [PubMed] [Google Scholar]
- Llewelyn JG, Thomas PK, Fonseca V, King RH, Dandona P. Acute painful diabetic neuropathy precipitated by strict glycaemic control. Acta Neuropatholo. 1985;72:157–163. doi: 10.1007/BF00685978. [DOI] [PubMed] [Google Scholar]
- Llewelyn JG, Thomas PK, Fonseca V, King RH, Dandona P. Acute painful diabetic neuropathy precipitated by strict glycaemic control. Acta Neuropathol. 1986;72:157–163. doi: 10.1007/BF00685978. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL Group DER. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzardo-Martins L, Martins DF, Stramosk J, Cidral-Filho FJ, Santos ARS. Glycogen synthase kinase 3-specific inhibitor AR-A014418 decreases neuropathic pain in mice: evidence for the mechanisms of action. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Morgado C, Silva L, Pereira-Terra P, Tavares I. Changes in serotoninergic and noradrenergic descending pain pathways during painful diabetic neuropathy: the preventive action of IGF1. Neurobiol Dis. 2011;43:275–284. doi: 10.1016/j.nbd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Mudaliar S, Henry RR. New oral therapies for type 2 diabetes mellitus: The glitazones or insulin sensitizers. Annu Rev Med. 2001;52:239–257. doi: 10.1146/annurev.med.52.1.239. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E, Rechler MM, Ishii DN. Effects of insulin, insulin-like growth factor-II, and nerve growth factor on neurite formation and survival in cultured sympathetic and sensory neurons. J Neurosci. 1986;6:1211–1219. doi: 10.1523/JNEUROSCI.06-05-01211.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibler J, Kleinridders A, Kuter-Luks B, Niehaves S, Bruning JC, Schwenk F. Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 2007;35:e54. doi: 10.1093/nar/gkm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J-y, Zheng X, Zukin SR, Bennett M VL. Insulin promotes rapid delivery of N-methyl-d- aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Murakawa Y, Zhang W, Xu G, Sima AA. Insulin receptor in rat peripheral nerve: its localization and alternatively spliced isoforms. Diabetes Metab Res Rev. 2000;16:354–363. doi: 10.1002/1520-7560(200009/10)16:5<354::aid-dmrr149>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sun R, Yan J, Willis WD. Activation of protein kinase B/Akt in the periphery contributes to pain behavior induced by capsaicin in rats. Neuroscience. 2007;144:286–294. doi: 10.1016/j.neuroscience.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Malik R, Harris N, Jakubowski JJ, Mody C, Rennie IG, Ward JD. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis) Diabetologia. 1996;39:329–335. doi: 10.1007/BF00418349. [DOI] [PubMed] [Google Scholar]
- Vital C, Vital A, Dupon M, Gin H, Rouanet-Larriviere M, Lacut JY. Acute painful diabetic neuropathy: two patients with recent insulin-dependent diabetes mellitus. J Peripher Nerv Syst. 1997;2:151–154. [PubMed] [Google Scholar]
- Weng H-RR, Gao M, Maixner DW. Glycogen synthase kinase 3 beta regulates glial glutamate transporter protein expression in the spinal dorsal horn in rats with neuropathic pain. Exper Neurol. 2014;252:18–27. doi: 10.1016/j.expneurol.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JL, Sokol DK, Smith LH, Snook RJ, Waguespack SG, Kincaid JC. Acute painful neuropathy (insulin neuritis) in a boy following rapid glycemic control for type 1 diabetes mellitus. J Child Neurol. 2003;18:365–367. doi: 10.1177/08830738030180051701. [DOI] [PubMed] [Google Scholar]
- Wuarin L, Guertin DM, Ishii DN. Early reduction in insulin-like growth factor gene expression in diabetic nerve. Exp Neurol. 1994;130:106–114. doi: 10.1006/exnr.1994.1189. [DOI] [PubMed] [Google Scholar]
- Xu J-T, Tu H-Y, Wen-Jun X, Xian-Guo L, Gui-Hong Z, Zhai C-H. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206:269279. doi: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Yorek MS, Obrosov A, Shevalye H, Lupachyk S, Harper MM, Kardon RH, Yorek MA. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst. 2014;19:205–217. doi: 10.1111/jns.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger DS, Rosoklija G, Hays AP, Trojaborg W, Latov N. Diabetic peripheral neuropathy: a clinicopathologic and immunohistochemical analysis of sural nerve biopsies. Muscle Nerve. 1996;19:722–727. doi: 10.1002/(SICI)1097-4598(199606)19:6<722::AID-MUS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]