Abstract
Aging is evident in most tissues and organ systems, but the mechanisms of aging are difficult to identify and poorly understood. Here, we test the hypothesis that aging results in uncorrected defects in stem cell and/or niche function, which lead to system failure. We used the spermatogonial stem cell (SSC) transplantation assay to determine the effect of aging on testis stem cell/niche function in mice. Between 12 and 24 months of age, male mice experienced a declining level of fertility associated with decreased testis weight, level of spermatogenesis, and total stem cell content. However, when stem cells were consecutively passaged at 3-month intervals to testes of young males, these stem cells continued to produce spermatogenesis for more than 3 years. Thus, SSC self-renewal continues long past the normal life span of the animal when the stem cell is continually maintained in a young niche/microenvironment. Moreover, these data suggest that infertility in old males results from deterioration of the SSC niche and failure to support an appropriate balance between stem cell self-renewal and differentiation.
Keywords: Spermatogonial stem cell, Stem cell self-renewal, Aging, Niche
Introduction
Stem cells are present in most, if not all, adult tissues and are generally located on the basement membrane, where they interact with surrounding stromal cells that produce a protected region called the stem cell niche [1]. Aberrant regulation of the stem cell/niche compartment can have dramatic consequences on tissue maintenance, which in some cases may manifest as aging [2–4]. A fundamental question is whether aging results from deterioration of the stem cell self-renewal capability, the niche regulatory function, or both. An essential requirement for unequivocal evaluation is a functional assay in which stem cells can be removed from their cognate young or aged niches, transplanted to a heterologous recipient environment, and cause complete replacement of the dependent tissue or system. This prerequisite currently exists for only hematopoiesis [5], epidermis/hair [6], and spermatogenesis [7, 8].
Because of the life-long replication of stem cells, it is tempting to speculate that they may be immortal. However, one theory posits that stem cells have a finite replicalive potential that becomes exhausted in old age [2–4, 9]. Indeed, when hematopoietic stem cells (HSCs) are serially transplanted to lethally irradiated recipients, their regenerative capacity is exhausted by the fifth passage, hematopoiesis is not restored to the normal state, and the stem cell population never recovers to the level found in the unmanipulated animal [10, 11]. Consequently, the potential immortality of HSCs, the best characterized of adult stem cells, has been questioned. In addition, studies of the stem cell/niche unit and clonal development in the hematopoietic system are complicated because of the location of the HSC within the medullary cavity.
Spermatogenesis is a productive self-renewing system that is maintained by spermatogonial stem cells (SSCs) and exhibits age-related deficits that ultimately result in infertility. In the testis, the SSC/niche compartment is readily accessible. In addition, the SSC transplantation technique allows clonal evaluation of stem cell quantity and quality. Therefore, we used this system to test the hypothesis that deficits in SSC number or function cause age-related infertility in mice.
Materials and Methods
Donor Mice and Cell Collection
Donor testis cells were obtained from young, aging, and old ROSA26 mice (stock no. 002073; The Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) that express the Escherichia coii lacZ gene in all germ cells. For the serial transplantation experiments, donor cells were obtained from 6-month-old crypt-orchid adult mice that express the green fluorescent protein (GFP) transgene under the control of the chicken α-actin promoter/CMV (cytomegalovirus) enhancer (stock no. 003291; The Jackson Laboratory), which drives expression in all germ cells. The cryptorchid procedure was performed as previously described and results in a 25-fold enrichment of SSCs [12]. Donor testis cell suspensions were prepared by two-step enzymatic digestion, as previously described [13, 14]. The Animal Care and Use Committee of the University of Pennsylvania approved all experimental procedures in accordance with The Guide for Care and Use of Laboratory Animals of the National Academy of Sciences (assurance no. A3079-01).
Recipient Mice and Transplantation Procedure
Recipient mice for SSC transplantation were W or C57BL/6 × 129/SvCP F1 hybrids (C57 × 129; The Jackson Laboratory). W mice are congenitally infertile and lack endogenous spermatogenesis due to a mutation in the c-kit receptor tyrosine kinase gene [15, 16]. C57 × 129 mice were treated with busulfan (55–60 mg/kg, i.p.) at 4–6 weeks of age and transplanted 6 weeks later. Busulfan-treated recipient testes are virtually devoid of endogenous spermatogenesis. Donor testis cells were introduced by efferent duct injection into the testes of recipient mice, as previously described [14].
Two to three months after transplantation, recipient mouse testes were collected and evaluated for colonies of donor-derived spermatogenesis. Testes transplanted with ROSA26 (lacZ) donor testis cells were stained with X-gal (5-bromo-4-chloro-3-indolyl β-d-galactoside) to visualize blue, donor-derived colonies of spermatogenesis. In whole testes, LacZ staining allows more accurate measurements than does GFP fluorescence. Colony number and colony length were determined using a dissecting microscope (Leica Microsystems, Bannockburn, IL, hup://www.leica.com).
For the serial transplantation experiments, green (GFP) donor spermatogenie colonies were visualized using an epifluorescent microscope (Leica Microsystems) with a GFP filter cube and a digital imaging system. This strain of mice was used because the testes do not require fixing to evaluate donor SSC colonization. Thus, live stem cells could be recovered for transplantation. After analysis, recipient testes were subjected to two-step enzymatic digestion, as described above, and the resulting cell suspension was retransplanted into new recipient mice.
Real-Time Polymerase Chain Reaction Analysis of Glial Cell-Derived Neurotrophic Factor (GDNF) and GDNF Family Receptor-α-1 Expression
To evaluate gene expression in the naturally aging mouse testis, males were divided into three groups based on age and testis weight: (a) young-fertile (2–4 months of age, n = 6, average testis weight of 92.5 ± 2.2 mg), (b) aging-fertile (15–19 months of age, n = 4, average testis weight of 81.1 ± 4.2 mg), and (c) aging-infertile (15–19 months of age, n = 4, average testis weight of 32.1 ± 0.9 mg). As a control, mature males treated with busulfan (60 mg/kg, n = 3, average testis weight of 24.7 ± 1.6 mg) were used to provide testes depleted of all germ cells, including stem cells, but with functional niches. Total cellular RNA was isolated using the Trizol method (Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and 1 μg of RNA from each sample was reverse-transcribed to cDNA using random hexamer primers and Superscript II reverse transcriptase (Invitrogen). Quality of RNA samples was determined by denaturing agarose gel electrophoresis and measurement of the 260/280 ratio. Only samples with a 260/280 ratio of 1.8 or higher were used for reverse transcription. Quality of the cDNA was determined by conventional polymerase chain reaction (PCR) for GAPDH (glyseraldehyde-3-phosphate dehydrogenase). Realtime PCR analyses were conducted using 20 ng of template cDNA/reaction and TaqMan MGB assays for GDNF, GDNF family receptor-α-1 (GFR-α-1), and GATA4 (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Samples were run in triplicate, and the average Ct (threshold cycle) values for GDNF and GFR-α-1 were normalized to GATA4. The ΔΔCt method was used to provide a quantitative assessment of fold change compared with the young-fertile animals.
Statistical Analysis
All statistical analyses were conducted using SPSS version 13 software (SPSS, Inc., Chicago, http://www.spss.com). Significant differences in average testis weight of males at different ages were determined using the Piecewise linear model. Stem cell content in the testes of young and aging males was analyzed with the general linear model, in which significant differences between ages was determined using Tukey's honesdy significant difference multiple comparisons test. Colony number and length from serially transplanted SSCs were analyzed using a linear regression and significant differences determined by single-factor analysis of variance (ANOVA). Analysis for significant differences between means in real-time PCR experiments was determined using the general linear model for ANOVA, and multiple comparisons were conducted using Tukey's post hoc test.
Results
Because the SSC transplantation assay allows quantitative evaluation of SSC number and clonal expansion, we examined the effect of aging on the stem cell/niche unit in this system. Male mice were maintained for periods up to 21 months in groups of four, prior to being individually housed with females for 3 months before sacrifice, and if a male sired progeny during this period, it was judged fertile. Males were considered infertile if no offspring were produced during the 3-month period. As expected from previous studies on mice [17, 18], male fertility was found to decline after approximately 12 months (Table 1). All males 12 months of age and younger were fertile; fertility deficits were evident between 14 and 18 months of age (only 4 of 11 males were fertile); from 20–24 months of age, all animals were infertile. Consistent with the fertility results, testis weight was constant during the first 12 months of life (p = .16) but underwent a significant decrease during the subsequent 12 months (p < .05; Fig. 1A). In an adult male, approximately 95% of testis weight arises from the germ cells in the seminiferous tubules [19]. Therefore, as anticipated from the decline in fertility and testis weight, the testes of older males were characterized by decreased number of seminiferous tubules with spermatogenesis (Fig. 1B, spermatogenesis is defined by the presence of multiple germ cell layers). However, the presence of SSCs is particularly difficult to ascertain histologically, because they cannot be easily distinguished in the seminiferous tubules and are extremely rare (one stem cell in 3,000–4,000 total testis cells [19]).
Table 1. Fertility in aging male mice.
| Age (months) | Fertile/total |
|---|---|
| 2 | 4/4 |
| 4 | 4/4 |
| 6 | 4/4 |
| 8 | 4/4 |
| 10 | 4/4 |
| 12 | 4/4 |
| 14 | 2/4 |
| 16 | 1/4 |
| 18 | 1/3 |
| 20 | 0/3 |
| 22 | 0/1 |
| 24 | 0/2 |
Four males (Fig. 1) were housed together and then mated individually with females 3 months before being sacrificed at the age indicated. Males in the 2-, 4-. and 6-month groups were housed with females for shorter periods of time. Group size at 18 months and older was reduced by age-related mortality.
Figure 1.
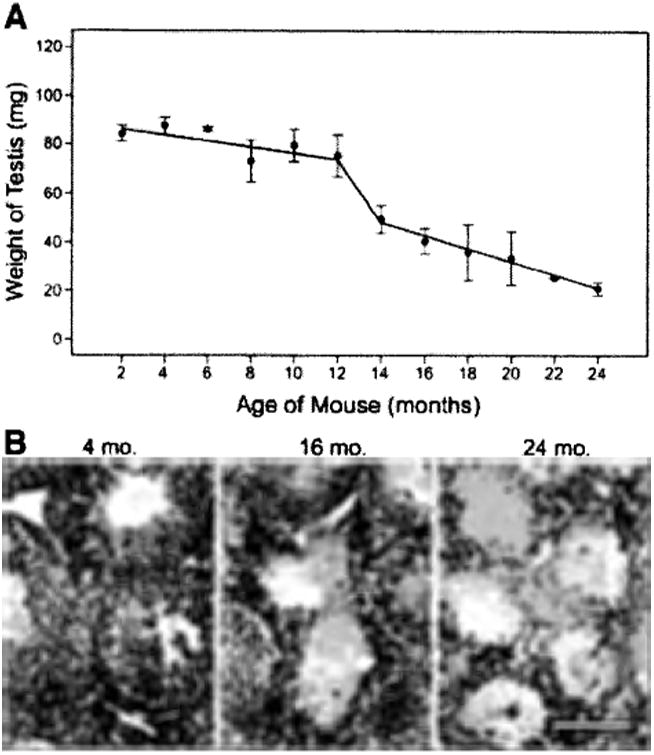
Testis weight and morphological appearance of normal spermatogenesis decrease with age. (A): Testis weights (mg) were determined for male ROSA26 mice from 2-24 months of age. (B): Histological evaluation of testes from aging mice. Left: Young males (4 months) had complete spermatogenesis in all seminiferous tubules. Center: Testes of aging males (16 months) had tubules with spermatogenesis as well as empty tubules. Right: Spermatogenesis was absent in the seminiferous tubules of old males (24 months). Stain = hematoxylin and eosin. Scale bar = 100 μm.
To determine stem cell activity, testes recovered at each time point were digested to a single-cell suspension and transplanted to testes of recipient males (∼3 months of age) in which endogenous spermatogenesis had been ablated by treatment with busulfan [7]. This assay provides a quantitative estimate of the stem cell content, because each transplanted donor stem cell generates a colony of spermatogenesis. Furthermore, clonal analysis is possible because each colony represents the expansion of germ cells (stem and differentiating) from a single transplanted SSC ([20]; Fig. 2A, inset). Based on the fertility data in Table 1 and morphological data in Figure 1, the transplantation results were divided into three groups, characterized as young (fertile, 2–12 months), aging (declining fertility, 14–18 months), and old (infertile, 20–24 months). All young animals were fertile with normal-size testes (80.35 ± 11 mg; mean ± SD, n = 26), whereas the aging group contained both normal and regressed testes (40.8 ± 22.5 mg, n = 14), and all males in the old group were infertile with regressed testes (26.3 ± 11.3 mg, n = 12). Both fertile and infertile animals were included in the aging group to provide a representation of the entire population. Analysis of recipient animals 3 months after transplantation (Fig. 2A) revealed that young animals had approximately 18.9 ± 2.3 × 103 stem cells per testis (mean ± SEM; see calculation in Fig. 2 legend). However, there was a dramatic and significant age-associated decline in the number of stem cells per testis, and only 3.7% of stem cells present in young males were recovered from the testes of the old males (20–24 months of age, 0.7 ± 0.6 × 103 stem cells per testis, p = .002). Interestingly, whereas there was a sharp decline in the number of stem cells recovered from the testes of old males, the capacity of each remaining stem cell to produce a colony of spermatogenesis remained robust beyond the time of reproductive decline, because stem cells in old-infertile animals produced colonies of similar size (3.0 ± 0.3 mm) as those from young-fertile animals (3.4 ± 0.1 mm, p = .089; Fig. 2B). If stem cells were aging, one would expect to observe a decrease in colony length; thus, our results suggest that failure of stem cell niche factors, rather than stem cells per se, are the primary cause of reproductive deficits observed in old males.
Figure 2.
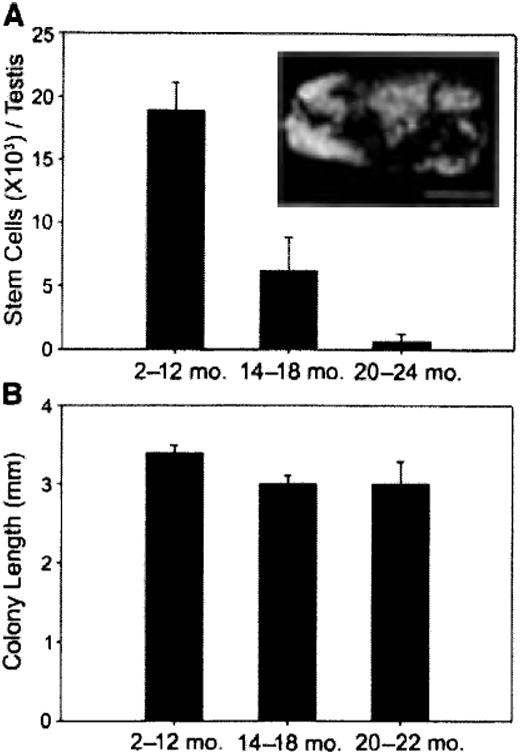
Stem cell activity in mouse testes. (A, inset): Macroscopic appearance of recipient testis transplanted with donor testis cell. Individual blue tubules indicate colonies of spermatogenesis arising from donor spermatogonial stem cells (SSCs). The SSC transplantation assay was used to evaluate stem cell activity in the testes of young (2–12 months), aging (14–18 months), and old (20–24 months) mice. Donor-derived colonies of spermatogenesis are unequivocally identified in recipient seminiferous tubules because they express a reporter gene (i.e., lacZ). Stem cell number (colony number) and kinetics of colony expansion (e.g., colony length) can be evaluated. Bar = 2 mm. (A, graph): Testis stem cell content for young (18.9 ± 2.3 ×103, n = 12 experiments, 145 recipient testes), aging (6.1 ± 2.6 ×103, n = 7 experiments, 84 recipient testes), and old (0.7 ± 0.6 7×103, n = 6 experiments, 68 recipient testes) ROSA26 mice (young vs, aging, p = .002; young vs. old, p = .001; aging vs. old, p = .329). Total stem cell number per donor testis = colonies per 105 cells transplanted × total cells recovered per donor testis 7 × 20 to account for transplantation efficiency [30]. (B): Colony length (mm) from donor testis cells of young (3.4 ± 0.1, n = 24 recipients, 248 colonies), aging (3.0 ± 0.1, n = 12 recipients, 120 colonies), and old (3.0 ± 0.3, n = 10 recipients, 128 colonies) males was determined in recipients by using a dissecting microscope and digital imaging system (young vs. aging, p = .111; young vs. old, p = .089; aging vs. old, p = .977). No stem cell activity was recovered from testes of 24-month-old males. Bars are mean ± SEM
The SSC niche is comprised, in part, of Sertoli cells and the factors they produce. GDNF, a member of the transforming growth factor-β (TGF-β) super family, is produced by Sertoli cells and signals through a receptor complex, including Ret receptor tyrosine kinase and GFR-α-1, that are expressed by spermatogonia. Previous research has demonstrated that both the GDNF ligand and GFR-α-1 receptor are required for normal regulation of SSC self-renewal and differentiation [21, 22]. We examined the gene expression of GDNF in the testes of males, as a potential measure of niche function, during aging using real-time PCR. The data revealed a biphasic change in GDNF expression (Fig. 3). Relative to young-fertile controls (2–4 months), aging-fertile animals (15–19 months) exhibited a 60% increase in GDNF expression (p ≤ .01), whereas in aging-infertile males (15–19 months) GDNF expression was reduced to 73% of young-fertile control values (p ≤ .01; Fig. 2). The initial increase in GDNF expression in aging-fertile animals may reflect a compensatory response by Sertoli cells to systemic age-related deficits (e.g., serum factors as recently described [23]) or changes in other germ cell types. However, GDNF expression ultimately declines and infertility appears in aging-infertile animals (Fig. 3). Interestingly, busulfan-treated testes (4 months) that are also devoid of endogenous germ cells but are known to have functional stem cell niches [7, 8] displayed a 430% increase in GDNF expression (p ≤ .001). This observation suggests that normal-functioning Sertoli cells in busulfan-treated males respond to a loss of germ cells by increasing niche factor production. However, this same response does not occur in aging-infertile testes (compare yellow and blue bars in Fig. 3), suggesting a Sertoli cell (niche) malfunction. Decreased GFR-α-1 expression in aging-infertile and busulfan-treated testes (p ≤ .01) appears to reflect the lack of germ cells that express this receptor.
Figure 3.
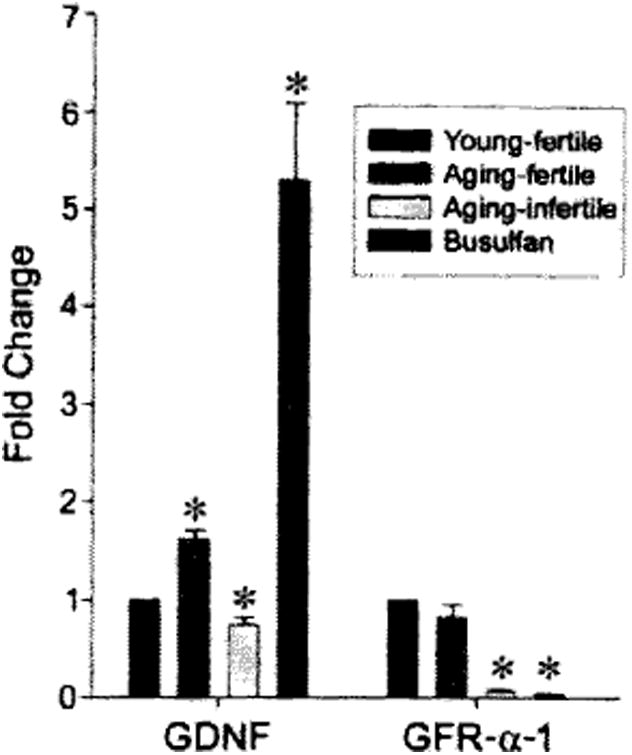
Real-time PCR analysis of GDNF and GFR-α-1 expression in the testes of young, aging, and busulfan-treated mice, using TaqMan MGB assays (Applied Biosystems). Young-fertile animals (black bars) were 2–4 months old, and testes weighed 92.5 ± 2.2 mg (n = 6). Aging-fertile animals (red bars) were 15–19 months old, and testes weighed 81.1 ± 4.2 mg (n = 4). Aging-infertile animals (yellow bars) were 15–19 months old, and testes weighed 32.1 ± 0.9 mg (n = 4). Busulfan-treated animals (blue bars) were 4 months old, and testes weighed 24.7 ±1.6 mg (n = 3). Data were normalized by comparison with the transcription factor GATA4, which is constitutively expressed in Sertoli cells, and reported as fold change (mean ± SEM) relative to the young-fertile control values. Asterisk indicates significant difference from control. Abbreviations: GDNF, glial cell line-derived neurotrophic factor; GFR, glial cell-derived neurotrophic factor family receptor; PCR, polymerase chain reaction.
To measure aging effects on the stem cell independent of the niche, SSCs from young (6 months of age), cryptorchid, GFP transgenic males were transplanted to young (3 months of age), busulfan-treated recipient males. Cryptorchid donor males were used to enrich the testis cell population for stem cells [12] and to standardize the starting cell suspension, because cryptorchid donor males contain no differentiating germ cells [24, 25]. Three months after donor cell transplantation, the recipient males were sacrificed and the testes were examined for the length (Fig. 4A) and number (Fig. 4B) of green donor-derived colonies of spermatogenesis. After analysis, testes from these recipients were digested to a single-cell suspension, which was transplanted to the testes of new young recipient males. This process was repeated seven more times at intervals of 91–104 days. Given that the age of stem cells from the original cryptorchid male was 278 days at the time of analysis (6 months of age at transplant + 98 days to analysis), at the time of the last recovery and analysis, the stem cells had been replicating for a total of 962 days or 32 months, always in the SSC niche of a young, busulfan-treated male (age 3–6 months). Average colony length in the first group of recipients was 5.4 ± 0.3 mm (n = 11 recipients, 310 colonies) and decreased to an average of 3.5 ± 0.1 mm (n = 60 recipients, 1,022 colonies) for the second to eighth recipient (Fig. 4A). The initial longer colony length probably reflects the origin of the stem cell population from cryptorchid testes in which differentiating germ cells that modulate stem cell replication are absent. Thus, these cells may be poised for an initial rapid expansion when placed in the permissive, busulfan-treated recipient testes. Most if not all subsequent stem cells came from areas of active donor cell spermatogenesis in busulfan-treated recipient testes, and in these transplantations donor cell-derived colony length remained constant (3.5 ± 0.1 mm, r2 = .003, p = .912).
Figure 4.
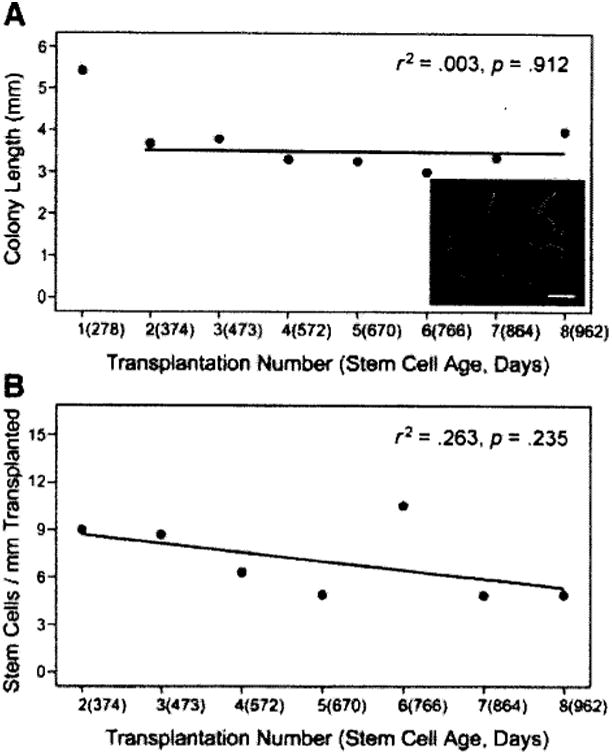
Serial transplantation of mouse spermatogonial stem cells. In the primary transplant, donor testis cells were obtained from 6-month-old, cryptorchid, green fluorescent protein (GFP) mice. (A): Three months after transplantation, the testes of recipient animals were evaluated for green colonies of donor spermatogenesis (inset, scale bar = 2 mm). After the initial transplant, seven more serial transplants were performed, resulting in a final donor stern cell age of 32 months (962 days). Colony number and colony length were recorded during each serial transfer. The length (mm) of each green colony was determined using an epifluorescent microscope, a GFP filter cube, and a digital imaging system. (B): Colonies per millimeter is a retrospective value that is determined based on the number of colonies produced per millimeter of green tubule observed in the previous transplant. Thus, there is no value for the first transplant.
Because colony length was determined at each passage, it was possible to calculate the number of colonies generated per millimeter of green tubule in the subsequent transplant (Fig. 4B). There was no significant change (r2 = .267, p = .235) in colonies generated per millimeter of donor-derived spermatogenesis during the 32-month study; each millimeter of donor testis tubule contained approximately seven stem cells (Fig. 4B). Although there is a small, but insignificant, downward tendency of the regression line for stem cells per millimeter of transplanted colony, the colony length does not change, consistent with a previous SSC serial transplantation study [26]. Therefore, the question of whether the SSC is immortal or just very long-lived remains open.
Stem cells are defined functionally by their capacity to self-renew and produce differentiated daughter cells. Serially transplanted donor stem cells (eighth transplant, 962 days) produce normal colonics of spermatogenesis when evaluated 3–4 months after the ninth transplant (total age, 1,060 and 1,102 days), as evidenced by the presence of green colonies of spermatogenesis (Fig. 5A) with multiple layers of green germ cells (Fig. 5B). Similarly, when serially transplanted donor stem cells (ninth transplant) were injected into W recipient mouse testes, which are genetically incapable of producing endogenous spermatogenesis, complete donor spermatogenesis was generated with multiple germ cell layers and sperm in the lumen of the seminiferous tubules (Fig. 5D).
Figure 5.
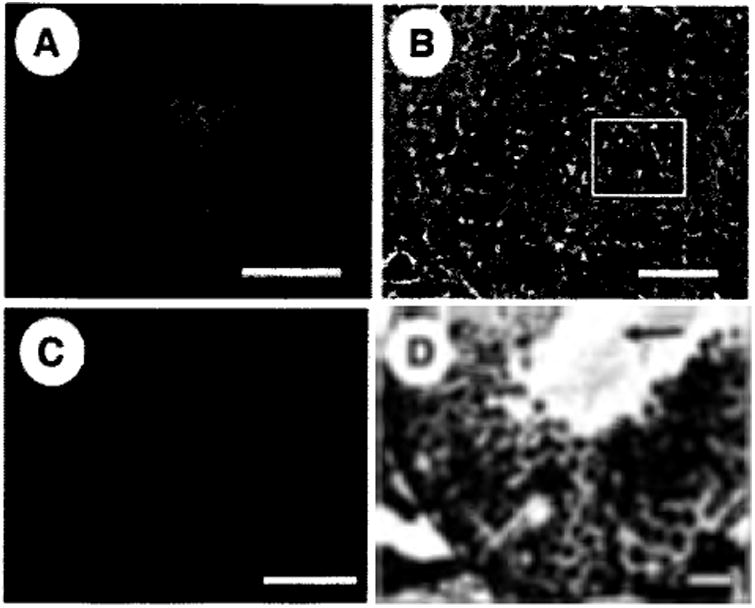
Histological evaluation of retransplanted seminiferous tubules. Serially retransplanted (eighth transplant, 962 days) donor spermatogonial stem cells produced normal colonies (∼3, 5, and 8 months after ninth transplant, total age 1,060, 1,102, and 1,216 days) of spermatogenesis as defined by the presence of multiple germ cell layers and sperm in the seminiferous tubule lumen of busulfan-treated (A, B) or W sterile (C, D) recipient mice. (A): Whole-mount image of green colony of donor spermatogenesis (1,102 days). (B): Frozen section through a colony of donor spermatogenesis (1,060 days). Fluorescent image showing multiple layers of green donor germ cells is overlayed on the phase-contrast image of the same section. The basement membrane is clearly visible on the left side of the section (arrow), and the boxed area indicates the center of the seminiferous tubule. (C): Whole-mount image of a green colony of donor spermatogenesis in a W recipient testis (1,216 days). (D): Cross-section through colony of donor spermatogenesis in (C). Complete spermatogenesis can be seen with sperm tails in the lumen of the seminiferous tubule. Arrow indicates sperm tails in the center of the seminiferous tubule. Scale bars = 2 mm (A, C), 50 μm (B, D).
Discussion
The separation of stem cell and niche function has been difficult for all adult stem cell systems. A variety of factors have contributed to this problem, including inaccessibility of the stem cell/niche unit, difficulty in identifying the rare stem cell, and variation in adequacy of functional assays. The SSC transplantation system provided a powerful tool that allowed us to demonstrate a dramatic decline in the number of functional stem cells in aging males. This decline might be attributed to failure of the stem cell to self-renew or of the niche to maintain stem cell function.
Although recent development of SSC culture techniques for the mouse indicates replication with an increase in numbers occurs in vitro [21, 27], the period of maintenance reported has been less than the life span of the mouse, and one cannot be certain that in vitro conditions have not modified the stem cell and facilitated continuous replication. However, continuous self-renewal of the SSC in vivo without significant loss in number observed in our experiments indicates that the cell is long-lived and potentially immortal. It is unclear whether this longevity is a characteristic shared with other adult tissue stem cells or unique to the germline, which is essential for species survival.
Stem cells are generally thought to be immortal, but this remains debatable. In the hematopoietic system, serial transplant studies indicate that HSCs are long-lived, but not immortal [10, 11]. However, it has been suggested that in those studies, the stress of transplantation may have decreased the potential life span of stem cell function [28]. Our experiments indicate that when SSCs are maintained, through serial transplantation, in a young, busulfan-treated testis environment, they can replicate well beyond the normal time of reproductive decline in vivo. These results are consistent with the observations of Ogawa and coworkers [26] and suggest that it is the niche, and not the stem cell, that deteriorates in the testes of aging males.
Finally, the SSC niche of aging males was directly assayed by Zhang and coworkers [29], who recently demonstrated that SSCs from young, fertile males were unable to establish colonies of spermatogenesis when transplanted into the testes of old, infertile males. A possible decline in SSC function due to aging irrespective of the niche was also suggested by Zhang et al. [29], because a decline in colony number and length was observed when SSCs from 2-year-old atrophied testes were transplanted into young, busulfan-treated recipients. In the current study, a similar finding for old testes was not observed (Fig. 2B), and colony length remained constant for more than 2 years after serial transplantation (Fig. 4A), suggesting that intrinsic aging of the SSC does not occur or proceeds more slowly than does aging of the niche. Further research will be needed to resolve the difference between the two studies.
Faithful GDNF signaling is required to maintain normal spermatogenesis in vivo [22] and is the critical factor for sustaining SSC proliferation in vitro [21]. Therefore, the observed fivefold increase in GDNF expression in the germ cell-depleted testes of busulfan-treated males may reflect a response by Sertoli cells to stimulate new germ cell proliferation. Thus, the busulfan-treated testis may be a particularly permissive environment for SSC engraftment and colony expansion and may not directly reflect the normal physiological microenvironment. However, our data indicate that, when continuously exposed to this altered environment, SSCs can divide for a very long time and intrinsic or normal aging does not occur. Thus, the theory that aging results from exhaustion of stem cell replicative potential is not supported in the case of spermatogenesis when SSCs are continually exposed to a high-GDNF environment. The biphasic GDNF expression in aging-fertile, as opposed to aging-infertile, testes may reflect an initial response by Sertoli cells to a deteriorating systemic milieu and/or stem cell/niche compartment (increased GDNF), followed by the ultimate demise of the stem cell niche (decreased GDNF).
Conclusion
The results of the current study focused on spermatogenesis are consistent with the observations of Conboy and coworkers [23], who demonstrated that aged liver and muscle stem/progenitor cells were rejuvenated upon exposure to young blood. Our studies suggest that declining function of supporting cells that constitute the stem cell niche is the major factor in age-related male infertility. This impaired function may be due to a lack of systemic (e.g., endocrine hormones) or local (e.g., paracrine factors) components. Also, an inability of the supporting Sertoli cells to respond to signals, such as a decline in FSH (follicle-stimulating hormone) responsiveness, or intrinsically produce important molecules (e.g., GDNF) may be causative reasons. Because aging is a phenomenon that affects all self-renewing tissues in the body and may result from defects in the local microenvironment, niche therapy could provide an avenue to retard the aging process of critical tissues.
Acknowledgments
We thank Dr. H. kubota for suggestions regarding the experiments and comments on the manuscript, C. Freeman and R. Naroznowski for assistance with animal maintenance and experimentation, J. Chittams for statistical analyses, and J. Hayden for photography. K.E.O. is supported by National Institutes of Health grant AG 024992. R.L.B. is supported by National Institutes of Health grants HD 044445 and AG 024992, the Commonwealth and General Assembly of Pennsylvania, and the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation. B.-Y.R. is currently affiliated with Department of Animal Science and Technology, Chung-Ang University, Gyungki-Do, Korea. K.E.O. is currently affiliated with Departments of OB/GYN and Reproductive Sciences and Molecular Genetics and Biochemistry, Pittsburgh Development Center of Magee-Women's Research Institute, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Footnotes
Disclosures: The authors indicate no potential conflicts of interest.
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Rao MS, Mattson MP. Stem cells and aging: Expanding the possibilities. Mech Ageing Dev. 2001;122:713–734. doi: 10.1016/s0047-6374(01)00224-x. [DOI] [PubMed] [Google Scholar]
- 3.Trosko JE. Human stem cells as targets for the aging and diseases of aging processes. Med Hypotheses. 2003;60:439–447. doi: 10.1016/s0306-9877(02)00446-2. [DOI] [PubMed] [Google Scholar]
- 4.Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DE. Competitive repopulation: A new assay for long-term stem cell functional capacity. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 6.Blanpain C, Lowry WE, Geoghegan A, et al. Self-Renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger H, Rennebeck G, Van Zant G. Regulation of hematopoietic stem cell aging in vivo by a distinct genetic element. Proc Natl Acad Sci U S A. 2005;102:5102–5107. doi: 10.1073/pnas.0408654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siminovitch L, Till JE, McCulloch EA. Decline in colony forming ability of marrow cells subjected to serial transplantation to irradiated mice. J Cell Physiol. 1964;64:23–31. doi: 10.1002/jcp.1030640104. 23-31. [DOI] [PubMed] [Google Scholar]
- 11.Ogden DA, Micklem HS. The fate of serially transplanted bone marrow cell populations from young and old donors. Transplantation. 1976;22:287–293. doi: 10.1097/00007890-197609000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara T, Avarbock MR, Brinstcr RL. Functional analysis of spermatogonial stem cells in steel and cryptorchid infertile mouse models. DevBiol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 13.Bellvé AR, Cavicchia JC, Millette CF, et al. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa T, Aréchaga JM, Avarbock MR, et al. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 15.Silvers WK. The Coat Colors of Mice. New York: Springer-Verlag; 1979. Dominant spotting, patch, and rump-white; pp. 206–241. [Google Scholar]
- 16.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- 17.Parkening TA, Collins TJ, Au WW. Paternal age and its effects on reproduction in C57BL/6NNia mice. J Gerontol. 1988;43:B79–B84. doi: 10.1093/geronj/43.3.b79. [DOI] [PubMed] [Google Scholar]
- 18.Tanemura K, Kurohmaru M, Kuramoto K, et al. Age-related morphological changes in the testis of the BDFl mouse. J Vet Med Sci. 1993;55:703–710. doi: 10.1292/jvms.55.703. [DOI] [PubMed] [Google Scholar]
- 19.Tegelenbosch RAJ, de Rooij DO. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Ebata KT, Nagano MC. Genetic analysis of the clonal origin of regenerating mouse spermatogenesis following transplantation. Biol Re-prod. 2003;69:1872–1878. doi: 10.1095/biolreprod.103.019273. [DOI] [PubMed] [Google Scholar]
- 21.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng X, Lindahl M, Hyvonen ME, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 23.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 24.Nishimune Y, Aizawa S, Komatsu T. Testicular germ cell differentiation in vivo. Fertil Steril. 1978;29:95–102. doi: 10.1016/s0015-0282(16)43045-1. [DOI] [PubMed] [Google Scholar]
- 25.de Rooij DG, Okabe M, Nishimune Y. Arrest of spermatogonial differentiation in jsd/jsd, S117H/S117H, and cryptorchid mice. Biol Reprod. 1999;61:842–847. doi: 10.1095/biolreprod61.3.842. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Ohmura M, Yumura Y, et al. Expansion of murine spermatogonial stem cells through serial transplantation. Biol Reprod. 2003;68:316–322. doi: 10.1095/biolreprod.102.004549. [DOI] [PubMed] [Google Scholar]
- 27.Kanatsu-Shinohara M, Ogonuki N, Inoue K, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Ebata KT, Robaire B, et al. Aging of male germ line stem cells in mice. Biol Reprod. 2005;74:119–124. doi: 10.1095/biolreprod.105.045591. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara T, Orwig KE, Avarbock MR, et al. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci U S A. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]


