Abstract
Purpose
Dysfunctional mitochondria are considered to be the major source of intracellular reactive oxygen species and play a central role in the pathophysiology of myocardial ischemia/reperfusion. This study sought to determine effects of mitochondria-targeted cytoprotective peptide SBT-20 on myocardial infarct size in two different models of ischemia/reperfusion.
Methods
For in vivo studies, anesthetized Sprague Dawley rats were subjected to 30 minutes of coronary artery occlusion followed by 3 hours of reperfusion. Rats received saline (control), low dose SBT-20 (0.3 mg/kg/hour) or high dose SBT-20 (3 mg/kg/hour) treatment (n=15 rats in each group). Saline or SBT-20 were delivered into the jugular vein starting 5 minutes after coronary artery occlusion and were continued for one hour post coronary artery reperfusion. Body temperature, heart rate and blood pressure were monitored during the procedure. At the end of 3 hours reperfusion, the ischemic risk area, no-reflow area, and infarct size were measured. In separate in vitro studies, isolated rat hearts were exposed to 20 minutes global ischemia, followed by SBT-20 administration (1µM) or no SBT-20 (control) throughout the 2h reperfusion. In vitro studies were conducted in cells and heart mitochondria to ascertain the mitochondrial effects of SBT-20 on mitochondrial respiration and reactive oxygen species production.
Results
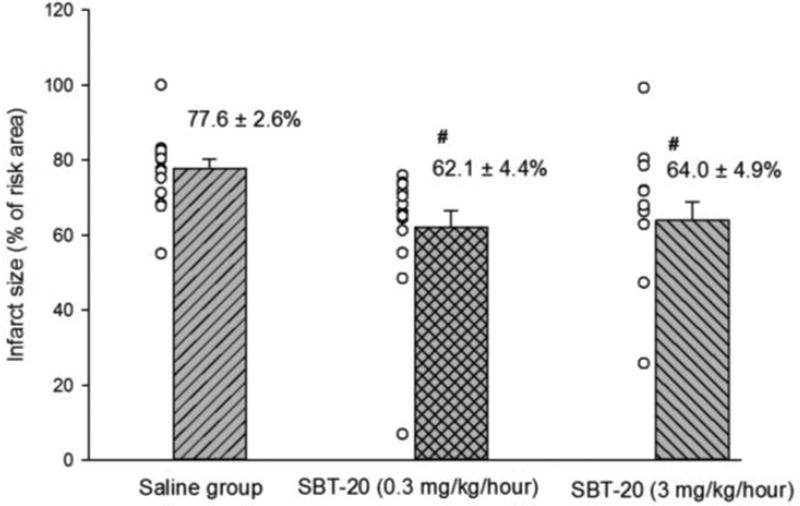
In the in vivo study, the ischemic risk areas (as a percentage of the left ventricle) were similar among the saline (49.5 ± 2.3%), low dose SBT-20 (48.6 ± 2.1%), and high dose SBT-20 groups (48.7 ± 3.0%). Treatment with SBT-20 significantly reduced infarct size (as a percentage of risk area) in low dose (62.1 ± 4.4%) and high dose (64.0 ± 4.9%) compared with saline treatment (77.6 ± 2.6%, p=0.001 for both doses). There was no difference in infarct size between low and high dose SBT-20 treatment. The no-reflow areas (as a percentage of the risk area) were comparable among the saline (23.9 ± 1.7%), low dose SBT-20 (23.7 ± 2.8%), and high dose groups (25.0 ± 2.1%). Body temperature, heart rate and blood pressure were comparable among the 3 groups at baseline, during ischemia, and at the end of 3 hours of reperfusion. In the in vitro study, infarct size was reduced from 43.3 ± 2.6% in control group (n=11) to 17.2 ± 2.8% in the SBT-20 treatment group (n=5, p<0.05). There were no benefits of SBT-20 on recovery of left ventricular developed pressure, coronary flow, or maximal rates of contraction/relaxation. In cell studies, treatment with SBT-20 significantly improved maximal mitochondrial respiration in response to an H2O2 challenge. In isolated mitochondria, reactive oxygen species production was significantly blunted following treatment with SBT-20.
Conclusions
In summary, SBT-20 significantly reduced infarct size in two different models of myocardial injury, but did not affect hemodynamics or no-reflow area in rat heart. The reduction in injury is postulated to involve stabilization of mitochondrial function and reduced mitochondrial production of ROS.
Keywords: myocardial infarction, mitochondria, peptide, cardioprotection
Although it has been recognized that early and successful reperfusion of the occluded coronary artery by percutaneous coronary interventions or thrombolysis is the most effective approach to limit ischemia-induced cardiac necrosis in the management of ST-segment elevation myocardial infarction, adjunctive pharmacologic or other therapies, together with reperfusion, may further salvage the ischemic jeopardized myocardium. The adjunctive therapeutic agents can be administered while the coronary artery is still occluded to increase the tolerance to ischemia, during reperfusion only to reduce lethal reperfusion injury, or by a combined reduction in both ischemic and reperfusion injury [1]. Numerous agents, such as atorvastatin, cyclosporine, nicorandil, adenosine, glucose-insulin-potassium, abciximab, etc., have been assessed as adjunctive therapies during reperfusion [2]. However, few adjunctive pharmacologic therapies have demonstrated any cardioprotective benefits in clinical trials so far.
Mitochondria are the major source of intracellular reactive oxygen species (ROS) in cardiomyocytes during ischemia and early reperfusion, and myocardial ischemia/reperfusion induces defects in mitochondrial complex I and complex III activity that can account for enhanced superoxide production and ischemia/reperfusion injury [3,4]. SBT-20 belongs to a class of cell-permeable peptides which target the inner mitochondrial membrane, reduce ROS, normalize electron transport chain function and ATP generation, and represent a promising therapeutic approach for treating ischemia/reperfusion induced mitochondrial dysfunction [5]. Previously, Cho et al [6] subjected rats to 60 minutes left coronary artery occlusion followed by 60 minutes reperfusion. Mitochondria-targeted peptide SBT-20, also known as SS-20 (3 mg/kg) was intraperitoneally injected at 30 minutes before left coronary occlusion, and another dose was given at 5 minutes before reperfusion. SBT-20 significantly reduced myocardial lipid peroxidation and infarct size compared to saline treatment in the control group. Because the acute coronary syndrome is unpredictable in most cases, administration of SBT-20 before the onset of ischemia is unrealistic. It is unknown whether administration of SBT-20 after the ischemic insult has already commenced can reduce myocardial infarct size or the zone of no-reflow (an area of microvascular obstruction that prevents myocardial perfusion even after patency of the infarct-related epicardial coronary artery is re-established). There are no previous data on the effect of SBT-20 on no-reflow. Therefore, the purpose of our present study is to investigate whether the administration of SBT-20 given after coronary artery occlusion and during reperfusion results in cardioprotective effects, in contrast to the preischemic treatment with SBT-20 in Cho’s study, and to examine the mechanism of its potential benefit.
Methods
This investigation was approved by the Institutional Animal Care and Use Committees at Huntington Medical Research Institutes and East Carolina University. All of the procedures outlined in this study were performed in accordance with the guidelines for the care and use of laboratory animals (NIH publication No. 85-23, National Academy Press, Washington DC, revised 2011).
In vivo myocardial ischemia/reperfusion model and experimental groups
Female Sprague Dawley rats (approximately 200 gram body weight) were anesthetized intraperitoneally with ketamine (75 mg/kg) and xylazine (5 mg/kg). The animals were intubated and mechanically ventilated with room air. A water circulating heating pad was used to keep the rat body temperature at 37°C. Their necks and chests were prepared and catheters were inserted via cut downs into the jugular vein (for drug delivery) and carotid artery (for arterial waveform monitoring). Under clean conditions the chest cavity was opened through an incision in the 4th left intercostal space to expose the heart. The pericardium was gently removed to expose the anterior surface of the left ventricle. A 4-0 silk suture was placed under the proximal portion of the left coronary artery within the interventricular groove, just under the tip of the left atrial appendage. The ends of the suture were threaded through a small plastic tube. At the time of coronary artery occlusion the artery was pulled through the plastic tube and the tube was clamped. Coronary artery reperfusion occurred by releasing the clamp and watching the surface of the heart for reactive hyperemia.
Rats received saline, low dose SBT-20 (0.3 mg/kg/hour) or high dose SBT-20 (3 mg/kg/hour) infusion treatment (n=15 rats in each group). Saline or SBT-20 were delivered into the jugular vein starting 5 minutes after coronary artery occlusion and were continued for one hour post coronary artery reperfusion. The coronary artery was occluded for 30 minutes followed by 3 hours of reperfusion.
Rectal temperature, heart rate and blood pressure were monitored throughout the protocol.
Assay of myocardial ischemic risk area, no-reflow area, and infarct area in in vivo model
At the end of 3 hours of reperfusion, Thioflavin S solution was injected into the jugular vein during the last one minute of reperfusion to assess the distribution of the no-reflow phenomenon (also known as the zone of microvascular obstruction). Areas receiving perfusion demonstrate a yellow green fluoresces of the Thioflavin S dye, while the no-reflow areas appear dark or non-fluorescent when heart slices are visualized under UV light. At the end of the period of reperfusion, the proximal coronary artery was briefly re-occluded and blue dye (Super imperse blue) was injected into the jugular vein with the artery re-occluded. Blue dye circulates only to perfused areas and does not reach the ischemic zone (which appears pink when viewing heart slices under standard white light). At the end of this step, IV KCL was administered while the rats were under deep anesthesia, in order to stop the heart in a relative diastolic or relaxed state. The heart was excised, excess fat cleared from the surface of the heart, the heart was gently washed in clear saline, and then transected into 4 transverse slices from apex to base. The heart slices were photographed under white light in order to delineate the ischemic risk zone (pink) in contrast to the nonischemic regions (blue) that received the blue dye. The heart slices were then photographed under UV light in order to delineate the areas of perfusion by Thioflavin S (yellow-green fluorescent areas) versus the no-reflow zones (non-fluorescent). Finally the hearts were incubated in 1% triphenyl tetrazolium choride (TTC), a chemical that stains viable cells brick red, while dead or necrotic cells appear white to yellow. The photographs were used for planimetry in order to determine the percentage of each heart slice that was at risk, infarcted, or contained no-reflow. Planimetered photos were corrected for the weight of each heart slice and then the percentage of each left ventricle that was at risk (ischemic), demonstrated no-reflow, and was necrotic, was calculated. Infarct size and no-reflow zone were expressed as the percentage of the left ventricular ischemic risk zone that went on to develop ischemic necrosis.
Infarct size and cardiac function in isolated rat heart model
Isolated rat hearts were exposed to ischemia/reperfusion per our established protocols [7,8]. Excised hearts were instrumented on a modified Langendorff apparatus for the simultaneous recording of left ventricular developed pressure, coronary flow, and rates of contraction/relaxation continuously throughout 20 minutes global ischemia and 120 minutes of reperfusion. A subset of hearts were perfused with 1µM SBT-20 beginning at the onset of reperfusion and lasting throughout the 2 hours of reperfusion. At the end of the protocol hearts were sliced for assessment of myocardial infarct size as previously described [9,10].
Cellular Respiration and Mitochondrial Studies
Oxygen consumption rate (OCR) was measured by XFe96 and XF96 Seahorse Extracellular Flux Analyzers (Agilent Technologies, Santa Clara, California, USA). C2C12 cells (approximately 80% confluent) and were seeded in XF96 cell culture microplates at 1.5 × 104 cells per well in 100uL of DMEM containing 4.5g/L D-glucose, L-glutamine, and 110mg/L sodium pyruvate supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin. After overnight incubation (37 °C, 5% CO2), all but 20uL of media was aspirated from each well, and 170uL of pre-warmed DMEM containing saline, SBT-20, or N-acetylcysteine (NAC) was added to each well for a final volume of 190uL. After 1 hour incubation (37 °C, 5% CO), 500uM H2O2 was added to stress the cells for 4 hours. Following the 4-hour incubation, all but 20uL of media was aspirated and 180uL of pre-warmed Seahorse XF Media (XF Base Medium, 10mM Pyruvate, 10mM Glucose, and 2mM Glutamax) was added to each well. After one more additional aspiration/wash cycle with pre-warmed XF Seahorse Media the microplates were placed into the XFe96 or XF96 analyzer. A XF Cell Mitochondrial Stress Test was completed to assess the bioenergetic status of the cell [11]. After OCR baseline measurements, sequential injections of the ATPase inhibitor oligomycin, proton uncoupler FCCP, and complex III inhibitor antimycin A were added to obtain final concentrations of 1ug/mL, 2uM, and 2uM, respectively. Antimycin-corrected data are expressed in the manuscript (pmol of O2 per minute).
For mitochondrial ROS studies, mitochondria were isolated from the left ventricles of male Sprague-Dawley rats [12], and 75ug/mL were placed in an Oroboros Oxygraph 2k-Fluorometer (Oroboros Instruments, Innsbruck, Austria) for assessment of mitochondrial ROS production using established protocols [13,14]. Mitochondrial ROS production (37°C) was obtained by monitoring the production of H2O2 in medium containing Buffer Z with creatine, 10µM Amplex UltraRed, 30 U/ml Superoxide Dismutase (SOD), 4 U/ml Horseradish Peroxidase (HRP), 10uM succinate, and 1mM EGTA (pH=7.1). For each mitochondrial experiment, H2O2 production after succinate was obtained by calculating the area under the curve for a 7 minute epoch.
Statistical analysis
All data are reported as mean ± SEM. In the in vivo study, values among the 3 groups were compared with one way analysis of variance with Tukey post hoc test. In the in vitro study, values between the 2 groups were compared with Student’s t-test. Statistically significant differences were established at p < 0.05.
Results
In the in vivo study with 30 minutes of coronary artery occlusion followed by 3 hours reperfusion, the ischemic risk areas, expressed as percentage of total left ventricle, were similar among the saline (49.5 ± 2.3%), low dose SBT-20 (48.6 ± 2.1%) and high dose SBT-20 treatment group (48.7 ± 3.0%). Infarct size, expressed as percentage of ischemic area, was significantly reduced in the low dose SBT-20 (62.1 ± 4.4%) and high dose SBT-20 (64.0 ± 4.9%) compared with the saline treatment group (77.6 ± 2.6%, p=0.001) (Fig 1). This represents a 20% reduction in infarct size in the low dose SBT-20 group and a 17.5% reduction in the infarct size in the high dose SBT-20 group. There was no difference in infarct size between the low and high dose SBT-20 treatment group. The no-reflow area expressed as percentage of ischemic risk area, was comparable among the saline (23.9 ± 1.7%), low dose SBT-20 (23.7 ± 2.8%) and high dose SBT-20 treatment group (25.0 ± 2.1%).
Figure 1.
The infarct size, which was expressed as percentage of left ventricle ischemic risk area, were significantly smaller in SBT-20 treatment groups (both low and high dose) compared to control saline group. # p=0.001 Saline group vs. both low and high dose SBT-20 group.
Body temperature was maintained at 37 °C during the procedure. Table 1 and 2 showed the body temperature, heart rate and blood pressure at 5 representative time points: at (1) 5 minutes prior coronary artery occlusion; (2) 1 minute prior to coronary artery reperfusion; (3) 1 hour after reperfusion; (4) 2 hours after reperfusion; and (5) 3 hours after reperfusion. There were no differences in body temperature (Table 1) during the procedure among the 3 groups. Heart rate, systolic blood pressure and diastolic blood pressure were comparable among the 3 groups at 5 minutes prior to coronary occlusion, 1 minute prior to coronary reperfusion and at 3 hours after reperfusion (Table 2). However, both systolic and diastolic blood pressure was significantly lower in high dose SBT-20 treatment compared to low dose SBT-20 at 1 and 2 hour after reperfusion (Table 2).
Table 1.
Body temperature (°C) at different time point (n=15 in each group)
| Time point | Saline group | Low dose group | High dose Group |
p value |
|---|---|---|---|---|
| 1 | 37.04 ± 0.03 | 37.04 ± 0.01 | 37.04 ± 0.01 | NS |
| 2 | 36.96 ± 0.04 | 36.98 ± 0.03 | 36.99 ± 0.06 | NS |
| 3 | 36.93 ± 0.05 | 36.95 ± 0.04 | 37.05 ± 0.05 | NS |
| 4 | 36.97 ± 0.05 | 36.97 ± 0.04 | 36.95 ± 0.05 | NS |
| 5 | 36.97 ± 0.06 | 37 ± 0.05 | 37 ± 0.04 | NS |
Time point: at (1) 5 minutes prior coronary artery occlusion; (2) 1 minute prior to coronary artery reperfusion; (3) 1 hour after reperfusion; (4) 2 hours after reperfusion; (5) 3 hours after reperfusion. NS: not significant.
Table 2.
Heart rate (beats/minute) and blood pressure (mmHg) at different time point (n=15 in each group)
| Time point | Saline group | Low dose group | High dose Group | p value | |
|---|---|---|---|---|---|
| Heart rate | |||||
| 1 | 287 ± 6 | 286 ± 8 | 274 ± 7 | NS | |
| 2 | 281 ± 9 | 273 ± 9 | 264 ± 7 | NS | |
| 3 | 275 ± 7 | 277 ± 9 | 267 ± 7 | NS | |
| 4 | 280 ± 5 | 284 ± 6 | 268 ± 7 | NS | |
| 5 | 287 ± 6 | 292 ±8 | 272 ± 9 | NS | |
| Systolic blood pressure | |||||
| 1 | 90 ± 4 | 90 ± 4 | 84 ± 3 | NS | |
| 2 | 75 ± 2 | 75 ± 3 | 70 ± 2 | NS | |
| 3 | 78 ± 3 | 84 ± 4 | 70 ± 2 | 0.037* | |
| 4 | 70 ± 2 | 84 ± 5 | 69 ± 3 | 0.016* | |
| 5 | 70 ± 3 | 76 ± 5 | 70 ±4 | NS | |
| Diastolic blood pressure | |||||
| 1 | 68 ± 3 | 70 ± 4 | 62 ± 3 | NS | |
| 2 | 55 ± 2 | 56 ± 2 | 50 ± 2 | NS | |
| 3 | 56 ± 2 | 62 ± 2 | 47 ± 2 | <0.001† | |
| 4 | 49 ± 2 | 60 ± 2 | 46 ± 2 | 0.007* | |
| 5 | 47 ± 2 | 53 ± 4 | 45 ± 2 | NS | |
Time point: at (1) 5 minutes prior coronary artery occlusion; (2) 1 minute prior to coronary artery reperfusion; (3) 1 hour after reperfusion; (4) 2 hours after reperfusion; (5) 3 hours after reperfusion. NS: not significant.
Low dose vs. high dose.
High dose vs. low dose and saline.
Results of infarct size and cardiac function in isolated rat heart model
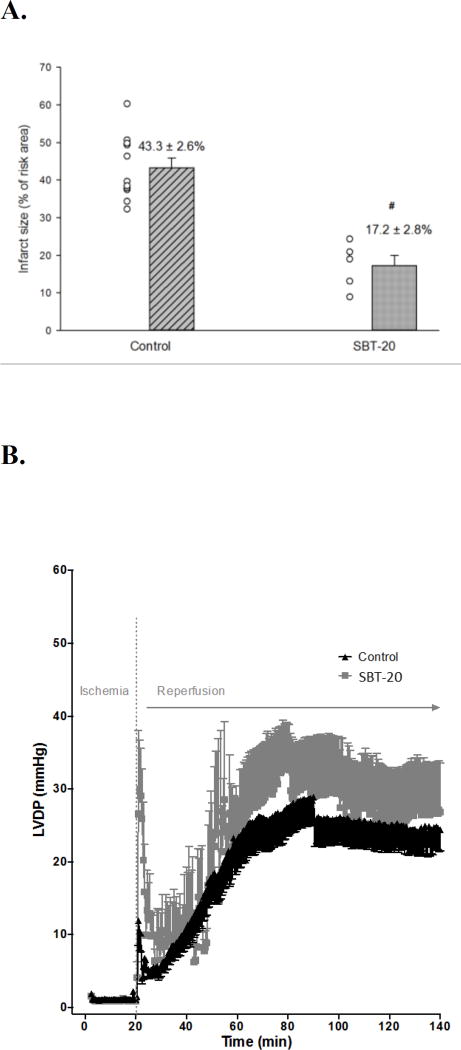
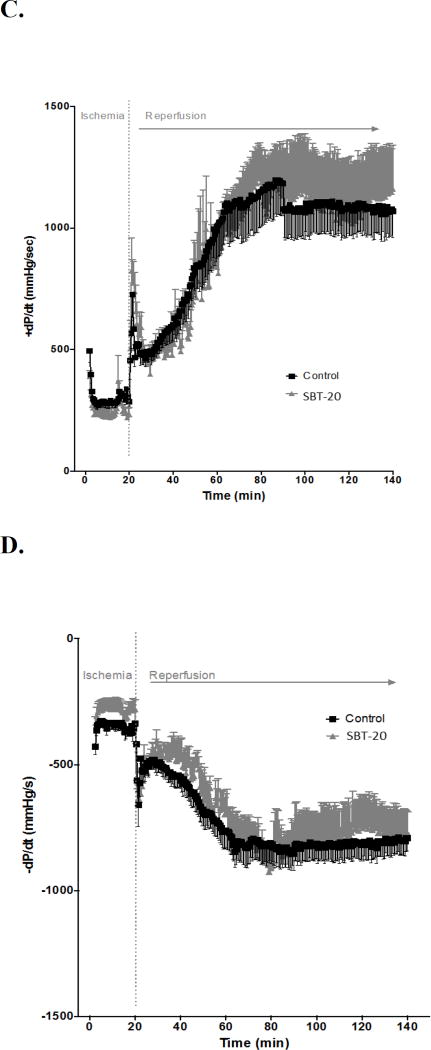
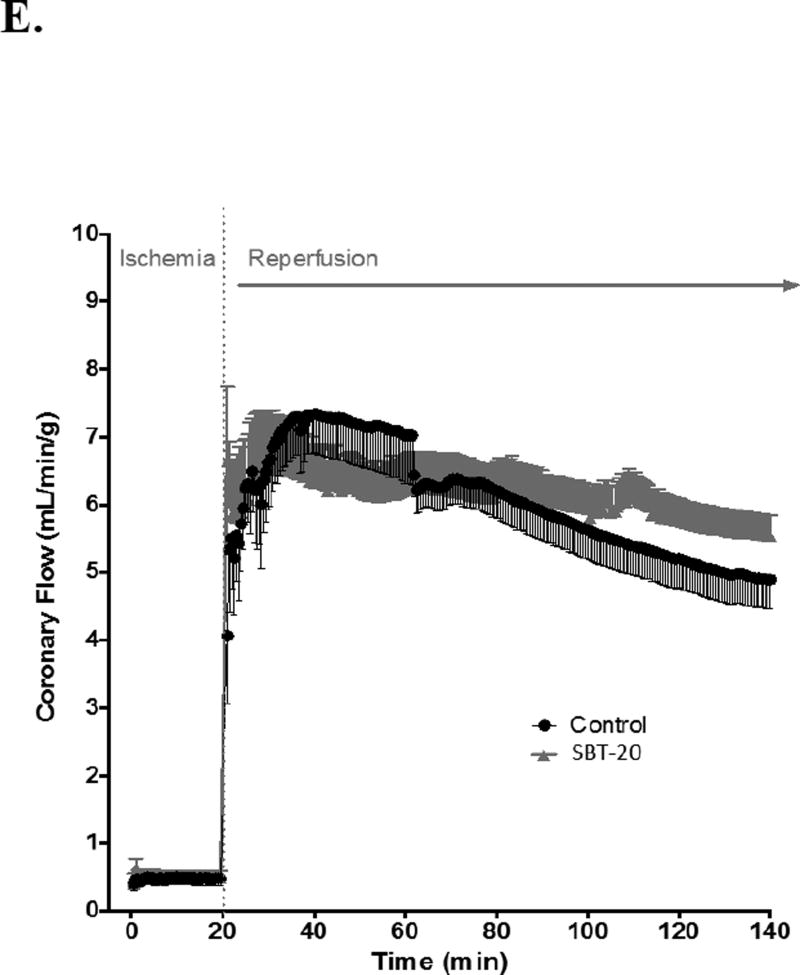
Results from isolated heart studies are presented in Figure 2. Infarct size was reduced from 43.3 ± 2.6% in control group (n=11) to 17.2 ± 2.8% in the SBT-20 treatment group (n=5, p < 0.05) (Figure 2A). This represents a 60.3% reduction in infarct size by SBT-20 treatment in the isolated rat heart model. There were no benefits of SBT-20 on recovery of left ventricular developed pressure, coronary flow, or maximal rates of contraction/relaxation. All of these parameters showed similar recovery to untreated controls throughout the duration of 2 hours reperfusion (Figure 2 B–E).
Figure 2.
Ischemia/reperfusion injury in isolated rat hearts subjected to global ischemia and reperfusion with 1 µM SBT-20. A: Infarct size assessed at the end of reperfusion in control and SBT-20-perfused hearts. B: Left ventricular developed pressure (LVDP) in hearts from the study. C and D: Maximal rate of contraction (+dP/dt) and relaxation (−dP/dt) throughout the course of reperfusion. E: Coronary flow rates, normalized to heart wet weight, for the reperfusion period. #, P<0.05 versus control.
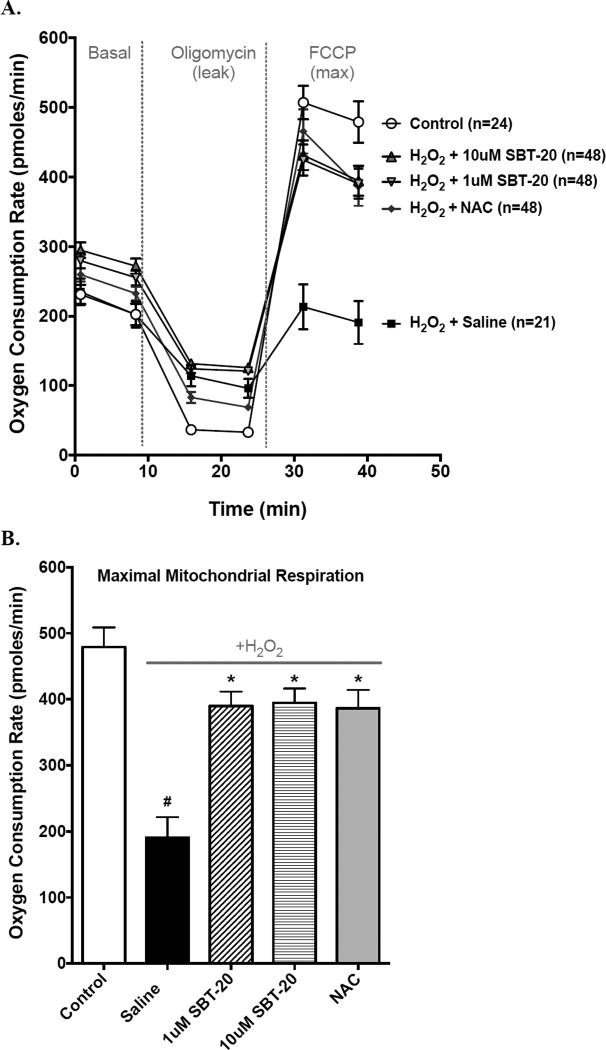
Mitochondrial Studies
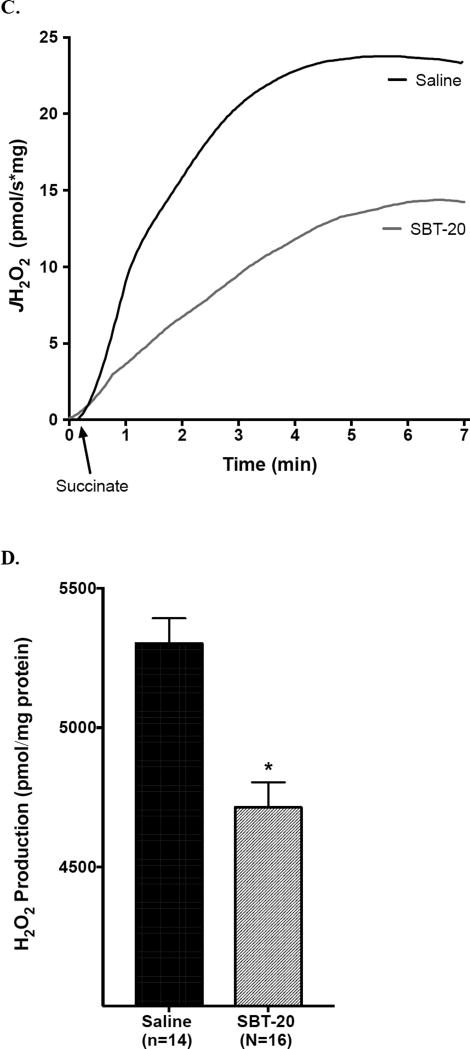
Data from cell and mitochondrial studies are presented in Figure 3. ROS-dependent mitochondrial dysfunction was determined in isolated cells stressed cells with H2O2. This injury paradigm significantly decreased maximal mitochondrial respiration in the untreated ‘saline’ group (Figure 3A and 3B). This decrease was prevented with either 1 or 10uM of SBT-20, as was incubation with a known ROS scavenger N-acetylcysteine (NAC). Each of these significantly improved mitochondrial function when compared to saline alone (P<0.05). To gain mechanistic insight into SBT-20’s mechanism of action, we also determined the effects of SBT-20 on mitochondrial ROS production in isolated heart mitochondria (Figure 3C and 3D). Treatment with SBT-20 significantly blunted ROS production by mitochondria respiring on succinate, which is postulated to mediate injury in early reperfusion [15].
Figure 3.
Mitochondrial effects of SBT-20 on mitochondrial respiration and reactive oxygen species production were determined in isolated stressed cells. A and B: This injury paradigm significantly decreased maximal mitochondrial respiration in the untreated ‘saline’ group. This decrease was prevented with either 1 or 10uM of SBT-20, as was incubation with a known ROS scavenger N-acetylcysteine (NAC). Each of these significantly improved mitochondrial function when compared to saline alone (P<0.05). C and D: Treatment with SBT-20 significantly blunted ROS production by mitochondria respiring on succinate, which is postulated to mediate injury in early reperfusion (P<0.05).
Discussion
Our present study demonstrated that administration of SBT-20 at 5 minutes after the onset of ischemia reduced myocardial necrosis but not no-reflow in the setting of 30 minutes of coronary artery occlusion followed by 3 hours reperfusion in rats. These data suggested that SBT-20 may be a promising adjunct pharmacologic agent to provide cardio-protective activity during ischemia and reperfusion therapy.
Ischemic stress-induced mitochondrial dysfunction is presently emerging as a specific pharmacological target in cardioprotective strategies in ischemic heart disease [16,17]. Mitochondria are considered to be the major source of intracellular reactive oxygen species (ROS) in cardiomyocytes during ischemia and early reperfusion. Recent studies have suggested that the intracellular accumulation of succinate during ischemia may be critically related to the propensity for mitochondria to produce ROS early in reperfusion through reverse electron transfer (RET) [15]. Our observation that succinate-derived mitochondrial ROS were significantly reduced with SBT-20 implies that this peptide reduces mitochondrial ROS production by blunting RET. Such a mechanism would improve electron transport ‘downstream’ from complex II (succinate dehydrogenase) to complexes III and ultimately complex IV, and would be consistent with improved mitochondrial oxygen consumption observed herein and by others (Szeto 2008 review article). Such a mechanism would be consistent with decreased lipid peroxidation observed in Cho et al [6], who occluded the rat left coronary for 60 minutes followed by 60 minutes, and administered SBT-20 (3 mg/kg) intraperitoneally twice (one at 30 min before coronary artery ligation and another dose at 5 minutes before reperfusion). SBT-20 significantly reduced myocardial lipid peroxidation and infarct size in ischemia/reperfusion injury. SBT-20 most likely reduces ROS production during ischemia/reperfusion since it does not have direct ROS scavenging properties [18]. Recently, it has been demonstrated that SBT-20, a water-soluble tetrapeptide, is capable of crossing the cell membrane without the need for specific transporters or receptors, and binds with high affinity to cardiolipin located in the inner mitochondrial membrane in the same manner as elamipretide (MTP-131) [16, 19, 20]. Cardiolipin is an anionic phospholipid expressed on the inner mitochondrial membrane that is required for cristae formation. SBT-20/cardiolipin complex inhibited cytochrome c peroxidase activity, protected mitochondrial damage, and optimized ATP production during ischemia [21].
Surprising, SBT-20 did not reduce no-reflow area in the present study, although it significantly reduced myocardial infarct size. However, studies using other models suggested that SBT-20 could protect the endothelium. In an in vitro Langendorff study with a model of prolonged cold ischemic storage/warm reperfusion of guinea pig hearts [5], SBT-20 treatment significantly increased coronary artery flow and prevented endothelial apoptosis compared to cardioplegic solution alone. In contrast, Han et al [22] described a study of cultured bovine aortic endothelial cells that were exposed to a steady laminar shear stress for 6 hours to cause a sustained increase in NO and a transient increase in ROS. Pre-incubation with culture medium containing SBT-20 for 1 hour and continued incubation with the same concentration of SBT-20 during shear stress had no effect on shear-induced ROS production of cultured endothelial cells. This difference in endothelial cell protection by SBT-20 may be the result of different experimental conditions. Because the causes of ischemic induced myocardial no-reflow in our model are likely multifactorial, including endothelial swelling that results in membrane-bound intraluminal blebs, swollen myocytes with subsarcolemmal blebs, that may compress adjacent capillaries, platelet plugs and fibrin tactoids, and neutrophil plugs [23], the specific reason that SBT-20 failed to prevent no-reflow needs further investigation.
Although SBT-20 induced significantly lower systemic arterial blood pressure in the high dose compared to the low dose group during some time points of this study; the infarct size reduction effect of high dose SBT-20 is probably not due to the afterload reduction alone, since low dose of SBT-20 also reduced infarct size to a similar degree under the condition of not decreasing blood pressure.
Elamipretide is the first member of this peptide family that entered into a clinical trial for reperfusion injury [16]. In experimental animal studies, Kloner et al [9] reported that elamipretide administered after the onset of ischemia reduced infarct size by 11% (p=nonsignificant) in rabbits and by 15.4% (P=0.02) in sheep (study by university of Pennsylvania) after coronary artery ligation. Elamipretide also attenuated the extent of no-reflow by 21.4% (P=0.0085) in rabbits, and reduced infarct size by 38% to 42% (P<0.05) in isolated perfused guinea pig hearts. In a multicenter, randomized, double-blind Phase 2a clinical trial [24], elamipretide or placebo was infused at a rate of 0.05 mg/kg/h among patients with first-time ST-elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) for a proximal or mid left anterior descending (LAD) artery occlusion. Elamipretide was administered ≥15 min, but <60 min prior to PCI and for 1 h following reperfusion. Although administration of elamipretide was well tolerated, elamipretide non-significantly reduced infarct size by approximately 11% assessed using MRI, which was a similar result to the reduction in infarct size observed in a rabbit model of reperfusion injury in Kloner’s study [9]. While elamipretide reduced infarct size by 11% in in vivo rabbit model and human patients, as well as by 38% to 42% in isolated perfused guinea pig hearts, SBT-20 treatment resulted in infarct size reduction by 20% in low dose and 17.5% in high dose in an in vivo model and by 60.3% in the isolated rat heart model in our present study. Moreover, in a direct comparison of effects of elamipretide and SBT-20 in an in vivo rat ischemia/reperfusion model, elamipretide resulted in 10% reduction in infarct size, while SBT-20 had a 21.4% reduction in infarct size [6]. Therefore, based on the above available data, compared with elamipretide, SBT-20 is more effective in reducing infarct size in myocardial ischemia/reperfusion situations.
In summary, SBT-20, administered starting at 5 minutes after coronary artery occlusion, significantly reduced infarct size by 20% in low dose and 17.5% in high dose treatment compared to saline treatment in a rat model with 30 minutes of coronary artery occlusion followed by 3 hours reperfusion. The infarct size limitation effects of SBT-20 are consistent with the results observed by Cho et al [6]: in their study SBT-20 was administrated at 30 minutes before coronary artery occlusion and reduced infarct size by 21.4%. In our present study, SBT-20 did not affect no-reflow area in a rat ischemia/reperfusion model, and the underlying mechanism remains unknown and needs to be further explored. SBT-20 is a promising adjunct pharmacologic agent for ischemia and reperfusion damage and deserves future trials.
Acknowledgments
These studies were supported by grants from NIH (R01 HL123647 and R15 HL122922 to D.A.B.) and Stealth BioTherapeutics (to D.A.B. and R.A.K).
Disclosures: D.A.B. and R.A.K has received consulting income from Stealth BioTherapeutics.
References
- 1.Kloner RA, Dai W, Hale SL, Shi J. Approaches to Improving Cardiac Structure and Function During and After an Acute Myocardial Infarction: Acute and Chronic Phases. J Cardiovasc Pharmacol Ther. 2015 Nov 25; doi: 10.1177/1074248415616187. pii: 1074248415616187. [DOI] [PubMed] [Google Scholar]
- 2.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the "dark side" of reperfusion. Circulation. 2009;120(21):2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber JI, Weis JN. Oxygen free radicals and cardiac reperfusion abnormalities. Hypertension. 1992;20:118–127. doi: 10.1161/01.hyp.20.1.118. [DOI] [PubMed] [Google Scholar]
- 4.Petrosillo G, Venosa ND, Pistolese M, et al. Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia–reperfusion: role of cardiolipin. FASEB J. 2006;20:269–276. doi: 10.1096/fj.05-4692com. [DOI] [PubMed] [Google Scholar]
- 5.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10(3):601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 6.Cho J, Won K, Wu D, et al. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18(3):215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 7.Frasier CR, Moukdar F, Patel HD, et al. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res. 2013;98(1):47–55. doi: 10.1093/cvr/cvt009. [DOI] [PubMed] [Google Scholar]
- 8.Brown DA, Hale SL, Baines CP, et al. Reduction of early reperfusion injury with the mitochondria-targeting peptide bendavia. J Cardiovasc Pharmacol Ther. 2014;19(1):121–132. doi: 10.1177/1074248413508003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloner RA, Hale SL, Dai W, et al. Reduction of ischemia/reperfusion injury with bendavia, a mitochondria-targeting cytoprotective Peptide. J Am Heart Assoc. 2012;1(3):e001644. doi: 10.1161/JAHA.112.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sloan RC, Moukdar F, Frasier CR, et al. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52(5):1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435(2):297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan RC, Moukdar F, Frasier CR, et al. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol. 2012;52(5):1009–1018. doi: 10.1016/j.yjmcc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Alleman RJ, Tsang AM, Ryan TE, et al. Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. Am J Physiol Heart Circ Physiol. 2016;310(10):H1360–1370. doi: 10.1152/ajpheart.00858.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, et al. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol. 2015;1264:245–261. doi: 10.1007/978-1-4939-2257-4_22. [DOI] [PubMed] [Google Scholar]
- 15.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szeto HH, Birk AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther. 2014;96(6):672–83. doi: 10.1038/clpt.2014.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown DA, Sabbah HN, Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther. 2013;140(3):258–66. doi: 10.1016/j.pharmthera.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279(33):34682–90. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao K, Luo G, Zhao GM, Schiller PW, Szeto HH. J. Pharmacol. Exp. Ther. 2003;304:425–432. doi: 10.1124/jpet.102.040147. [DOI] [PubMed] [Google Scholar]
- 20.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250–61. doi: 10.1681/ASN.2012121216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birk AV, Chao WM, Liu S, Soong Y, Szeto HH. Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim Biophys Acta. 2015;1847(10):1075–84. doi: 10.1016/j.bbabio.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther. 2009;329(1):94–101. doi: 10.1124/jpet.108.145557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kloner RA. No-reflow phenomenon: maintaining vascular integrity. J Cardiovasc Pharmacol Ther. 2011;16(3–4):244–50. doi: 10.1177/1074248411405990. [DOI] [PubMed] [Google Scholar]
- 24.Gibson CM, Giugliano RP, Kloner RA, et al. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J. 2016;37(16):1296–1303. doi: 10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]