Abstract
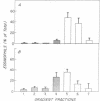
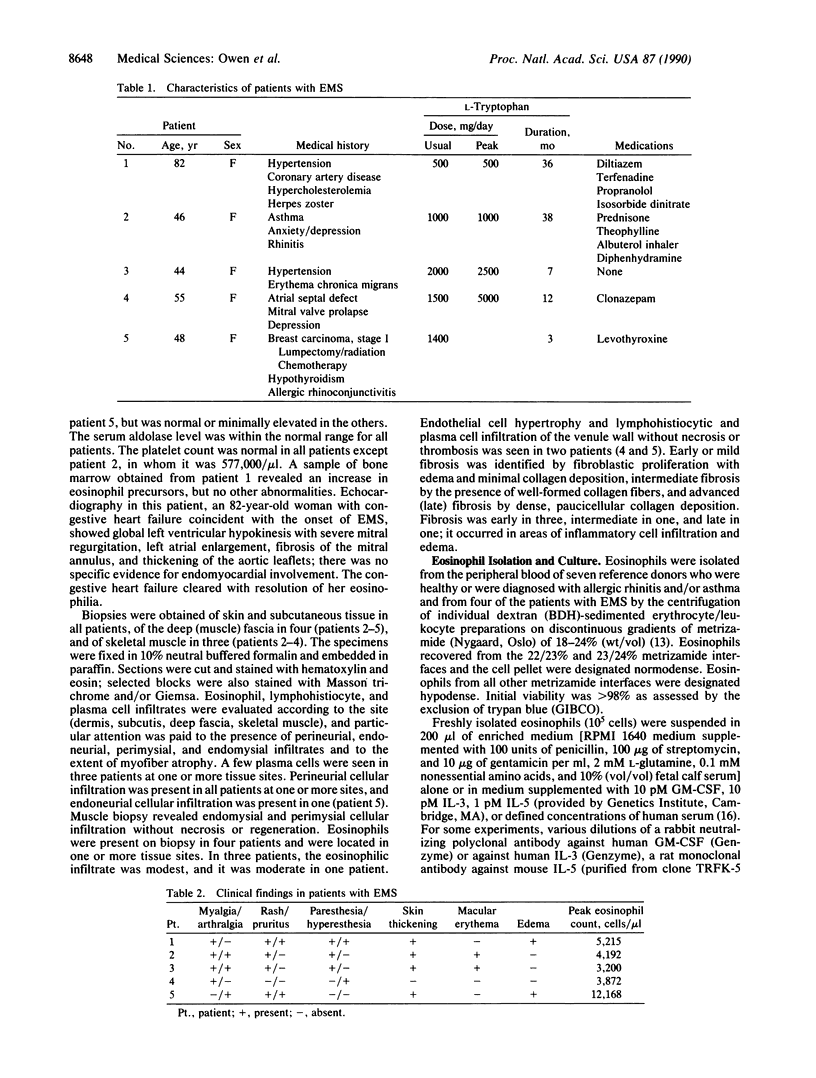
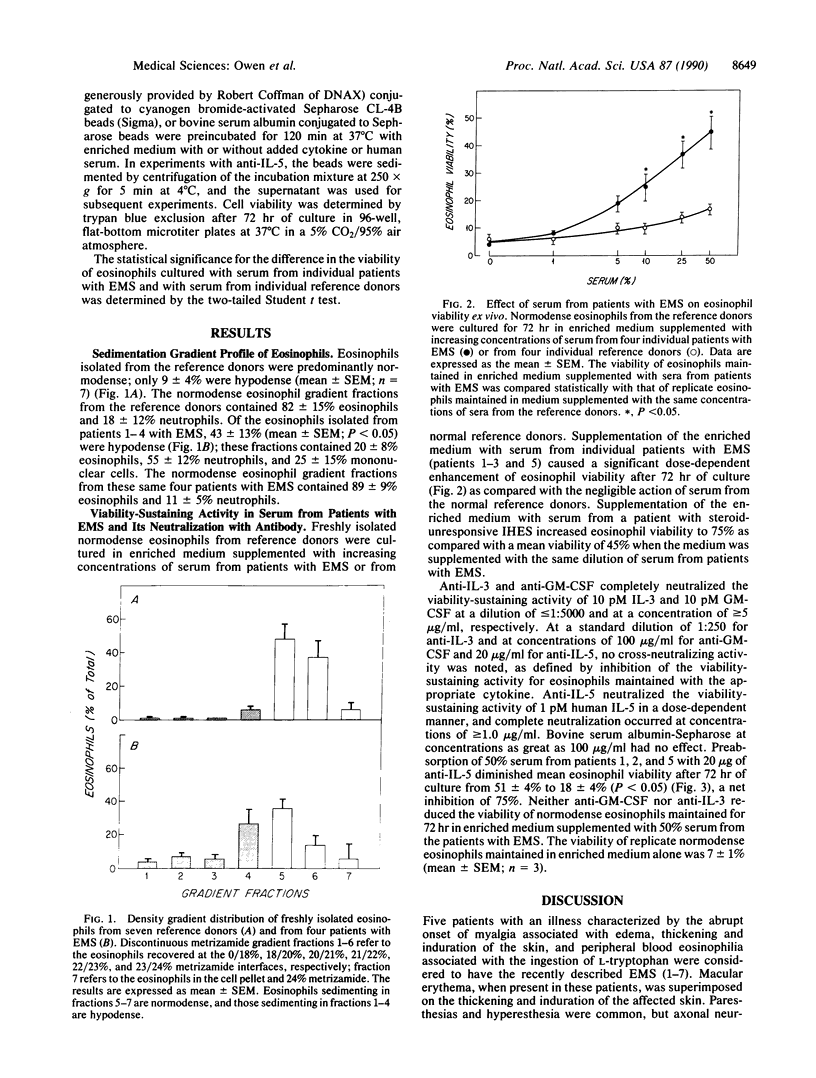
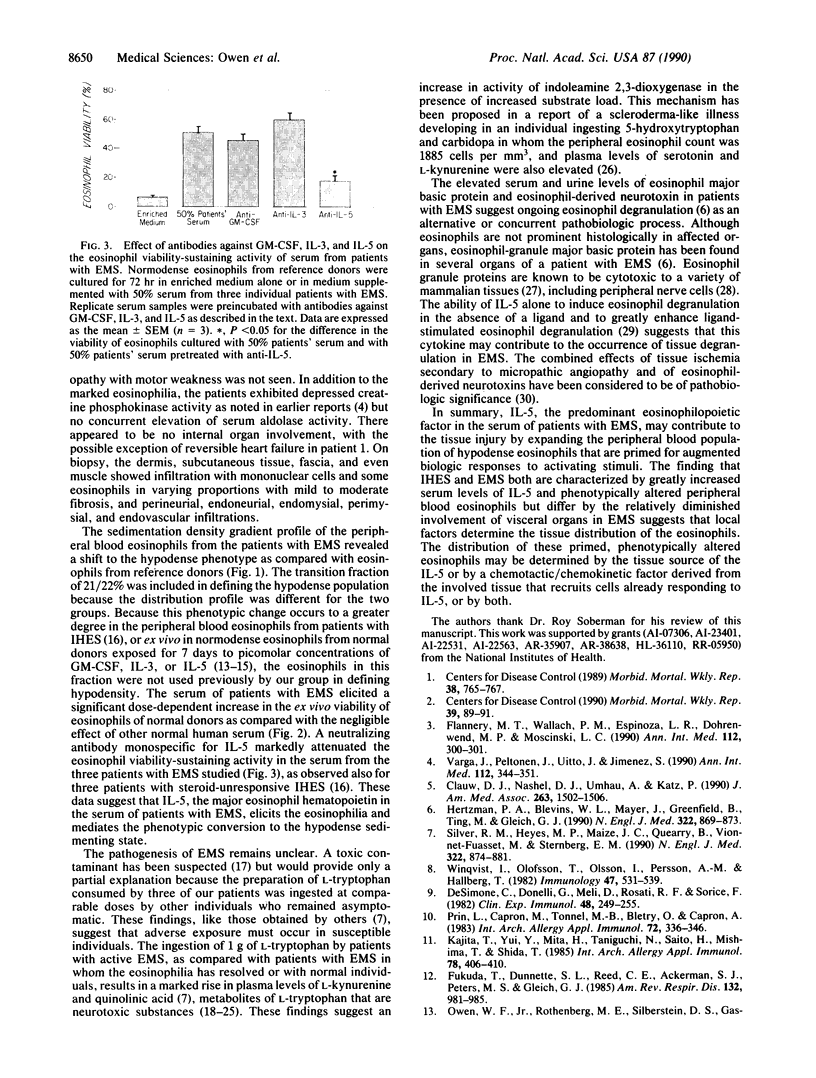
The recent recognition of the eosinophilia-myalgia syndrome (EMS) associated with the ingestion of L-tryptophan prompted an analysis of the peripheral blood eosinophil phenotypes and of the serum eosinophil hematopoietins in this disorder. Five patients with an illness characterized by the abrupt onset of aching skeletal muscles, edema, thickening and induration of the skin, and marked blood eosinophilia associated with L-tryptophan ingestion provided eosinophils, serum, or both, for evaluation. Gradient sedimentation density analysis of the peripheral blood eosinophils from four of these patients revealed that 43 +/- 13% (mean +/- SEM) of the cells had converted to the abnormal (hypodense) sedimenting phenotype. When normodense eosinophils from the reference donors were cultured for 3 days in medium supplemented with increasing concentrations of serum from the patients with EMS, their viability increased in a dose-dependent manner to 45%, which was significantly augmented over the effect of normal serum. This eosinophil viability-sustaining activity was inhibited by 76 +/- 7% (mean +/- SEM; n = 3) by the addition of anti-interleukin 5 (IL-5) but not by neutralizing antibodies monospecific for either granulocyte/macrophage colony-stimulating factor (GM-CSF) or IL-3. IL-5, an eosinophilopoietic factor, converts normodense peripheral blood eosinophils in vitro to a hypodense sedimenting form with extended viability and augmented biologic responses to activating stimuli. Thus, the presence of IL-5 in the sera of patients with EMS may contribute to the development and maintenance of the eosinophilia and may regulate the conversion of the peripheral blood eosinophils to the hypodense phenotype with augmented pathobiologic potential.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clauw D. J., Nashel D. J., Umhau A., Katz P. Tryptophan-associated eosinophilic connective-tissue disease. A new clinical entity? JAMA. 1990 Mar 16;263(11):1502–1506. [PubMed] [Google Scholar]
- De Simone C., Donneli G., Meli D., Rosati F., Sorice F. Human eosinophils and parasitic diseases. II. Characterization of two cell fractions isolated at different densities. Clin Exp Immunol. 1982 Apr;48(1):249–255. [PMC free article] [PubMed] [Google Scholar]
- Eastman C. L., Guilarte T. R. Cytotoxicity of 3-hydroxykynurenine in a neuronal hybrid cell line. Brain Res. 1989 Aug 28;495(2):225–231. doi: 10.1016/0006-8993(89)90216-3. [DOI] [PubMed] [Google Scholar]
- Flannery M. T., Wallach P. M., Espinoza L. R., Dohrenwend M. P., Moscinski L. C. A case of the eosinophilia-myalgia syndrome associated with use of an L-tryptophan product. Ann Intern Med. 1990 Feb 15;112(4):300–301. doi: 10.7326/0003-4819-112-4-300. [DOI] [PubMed] [Google Scholar]
- Fujisawa T., Abu-Ghazaleh R., Kita H., Sanderson C. J., Gleich G. J. Regulatory effect of cytokines on eosinophil degranulation. J Immunol. 1990 Jan 15;144(2):642–646. [PubMed] [Google Scholar]
- Fukuda T., Dunnette S. L., Reed C. E., Ackerman S. J., Peters M. S., Gleich G. J. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am Rev Respir Dis. 1985 Nov;132(5):981–985. doi: 10.1164/arrd.1985.132.5.981. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Garthwaite J. Quinolinate mimics neurotoxic actions of N-methyl-D-aspartate in rat cerebellar slices. Neurosci Lett. 1987 Aug 18;79(1-2):35–39. doi: 10.1016/0304-3940(87)90668-9. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Hertzman P. A., Blevins W. L., Mayer J., Greenfield B., Ting M., Gleich G. J. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990 Mar 29;322(13):869–873. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- Kajita T., Yui Y., Mita H., Taniguchi N., Saito H., Mishima T., Shida T. Release of leukotriene C4 from human eosinophils and its relation to the cell density. Int Arch Allergy Appl Immunol. 1985;78(4):406–410. doi: 10.1159/000233922. [DOI] [PubMed] [Google Scholar]
- Kim J. P., Choi D. W. Quinolinate neurotoxicity in cortical cell culture. Neuroscience. 1987 Nov;23(2):423–432. doi: 10.1016/0306-4522(87)90066-2. [DOI] [PubMed] [Google Scholar]
- Lapin I. P. Kynurenines and seizures. Epilepsia. 1981 Jun;22(3):257–265. doi: 10.1111/j.1528-1157.1981.tb04108.x. [DOI] [PubMed] [Google Scholar]
- Martin R. W., Duffy J., Engel A. G., Lie J. T., Bowles C. A., Moyer T. P., Gleich G. J. The clinical spectrum of the eosinophilia-myalgia syndrome associated with L-tryptophan ingestion. Clinical features in 20 patients and aspects of pathophysiology. Ann Intern Med. 1990 Jul 15;113(2):124–134. doi: 10.7326/0003-4819-113-2-124. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., Singh E. Neurotoxic effects of endogenous materials: quinolinic acid, L-pyroglutamic acid, and thyroid releasing hormone (TRH). Exp Neurol. 1984 Nov;86(2):410–413. doi: 10.1016/0014-4886(84)90197-3. [DOI] [PubMed] [Google Scholar]
- Owen W. F., Jr, Rothenberg M. E., Silberstein D. S., Gasson J. C., Stevens R. L., Austen K. F., Soberman R. J. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987 Jul 1;166(1):129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Rothenberg M. E., Petersen J., Weller P. F., Silberstein D., Sheffer A. L., Stevens R. L., Soberman R. J., Austen K. F. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989 Jul 1;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli A., Ossi C., Colombo R., Tofanetti O., Spazzi L. Experimental convulsions in rats induced by intraventricular administration of kynurenine and structurally related compounds. Neuropharmacology. 1984 Mar;23(3):333–337. doi: 10.1016/0028-3908(84)90196-5. [DOI] [PubMed] [Google Scholar]
- Prin L., Capron M., Tonnel A. B., Bletry O., Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72(4):336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Petersen J., Stevens R. L., Silberstein D. S., McKenzie D. T., Austen K. F., Owen W. F., Jr IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989 Oct 1;143(7):2311–2316. [PubMed] [Google Scholar]
- Silver R. M., Heyes M. P., Maize J. C., Quearry B., Vionnet-Fuasset M., Sternberg E. M. Scleroderma, fasciitis, and eosinophilia associated with the ingestion of tryptophan. N Engl J Med. 1990 Mar 29;322(13):874–881. doi: 10.1056/NEJM199003293221302. [DOI] [PubMed] [Google Scholar]
- Slutsker L., Hoesly F. C., Miller L., Williams L. P., Watson J. C., Fleming D. W. Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer. JAMA. 1990 Jul 11;264(2):213–217. [PubMed] [Google Scholar]
- Sternberg E. M., Van Woert M. H., Young S. N., Magnussen I., Baker H., Gauthier S., Osterland C. K. Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa. N Engl J Med. 1980 Oct 2;303(14):782–787. doi: 10.1056/NEJM198010023031403. [DOI] [PubMed] [Google Scholar]
- Sunohara N., Furukawa S., Nishio T., Mukoyama M., Satoyoshi E. Neurotoxicity of human eosinophils towards peripheral nerves. J Neurol Sci. 1989 Aug;92(1):1–7. doi: 10.1016/0022-510x(89)90170-6. [DOI] [PubMed] [Google Scholar]
- Varga J., Peltonen J., Uitto J., Jimenez S. Development of diffuse fasciitis with eosinophilia during L-tryptophan treatment: demonstration of elevated type I collagen gene expression in affected tissues. A clinicopathologic study of four patients. Ann Intern Med. 1990 Mar 1;112(5):344–351. doi: 10.7326/0003-4819-112-5-344. [DOI] [PubMed] [Google Scholar]
- Whetsell W. O., Jr, Schwarcz R. Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci Lett. 1989 Feb 27;97(3):271–275. doi: 10.1016/0304-3940(89)90609-5. [DOI] [PubMed] [Google Scholar]
- Winqvist I., Olofsson T., Olsson I., Persson A. M., Hallberg T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology. 1982 Nov;47(3):531–539. [PMC free article] [PubMed] [Google Scholar]
- el-Defrawy S. R., Boegman R. J., Jhamandas K., Beninger R. J. The neurotoxic actions of quinolinic acid in the central nervous system. Can J Physiol Pharmacol. 1986 Mar;64(3):369–375. doi: 10.1139/y86-060. [DOI] [PubMed] [Google Scholar]