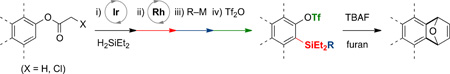
Abstract
Diversely substituted arylsilyl triflates, as aryne precursors for aryne cycloaddition reactions, were accessed from benzodioxasilines. Catalytic reductive C–H ortho-silylation of phenols with traceless acetal directing groups was exploited to prepare benzodioxasilines. Sequential addition of MeLi and then trifluoromethanesulfonic anhydride to benzodioxasilines provided arylsilyl triflates in a single pot. Notably, this approach was successfully utilized to prepare sterically hindered 1,2,3-trisubstituted arylsilyl triflates, which ultimately underwent fluoride-mediated aryne cycloaddition.
Keywords: AryneArrylsilyl triflatesBenzodioxasilinesC-H, activationCycloaddition, Silyl acetals
Graphical Abstract
1. Introduction
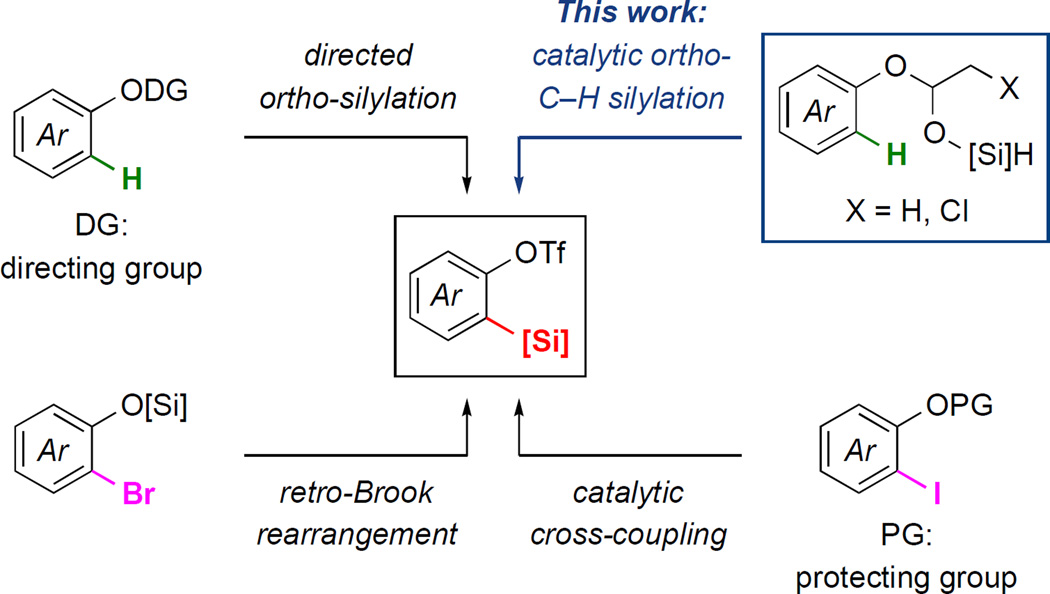
Ever since the discovery of benzynes in the mid 1950s,1 these highly reactive intermediates have been employed in a wide variety of important applications, such as the synthesis of bioactive molecules,2 organic materials,3 and catalysts.4 Additionally, benzynes enable the formation of diverse heterocyclic frameworks, which are difficult to obtain by conventional methods, via reactions with various arynophiles.5 Among the several methods that have been developed for the efficient generation of arynes,6 the reaction of silylaryl triflates with fluoride is one of the most widely used approaches.7 These stable benzyne precursors, which can undergo aryne cycloadditions under mild conditions, have led to a resurgence in aryne chemistry. Generally, these highly useful 1,2-silylaryl triflates have been prepared by one of the following three methods: 1) a sequence of directed ortho metalation (DoM),8 silylation and triflation, 2) catalytic cross-coupling of ortho-haloarenes9 and triflation, and 3) ortho-halo-phenols via retro-Brook rearrangement10 followed by triflation (Scheme1). However, each of these methods suffer from challenges and/or limitations: the commercial availability of starting materials, challenging post-directing group manipulation due to potential protodesilylation, the need for either pre-functionalization of arene substrates or stoichiometric basic reagents, and limited functional group compatibility.11 Furthermore, preparation of site-selectively installed silyl and triflate moieties within multi-substituted arenes for regioselective aryne cycloaddition is often difficult to access by these methods.
Scheme 1.
Methods to prepare silylaryl triflates
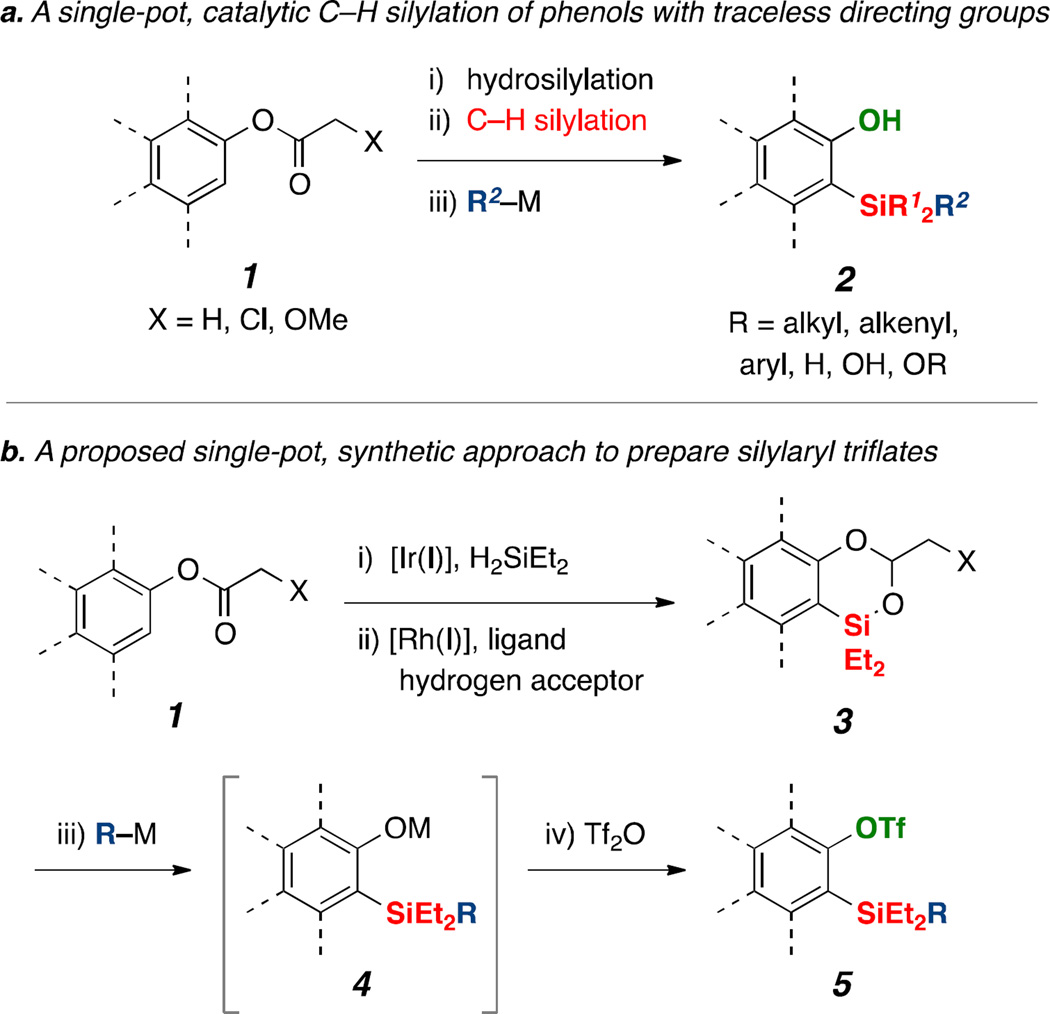
We recently developed a method to access versatile benzodioxasilines 3 from readily accessible phenyl acetates, derived from simple phenol, via catalytic reductive C–H ortho-silylation with traceless acetal directing groups (Scheme 2a).12 Specifically, a relay of iridium and rhodium catalysts involving hydrosilylation of esters with dihydrosilanes and arene ortho-C–H silylation, respectively, which were followed by a subsequent nucleophile addition to the electrophilic silicon to remove the acetal directing group, directly provides unmasked ortho-silyl phenol products in a single vessel. This strategy was successfully applied to the preparation of multi-substituted arenes by exploiting a new, formal α-chloroacetyl directing group. In particular, this new directing group tactic permits access to sterically hindered ortho-silyl phenols. Importantly the synthesis of 1,2,3-trisubstituted phenolic arenes, which are difficult to obtain by other catalytic means, was established. Following this study, we speculated that diversely substituted benzodioxasilines 3 could be excellent precursors for preparation of silylaryl triflates. For example, addition of organometallic agents to silicon and then subsequent trapping of the resulting ortho-silyl oxyanion intermediates 4 by trifluoromethanesulfonic anhydride can directly afford diversely substituted silylaryl triflates (Scheme 2b).
Scheme 2.
Synthetic approach to silylaryl triflates via ortho-C–H silylation
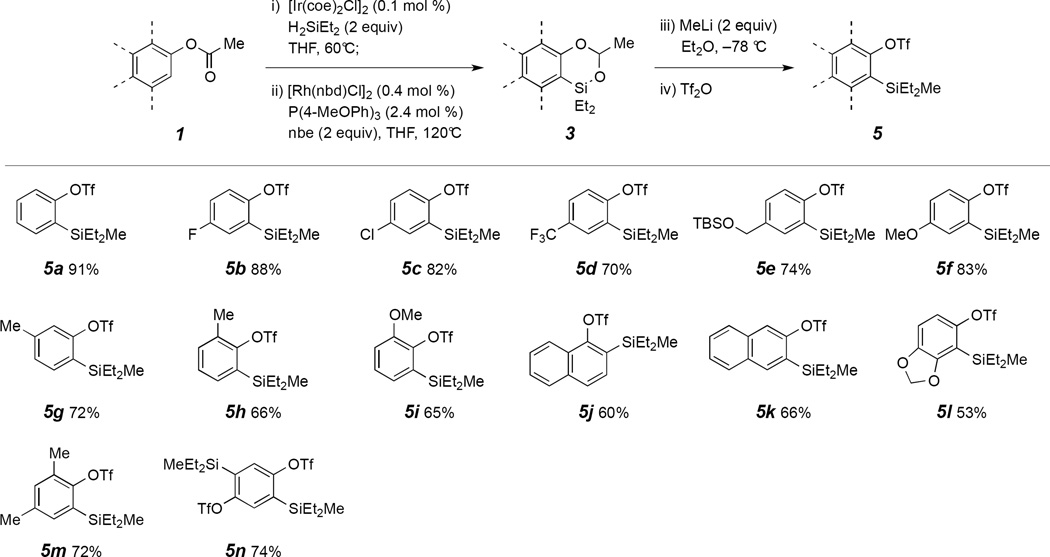
2. Results and Discussion
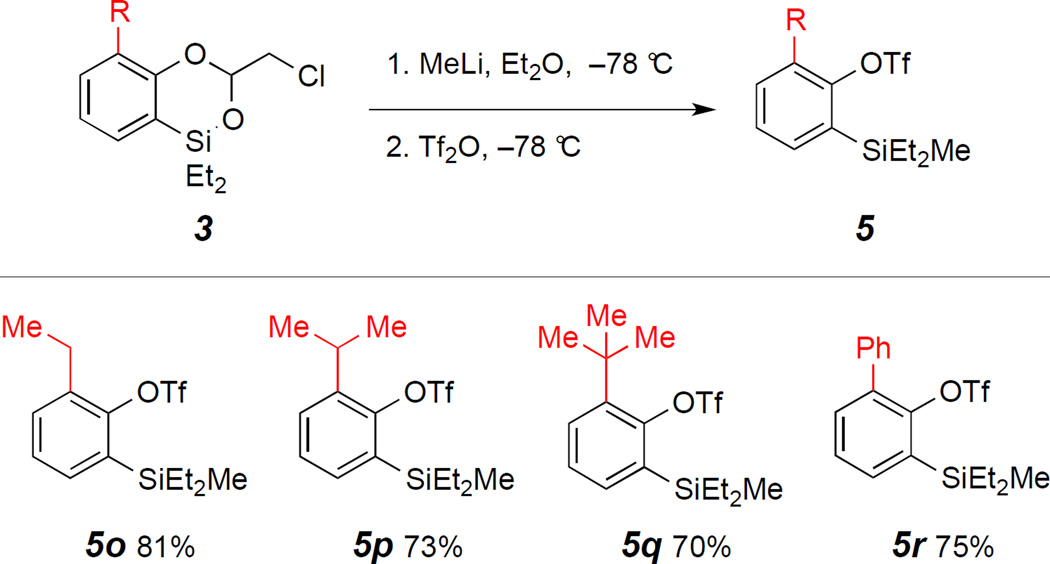
Benzodioxasilines were efficiently prepared through Ircatalyzed hydrosilylation of phenyl acetates followed by Rhcatalyzed C–H silylation in a single pot. We then examined the efficiency of the nucleophilic ring-opening reaction of benzodioxasilines 3 with simple, readily available organometallic reagents (e.g., organolithium reagents), followed by direct triflation with trifluoromethanesulfonic anhydride. The single-pot, sequential reactions with benzodioxasiline 3a, generated from phenyl acetate 1a via catalytic reductive ortho-C–H silylation and used without purification, produced the desired silylaryl triflate 5a by MeLi ring-opening and trifilation reactions in excellent yield (91% from 1a) (Table 1). Electronically differentiated, diverse substituted benzodioxasilines provided silylaryl triflates 5 in moderate to excellent yields (four steps from 1) under this reaction conditions. Specifically, halogens, trifluoromethyl, TBS-protected primary alcohol group, ortho-methyl, methoxy within benzodioxasilines 3 were tolerated by the four-step reaction conditions to furnish silylaryl triflates (5b–5i). 1- and 2-naphthyl silyl triflates (5j and 5k) were produced from 1j and 1k in modest yields via the reaction sequence. Moreover, disubstituted benzodioxasilines 3l and 3m afforded 5l and 5m in 53% and 72% yields, respectively. Finally, dual functionalization produced bis-silylaryl triflate 5n in 74% yield.
Table 1.
Preparation of silylaryl triflates 5 from benzodioxasilines 3
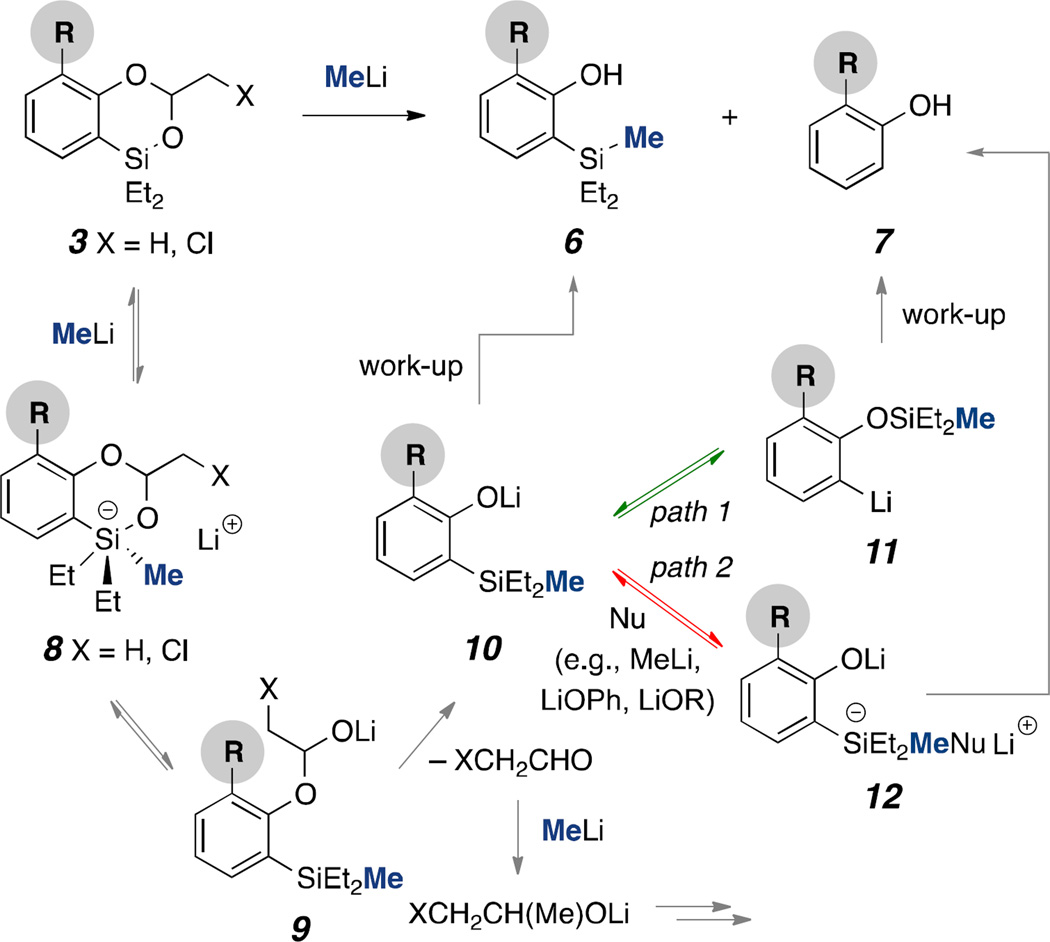
During these processes, we found that noticeable, yet minor desilylation phenol byproducts 7 were also produced with most substrates (Scheme 3). Especially, substrates such as 5d, 5j, and 5k produced a significant amount of 7. For the desilylation event, we reasoned that a nucleophilic attack of MeLi to dioxasilines first generates putative penta-coordinate silicate species 8, which undergoes a fragmentation process to afford lithium ortho-silyl phenoxide 10 (via lithio acetal 9) and acetaldehyde. A nucleophilic addition of MeLi to acetaldehyde can then furnish XCH2CH(Me)OLi. At this moment two possible scenarios explaining the observed desilylation are feasible: 1) Intramolecular silyl transfer–a [1,3]-silatropic rearrangement can be attributed to this issue. For example, a [1,3]-Brook rearrangement13 of ortho-silyl phenols to afford silyl ethers has been reported, however, this process was explored mainly under acidic conditions,14 elevated temperatures15 or catalytic aerobic conditions.16 Additionally, under strong basic conditions a retro-[1,3]-silatropic process within ortho-silyl phenols has been well reported.10 2) Intermolecular silyl transfer–nucleophiles (e.g., MeLi, LiOPh, LiOR), present in the reaction, can engage with 10 to produce dianions of type 12, which eventually afford phenols 7 upon work-up. To minimize the potential rearrangement (10 to 11, path 1) or reversible association of nucleophiles (10 to 12, path 2) to silyl moiety after the first fragmentation (3 to 10), we quickly quenched the reaction within <1 min with Tf2O at −78 °C (achieving a full consumption of 3). This procedure substantially reduced the formation of 7, thereby enhancing yields of desirable products 6.
Scheme 3.
Proposed mechanism of desilylation
Next we investigated the single-pot, sequential strategy concerning nucleophilic attack of MeLi and triflation of the sterically demanding benzodioxasilines 3 bearing substituents at ortho position relative to the hydroxyl group (Table 2). These substrates consistently produced substantial desilylation adducts 7 even in a short period of reaction time. We found that the purity of the dioxasilines 3 was crucial to affect the MeLi addition reaction. Specifically, when purified benzodioxasilines 3o–r, obtained by a column chromatography after the reductive C–H silylation, were employed under the reaction conditions, we were able to isolate 5o–r in good yields. Of note, these 1,2,3-trisubstituted arylsilyl triflates are difficult to access by other catalytic means.
Table 2.
Preparation of sterically hindered silylaryl triflates 5 from benzodioxasilines 3
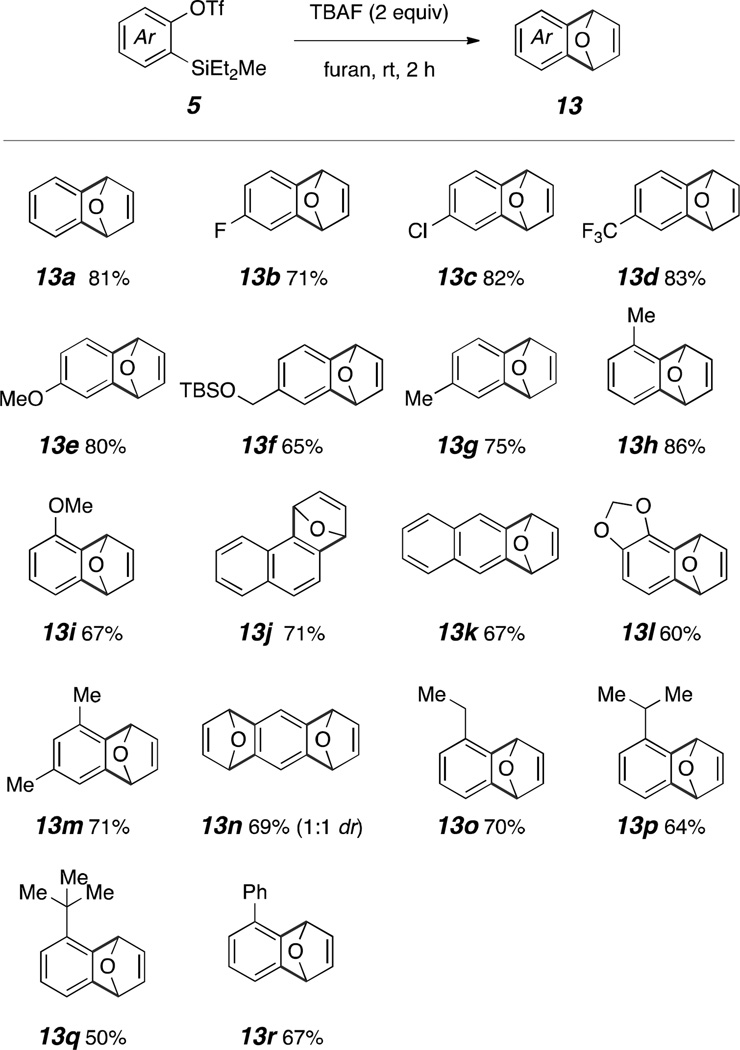
We next explored fluoride-mediated [4+2] aryne cycloaddition reactions of diethylmethylsilylaryl triflates 5 with furan (Table 3). Cycloaddition reactions with electron-rich and -deficient arylsilyl triflates with furan (solvent) provided a variety of 1,4-dihydro-1,4-epoxynaphthalenes 13, demonstrating good functional group tolerance. Under these reaction conditions, meta and para-substituted arylsilyl triflates (5b–g) produced the corresponding 6-substituted 1,4-dihydro-1,4-epoxynaphthalenes (13b–g) in good yields. In particular, a TBS-protected primary alcohol group within 5f survived under the reaction conditions at 0 °C. The ortho-substituted arylsilyl triflates (5h and 5i) underwent aryne cycloadditions to provide 13h and 13i in 86% and 67% yields, respectively. This approach is also successful with 1- and 2-silylnaphthyl triflates (5j and 5k, respectively) to afford 13j and 13k in modest yields. Furthermore, cycloadditions with di-substituted arylsilyl triflates (i.e., 5l and 5m) and tetra-substituted arylsilyl triflate (5n) produced 13l, 13m, and 13n (1:1 dr) in good yields. Next, we studied the cycloaddition of sterically hindered, 1,2,3-trisubstituted arylsilyl triflates 5o–r. The corresponding cycloadducts 13o–r were successfully produced in moderate yields.
Table 3.
Fluoride-mediated [4+2] aryne cycloaddition reaction of diethylmethylsilylaryl triflates 5
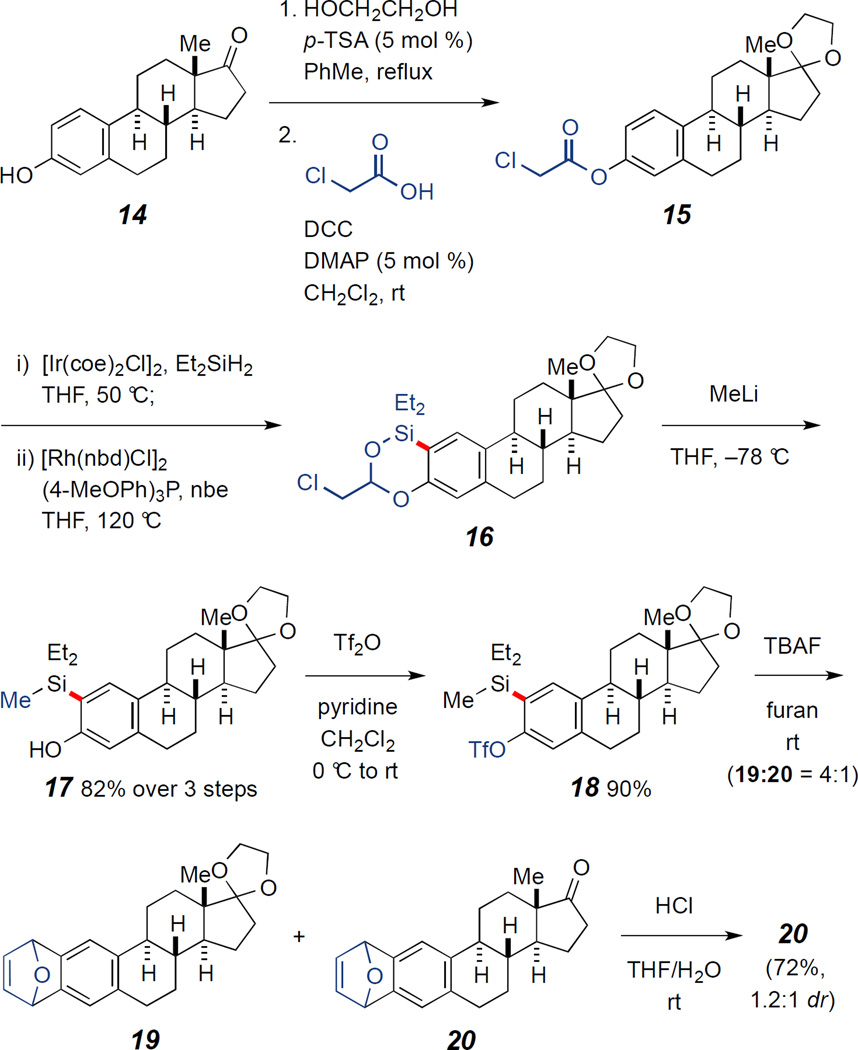
An important component of this project was broadening the scope of the approach towards the synthesis of bioactive molecules. For this purpose, we examined the [4+2] aryne cycloaddition of arylsilyl triflates derived from estrone 14. The C17 ketone in estrone, which was not compatible in Ircatalyzed ester hydrosilylation, was first protected with a ketal group. Next, the α-Chloroacetyl group was installed to the phenol for an effective arene C–H silylation reaction in a hindered environment. Catalytic reductive ortho-C–H bond silylation of the resulting phenyl acetate with the α-chloroacetyl formal directing group provided dioxasiline 16 (only C2). A MeLi addition to 16 afforded C2-silyl phenol 17 in 82% yield over 3 steps. The reaction of 17 and Tf2O in the presence of pyridine gave arylsilyl triflate 18, which in turn was utilized for the aryne cycloaddition with furan to afford cycloadduct 19. During the course of the cycloaddition reaction partial deprotection of the ketal group was observed (19:20 = 4:1). ketal deprotection of 19 afforded 20 (72% yield, 1.2:1 dr). (scheme 4)
Scheme 4.
Synthetic approach to estrone derivative 20
3. Conclusion
To summarize, we have developed an efficient strategy to prepare diversely substituted arylsilyl triflates. The catalytic reductive ortho-C–H silylation of phenols via a traceless acetal directing group to afford benzodioxasilines 3, followed by a sequential addition of MeLi and trifluoromethanesulfonic anhydride, furnished arylsilyl triflates 5 in a single pot. In particular, α-chloroacetyl formal directing group was required for the effective reductive ortho-C–H silylation to afford 1,2,3-trisubstitued arylsilyl triflates 5. Furthermore, this class of arylsilyl triflates demanded purification of benzodioxasilines 3o–r for subsequent nucleophilic ring-opening and triflation processes, in order to minimize unwanted desilylation byproduct 7. We demonstrated that fluoride-mediated [4+2] aryne cycloaddition reaction of the resulting diethylmethylsilylaryl triflates 5 with furan to afford distinctly substituted cycloadducts 13, some of which were not accessed previously.
4. Experimental section
4.1. General
Reactions requiring anhydrous conditions were performed under an atmosphere of nitrogen or argon in flame or oven-dried glassware. Anhydrous toluene and dichloromethane (DCM) were distilled from CaH2. Anhydrous tetrahydrofuran (THF) and diethyl ether (Et2O) were distilled from sodium and benzophenone. Triethylamine and pyridine were distilled from KOH. DMF and DMSO were stored over 4 Å molecular sieves. All other solvents and reagents from commercial sources were used as received. NMR spectra were recorded on a 500 or 300 MHz NMR spectrometer. 1H NMR chemical shifts are referenced to chloroform (7.26 ppm) and DMSO-d6 (2.50 ppm). 13C NMR chemical shifts are referenced to 13CDC13 (77.23 ppm) and DMSO-d6 (39.52 ppm). The following abbreviations are used to describe multiplets: s (singlet), d (doublet), t (triplet), q (quartet), pent (pentet), m (multiplet), nfom (nonfirst-order multiplet), and br (broad). The following format was used to report peaks: chemical shift in ppm [multiplicity, coupling constant(s) in Hz, integral, and assignment]. 1H NMR assignments are indicated by structure environment (e.g., CHaHb). 1H NMR and 13C NMR were processed with the iNMR software program. Infrared (IR) spectra were recorded using neat (for liquid compound) or a thin film from a concentrated DCM solution. Absorptions are reported in cm−1. Only the most intense and/or diagnostic peaks are reported. MPLC refers to medium pressure liquid chromatography (25–200 psi) using hand-packed columns of silica gel (20–45 µm, spherical, 70 Å pore size), an HPLC pump, and a differential refractive index detector. High-resolution mass spectra (HRMS) were recorded in atmospheric-pressure chemical ionization and time-of-flight (APCI/TOF) mode. Samples were introduced as solutions in a mixed solution of methanol and DCM. Analytical TLC experiments were performed on an F254 plate with 250 µm thickness. Detection was performed by UV light or potassium phosphomolybdic acid, potassium permanganate, and p-anisaldehyde staining.
4.2. General Procedure for Preparation of Arylsilyl triflates from Phenyl Acetates (5)
(i) [Ir(coe)2Cl]2 (0.9 mg, 0.1 mol %) and aryl acetates 1 (1 mmol) were added to a flame-dried, nitrogen-purged septum-capped vial. The mixture was dissolved with THF (0.3 mL, 3.3 M), and diethylsilane (0.26 mL, 2 mmol) was added to the mixture. The septum on the vial was replaced by a screw cap with a Teflon liner under a N2 atmosphere [note: diethylsilane (bp 56 °C and density 0.686 g/mL) is volatile]. The reaction mixture was stirred for 3–12 h at 60 °C. Volatiles were removed in vacuo to afford silyl acetals, which were directly used for subsequent reactions without further purification. (ii) [Rh(nbd)Cl]2 (1.8 mg, 0.4 mol %), tris(4-methoxyphenyl)phosphine (8.4 mg, 2.4 mol %), norbornene (188 mg, 2 mmol), and THF (1 mL, 1 M) were added to the crude silyl acetals (1 mmol). The septum on the vial was replaced by a screw cap with a Teflon liner, and the mixture was stirred at 120 °C for 15 min. The reaction progress was monitored by GC/MS spectrometry. The resulting benzodioxasilines 3 were directly used for a subsequent reaction without further purification. For hindered substrates 3o–r, the resulting benzodioxasilines 3 were purified for the subsequent reactions; volatiles were removed in vacuo, and the resulting mixture was dissolved with pentane, filtered through a pad of Celite®, and concentrated in vacuo. The crude product was purified by MPLC (hexanes/EtOAc = 80:1, 5 mL/min, retention time 5–15 min). (iii) The crude benzodioxasilines 3 (1 mmol, THF, 1 M) were diluted with diethyl ether (3 mL, 0.33 M) and cooled to −78 °C. MeLi (3 mmol, 1.6 M in Et2O) were added into the reaction mixture at −78 °C and stirred for 1 min. (iv) Trifluoromethanesulfonyl anhydride (1.2 mmol, 0.2 mL) was added into the reaction mixture. The reaction mixture was warmed to rt and stirred for 30 min. The reaction mixture was cooled to 0 °C and saturated aqueous ammonium chloride solution was added. The mixture was extracted with diethyl ether three times. The combined organic layer was washed with water and brine, and dried over anhydrous sodium sulfate. Volatiles were removed in vacuo, and the crude mixture was purified by MPLC to afford arylsilyl triflates 5 (hexanes/EtOAc = 40:1, 5 mL/min, retention time 6–20 min).
4.2.1. 2-[Diethyl(methyl)silyl]-4-(trifluoromethyl)phenyl trifluoromethanesulfonate (5d)
Yield (70%, 276 mg); colorless oil; 1H NMR (CDC13, 500 MHz) δ 7.76 (d, J = 2.4 Hz, 1H, Ar-H), 7.71 (dd, J = 8.7, 2.4 Hz, 1H, Ar-H), 7.49 (d, J = 8.7 Hz, 1H, Ar-H), 0.98–0.90 [m, 10H, Si(CH2CH3)2], and 0.38 (s, 3H, SiCH3); 13C NMR (CDC13, 125 MHz) δ 157.3, 134.0 (q, 3JF-C = 3.6 Hz), 132.6, 129.9 (q, 2JF-C = 32.5 Hz), 128.7 (q, 3JF-C = 3.6 Hz), 123.8 (q, 1JF-C = 273.2 Hz), 119.9, 118.8 (q, 1JF-C = 320.3 Hz), 7.3, 5.3, and −5.6; IR (neat) 2962 (m), 2857 (w), 1566 (w), 1421 (s), 1201 (s), 1131 (s), 1045 (m), 889 (s), and 773 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C13H16F6O3SSi)+: 394.0494. Found: 394.0463.
4.2.2. 4-{[(tert-Butyldimethylsilyl)oxy]methyl}-2-(diethyl[methyl] silyl)phenyl trifluoromethanesulfonate (5e)
Yield (74%, 348 mg); colorless oil; 1H NMR (CDC13, 500 MHz) δ 7.48 (d, J = 2.2 Hz, 1H, Ar-H), 7.37 (dd, J = 8.6, 2.2 Hz, 1H, Ar-H), 7.30 (d, J = 8.6 Hz, 1H, Ar-H), 4.76 (s, 2H, Ar-CH2OTBS), 0.95 [s, 9H, OSiC(CH3)3], 0.97–0.85 [m, 10H, Si(CH2CH3)2], 0.34 (s, 3H, SiCH3), and 0.11 [s, 6H, OSi(CH3)2]; 13C NMR (CDC13, 125 MHz) δ 154.3, 140.7, 134.3, 130.4, 128.8, 119.4, 118.7 (q, 1JF-C = 320.3 Hz), 64.3, 26.1, 18.5, 7.5, 5.6, −5.1, and −5.3; IR (neat) 2954 (m), 2873 (w), 1571 (w), 1454 (s), 1244 (s), 1133 (s), 1061 (m), 888 (s), and 621 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C19H34F3O4SSi2)+: 471.1663. Found: 471.1689.
4.2.3. 2-[Diethyl(methyl) silyl]-4-methoxyphenyl trifluoromethanesulfonate (5f)
Yield (83%, 295 mg); colorless oil; 1H-NMR (CDC13, 500 MHz) δ 7.26 (d, J = 9.0 Hz, 1H, Ar-H), 7.00 (d, J = 3.2 Hz, 1H, Ar-H), 6.90 (dd, J = 9.0, 3.2 Hz, 1H, Ar-H), 3.82 (s, 3H, Ar-OCH3), 0.98–0.84 [m, 10H, Si(CH2CH3)2], and 0.34 (s, 3H, SiCH3); 13C NMR (CDC13, 125 MHz) δ 158.1, 148.6, 132.4, 122.2, 120.8, 118.7 (q, 1JF-C = 320.3 Hz), 115.0, 55.6, 7.3, 5.4, and −5.5; IR (neat) 2957 (m), 2878 (w), 1576 (w), 1467 (s), 1211 (s), 1121 (s), 1034 (m), 889 (s), and 618 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes: EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C13H20F3O4SSi)+: 357.0798. Found: 357.0815.
4.2.4. 2-[Diethyl(methyl)silyl]-5-methylphenyl trifluoromethanesulfonate (5g)
Yield (72%, 245 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.39 (d, J = 7.9 Hz, 1H, Ar-H), 7.17–7.14 (m, 2H, Ar-H), 2.39 (s, 3H, Ar-CH3), 0.95–0.83 [m, 10H, Si(CH2CH3)2], and 0.32 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 155.6, 142.2, 136.9, 128.5, 127.0, 120.3, 118.7 (q, 1JF-C = 320.3 Hz), 21.5, 7.5, 5.6, and −5.3; IR (neat) 2957 (m), 2877 (w), 1610 (w), 1417 (s), 1204 (s), 1139 (s), 1046 (m), 839 (s), and 597 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C13H20F3O3SSi)+: 341.0849. Found: 341.0873.
4.2.5. 2-[Diethyl(methyl)silyl]-6-methylphenyl trifluoromethanesulfonate (5h)
Yield (66%, 224 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.39 (dd, J = 6.9, 2.2 Hz, 1H, Ar-H), 7.31 (dd, J = 7.5, 2.2 Hz, 1H, Ar-H), 7.28 (dd, J = 7.5, 6.9 Hz, 1H, Ar-H), 2.40 (s, 3H, Ar-CH3), 0.99–0.87 [m, 10H, Si(CH2CH3)2], and 0.37 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 151.6, 135.3, 134.0, 132.8, 131.7, 128.1, 118.9 (q, 1JF-C = 319.8 Hz), 17.6, 7.5, 6.1, and −4.5; IR (neat) 2957 (m), 2875 (w), 1587 (w), 1455 (s), 1232 (s), 1115 (s), 1026 (m), 881 (s), and 610 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (13H20F3O3SSi)+: 341.0849. Found: 341.0818.
4.2.6. 2-[Diethyl(methyl)silyl]-6-methoxyphenyl trifluoromethanesulfonate (5i)
Yield (65%, 231 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.30 (dd, J = 7.8, 7.8 Hz, 1H, Ar-H), 7.04 (dd, J = 7.8, 1.5 Hz, 1H, Ar-H), 7.03 (dd, J = 7.8, 1.5 Hz, 1H, Ar-H), 3.86 (s, 3H, Ar-OCH3), 0.96–0.86 [m, 10H, Si(CH2CH3)2], and 0.35 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 150.3, 143.6, 133.4, 128.7, 127.6, 119.2 (q, 1JF-C = 321.6 Hz), 114.1, 55.7, 7.5, 5.6, and −5.1; IR (neat) 2957 (m), 2879 (w), 1575 (w), 1461 (s), 1203 (s), 1137 (s), 1030 (m), 870 (s), and 631 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C13H20F3O4SSi)+: 357.0798. Found: 357.0776.
4.2.7. 2-[Diethyl(methyl)silyl]naphthalen-1-yl trifluoromethanesulfonate (5j)
Yield (60%, 226 mg) colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.90 (dd, J = 7.0, 1.3 Hz, 1H, Ar-H), 7.87 (ddd, J = 8.1, 1.1, 0.4 Hz, 1H, Ar-H), 7.82 (dd, J = 8.2, 1.4 Hz, 1H, Ar-H), 7.77 (dd, J = 8.1, 1.1 Hz, 1H, Ar-H), 7.53 (dd, J = 8.1, 7.0 Hz, 1H, Ar-H), 7.45 (dd, J = 8.1, 8.1 Hz, 1H, Ar-H), 0.99–0.90 [m, 10H, Si(CH2CH3)2], and 0.40 (s, 3H, SiCH3); 13C NMR (CDCl3, 76 MHz) δ 148.6, 137.9, 136.0, 132.2, 129.9, 129.3, 129.0, 126.2, 124.6, 118.9 (q, 1JF-C = 320.2 Hz), 114.9, 7.9, 7.3, –2.7; IR (neat) 2956 (m), 2877 (w), 1485 (s), 1192 (s), 1111 (s), 1051 (w), 890 (s), and 650 (s) cm−1; TLC Rf = 0.5 in 80:1 hexanes: EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C16H20F3O3SSi)+: 376.0776. Found: 376.0759.
4.2.8. 3-[Diethyl(methyl)silyl]naphthalen-2-yl trifluoromethanesulfonate (5k)
Yield (66%, 248 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 8.00 (s, 1H, Ar-H), 7.88 (dd, J = 6.8, 2.2 Hz, 1H, Ar-H), 7.85 (dd, J = 6.8, 2.2 Hz, 1H, Ar-H), 7.82 (s, 1H, Ar-H), 7.59–7.53 (m, 2H, Ar-H), 1.01–0.92 [m, 10H Si(CH2CH3)2], and 0.42 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 152.9, 138.5, 134.3, 131.9, 129.2, 128.15, 127.99, 127.95, 127.1, 118.8 (q, 1JF-C = 320.3 Hz), 116.6, 7.5, 5.6, and −5.2; IR (neat) 2957 (m), 2874 (w), 1532 (w), 1474 (s), 1202 (s), 1109 (s), 1046 (m), 874 (s), and 637 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc. HRMS (APCI/TOF) calcd for (M+H)+ (C16H20F3O3SSi)+: 377.0849. Found: 377.0817.
4.2.9. 4-[Diethyl(methyl)silyl]benzo[d][1,3]dioxol-5-yl trifluoromethanesulfonate (5l)
Yield (53%, 196 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 6.79 (app s, 2H, Ar-H), 5.98 (s, 2H, OCH2O), 0.98–0.84 (m, 10H, Si(CH2CH3)2], and 0.38 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 153.8, 148.7, 145.8, 118.7 (q, 1JF-C = 320.3 Hz), 113.5, 112.7, 109.1, 101.6, 7.4, 6.0, and −4.6; IR (neat) 2955 (m), 2865 (w), 1581 (w), 1465 (s), 1208 (s), 1124 (s), 1030 (m), 869 (s), and 661 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (13H18F3O5SSi)+: 371.0591. Found: 371.0585.
4.2.10. 2-[Diethyl(methyl)silyl]-4,6-dimethylphenyl trifluoromethanesulfonate (5m)
Yield (72%, 255 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.18 (d, J = 2.3 Hz, 1H, Ar-H), 7.13 (d, J = 2.3 Hz, 1H, Ar-H), 2.38 (s, 3H, Ar-CH3), 2.36 (s, 3H, Ar-CH3), 1.01–0.92 [m, 10H Si(CH2CH3)2], and 0.39 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 149.6, 137.7, 135.7, 134.6, 132.4, 131.2, 119.0 (q, 1JF-C = 320.1 Hz), 20.9, 17.5, 7.6, 6.1, and −4.5; IR (neat) 2956 (m), 2877 (w), 1460 (s), 1221 (s), 900 (s), 1039 (m), 865 (s), and 633 (s) cm−1; TLC Rf = 0.5 in 40:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H22F3O3SSi)+: 355.1006. Found: 355.1013.
4.2.11. 2,5-bis[Diethyl(methyl)silyl]-1,4-phenylene bis(trifluoromethanesulfonate) (5n)
Yield (74%, 425 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.47 (s, 2H, Ar-H), 0.99–0.88 [m, 20H, Si(CH2CH3)2], and 0.37 (s, 6H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 153.5, 135.8, 127.7, 118.8 (q, 1JF-C = 320.6 Hz), 7.2, 5.2, and −5.7; IR (neat) 2958 (m), 2879 (w), 1461 (w), 1422 (s), 1223 (s), 1137 (s), 1078 (m), 884 (s), and 615 (s) cm−1; TLC Rf = 0.5 in 20:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (8H29F6O6S2Si2)+: 575.0843. Found: 575.0817.
4.2.12. 2-[Diethyl(methyl)silyl]-6-ethylphenyl trifluoromethanesulfonate (5o)
Yield (81%, 287 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.40 (dd, J = 7.3, 2.1 Hz, 1H, Ar-H), 7.37 (dd, J = 7.3, 2.1 Hz, 1H, Ar-H), 7.33 (dd, J = 7.3, 7.3 Hz, 1H, Ar-H), 2.79 (q, J = 7.6 Hz, 2H, Ar-CH2CH3), 1.25 (t, J = 7.6 Hz, 3H, Ar-CH2CH3), 0.99–0.87 [m, 10H, Si(CH2CH3)2], and 0.37 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 150.7, 137.5, 135.3, 132.9, 131.9, 128.3, 118.9 (q, 1JF-C = 319.35 Hz), 23.5, 14.2, 7.5, 6.2, and −4.4; IR (neat) 2957 (m), 2878 (w), 1576 (w), 1467 (s), 1211 (s), 1121 (s), 1034 (m), 889 (s), and 618 (s) cm−1; TLC Rf = 0.5 in 80:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H22F3O3SSi)+: 355.1006. Found: 355.1018.
4.2.13. 2-[Diethyl(methyl)silyl]-6-isopropylphenyl trifluoromethanesulfonate (5p)
Yield (269 mg, 73%); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.42 (dd, J = 7.0, 2.5 Hz, 1H, Ar-H), 7.38–7.33 (m, 2H, Ar-H), 3.31 [hept, J = 6.8 Hz, 1H, Ar-CH(CH3)2], 1.24 [d, J = 6.8 Hz, 6H, Ar-CH(CH3)2], 0.96–0.87 [m, 10H, Si(CH2CH3)2], and 0.35 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 149.5, 142.2, 135.2, 133.0, 129.3, 128.4, 118.8 (q, 1JF-C = 317.3 Hz), 27.3, 23.7, 7.6, 6.2, and − 4.3; IR (neat) 2958 (m), 2877 (w), 1571 (w), 1447 (s), 1201 (s), 1154 (s), 1027 (m), 892 (s), and 636 (s) cm−1; TLC Rf = 0.5 in 80:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C15H24F3O3SSi)+: 369.1162. Found: 369.1145.
4.2.14. 2-(tert-Butyl)-6-[diethyl(methyl)silyl]phenyl trifluoromethanesulfonate (5q)
Yield (70%, 267 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.55 (dd, J = 7.8, 1.9 Hz, 1H, Ar-H), 7.37 (dd, J = 7.2, 1.9 Hz, 1H, Ar-H), 7.30 (dd, J = 7.8, 7.2 Hz, 1H, Ar-H), 1.43 [s, 9H, C(CH3)3], 0.95–0.84 [m, 10H Si(CH2CH3)2], and 0.32 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 148.2, 143.8, 135.6, 134.6, 131.8, 127.6, 118.6 (q, 1JF-C = 320.3 Hz), 36.5, 32.1, 7.6, 6.9, and −3.6; IR (neat) 2957 (m), 2878 (w), 1564 (w), 1443 (s), 1236 (s), 1163 (s), 1041 (m), 874 (s), and 608 (s) cm−1; TLC Rf = 0.5 in hexanes. HRMS (APCI/TOF) calcd for (M+H)+ (C16H26F3O3SSi)+: 383.1319. Found: 383.1304.
4.2.15. 3-[Diethyl(methyl)silyl]-(1,1’-biphenyl)-2-yl trifluoromethanesulfonate (5r)
Yield (75%, 301 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.54–7.52 (m, 1H, Ar-H), 7.45–7.37 (m, 7H, Ar-H), 1.02–0.93 [m, 10H, Si(CH2CH3)2], and 0.42 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 150.1, 137.1, 136.5, 133.96, 133.85, 129.8, 128.6, 128.2, 118.2 (q, 1JF-C = 320.7 Hz), 7.6, 6.2, and −4.3; IR (neat) 2957 (m), 2878 (w), 1556 (w), 1435 (s), 1221 (s), 1133 (s), 1024 (m), 869 (s), and 798 (s) cm−1; TLC Rf = 0.5 in 80:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C18H22F3O3SSi)+: 403.1006. Found: 403.9985.
4.3. General Procedure for Fluoride-Mediated Benzyne Cycloaddition Reactions (13)
Arylsilyl triflate (0.5 mmol) 5 was dissolved in furan (0.2 mL) and placed in a 4 mL vial. TBAF (1.2 equiv, 1 M in THF) was added into the reaction mixture at rt. The septum on the vial was replaced by a screw cap with a Teflon liner, and the mixture was stirred at rt for 2 h. The reaction was quenched by adding saturated aqueous ammonium chloride. The reaction mixture was extracted with diethyl ether and concentrated in vacuo to afford the crude mixture, which was purified by MPLC (hexanes/EtOAc = 10:1, 5 mL/min, retention time 7–15 min) to afford 1,4-dihydro-1,4-epoxynaphthalenes 13.
4.3.1. 6-(Trifluoromethyl)-1,4-dihydro-1,4-epoxynaphthalene (13d)
Yield (83%, 88 mg) colorless oil; 1H NMR (CDCl3, 300 MHz) δ 7.46 (s, 1H, Ar-H), 7.33 (d, J = 7.4 Hz, 1H, Ar-H), 7.29 (d, J = 7.4 Hz, 1H, Ar-H), 7.06 (dd, J = 5.5, 1.7 Hz, 1H, CH(O)CH=CH), 7.04 (dd, J = 5.5, 1.8 Hz, 1H CH(O)CH=CH), and 5.77 (app s, 2H, CHOCH); 13C NMR (CDCl3, 76 MHz) δ 153.4, 150.5, 143.3, 142.9, 127.7 (q, 2JF-C = 32.3 Hz), 124.3 (q, 1JF-C = 271.6 Hz), 123.2 (q, 3JF-C = 4.3 Hz), 120.2, 117.2 (q, 3JF-C = 3.6 Hz), and 82.3 (2). IR (neat): 3035 (w), 2956 (m), 2924 (m), 2853 (m), 1703 (w), 1631 (m), 1527 (m), 1222 (s), 1087 (s), 1031 (s), and 826 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H8F3O)+: 213.0522. Found: 213.0514.
4.3.2. tert-Butyl[(1,4-dihydro-1,4-epoxynaphthalen-6-yl)methoxy]dimethylsilane (13e)
Yield (80%, 47 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.24 (s, 1H, Ar-H), 7.18 (d, J = 7.2 Hz, 1H, Ar-H), 7.01 [nfom, 2H, CH(O)CH=CH], 6.91 (d, J = 7.2 Hz, 1H, Ar-H), 5.70 (s, 2H, CHOCH), 4.67 (s, 2H, Ar-CH2OTBS), 0.93 [s, 9H, OSiC(CH3)3], and 0.09 [s, 6H, OSi(CH3)2]; 13C NMR (CDCl3, 125 MHz) δ 149.5, 147.9, 143.27, 143.12, 138.8, 122.7, 120.0, 118.8, 82.58, 82.41, 65.2, 26.2, 18.6, and −5.0; IR (neat) 3053 (m), 2874 (w), 1600 (w), 1460 (m), 1415 (s), 1278 (m), 1209 (s), 1139 (s), 1045 (s), 845 (w), 695 (s), and 571 (m) cm−1. TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C17H25O2Si)+: 289.1618. Found: 289.1602.
4.3.3. 6-Methoxy-1,4-dihydro-1,4-epoxynaphthalene (13f)
Yield (65%, 94 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.13 (d, J = 7.8 Hz, 1H, Ar-H), 7.03 [dd, J = 5.6, 1.8 Hz, 1H CH(O)CH=CH], 6.99 [dd, J = 5.6, 1.8 Hz, 1H CH(O)CH=CH], 6.91 (d, J = 2.2 Hz, 1H, Ar-H), 6.42 (dd, J = 7.8, 2.2 Hz, 1H, Ar-H), 5.68 (app s, 1H, CHOCH), 5.66 (app s, 1H, CHOCH), and 3.77 (s, 3H, Ar-OCH3); 13C NMR (CDCl3, 125 MHz) δ 157.6, 151.2, 143.7, 142.4, 140.6, 120.6, 109.9, 107.4, 82.6, 82.2, and 55.8; IR (neat) 3036 (w), 2961 (w), 2909 (w), 1632 (m), 1516 (m), 1481 (m), 1264 (s), 1217 (s), 1030 (m), 812 (s), and 560 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H11O2)+: 175.0754. Found: 175.0737.
4.3.4. 6-Methyl-1,4-dihydro-1,4-epoxynaphthalene (13g)
Yield (75%, 35 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.13 (d, J = 7.2 Hz, 1H, Ar-H), 7.10 (s, 1H, Ar-H), 7.02 [dd, J = 5.5, 1.7 Hz, 1H CH(O)CH=CH], 7.01 [dd, J = 5.5, 1.7 Hz, 1H CH(O)CH=CH], 6.77 (d, J = 7.2 Hz, 1H, Ar-H), 5.69 (app s, 1H, CHOCH), 5.67 (app s, 1H, CHOCH), and 2.30 (s, 3H, Ar-CH3); 13C NMR (CDCl3, 125 MHz) δ 149.5, 146.2, 143.4, 143.0, 135.0, 125.2, 121.8, 120.1, 82.50, 82.38, and 21.5; IR (neat) 3038 (m), 2954(w), 1620 (w), 1599 (w), 1459 (m), 1279 (s), 1165 (m), 1020 (s), 845 (s), 641 (s), and 573 (s) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H11O)+: 159.0804. Found: 159.0821.
4.3.5. 5-Methyl-1,4-dihydro-1,4-epoxynaphthalene (13h)
Yield (86%, 68 mg); colorless oil; 1H NMR (CDCl3, 300 MHz) δ 7.09 (d, J = 6.9 Hz, 1H, Ar-H), 7.07–6.99 [m, 2H, CH(O)CH=CH], 6.88 (dd, J = 7.6, 6.9 Hz, 1H, Ar-H), 6.78 (d, J = 7.6 Hz, 1H, Ar-H), 5.81 (app s, 1H, CHOCH), 5.71 (app s, 1H, CHOCH), and 2.32 (s, 3H, ArCH3); 13C NMR (CDCl3, 76 MHz) δ 148.7, 147.4, 143.2, 142.7, 130.1, 126.8, 125.1, 117.9, 82.6, 80.9, and 18.2; IR (neat) 3036 (m), 2841 (w), 1632 (w), 1465 (m), 1522 (s), 1216 (s), 1347 (s), 1014 (s), 835 (m), 662 (s), and 573 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H11O)+: 159.0804. Found: 159.0791.
4.3.6. 5-Methoxy-1,4-dihydro-1,4-epoxynaphthalene (13i)
Yield (60%, 56 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.07 [dd, J = 5.5, 1.8 Hz, 1H, CH(O)CH=CH], 7.03 [dd, J = 5.5, 1.8 Hz, 1H CH(O)CH=CH], 6.97 (dd, J = 8.0, 7.0 Hz, 1H, Ar-H), 6.93 (dd, J = 7.0, 0.8 Hz, 1H, Ar-H), 6.59 (dd, J = 8.0, 0.8 Hz, 1H, Ar-H), 5.95 (app s, 1H, CHOCH), 5.70 (app s, 1H, CHOCH), and 3.83 (s, 3H, Ar-CH3); 13C NMR (CDCl3, 76 MHz) δ 153.1, 151.7, 143.23, 143.08, 135.2, 127.2, 113.9, 110.5, 82.7, 80.3, and 55.9; IR (neat) 3042 (m), 2874 (w), 1642 (w), 1453 (m), 1538 (s), 1312 (m), 1167 (s), 1041 (m), 844 (m), and 521 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H11O2)+: 175.0754. Found: 175.0731.
4.3.7. 1,4-Dihydro-1,4-epoxyphenanthrene (13j)
Yield (71%, 69 mg); pale yellow solid, mp 85–87 °C; 1H NMR (CDCl3, 300 MHz) δ 7.83 (dd, J = 8.3, 8.3 Hz, 2H, Ar-H), 7.56 (ddd, J = 7.9, 7.9, 7.9 Hz, 2H, Ar-H), 7.47 (dd, J = 8.1, 7.4 Hz, 1H, Ar-H), 7.39 (dd, J = 8.1, 7.6 Hz, 1H, Ar-H), 7.31–7.12 [m, 2H, CH(O)CH=CH], 6.28 (app s, 1H, CHOCH), and 5.94 (app s, 1H, CHOCH).; 13C NMR (CDCl3, 76 MHz) δ 148.5, 148.0, 145.1, 143.6, 132.0, 129.0, 127.8, 126.4, 125.6, 125.3, 122.9, 119.5, 83.6, and 81.4; IR (neat) 3050 (m), 2855 (w), 1655 (w), 1517 (m), 1451 (m), 1345 (s), 1147 (s), 1039 (s), 683 (s), and 482 (s); TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H11O)+: 194.0732. Found: 194.0741.
4.3.8. 1,4-Dihydro-1,4-epoxyanthracene (13k)
Yield (67%, 65 mg) pale yellow solid, mp 160–162 °C; 1H NMR (CDCl3, 500 MHz) δ 7.76–7.67 (m, 2H, Ar-H), 7.59 (s, 2H, Ar-H), 7.45–7.42 (m, 2H, Ar-H), 7.01–6.94 [m, 2H, CH(O)CH=CH], and 5.81 ( app s, 2H, CHOCH); 13C NMR (CDCl3, 125 MHz) δ 144.3, 141.9, 132.1, 128.3, 126.3, 118.8, and 82.0; IR (neat) 3024 (m), 2929 (w), 1666 (w), 1538 (m), 1454 (s), 1193 (s), 1025 (s), 843 (s), 744 (s), and 496 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H11O)+: 195.0804. Found: 195.0788.
4.3.9. 6,9-Dihydro-6,9-epoxynaphtho[1,2-d][1,3]dioxole (13l)
Yield (60%, 56 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.00 [dd, J = 5.6, 1.7 Hz, 1H, CH(O)CH=CH], 6.97 [dd, J = 5.6, 1.7 Hz, 1H, CH(O)CH=CH], 6.72 (d, J = 7.2 Hz, 1H, Ar-H), 6.38 (d, J = 7.2 Hz, 1H, Ar-H), 5.90 (d, J = 10.7 Hz, 1H, OCHaHbO), 5.90 (d, J = 10.7 Hz, 1H, OCHaHbO), 5.85 (app s, 1H, CHOCH), and 5.65 (app s, 1H, CHOCH); 13C NMR (CDCl3, 125 MHz) δ 146.5, 143.2 (2), 141.5, 140.6, 127.3, 113.3, 103.5, 101.1, 82.5, and 79.7; IR (neat) 3053 (m), 2960 (w), 2777 (w), 1650 (w), 1572 (m), 1458 (s), 1232 (s), 1042 (s), 954 (s), 829 (m), 638 (s), and 522 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C11H9O3)+: 189.0546. Found: 189.0565.
4.3.10. 5,7-Dimethyl-1,4-dihydro-1,4-epoxynaphthalene (13m)
Yield (71%, 61 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.03 [dd, J = 5.5, 1.7 Hz, 1H, CH(O)CH=CH], 7.02 [dd, J = 5.5, 1.7 Hz, 1H, CH(O)CH=CH], 6.94 (s, 1H, Ar-H), 6.60 (s, 1H, Ar-H), 5.78 (app s, 1H, CHOCH), 5.66 (app s, 1H, CHOCH), 2.28 (s, 3H, Ar-CH3), and 2.27 (s, 3H, Ar-CH3); 13C NMR (CDCl3, 125 MHz) δ 149.2, 144.5, 143.08, 143.03, 134.9, 129.8, 127.0, 119.4, 82.7, 80.9, 21.3, and 18.2; IR (neat) 3047 (m), 2964 (m), 2872 (w), 1612 (w), 1481 (m), 1532 (s), 1214 (s), 1121 (s), 644 (s), and 521 (m) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C12H13O)+: 173.0961. Found: 173.0942.
4.3.11. 1,4,5,8-Tetrahydro-1,4:5,8-diepoxyanthracene (13n)
Yield (69%, 72 mg) (1:1 dr); white sold, mp 189–191 °C ; 1H NMR (CDCl3, 500 MHz) δ 7.20 (s, 2H, Ar-H), 7.06–6.99 (m, 4H, CH(O)CH=CH], and 5.63 (app s, 4H, CHOCH); 13C NMR (CDCl3, 125 MHz) δ 148.0, 143.6, 114.3, and 82.6; IR (neat) 3047 (w), 2963 (m), 2877 (w), 1640 (w), 1603 (w), 1485 (m), 1528 (s), 1222 (s), 1147 (s), 1029 (s), 845 (m), 635 (s), and 516 (m) cm−1; TLC Rf = 0.5 in 3:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H11O2)+: 211.0754. Found: 211.0767.
4.3.12. 5-Ethyl-1,4-dihydro-1,4-epoxynaphthalene (13o)
Yield (70%, 60 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.12 (d, J = 7.0 Hz, 1H, Ar-H), 7.05–7.02 [nfom, 2H, CH(O)CH=CH], 6.92 (dd, J = 7.9, 7.0 Hz, 1H, Ar-H), 6.81 (d, J = 7.9 Hz, 1H, Ar-H), 5.84 (app s, 1H, CHOCH), 5.72 (app s, 1H, CHOCH), 2.70 (dq, J = 14.1, 7.6 Hz, 1H, Ar-CHaHbCH3), 2.63 (dq, J = 14.1, 7.6 Hz, 2H, Ar-CHaHbCH3), and 1.21 (dd, J = 7.7, 7.7 Hz, 3H, Ar-CHaHbCH3); 13C NMR (CDCl3, 125 MHz) δ 148.7, 146.9, 143.4, 142.9, 136.7, 125.40, 125.34, 118.2, 82.6, 81.0, 26.2, and 16.0; IR (neat) 3050 (m), 2963 (m), 2871 (w), 1643 (w), 1609 (w), 1470 (m), 1278 (m), 1141 (m), 1087 (m), 1003 (s), 870 (s), 675 (s), and 603 (m) cm−1; TLC Rf = 0.5 in 10:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C12H13O)+: 173.0961. Found: 173.0942.
4.3.13. 5-Isopropyl-1,4-dihydro-1,4-epoxynaphthalene (13p)
Yield (64%, 60 mg); colorless oil; 1H NMR (CDCl3, 500 MHz) δ 7.11 (d, J = 6.9 Hz, 1H, Ar-H), 7.05–7.01 [nfom, 2H, CH(O)CH=CH], 6.94 (dd, J = 7.9, 6.9 Hz, 1H, Ar-H), 6.86 (d, J = 7.9, 0.5 Hz, 1H, Ar-H), 5.91 (app s, 1H, CHOCH), 5.70 (app s, 1H, CHOCH), 3.05 (hept, J = 6.9 Hz, 1H), 1.29 (d, J = 6.9 Hz, 3H), and 1.21 (d, J = 6.9 Hz, 3H); 13C NMR (CDCl3, 125 MHz) δ 148.7, 146.4, 143.5, 142.9, 141.2, 125.5, 122.8, 118.3, 82.6, 81.2, 31.8, 24.3, and 23.2; IR (neat) 3052 (m), 2956 (m), 2872 (w), 1641 (w), 1611 (w), 1475 (m), 1274 (m), 1121 (m), 1065 (m), 671 (s), and 653 (m) cm−1; TLC Rf = 0.5 in 10:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C13H15O)+: 187.1117. Found: 187.1103.
4.3.14. 5-(Tert-butyl)-1,4-dihydro-1,4-epoxynaphthalene (13q)
Yield (50%, 50 mg); white solid, mp 53–54 °C; 1H NMR (CDCl3, 500 MHz) δ 7.12 (d, J = 6.8, 1.1 Hz, 1H, Ar-H), 7.06–7.02 [nfom, 2H, CH(O)CH=CH], 6.97 (dd, J = 8.1, 1.1 Hz, 1H, Ar-H), 6.93 (dd, J = 8.1, 6.8 Hz, 1H, Ar-H), 6.15 (app s, 1H, CHOCH), 5.68 (app s, 1H, CHOCH), and 1.36 (s, 9H); 13C NMR (CDCl3, 125 MHz) δ 149.2, 146.7, 144.1, 143.8, 142.8, 125.2, 122.6, 118.4, 83.2, 82.1, 35.5, and 31.5; IR (neat) 3066 (m), 2963 (m), 2869 (w), 1686 (w), 1588 (w), 1470 (m), 1279 (m), 1187 (m), 1120 (m), 1008 (m), 878 (s), 710 (s), and 658 (m) cm−1; TLC Rf = 0.5 in 10:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C14H17O)+: 201.1274. Found: 201.1294.
4.3.15. 5-Phenyl-1,4-dihydro-1,4-epoxynaphthalene (13r)
Yield (67%, 74 mg); pale yellow solid, mp 63–65 °C; 1H NMR (CDCl3, 500 MHz) δ 7.51–7.45 (m, 2H, Ar-H), 7.42–7.35 (m, 3H, Ar-H), 7.29–7.25 (m, 1H, Ar-H), 7.20–7.10 [m, 2H, CH(O)CH=CH], 7.09–7.04 (m, 2H, Ar-H), 5.84 (app s, 1H, CHOCH), and 5.78 (app s, 1H, CHOCH). 13C NMR (CDCl3, 125 MHz) δ 149.3, 147.1, 143.5, 143.0, 139.8, 135.1, 128.9, 128.3, 127.6, 125.6, 125.4, 119.5, 82.7, and 82.0; IR (neat) 3063 (m), 2962 (m), 2872 (w), 1662 (w), 1581 (w), 1473 (m), 1282 (m), 1193 (m), 1117 (m), 1003 (m), 871 (s), and 710 (s), cm−1; TLC Rf = 0.5 in 10:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C16H13O)+: 221.0961. Found: 221.0945.
4.4. Procedure for Preparation of (8R,9S,13S,14S)-13-Methyl-6,7,8,9,11,12,13,14,15,16-decahydrospiro[cyclopenta[a]phenanthrene-17,2’-[1,3]dioxolan]-3-yl 2-chloroacetate (15)
A round-bottom flask (100 mL) with a magnetic stir bar and a Dean-Stark apparatus was charged with estrone 14 (3.0 g, 11.0 mmol) and p-TsOH (105 mg, 5 mol %). Toluene (60 mL) and ethylene glycol (3.1 ml, 55 mmol) were added and the reaction mixture was heated at reflux for 14 h. The reaction was cooled to rt, and the solvent was removed in vacuo, ethyl acetate (30 mL) and brine (50 mL) were added. The mixture was extracted with ethyl acetate three times, and the combined organic extract was washed with water and brine, and dried over anhydrous sodium sulfate. The volatiles were removed in vacuo to afford the crude product, which was directly used for a subsequent reaction without further purification. The crude product (11 mmol) and chloroacetic acid (1.9 g, 20 mmol) and DMAP (67 mg, 5 mol %) were dissolved with CH2Cl2 (20 mL), the reaction mixture was cooled to 0 °C with an ice bath. DCC (2.7 g, 13 mmol) was added into the reaction mixture slowly. The reaction mixture was warmed to rt and stirred for 10 h. Diethyl ether (30 mL) was added to precipitate the urea byproduct. The mixture was filtered and the filtrate was treated with saturated aqueous sodium bicarbonate. Aqueous phase was extracted with ethyl acetate three times. The combined organic extracts were washed with water and brine, and dried over anhydrous sodium sulfate. The volatiles were removed in vacuo, and the crude mixture was purified by flash column (hexanes: EtOAc 3:1) to afford the ester 15 (3.27 g, 76% over two steps). White solid, mp 113–115 °C; 1H NMR (CDCl3, 500 MHz) δ 7.30 [d, J = 8.1 Hz, 1H, H(1)], 6.88 [dd, J = 8.1, 2.6 Hz, 1H, H(2)], 6.83 [d, J = 2.6 Hz, 1H, H(4)], 4.28 (s, 2H, CH2Cl), 3.98–3.88 (m, 4H, OCH2CH2O), 2.88–2.84 (m, 2H, alkyl-H), 2.32 (dddd, J = 13.2, 4.2, 4.2, 2.7 Hz, 1H, alkyl-H), 2.26 (ddd, J = 10.6, 10.6, 4.1 Hz, 1H, alkyl-H), 2.03 (ddd, J = 14.0, 11.2, 3.1 Hz, 1H, alkyl-H), 1.93–1.74 (m, 4H, alkyl-H), 1.63 (ddd, J = 12.1, 10.8, 7.0 Hz, 1H, alkyl-H), 1.55–1.31 (m, 5H, alkyl-H), and 0.88 [s, 3H, CH(18)3]; 13C NMR (CDCl3, 125 MHz) δ 166.4, 148.2, 138.9, 138.8, 126.8, 121.2, 119.6, 118.2, 65.5, 64.8, 49.6, 46.3, 44.0, 41.1, 38.8, 34.4, 30.9, 29.7, 26.9, 26.2, 22.6, and 14.5; IR (neat) 2935 (m), 2864 (m), 1775 (s), 1724 (m), 1493 (m), 1304 (m), 1190 (s), 1042 (m), and 804 (m) cm−1. m.p. 76–78 °C; TLC Rf = 0.5 in 3:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C22H28ClO4)+: 391.1671. Found: 391.1659.
4.5. Procedure for Preparation of (8R,9S,13S,14S)-3-[Diethyl(methyl)silyl)]-13-methyl-6,7,8,9,11,12,13,14,15,16-decahydrospiro(cyclopenta[a]phenanthrene-17,2’-[1,3]dioxolan)-2-yl trifluoromethanesulfonate (18)
C2-silyl phenol 17 (414 mg, 1 mmol) and pyridine (0.12 mL, 1.5 mmol) in CH2Cl2 (2 mL, 1 M) were cooled to 0 °C with an ice bath. Trifluoromethanesulfonyl anhydride (0.25 mL, 1.5 mmol) was added into the reaction mixture dropwise. The reaction mixture was stirred at 0 °C for 1 h and an additional 30 min at rt. The reaction was quenched by saturated aqueous sodium bicarbonate and was extracted with diethyl ether three times. The combined organic extracts were washed with water two times and brine, and dried over anhydrous sodium sulfate. The volatiles were removed in vacuo, and the crude mixture was purified by MPLC (hexanes/EtOAc = 5:1, 5 mL/min) to afford arylsilyl triflate 18. White solid; Yield (90%, 491 mg) 1H NMR (CDCl3, 500 MHz) δ 7.39 (s, 1H, Ar-H), 7.01 (s, 1H, Ar-H), 3.99–3.88 (m, 4H, OCH2CH2O), 2.87 (dd, J = 8.4, 4.0 Hz, 2H, alkyl-H), 2.37–2.31 (m, 1H, alkyl-H), 2.30–2.25 (m, 1H, alkyl-H), 2.04 (ddd, J = 14.1, 11.6, 2.7 Hz, 1H, alkyl-H), 1.94–1.76 (m, 4H alkyl-H), 1.64 (ddd, J = 11.9, 10.9, 7.0 Hz, 1H alkyl-H), 1.56 (ddd, J = 12.6, 3.3, 2.6 Hz, 1H alkyl-H), 1.55–1.33 (m, 4H), 0.89 [s, 3H CH(18)3], 0.97–0.80 [m, 10H, Si(CH2CH3)2], and 0.30 (s, 3H, SiCH3); 13C NMR (CDCl3, 125 MHz) δ 153.5, 141.0, 139.8, 134.1, 126.8, 119.58, 119.51, 118.7 (q, 1JF-C = 319.6 Hz), 65.5, 64.8, 49.5, 46.3, 44.0, 38.8, 34.4, 30.8, 29.9, 26.8, 26.1, 22.6, 14.5, 7.6, 5.68, 5.59, and −5.2; IR (neat) 2957 (m), 2874 (w), 1493 (m), 1304 (m), 1190 (s), 1042 (m), and 804 (m) (s) cm−1; TLC Rf = 0.5 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C26H38F3O5SSi)+: 547.2156. Found: 547.2139.
4.6. (3aS,3bR,11bS,13aS)-13a-Methyl-2,3,3a,3b,4,5,7,10,11b,12,13,13a-dodecahydrospiro[7,10-epoxycyclopenta[c]tetraphene-1,2’-[1,3]dioxolane] (19)
C2-Silyl triflate 18 (109 mg, 0.2 mmol) was dissolved in furan (0.2 mL). TBAF (0.6 mL, 0.24 mmol, 1 M in THF) was added to the reaction mixture at rt. The septum on the vial was replaced by a screw cap with a Teflon liner, and the mixture was stirred at rt for 2 h. Reaction progress was monitored by TLC until full conversion of 18. TLC showed two spots, indicating formation of compound 19 and 20. The reaction was quenched by adding saturated aqueous ammonium chloride. The reaction mixture was extracted with diethyl ether and concentrated in vacuo to afford a mixture of 19 and 20 (4:1), which was used for next step without further purification. White solid; Yield (60%, 44 mg) (1.2:1 dr). 1H NMR (CDCl3, 500 MHz) δ 7.25 (s, 0.45H, Ar-H), 7.24 (s, 0.55H, Ar-H), 7.02–6.98 [m, 3H, Ar-H and CH(O)CH=CH], 5.66–5.65 (m, 2H, CHOCH), 3.98–3.87 (m, 4H, OCH2CH2O), 2.85–2.72 (m, J = 2.9 Hz, 2H, Alkyl-H), 2.32–2.28 (m, 1H, Alkyl-H), 2.27–2.19 (m, 1H, Alkyl-H), 2.05–1.99 (m, 1H, Alkyl-H), 1.89–1.73 (m, 4H, Alkyl-H), 1.65–1.59 (m, 1H, Alkyl-H), 1.56–1.51 (m, 1H, Alkyl-H), 1.49–1.31 (m, 4H, Alkyl-H), 0.88 [s, 1.35H, CH(18)3], and 0.86 [s, 1.65H CH(18)3]; 13C NMR (CDCl3, 125 MHz) δ 146.34, 146.32, 146.21, 146.09, 143.24, 143.20, 143.01 (2), 136.84, 136.73, 133.31, 133.27, 121.57, 121.52, 119.62, 119.60, 118.0 (2), 82.58, 82.55, 82.36, 82.28, 65.5 (2), 64.8 (2), 49.61, 49.57, 46.34, 46.32, 44.47, 44.39, 39.18, 39.13, 34.4 (2), 31.0 (2), 30.1 (2), 27.11, 27.09, 26.44, 26.30, 22.6 (2), and 14.5 (2); IR (neat) 3061 (m), 2957 (m), 2873 (w), 1491 (m), 1205 (m), 1192 (s), 1041 (m), and 814 (m) (s) cm−1; TLC Rf = 0.4 in 5:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (C24H29O3)+: 365.2111. Found: 365.2085.
4.7. Procedure for Preparation of (3aS,3bR,11bS,13aS)-13a-Methyl-2,3,3a,3b,4,5,7,10,11b,12,13,13a-dodecahydro-1H-7,10-epoxycyclopenta[c]tetraphen-1-one (20)
The crude mixture of 19 was dissolved in a mixture of THF/H2O (0.5 mL, 1:1, 0.1 M). Hydrochloric acid (0.5 ml, 1 M) was added and the mixture was stirred at rt for 5 h. The reaction was quenched by adding saturated aqueous sodium bicarbonate. The reaction mixture was extracted with diethyl ether three times and concentrated in vacuo to afford the crude mixture, which was purified by MPLC (hexanes/EtOAc = 5:1, 5 mL/min, retention time 15 min) to provide 20. Yield (72%, 46 mg) (1.2:1 dr); White solid; 1H NMR (CDCl3, 500 MHz) δ 7.24 (s, 0.45H, Ar-H), 7.24 (s, 0.55H, Ar-H), 7.02–6.98 [m, 3H, Ar-H and CH(O)CH=CH], 5.66 (app s, 2H, CHOCH), 2.86–2.82 (m, 2H, Alkyl-H), 2.50 (ddd, J = 19.0, 8.7, 2.3 Hz, 1H, Alkyl-H), 2.41–2.35 (m, 1H, Alkyl-H), 2.27–2.24 (m, 1H, Alkyl-H), 2.18–2.10 (m, 1H, Alkyl-H), 2.07–1.94 (m, 3H, Alkyl-H), 1.63–1.42 (m, 6H, Alkyl-H), 0.91 [s, 1.35H, CH(18)3], and 0.89 [s, 1.65H, CH(18)3]; 13C NMR (CDCl3, 125 MHz) δ 221.1 (2), 146.60, 146.58, 146.44 (2), 143.25, 143.23, 142.99, 142.96, 136.17, 136.07, 133.08, 133.05, 121.58, 121.54, 117.9 (2), 82.57, 82.54, 82.35, 82.27, 50.6 (2), 48.21, 48.19, 44.81, 44.73, 38.51, 38.47, 36.1 (2), 31.8 (2), 30.0 (2), 26.7 (2), 26.23, 26.10, 21.8 (2), and 14.1 (2); IR (neat) 3053 (m), 2961 (m), 2874 (w), 1631 (w), 1442 (m), 1222 (m), 1191 (m), 1115 (m), 1001 (m), 851 (s), 706 (s), cm−1; TLC Rf = 0.5 in 3:1 hexanes:EtOAc; HRMS (APCI/TOF) calcd for (M+H)+ (22H25O2)+: 321.1849. Found: 321.1855.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM116031) and the ACS Petroleum Research Fund (PRF# 54831-DNI1) for support. The NSF (CHE-0234811 and CHE-0840509) is acknowledged for partial funding of the purchases of the NMR spectrometers used in this work. This paper is dedicated to the memory of his former colleague and friend, Dr. James P. Bacci (1979–2015).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data related to this article can be found at
References and notes
- 1.Roberts JD, Simmons HE, Carlsmith L, Vaughan CW. J. Am. Chem. Soc. 1953;75:3290–3291. [Google Scholar]
- 2.(a) Goetz AE, Shah TK, Garg NK. Chem. Commun. 2015;51:34–45. doi: 10.1039/c4cc06445c. [DOI] [PubMed] [Google Scholar]; (b) Tadross PM, Stoltz BM. Chem. Rev. 2012;112:3550–3577. doi: 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]
- 3.(a) Pérez D, Peña D, Guitián E. Eur. J. Org. Chem. 2013;2013:5981–6013. [Google Scholar]; (b) Wu D, Ge H, Liu SH, Yin J. RSC Advances. 2013;3:22727–22738. [Google Scholar]
- 4.Truong T, Daugulis O. Chem. Sci. 2013;4:531–535. doi: 10.1039/c2sc21288a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Allan KM, Gilmore CD, Stoltz BM. Angew. Chem. 2011;123:4580–4583. doi: 10.1002/anie.201100911. [DOI] [PubMed] [Google Scholar]; (b) Smith AB, Kim W-S. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6787–6792. doi: 10.1073/pnas.1015265108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Goetz AE, Garg NK. Nat. Chem. 2013;5:54. doi: 10.1038/nchem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yoshida H, Yoshida R, Takaki K. Angew. Chem., Int. Ed. 2013;52:8629–8632. doi: 10.1002/anie.201302783. [DOI] [PubMed] [Google Scholar]; (e) Hendrick CE, McDonald SL, Wang Q. Org. Lett. 2013;15:3444–3447. doi: 10.1021/ol401518c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Bhojgude SS, Thangaraj M, Suresh E, Biju AT. Org. Lett. 2014;16:3576–3579. doi: 10.1021/ol501579d. [DOI] [PubMed] [Google Scholar]; (g) Liu F-L, Chen J-R, Zou Y-Q, Wei Q, Xiao W-J. Org. Lett. 2014;16:3768–3771. doi: 10.1021/ol501638x. [DOI] [PubMed] [Google Scholar]; (h) Pandya VG, Mhaske SB. Org. Lett. 2014;16:3836–3839. doi: 10.1021/ol5018646. [DOI] [PubMed] [Google Scholar]; (i) Sumida Y, Harada R, Kato-Sumida T, Johmoto K, Uekusa H, Hosoya T. Org. Lett. 2014;16:6240–6243. doi: 10.1021/ol5031734. [DOI] [PubMed] [Google Scholar]; (j) Hendrick CE, Wang Q. J. Org. Chem. 2014;80:1059–1069. doi: 10.1021/jo502541t. [DOI] [PubMed] [Google Scholar]; (k) Yoshida S, Yano T, Misawa Y, Sugimura Y, Igawa K, Shimizu S, Tomooka K, Hosoya T. J. Am. Chem. Soc. 2015;137:14071–14074. doi: 10.1021/jacs.5b10557. [DOI] [PubMed] [Google Scholar]; (l) Peng X, Ma C, Tung C-H, Xu Z. Org. Lett. 2016 doi: 10.1021/acs.orglett.6b02027. [DOI] [PubMed] [Google Scholar]; (m) Shu W-M, Zheng K-L, Ma J-R, Wu A-X. Org. Lett. 2016 [Google Scholar]; (n) Dhokale RA, Mhaske SB. Org. Lett. 2016 doi: 10.1021/acs.orglett.6b01384. [DOI] [PubMed] [Google Scholar]; (o) Shah TK, Medina JM, Garg NK. J. Am. Chem. Soc. 2016;138:4948–4954. doi: 10.1021/jacs.6b01986. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Karmakar R, Lee D. Chem. Soc. Rev. 2016 [Google Scholar]
- 6.(a) Stoermer R, Kahlert B. Berichte der deutschen chemischen Gesellschaft. 1902;35:1633–1640. [Google Scholar]; (b) Gilman H, Avakian S. J. Am. Chem. Soc. 1945;67:349–351. [Google Scholar]; (c) Huisgen R, Sauer J, Hauser A. Chem. Ber. 1958;91:2366–2374. [Google Scholar]; (d) Wittig G, Hoffmann RW. Chem. Ber. 1962;95:2718–2728. [Google Scholar]; (e) Stiles M, Miller RG, Burckhardt U. J. Am. Chem. Soc. 1963;85:1792–1797. [Google Scholar]; (f) Campbell C, Rees C. Journal of the Chemical Society C: Organic. 1969:742–747. [Google Scholar]; (g) Meyers A, Pansegrau PD. J. Chem. Soc., Chem. Commun. 1985:690–691. [Google Scholar]; (h) Hart H, Harada K, Du CJF. J. Org. Chem. 1985;50:3104–3110. [Google Scholar]; (i) Wenk HH, Winkler M, Sander W. Angew. Chem., Int. Ed. 2003;42:502–528. doi: 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]; (j) Gampe C, Carreira E. Angew. Chem., Int. Ed. 2012;51:3766–78. doi: 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]; (k) Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP. Nature. 2012;490:208–212. doi: 10.1038/nature11518. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Niu D, Willoughby PH, Woods BP, Baire B, Hoye TR. Nature. 2013;501:531–534. doi: 10.1038/nature12492. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Yun SY, Wang K-P, Lee N-K, Mamidipalli P, Lee D. J. Am. Chem. Soc. 2013;135:4668–4671. doi: 10.1021/ja400477r. [DOI] [PubMed] [Google Scholar]; (n) Goetz AE, Garg NK. J. Org. Chem. 2014;79:846–851. doi: 10.1021/jo402723e. [DOI] [PubMed] [Google Scholar]; (o) Mesgar M, Daugulis O. Org. Lett. 2016;18:3910–3913. doi: 10.1021/acs.orglett.6b01952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Himeshima Y, Sonoda T, Kobayashi H. Chem. Lett. 1983;12:1211–1214. [Google Scholar]; (b) Kitamura T, Yamane M. J. Chem. Soc., Chem. Commun. 1995:983–984. [Google Scholar]
- 8.(a) Beak P, Snieckus V. Acc. Chem. Res. 1982;15:306–312. [Google Scholar]; (b) Beak P, Meyers A. Acc. Chem. Res. 1986;19:356–363. [Google Scholar]; (c) Snieckus V. Chem. Rev. 1990;90:879–933. [Google Scholar]; (d) Whisler MC, MacNeil S, Snieckus V, Beak P. Angew. Chem., Int. Ed. 2004;43:2206–2225. doi: 10.1002/anie.200300590. [DOI] [PubMed] [Google Scholar]
- 9.Yamanoi Y, Nishihara H. J. Org. Chem. 2008;73:6671–6678. doi: 10.1021/jo8008148. [DOI] [PubMed] [Google Scholar]
- 10.(a) Billedau R, Sibi M, Snieckus V. Tetrahedron Lett. 1983;24:4515–4518. [Google Scholar]; (b) He H-M, Fanwick PE, Wood K, Cushman M. J. Org. Chem. 1995;60:5905–5909. [Google Scholar]
- 11.(a) Peña D, Cobas A, Pérez D, Guitián E. Synthesis. 2002;2002:1454–1458. [Google Scholar]; (b) Bronner SM, Garg NK. J. Org. Chem. 2009;74:8842–8843. doi: 10.1021/jo9020166. [DOI] [PubMed] [Google Scholar]; (c) Atkinson DJ, Sperry J, Brimble MA. Synthesis. 2010;2010:911–913. [Google Scholar]
- 12.(a) Simmons EM, Hartwig JF. J. Am. Chem. Soc. 2010;132:17092–17095. doi: 10.1021/ja1086547. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheng C, Brookhart M. Angew. Chem., Int. Ed. 2012;51:9422–9424. doi: 10.1002/anie.201205154. [DOI] [PubMed] [Google Scholar]; (c) Hua Y, Asgari P, Dakarapu US, Jeon J. Chem. Commun. 2015;51:3778–3781. doi: 10.1039/c4cc09850a. [DOI] [PubMed] [Google Scholar]; (d) Hua Y, Asgari P, Avullala T, Jeon J. J. Am. Chem. Soc. 2016;138:7982–7991. doi: 10.1021/jacs.6b04018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Brook AG. Acc. Chem. Res. 1974;7:77–84. [Google Scholar]; (b) Moser WH. Tetrahedron. 2001;57:2065–2084. [Google Scholar]
- 14.Austin WF, Zhang Y, Danheiser RL. Tetrahedron. 2008;64:915–925. doi: 10.1016/j.tet.2007.10.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Cooper GD. J. Org. Chem. 1961;26:925–929. [Google Scholar]; (b) Eastham SA, Ingham SP, Hallett MR, Herbert J, Quayle P, Raftery J. Tetrahedron Lett. 2006;47:2299–2304. [Google Scholar]; (c) Schön U, Messinger J, Solodenko W, Kirschning A. Synthesis. 2012;44:3822–3828. [Google Scholar]
- 16.Esguerra KVN, Fall Y, Petitjean Ln, Lumb J-P. J. Am. Chem. Soc. 2014;136:7662–7668. doi: 10.1021/ja501789x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.