Abstract
Primary progressive aphasia (PPA) is clinically defined by an initial loss of language function and preservation of other cognitive abilities, including episodic memory. While PPA primarily affects the left-lateralized perisylvian language network, some clinical neuropsychological tests suggest concurrent initial memory loss. The goal of this study was to test recognition memory of objects and words in the visual and auditory modality to separate language-processing impairments from retentive memory in PPA. Individuals with non-semantic PPA had longer reaction times and higher false alarms for auditory word stimuli compared to visual object stimuli. Moreover, false alarms for auditory word recognition memory were related to cortical thickness within the left inferior frontal gyrus and left temporal pole, while false alarms for visual object recognition memory was related to cortical thickness within the right-temporal pole. This pattern of results suggests that specific vulnerability in processing verbal stimuli can hinder episodic memory in PPA, and provides evidence for differential contributions of the left and right temporal poles in word and object recognition memory.
Keywords: Primary Progressive Aphasia (PPA), Recognition, Memory, Cortical Atrophy, Inferior Frontal Gyrus, Temporal Pole
1. Introduction
Primary progressive aphasia (PPA) is a neurodegenerative dementia syndrome clinically characterized by the selective loss of language and initial preservation of other cognitive abilities, including episodic memory (M. M. Mesulam, 2003). Despite the prevailing notion that PPA primarily affects language, some studies have reported initial memory deficits (Hutchinson & Mathias, 2007; Zakzanis, 1999). However, the verbal assessment methods used in these studies could not determine whether the problem was secondary to the aphasia or also indicative of a general episodic memory failure.
Episodic memory depends on effective stimulus processing and successful binding into a durable representation (Eichenbaum, 2000). Poor verbal memory in PPA could therefore be due to upstream deficiencies in stimulus processing (a language-driven impairment) or due to more downstream deficiencies in relational binding (a memory-driven impairment) (Neary & Snowden, 1996; Osher, Wicklund, Rademaker, Johnson, & Weintraub, 2007).
Based on the nature of the language impairment, PPA has been subdivided into three clinical variants (Gorno-Tempini, et al., 2011). Relative to individuals with semantic PPA, non-semantic variants of PPA (agrammatic and logopenic subtypes) have preserved single word comprehension. Instead, individuals with non-semantic PPA can exhibit agrammatism, loss of fluency, and poor repetition (Gorno-Tempini, et al., 2011; Grossman, 2012; M. Mesulam, et al., 2009; Weintraub, Rubin, & Mesulam, 1990). In concert with these language impairments, individuals with non-semantic PPA can show peak cortical atrophy within the left inferior frontal gyrus (IFG) or temporal parietal junction (TPJ). As the disease progresses, atrophy spreads to other components of the language network (Rogalski, et al., 2011; Rogalski, et al., 2014). The hippocampus is not an initial site of peak atrophy in PPA.
The left IFG, known as Broca’s area, is specialized for phonological encoding and fluency (Hickok & Poeppel, 2007; Xiang, Fonteijn, Norris, & Hagoort, 2010). The left IFG is structurally connected via the arcuate fasciculus to the left temporal parietal junction, a site that is likely to be important for integrating visual and auditory information (Raij, Uutela, & Hari, 2000). The adjacent superior temporal gyrus acts as an auditory association area, serving the phonological loop and auditory working memory (Leff, et al., 2009). The inclusion of these areas within the regions of peak atrophy in non-semantic PPA suggests that individuals with PPA may have vulnerability in processing auditory stimuli in the verbal modality.
The role of the temporal poles in object and language processing is debated. Studies of patients with semantic dementia (with bilateral anterior temporal lobe atrophy), have proposed that both the left and right temporal lobe contain domain-independent object representations (Patterson, Nestor, & Rogers, 2007; Pobric, Jefferies, & Ralph, 2007, 2010). In contrast, other studies have dissociated verbal from non-verbal markers of object knowledge and suggest disparate domain-specific roles for the temporal poles. This model suggests that the left temporal pole acts in part with the left lateralized language network and is critical for verbal and semantic associations of objects; whereas the right temporal pole acts within the predominantly right-lateralized or bilateral object recognition network (Hurley, Paller, Rogalski, & Mesulam, 2012; M. M. Mesulam, Thompson, Weintraub, & Rogalski, 2015; M. M. Mesulam, et al., 2013).
Our group has previously studied recognition memory in PPA. We found a higher incidence of false alarms for visually presented words compared to objects, especially among semantically related foils (Rogalski, Blum, Rademaker, & Weintraub, 2007). The present study aims to extend these findings by examining the anatomical substrates related to language processing and subsequent memory performance using object and word stimuli in both the visual and auditory modalities. The goal was to dissociate language-processing impairments from episodic memory impairments in mild non-semantic PPA.
2. Methods
2.1 Participants
Twenty-two participants with PPA and fourteen age-matched cognitively normal controls participated in this experiment. All participants were right-handed.
PPA participants were recruited from the PPA Research Program at the Cognitive Neurology and Alzheimer’s Disease Center (CNADC) at Northwestern University. PPA participants were clinically diagnosed by a neurologist (MMM), and subtyped as non-semantic by established criteria (Gorno-Tempini, et al., 2011). Eight participants were characterized as logopenic, 12 participants were characterized as agrammatic, and 2 participants were unclassifiable as either strictly agrammatic or logopenic. All PPA participants therefore had prominent deficits in grammatical processing or fluency with preserved single word comprehension. Participation in the research program included a series of neuropsychological tests assessing overall cognition, and structural neuroimaging. Normal controls were recruited from the Clinical Core at the Northwestern University Alzheimer’s Disease Center. All control participants reported no history of neurologic or psychiatric condition. Through participation in the Clinical Core, control participants also received series of neuropsychological tests confirming normal cognitive functioning (defined as within 1 standard deviation of age normed scores) within 3 months of participation in our experiment. All participants gave written informed consent, and were monetarily compensated for their time. Northwestern University Institutional Review Board approved all study procedures.
2.2 Neuropsychological Measures
To better characterize the participants included in this study, a series of neuropsychological tests were conducted during each study visit. To characterize aphasia, language specific measures were collected within the PPA cohort. Western Aphasia Battery - Aphasia Quotient (WAB) (Kertesz, 1982) is a composite measure of aphasia severity based on auditory comprehension, naming, repetition and spontaneous speech production. The 60-item Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, 1983) was used to measure object naming. A subset of 36 moderately difficult items (#157–192) from PPVT (Peabody Picture Vocabulary Test) (Dunn, 2007) was also used to measure auditory lexical-semantic processing and verbal comprehension.
Diadochokinetic rate (how quickly a participant can accurately repeat a series of phonetic sounds [puh/tuh/kuh] over 5 seconds), and deterioration in articulation with the increase of syllable word length [thick, thicken, thickening], were used to characterize the presence of motor speech impairments, based on standardized tests from an apraxia battery (Dabul, 2000; Wertz, LaPointe, & Rosenbek, 1984). In all study participants, the Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975) was used to measure overall dementia severity. Table 1 presents the neuropsychological data comparing and characterizing each diagnostic group. Two control participants received the Montreal Cognitive Assessment (MoCA) (Nasreddine, et al., 2005) rather than the MMSE, and their scores were accordingly converted (Roalf et al., 2012). Independent sample t-tests and chi-square tests were used to compare both groups where appropriate. Age between both groups (t(1,34)=0.08, p=0.94) and gender distribution (χ2=0.77, p=0.38) were matched. As expected in an aphasic sample (Osher, et al., 2007), MMSE was significantly lower for PPA patients (t(1,34)=6.35, p<0.001), as was performance on the PPVT (t(1,34)=3.52, p=0.002).
Table 1. Neuropsychological measures and participant demographics.
Means ± standard deviations are reported for neuropsychological measures and participant demographics for controls and non-semantic primary progressive aphasia. Abbreviations: PPA = Primary progressive aphasia, MMSE = Mini Mental State Exam, PPVT = Peabody Picture Vocabulary Test, WAB-AQ = Western Aphasia Battery Aphasia Quotient, BNT = Boston Naming Test.
| PPA (n=22) | Control (n=14) | |
|---|---|---|
| Age (years) | 65.81 ± 7.26 | 65.64 ± 6.40 |
| Gender | 14M:8F | 6M:8F |
| Symptom duration (years) | 2.90 ± 1.20 | -- |
| MMSE | 25.95 ± 2.40 | 29.53 ± 0.97 |
| PPVT | 33.55 ± 2.13 | 35.29 ± 0.73 |
| WAB-AQ | 86.55 ± 7.63 | -- |
| BNT | 58.9 ± 2.18 | -- |
| Diadochokinetic Rate | 4.27 ± 2.73 | -- |
| Deterioration Articulation | 0.15 ± 0.28 | -- |
2.3 Stimuli
This study examined recognition memory performance over three modality conditions: auditory words, visual words, and visual objects. All stimuli were high-frequency concrete namable items presented via SuperLab Pro v2.0.3. Frequency was matched for each condition. Mean frequency was 15.710 as measured by the Corpus of Contemporary American English (Davies, 2008).
Visual stimuli were presented at the center of the screen (1920×1080 pixel resolution) for 2000ms. Objects were a subset of color-line drawings (derived from (Rossion & Pourtois, 2004)). Words (6.25±2.11 letters) were horizontally presented and appeared in 72-point font. Auditory words were presented in a male native English voice for variable times (723.086±192.25ms) depending on the length of the word (6.35±2.19 letters). During the presentation of an auditory stimulus, a sound icon would visually appear at the center of the screen to indicate stimulus onset.
Each condition had distinct stimuli. For example, if a rooster appeared as a visual object, the visual word “rooster” and the auditory word “rooster” were not used. Stimulus presentation order within each modality was randomized.
2.4 Task Design
Instructions were presented both visually and orally. The main task consisted of two phases: study phase and test phase. At the onset of each study phase, three stimuli were presented as practice trials. The practice trials were implemented to ensure adequate volume for auditory stimuli and complete comprehension of task instructions.
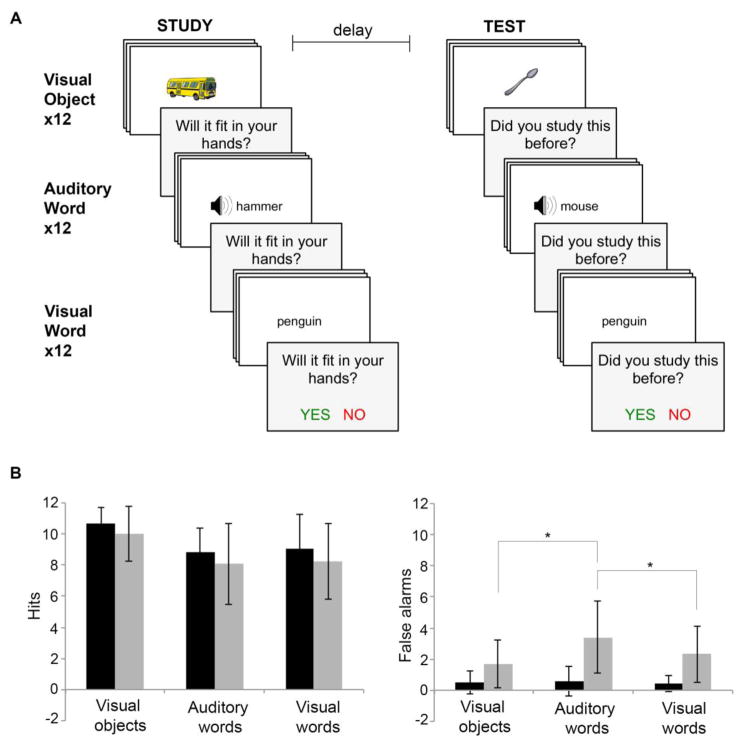
During the study phase, participants were presented with a total of 36 stimuli (12 in each condition). Participants were not explicitly instructed to remember the item. Rather, participants were asked to view each target item (one at a time; 1000ms ISI) and make a perceptual judgment. Participants responded ‘YES’ or ‘NO’ (colored green and red respectively) via buttons to the question: “Does the (target item) fit in one hand?” This was implemented to ensure adequate attention and encoding to each stimulus presentation. Following a 20-minute delay, participants were given an incidental recognition test in which they had to again respond ‘YES’ or ‘NO’ (again, colored green and red respectively) to whether or not each item was one they had seen or heard before. Thirty-six additional novel items (12 for each condition) were included as foils within the test phase. Stimuli were presented for the same duration in both the study and test phase. Participants had up to five seconds to respond before the onset of the next stimulus. For both the study and test phase, participants were asked to respond as accurately and quickly as possible. All responses were made using clearly labeled buttons on a computer keyboard. Trials during study and test were blocked by format. Format condition was randomized; however condition order was maintained between study and test phases for each participant to ensure consistent delay intervals (Figure 1A).
Figure 1. Behavioral hit and false alarm performance.
(A) Participants are presented with a total of 36 target items across three modalities (visual objects, auditory words, and visual words). To ensure adequate encoding, participants are asked to make a perceptual judgment for each stimulus. After a 20-minute delay, participants are given a surprise (i.e., incidental) yes/no recognition test to identify 36 targets among 36 novel distractors. (B) Mean hits and false alarms for PPA (n=22) and control (n=14) participants across each stimulus modality. Significant within-group pair-wise comparisons between stimulus modalities are indicated by an asterisk (*). Error bars represent standard deviation of the mean.
After completion of the test phase, each participant also completed a comprehension assessment for each stimulus format condition. As each target stimulus was presented, participants were asked to correctly identify the matching visual word from a set of eight choices. These eight items included foil stimuli used in the main memory experiment. For the visual word condition, participants identified the matching visual object. Comprehension data were not collected for two participants.
2.5 Behavioral Analysis
Responses were converted to total hits (correctly identifying a studied target) and false alarms (incorrectly identifying a non-studied foil as a studied target). Overall recognition was first assessed using d′, a measure of signal sensitivity. The degree to which old (target) items could be discriminated from new (foil) items was calculated using d′ = z(H) − z(FA) (where H = hit rate, FA = false alarm rate). We also calculated response bias, the tendency to only produce ‘old’ or ‘new’ responses, using c = (−0.5)[z(H) + z(FA)], where a positive c indicates a conservative bias, or the tendency to produce a ‘new’ response.
In order to understand how recognition memory differed by stimulus modality in PPA and control, ANOVAs were conducted separately for signal sensitivity, response bias, total hits, false alarms, and reaction time. Each ANOVA was designed with one between-subjects factor of diagnosis (PPA, control) and one within subject factor of stimulus modality (visual object, visual word, auditory word). For all significant effects, post-hoc Bonferroni-corrected parametric t-tests were completed and presented.
For all dependent variables (for each diagnosis group, at each stimulus modality condition), Shapiro-Wilk tests of normality were conducted. Reaction time was logarithmically transformed prior to analysis in order to use parametric statistics. In the case of total hits and false alarms, for which at least one distribution (modality x group) deviated from normality, a non-parametric mixed-design permutation test with one between-subjects factor of diagnosis (PPA, control) and one within subject factor of stimulus modality (visual object, visual word, auditory word) were performed. Similarly, for all significant effects, post-hoc nonparametric Wilcoxon paired t-tests were used. When applicable, both parametric and non-parametric results are reported.
2.6 MRI acquisition
MRI data were collected for 21/22 PPA participants using a Siemens 3T TIM Trio whole-body magnet (Siemens AG, Erlangen, Germany) with a 12-channel head coil, provided by Northwestern University Center for Translational Imaging (CTI) Facility, supported by Northwestern University Department of Radiology. A T1-weighted 3D MPRAGE structural sequence was acquired (with TR=2300ms, TE=2.91ms, FOV=256 mm, flip angle=9°, TI=900ms, and 1mm3 voxel resolution collected over 176 sagittal slices) within 24 hours of completion of the memory task.
2.7 MRI analysis
Structural MRIs were pre-processed using FreeSurfer (v5.1.0; http://surfer.nmr.mgh.harvard.edu/). Surface errors were removed by manual iterative correction directed by established guidelines (Segonne, Pacheco, & Fischl, 2007). Cortical thickness was calculated by measuring the distance between modeled representations of the white-grey boundary and pial-CSF boundary (Dale, Fischl, & Sereno, 1999). To identify patterns of peak atrophy within our PPA sample, whole-brain vertex-wise cortical thickness in PPA participants was compared to a separate group of 35 right-handed cognitively healthy normal-controls (62.26±7.22, 17M:18F, MMSE: 29.51±0.74), described previously in (Rogalski, et al., 2014). A vertex-wise general linear model was used to detect peak cortical atrophy using a relatively stringent FDR (False Discovery Rate) of 0.001 (Genovese, Lazar, & Nichols, 2002).
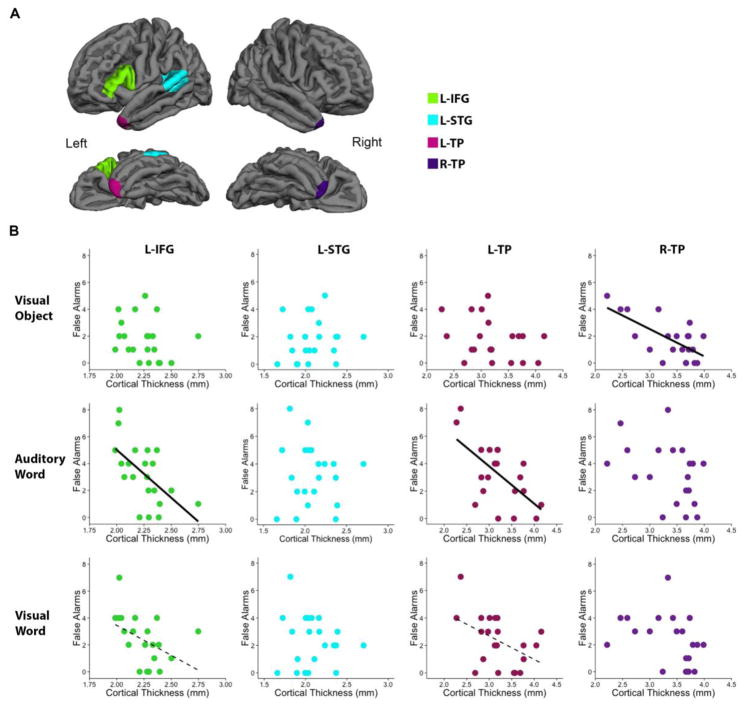
Thickness was then extracted from four a priori regions of interest (ROIs; Figure 3A) using standard FreeSurfer algorithms in subject-native space: the left inferior frontal gyrus (L-IFG), left posterior superior temporal gyrus (L-STG), left temporal pole (L-TP), and right temporal pole (R-TP). The left inferior frontal gyrus ROI was created by merging the left pars triangularis and left pars opercularis using the Desikan-Killiany atlas embedded within the FreeSurfer software suite (Desikan, et al., 2006). Similarly, the superior temporal gyrus ROI included both the posterior third of the superior temporal gyrus and the superior temporal sulcus from the Desikan atlas.
Figure 3. Relationship between mean cortical thickness and false alarms.
(A) Four a priori ROIs are presented in the lateral and inferior view of the left and right hemisphere. (B) Within the PPA cohort (n=21), mean cortical thickness was correlated with false alarms for each stimulus modality. Notably, L-IFG thickness and L-TP thickness correlated with false recognition memory of auditory words and R-TP thickness correlated with false recognition memory of visual objects. Solid lines represent significant correlations (p<0.0125), while dashed lines represent trends (p<0.05). Abbreviations: ROI = Region of Interest, L-IFG = Left Inferior Frontal Gyrus, L-STG = Left Superior Temporal Gyrus, L-TP = Left Temporal Pole, R-TP = Right Temporal Pole.
For each ROI, mean thickness was first compared between our sample of 21 PPA and 35 normal controls using independent t-tests, Bonferroni corrected for multiple ROI tests. Then, to investigate the relationship between false alarms and cortical thickness within PPA, pairwise Pearson correlations were conducted separately for each condition (visual objects, visual words, and auditory words) with each ROI. Residuals of each regression were first tested for normality using Shapiro-Wilk test. If normality was violated, a non-parametric Spearman rank correlation was performed. A significance criterion of p=0.0125 was applied based on a Bonferroni correction for multiple ROIs.
3. Results
3.1 Overall Recognition
Compared to controls, participants with PPA were less able to discriminate between targets and foils (Table 2). Analysis of d′ revealed a main effect of diagnosis (F(1,34) = 28.96, p<0.001), such that across all conditions, d′ significantly differed between diagnosis groups, such that PPA had significantly lower d′ than controls (t(1,34)=5.38, p<0.001). There was also a main effect of stimulus modality (F(2,68) = 17.19, p<0.001), such that all participants had greater d′ for visual objects compared to auditory words (t(1,35)=5.88, p<0.001) and compared to visual words (t(1,35)=3.65, p<0.001). There was no significant interaction between diagnosis and stimulus modality (p=0.071).
Table 2. Signal Sensitivity and Response Bias.
Mean ± standard deviations are reported for sensitivity (d′) and response bias (c) for each stimulus modality condition for controls and non-semantic primary progressive aphasia.
| d′ | Control | PPA |
|---|---|---|
| Visual Objects | 1.64 ± 0.17 | 1.31 ± 0.39 |
| Auditory Words | 1.42 ± 0.22 | 0.71 ± 0.51 |
| Visual Words | 1.50 ± 0.28 | 0.95 ± 0.51 |
| c | Control | PPA |
| Visual Objects | 0.86 ± 0.09 | 0.80 ± 0.19 |
| Auditory Words | 0.96 ± 0.14 | 0.75 ± 0.30 |
| Visual Words | 0.97 ± 0.12 | 0.82 ± 0.25 |
Both participants with PPA and controls had a conservative bias, and were more likely to respond ‘new’ instead of ‘old’ (Table 2). Analysis of bias revealed a main effect of diagnosis, such that across all conditions, PPA had less of a tendency to respond ‘new’ than controls (t(1,34)=2.38, p=0.02). There was no main effect of stimulus modality (p=0.17) and no significant interaction for bias between modality and diagnosis (p=0.12). Thus, both signal sensitivity and response bias did not capture any specific effect of stimulus modality on recognition memory in PPA.
It is important to note that PPA participants scored 100% on comprehension for target items for each condition. High scores are consistent with preserved single-word comprehension characteristic of non-semantic PPA and suggest that comprehension for the stimuli involved in this task did not affect recognition memory performance across conditions.
3.2 Hits and False Alarms
In order to understand how recognition memory differed in PPA and control, we next tested the effect of stimulus modality on hits and false alarms. Parametric analysis of hits (Figure 1B) revealed a main effect of stimulus modality (F(2,68)=14.63, p<0.001) such that irrespective of diagnosis, all participants had more hits for visual objects compared to visual words (t(1,35)=4.79, p<0.001) and compared to auditory words (t(1,35)=5.62, p<0.001). There was no main effect of diagnosis (p=0.18) and no interaction between diagnosis and stimulus modality (p=0.96).
False alarm analysis revealed a main effect of diagnosis (F(1,34)=20.12, p<0.001), such that participants with PPA had greater false alarms than controls irrespective of stimulus modality (t(1,34)=4.48, p<0.001). There was also a main effect of stimulus modality (F(2,68)=8.98, p<0.001). Interestingly, there was a significant interaction of diagnosis and stimulus modality (F(2,68)=4.84, p=0.01). Post-hoc t-tests indicated that that participants with PPA had greater false alarms for auditory words compared to visual objects (t(1,21)=3.91, p=0.002) and compared to written words (t(1,21)=3.25, p=0.01; all other pairwise comparisons p>0.1) whereas controls showed no difference across all stimulus modalities (all pairwise comparisons p>0.1).
Results were consistent using non-parametric permutation tests. Analysis of hits revealed a main effect of stimulus modality (p<0.001). There was no main effect of diagnosis (p=0.2) and no interaction between diagnosis and stimulus modality (p=0.93). A non-parametric analysis of false alarms revealed a main effect of diagnosis (p<0.001), a main effect of stimulus modality (p=0.003), and a significant interaction between stimulus and modality (p=0.02), such that participants with PPA had greater false alarms for auditory words compared to visual objects (p=0.0027), and visual words (p=0.028) whereas controls showed no differences across all stimulus modalities (all pairwise comparisons p>0.1). Thus, while total hits did not differ from controls, individuals with PPA more false alarms across all conditions, and notably, had more false alarms for auditory words.
3.3 Reaction Time
Analysis of reaction time revealed a main effect of diagnosis (F(1,34)=28.31, p<0.001), such that participants with PPA had longer response times than controls irrespective of stimulus modality (t(1,34)=13.73, p<0.001). There was also a main effect of stimulus modality (F(2,68) = 4.92, p<0.001). Consistently, there was a significant interaction of diagnosis and stimulus modality (F(2,68)=4.84, p=0.01). Post-hoc t-tests indicated that that participants with PPA had longer reaction times for auditory words compared to visual objects (t(1,21)=8.362, p<0.001), and compared to written words (t(1,21)=3.30, p=0.024; all other pairwise comparisons p>0.1), whereas controls showed no difference across all stimulus modalities (all pairwise comparisons p>0.1). These results are consistent with increased false alarms of auditory word recognition memory and highlight the selective verbal memory impairment in PPA.
3.4 Correlations and Cortical Atrophy
Vertex-wise thickness analyses across the left and right hemispheres were used to identify areas of neurodegenerative cortical atrophy within our agrammatic PPA participants. Consistent with previous reports (Rogalski, et al., 2011), as a group, participants with non-semantic PPA showed atrophy through the left perisylvian cortex, including atrophy in the inferior frontal gyrus, the superior and middle temporal gyri, temporal parietal junction and the inferior temporal gyrus (Figure 2).
Figure 2. Peak PPA group atrophy patterns.
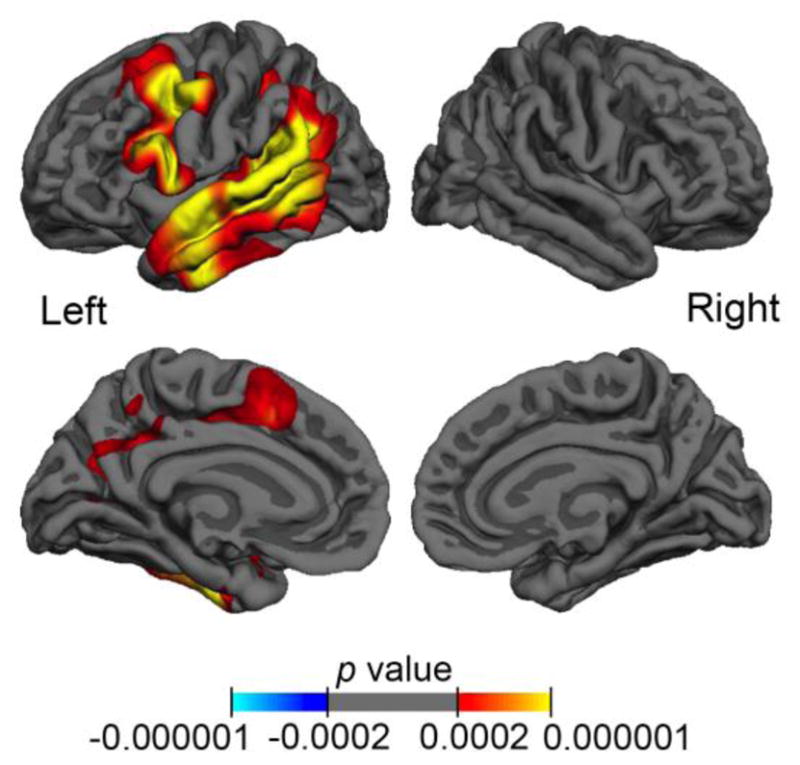
Significant peak atrophy patterns, as measured by cortical thickness, for PPA participants (n=21) compared to controls (n=35) are represented on the lateral and medial views of the left and right hemisphere. False discovery rate was stringently set to 0.001 to highlight peak atrophy; warmer colors represent significant thinning and corresponding p-values are displayed by the color bar.
Thickness was extracted from our four a priori ROIs (IFG, STG, L-TP and R-TP). Using these ROIs, our sample of non-semantic PPA had significantly more atrophy within the left IFG (t(1,54)=4.59, p<0.001) and left STG (t(1,54)=8.28, p<0.001), but not within the left (t(1,54)=2.33, p>0.1) or right (t(1,54)=0.52, p>0.1) temporal pole. Thus, cortical thinning measured by ROI is consistent with patterns of atrophy typical in non-semantic PPA (Rogalski, et al., 2011).
Mean thickness for each ROI was then correlated with false alarms for each stimulus modality (visual object, auditory word, visual word) (Figure 3B). All correlations are employed Pearson statistics except when specifically additionally noted as Spearman rank. False alarms within the auditory word condition correlated with cortical thickness in the L-IFG (r=−0.591, p=0.0047), and L-TP (r=−0.62, p=0.0027) but not with thickness in the L-STG (r=−0.06, ns), or the R-TP(r=−0.41, ns). Thus, PPA participants with greater cortical thinning within the left inferior frontal gyrus and left temporal pole had worse recognition of auditory words.
False alarms within the visual word condition did not significantly correlate with cortical thickness extracted from any of ROIs (L-STG: r=−0.11, ns; R-TP: r=−0.38, ns; Spearman r=−0.45; ns) though there was a trending relationship with the left temporal pole (L-TP: r=−0.46, p=0.034) and left inferior frontal gyrus (L-IFG: r= −0.44, p=0.045; Spearman-r=−0.53, p=0.014).
False alarms within the visual object condition correlated with cortical thickness in the R-TP (r=−0.68, p=0.0007), but not with thickness in the L-TP (r=−0.33, ns), the L-STG (r=0.12, ns) or the L-IFG (r=−0.12, ns). Thus, PPA participants with greater cortical thinning of the right temporal pole had worse performance within the visual object condition.
4. Discussion
The present study examined memory for objects and words as tested by recognition accuracy. Compared to healthy controls, individuals with PPA showed similar levels of true (hit) recognition. Both healthy controls and individuals with PPA also showed greater true recognition for objects compared to word stimuli. This result was not surprising given the abundance of research in control populations suggesting that deeper perceptual encoding allows more accurate recognition and recall memory for objects compared to words (Grady, McIntosh, Rajah, & Craik, 1998; Schacter, Koutstaal, & Norman, 1997).
While true hits did not differ from controls, individuals with PPA had higher levels of false alarms across all conditions, and critically, had higher levels of false alarms for auditory word stimuli compared to visual object stimuli. Higher incidence of false alarms has been used as a marker of episodic memory impairments in aging (Duarte, Graham, & Henson, 2010) and Alzheimer’s dementia (Abe, et al., 2011; Budson, Wolk, Chong, & Waring, 2006). However, the selective impairment in recognition of auditory word stimuli and not visually presented object stimuli presented in this study coincides with the selective language impairments of non-semantic PPA and supports the contention that poor memory is a secondary manifestation of the language impairment.
More false alarms in the auditory word condition were related to greater cortical thinning within the inferior frontal gyrus, a common region of peak atrophy in non-semantic PPA. Functional imaging studies have suggested that preserved auditory word recognition in healthy older adults is related to inferior frontal gyrus activity (Roxbury, McMahon, Coulthard, & Copland, 2015). As older adults engaged in word-learning tasks rely more on phonological encoding than semantic knowledge (Service E, 1993), the L-IFG atrophy in PPA could directly affect the ability to engage verbal strategies, resulting in poor encoding and ultimately poor recognition memory performance.
Another finding in this study is that higher false alarms for auditory words was associated with left temporal pole thickness, while higher false alarm for visual objects was associated with right temporal pole thickness. Although the relationship between visual word recognition memory performance and atrophy of the left temporal pole was only trending, the relationship between recognition performance and cortical thickness across all conditions supports the dissociation of verbal and object recognition in the left and right temporal poles. These results are consistent with the dual-route model suggesting a domain-specific (rather than domain-independent) roles for the anterior temporal poles (M. M. Mesulam, et al., 2013). Non-verbal object recognition impairments may actually arise from bilateral damage to the temporal poles. This is consistent with other studies in which non-verbal object recognition was worse in PPA cases with bilateral temporal pole atrophy compared to those with unilateral left temporal pole atrophy (M. M. Mesulam, et al., 2015).
One study has found hippocampal atrophy within non-semantic PPA (Gorno-Tempini, et al., 2004), though only in the logopenic subtype where underlying pathology is often Alzheimer’s disease. In contrast, other studies found no differences in hippocampal volume in PPA compared to healthy controls (van de Pol, et al., 2006), and found no relationship between medial temporal volume and memory performance across subtypes and related syndromes (Irish, et al., 2015; Mansoor, et al., 2015). Another study including a mix of agrammatic and logopenic non-semantic PPA patients found that even in the absence of overt deficits in memory when compared to controls, subtle hippocampal shape surface deformities were related to memory performance (Christensen, et al., 2015). Future longitudinal studies will show whether the memory perturbations in PPA are exclusively caused by damage within the language network or whether they also relate to the eventual spread of pathology to the hippocampal complex.
In a smaller study in a cohort including all subtypes, we had previously shown that PPA was associated with higher false alarms of visually presented words compared to objects (Rogalski, et al., 2007). The previous study did not include enough participants across each subtype to differentiate memory performance by specific language impairments. As such, worse recognition memory in the auditory modality compared to the visual modality could represent initial speech and auditory vulnerabilities (Grube, et al., 2016) specific to mild non-semantic PPA.
Additional support for the material-specificity of memory impairments in PPA comes from the Three Words Three Shapes test, designed to test recall and recognition memory for non-verbal abstract objects compared to words (Weintraub, et al., 2000). PPA patients showed specific impairments in online encoding and retrieval of verbal, but not non-verbal items; while patients with dementia of the Alzheimer type showed impairments for both verbal and non-verbal items (Kielb, et al., 2016; Weintraub, et al., 2013) reinforcing the notion that learning and memory impairments observed in PPA are a secondary result of aphasia.
In summary, this study further demonstrates that memory impairments in PPA can be attributed largely to deficiencies in stimulus encoding within the language network of the left hemisphere.
Table 3. Reaction Time.
Mean reaction time (RT) ± standard deviations reported in milliseconds for each stimulus modality condition for controls and non-semantic primary progressive aphasia.
| RT | Control | PPA |
|---|---|---|
| Visual Objects | 662.3 ± 141.6 | 953.6 ± 270.0 |
| Auditory Words | 769.9 ± 322.9 | 1607.0 ± 600.5 |
| Visual Words | 721.3 ± 220.1 | 1186.2 ± 599.6 |
Highlights.
Recognition memory of auditory words is impaired in non-semantic PPA.
Atrophy of left inferior frontal gyrus correlated with auditory word false alarms.
Processing verbal stimuli can hinder episodic memory in PPA.
Correlations provide evidence for distinct roles of the left and right temporal pole.
Acknowledgments
This research was supported by: the National Institute on Aging through the Northwestern University Alzheimer’s Disease Care Center grant [AG13854] and an individual training grant [T32AG20506], the National Institute on Deafness and Communication Disorders [DC00855], and the National Institute of Neurological Disorders and Stroke [NS075075 and NS069788].
We would like to thank Jaiashre Sridhar and Adam Martersteck for assistance with FreeSurfer analyses, and Benjamin Rader, Hannah McKenna, Emmaleigh Taylor, and Marie Saxon for assistance with data collection. Neuroimaging was preformed and supported by Northwestern University Center for Translational Imaging in the Department of Radiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Fujii T, Nishio Y, Iizuka O, Kanno S, Kikuchi H, Takagi M, Hiraoka K, Yamasaki H, Choi H, Hirayama K, Shinohara M, Mori E. False item recognition in patients with Alzheimer’s disease. Neuropsychologia. 2011;49:1897–1902. doi: 10.1016/j.neuropsychologia.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer’s disease: separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Christensen A, Alpert K, Rogalski E, Cobia D, Rao J, Beg MF, Weintraub S, Mesulam MM, Wang L. Hippocampal subfield surface deformity in non-semantic primary progressive aphasia. Alzheimers Dement (Amst) 2015;1:14–23. doi: 10.1016/j.dadm.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabul BL. Apraxia battery for adults. Austin, TX: 2000. Pro-ed. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davies M. The Corpus of Contemporary American English: 520 million words, 1990-present. 2008 In (pp. Available online at http://corpus.byu.edu/coca/.)
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duarte A, Graham KS, Henson RN. Age-related changes in neural activity associated with familiarity, recollection and false recognition. Neurobiol Aging. 2010;31:1814–1830. doi: 10.1016/j.neurobiolaging.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Dunn LMD, Douglas M. Peabody Picture Vocabulary Test. Toronto, Ontario, Canada: Pearson Assessments; 2007. [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Craik FI. Neural correlates of the episodic encoding of pictures and words. Proc Natl Acad Sci U S A. 1998;95:2703–2708. doi: 10.1073/pnas.95.5.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Bruffaerts R, Schaeverbeke J, Neyens V, De Weer AS, Seghers A, Bergmans B, Dries E, Griffiths TD, Vandenberghe R. Core auditory processing deficits in primary progressive aphasia. Brain. 2016;139:1817–1829. doi: 10.1093/brain/aww067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Paller KA, Rogalski EJ, Mesulam MM. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci. 2012;32:4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AD, Mathias JL. Neuropsychological deficits in frontotemporal dementia and Alzheimer’s disease: a meta-analytic review. J Neurol Neurosurg Psychiatry. 2007;78:917–928. doi: 10.1136/jnnp.2006.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Bunk S, Tu S, Kamminga J, Hodges JR, Hornberger M, Piguet O. Preservation of episodic memory in semantic dementia: The importance of regions beyond the medial temporal lobes. Neuropsychologia. 2015;81:50–60. doi: 10.1016/j.neuropsychologia.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kertesz A. Western Aphasia Battery. The Psychological Corporation; 1982. [Google Scholar]
- Kielb S, Cook A, Wieneke C, Rademaker A, Bigio EH, Mesulam MM, Rogalski E, Weintraub S. Neuropathologic Associations of Learning and Memory in Primary Progressive Aphasia. JAMA Neurol. 2016;73:846–852. doi: 10.1001/jamaneurol.2016.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor Y, Jastrzab L, Dutt S, Miller BL, Seeley WW, Kramer JH. Memory profiles in pathology or biomarker confirmed Alzheimer disease and frontotemporal dementia. Alzheimer Dis Assoc Disord. 2015;29:135–140. doi: 10.1097/WAD.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia--a language-based dementia. N Engl J Med. 2003;349:1535–1542. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138:2423–2437. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136:601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J. Fronto-temporal dementia: nosology, neuropsychology, and neuropathology. Brain Cogn. 1996;31:176–187. doi: 10.1006/brcg.1996.0041. [DOI] [PubMed] [Google Scholar]
- Osher JE, Wicklund AH, Rademaker A, Johnson N, Weintraub S. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2007;22:468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci U S A. 2007;104:20137–20141. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MA. Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2010;48:1336–1342. doi: 10.1016/j.neuropsychologia.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Raij T, Uutela K, Hari R. Audiovisual integration of letters in the human brain. Neuron. 2000;28:617–625. doi: 10.1016/s0896-6273(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Blum D, Rademaker A, Weintraub S. False recognition of incidentally learned pictures and words in primary progressive aphasia. Neuropsychologia. 2007;45:368–377. doi: 10.1016/j.neuropsychologia.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Cobia D, Martersteck A, Rademaker A, Wieneke C, Weintraub S, Mesulam MM. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology. 2014;83:1184–1191. doi: 10.1212/WNL.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Roxbury T, McMahon K, Coulthard A, Copland DA. An fMRI Study of Concreteness Effects during Spoken Word Recognition in Aging. Preservation or Attenuation? Front Aging Neurosci. 2015;7:240. doi: 10.3389/fnagi.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Koutstaal W, Norman KA. False memories and aging. Trends Cogn Sci. 1997;1:229–236. doi: 10.1016/S1364-6613(97)01068-1. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Service E CFIM. Differences Between Young and Older Adults in Learning A Foreign Vocabulary. Journal of Memory and Language. 1993;32:608–623. [Google Scholar]
- Van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, Gertz HJ, Scheltens P. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77:439–442. doi: 10.1136/jnnp.2005.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Peavy GM, O’Connor M, Johnson NA, Acar D, Sweeney J, Janssen I. Three words three shapes: A clinical test of memory. J Clin Exp Neuropsychol. 2000;22:267–278. doi: 10.1076/1380-3395(200004)22:2;1-1;FT267. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Rogalski E, Shaw E, Sawlani S, Rademaker A, Wieneke C, Mesulam MM. Verbal and nonverbal memory in primary progressive aphasia: the Three Words-Three Shapes Test. Behav Neurol. 2013;26:67–76. doi: 10.3233/BEN-2012-110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- Wertz RT, LaPointe LL, Rosenbek JC. Apraxia of speech in adults: The disorder and its management. Orlando, FL: Grune & Stratton; 1984. [Google Scholar]
- Xiang HD, Fonteijn HM, Norris DG, Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cereb Cortex. 2010;20:549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK. The neuropsychological signature of primary progressive aphasia. Brain Lang. 1999;70:70–85. doi: 10.1006/brln.1999.2140. [DOI] [PubMed] [Google Scholar]