Summary
Tissue homeostasis requires production of newly differentiated cells from resident adult stem cells. Central to this process is the expansion of undifferentiated intermediates known as transit-amplifying (TA) cells, but how stem cells are triggered to enter this proliferative TA state remains an important open question. Using the continuously growing mouse incisor as a model of stem cell-based tissue renewal, we found that the transcriptional cofactors YAP and TAZ are required both to maintain TA cell proliferation and to inhibit differentiation. Specifically, we identified a pathway involving activation of Integrin α3 in TA cells that signals through a LATS-independent FAK/CDC42/PP1A cascade to control YAP-S397 phosphorylation and nuclear localization. This leads to Rheb expression and potentiates mTOR signaling to drive proliferation of TA cells. These findings thus reveal a YAP/TAZ signaling mechanism that coordinates stem cell expansion and differentiation during organ renewal.
Keywords: adult stem cells, tooth, YAP, TAZ, ITGA3, FAK, CDC42, PP1A
Graphical abstract
Introduction
As an organ ages, replacement of worn or injured tissue depends on resident somatic stem cells that have the ability to self-renew and generate differentiated cells. This stem cell-based renewal is particularly important for maintaining the homeostasis of tissues with constant cell turnover, such as the hematopoietic system, the intestinal epithelium, germ cells in the testis, and various epidermal appendages such as hair follicles and teeth (Wabik and Jones, 2015). During tissue renewal, stem cells or their proliferative descendants, known as transit-amplifying (TA) cells, divide regularly in order to meet the homeostatic demands of each tissue. The induction of stem and progenitor cell proliferation, as well as the differentiation of their progeny, must therefore be tightly regulated. Uncontrolled proliferation can lead to tissue hyperplasia (White et al., 2014; Zhou et al., 2011) and/or exhaustion of the stem cell pool (Waikel et al., 2001; Yilmaz et al., 2006), whereas loss of stem cells’ proliferative capacity disrupts normal tissue maintenance (Chen et al., 2012; Schlegelmilch et al., 2011). Thus, a central goal in stem cell biology is to understand the mechanisms that govern proliferation and differentiation of stem and TA cells in vivo.
The adult mouse incisor provides a paradigm for studying tissue renewal and regeneration. This organ continuously replaces tissues lost as a result of abrasion from gnawing through the activity of epithelial and mesenchymal stem cells that give rise to all adult tooth cell types, including ameloblasts and odontoblasts that produce enamel and dentin respectively (Biehs et al., 2013; Harada et al., 1999; Juuri et al., 2012; Kaukua et al., 2014; Seidel et al., 2010). In particular, ameloblasts are derived from dental epithelial stem cells (DESCs) in the labial cervical loop (laCL), the niche region at the proximal end of the incisor (Figure 1A). Lineage tracing has shown that DESCs marked by Gli1, Bmi1, and Sox2 reside in the outer enamel epithelium (OEE) and the underlying stellate reticulum (SR) of the laCL (Figure 1B) and have the capacity to both self-renew and give rise to ameloblasts and stratum intermedium cells (Biehs et al., 2013; Juuri et al., 2012; Seidel et al., 2010). The production of ameloblasts from progenitors thus resembles a conveyor belt, where the less proliferative DESCs originating from the OEE first give rise to rapidly dividing TA cells in the inner enamel epithelium (IEE) that then move distally along the length of the epithelium as they cease proliferation and undergo differentiation. Therefore, as in other tissues with constant cell turnover, the function of the incisor depends on proper regulation of TA cell proliferation and differentiation. However, what mechanisms control these processes remains an open question.
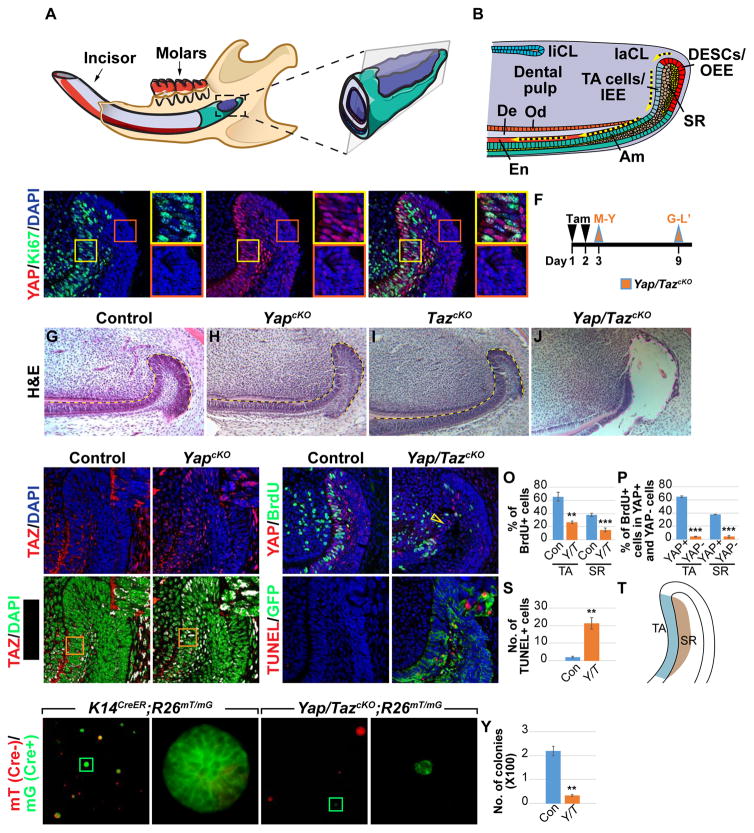
Figure 1. YAP/TAZ are required for the maintenance of laCLs.
(A) Schematic diagram of the mouse lower jaw.
(B) Cross section of the proximal incisor showing that in the labial cervical loop (laCL) dental epithelial stem cells (DESCs) in the outer enamel epithelium (OEE) give rise first to transitamplifying (TA) cells in the inner enamel epithelium (IEE) and then differentiated enamel (En)- secreting ameloblasts (Am). There are two morphologically distinct cell types in the stellate reticulum (SR) and inner SR cells underneath the TA cells also act as TA cells. De, dentin; liCL, lingual cervical loop; and Od, odontoblasts.
(C–E) Immunostaining of YAP and Ki67 in the laCL. Enlarged images of TA (yellow boxes) and OEE (red boxes) regions are also displayed.
(F) Timeline depicting Cre induction (tamoxifen (Tam) injection, black arrowheads) and sample collection (orange arrowheads).
(G–J) H&E staining of control (G), YapcKO (H), TazcKO (I), and Yap/TazcKO (J) laCLs. Pink dashed line outlines the tissue loss in (J).
(K–L’) TAZ immunostaining in control and YapcKO laCLs. Overlapping TAZ and DAPI staining is shown in white (K’ and L’). Insets are enlargements of the TA region.
(M–P) BrdU labeling in control and Yap/TazcKO laCLs. Open yellow arrowhead in (N) marks reduced proliferation. Quantification was performed by calculating the percentage of BrdUpositive (+) cells per section in control and Yap/TazcKO laCLs (O) and by comparing the percentage of BrdU+ cells between YAP+ and YAP- cells in mutant laCLs (P).
(Q–S) TUNEL staining shows increased cell death in Yap/TazcKO. GFP marks Cre active cells.
(T) Schematic diagram of the TA and SR regions used for quantification.
(U–Y) Colony formation assays in 3D matrigel. (V and X) are enlarged images of Cre active cells in (U and W). The average number of colonies (excluding single cells) per well is quantified (Y).
Dashed lines outline laCLs. Representative images and quantitative data are shown. Scale bar in (X) represents 50 μm in (C–E, K–N, Q, and R), 90 μm in (G–J), 240 μm in (U and W), 15 μm in
(V and X). All quantitative data are presented as mean ± SD. **p<0.01; ***p<0.001.
See also Figure S1
Yes-associated protein (YAP) and its homolog, transcriptional co-activator with PDZ-binding motif (TAZ), are effectors of the evolutionarily conserved Hippo signaling pathway that play key roles in coordinating cell proliferation and differentiation (Yu et al., 2015). For example, overexpression of activated YAP results in progenitor pool expansion, tissue hyperplasia, and altered differentiation in the skin, intestine, liver, and lung (Schlegelmilch et al., 2011; Camargo et al., 2007; Lange et al., 2015; Lu et al., 2010). Conversely, epidermal deletion of Taz and/or Yap undermines the proliferative potential of stem cells both during homeostasis and wound healing (Elbediwy et al., 2016; Schlegelmilch et al., 2011), while Yap is specifically required for injury repair in the intestine, mammary gland, and liver, (Bai et al., 2012; Cai et al., 2010; Chen et al., 2014).
Mechanistically, the transcriptional activity of YAP/TAZ depends on their localization in the nucleus or cytoplasm, which can be regulated by diverse extracellular inputs, including cell-cell contact, mechanical stimuli, cell polarity, energy stress, and G-protein coupled receptor (GPCR) signaling (Zhao et al., 2007; Dupont et al., 2011; Szymaniak et al., 2015; Mo et al., 2015; Yu et al., 2012). These signals are in part relayed through the MAP4K/MST1/2-LATS1/2 kinase cascade, where activated phospho-LATS1/2 phosphorylate YAP/TAZ on several serine residues, including serine 127 (S127; S89 in TAZ), leading to YAP/TAZ translocation to the cytoplasm, and serine 397 (S397; S311 in TAZ), resulting in protein degradation (Zhao et al., 2010). In addition to LATS-dependent regulation, phosphorylation of YAP/TAZ can also be controlled by non-LATS kinases (e.g. SRC kinase) and phosphatases (e.g. Protein Phosphatase 1A (PP1A) and PP2A) (Li et al., 2016; Schlegelmilch et al., 2011). However, as many of the studies to date focusing on YAP/TAZ regulation have been conducted in cell culture, a critical question that remains to be addressed is whether these upstream signals and regulations are physiologically relevant and how they control YAP/TAZ function to drive proper stem cell proliferation and differentiation in a tissue.
Here, we report that YAP and TAZ play functionally redundant roles in the adult incisor laCL to maintain TA cell proliferation and survival, as well as to inhibit precocious differentiation. This occurs in part through the control of Rheb expression and subsequent effects on mTOR activation. The regulation of YAP in TA cells depends on induction of the ITGA3-FAK-CDC42 signaling axis specifically in the TA region, which promotes interaction between PP1 and YAP and dephosphorylation on YAP-S397 in a LATS-independent manner that is distinct from the S127-guided regulation described previously. This novel regulatory pathway thus drives YAP accumulation in the TA cell nuclei, enabling the transition of stem cells into a high proliferation TA state in order to maintain proper tissue homeostasis.
Results
YAP and TAZ are expressed in the nucleus and the cytoplasm, respectively, in epithelial TA cells of the mouse incisor
Because YAP and TAZ are important regulators of cell proliferation and differentiation, we set out to study the roles of these proteins in the regulation of adult incisor renewal. We first assessed their expression in wild type laCLs by in situ hybridization and found that both Yap and Taz are abundantly expressed in the laCL (Figures S1A and S1B), with the strongest expression detected in the TA cells. As the localization of YAP and TAZ in the nucleus or cytoplasm is a key determinant of their function, we next carried out immunostaining to examine their subcellular distribution in the laCL. In accordance with the notion that nuclear YAP tends to promote proliferation, we observed high levels of nuclear YAP in the proliferating TA cells that are marked by Ki67 immunostaining and BrdU incorporation (Figures 1C–1E and 1M). This was in contrast to the low proliferating DESC/OEE region, where we observed minimal nuclear YAP and weak cytoplasmic staining (Figures 1C–1E). A similar YAP expression pattern was also observed in the lingual CL (Figure S1C). Interestingly, the expression pattern of YAP subcellular localization in the laCL is not mirrored by TAZ, which is expressed exclusively in the cytoplasm (Figures 1K and 1K’), suggesting that YAP and TAZ are regulated differently in the laCL.
YAP/TAZ are required for maintaining the laCL
To investigate the functional requirement of YAP in the laCL, we genetically deleted Yap in the adult dental epithelium. We crossed a Yap conditional allele (Yapf/f) (Xin et al., 2011) with Keratin 14CreER (K14CreER) (Li et al., 2000), in which tamoxifen inducible Cre recombinase is expressed in the incisor epithelium (Figures S1D–S1F), to generate K14CreER;Yapf/f conditional knockout (cKO) mutants (YapcKO). We first examined the general architecture of the laCL by hematoxylin and eosin (H&E) staining one week after injection of 8-week-old mice with tamoxifen (Figure 1F). To our surprise, most YapcKO mutant laCLs were morphologically indistinguishable from the Cre-negative controls (n = 9/12) (Figures 1G and 1H), although in a minority of samples (n = 3/12) the laCLs were disorganized and exhibited small holes in the tissue (Figures S1K and S1L). We therefore considered the possibility that loss of YAP could be compensated by TAZ, and this was supported by increased nuclear TAZ in YapcKO laCLs (Figures 1L and 1L’). As TAZ single deletion (TazcKO) had no effect on the laCL (Figure 1I), we generated Yap/TazcKO double mutants. Deletion of both Yap and Taz caused cells in the TA and SR regions to detach from one another by 4 days after Cre induction (Figures S1M and S1N), and by 7 days after Cre induction there was a remarkable tissue loss in the SR and TA regions (n = 12/12) (Figures S1O and S1P). In the most severe cases, the entire laCL was lost and a large hole developed (Figure 1J). A similar phenotype was also observed in the lingual CL (Figure S1S and S1T), suggesting a conserved YAP/TAZ function in different populations of DESCs. The loss of tissue was confirmed by computed microtomography (μCT), which enables visualization of tissues without the potential for causing histological artifacts (Figures S1Q and S1R). Because Yap/TazcKO animals gradually ceased eating and became moribund 7 – 10 days after Cre initiation, likely due to the requirement for YAP/TAZ function in other epithelial organs, we were unable to assess the long-term consequences on mineral deposition in the distal incisor.
The dramatic loss of the laCL in Yap/TazcKO could be attributed to decreased proliferation, increased cell death, or both. We first measured the percentage of proliferating cells by BrdU labeling 2 days after Cre induction (Figure 1F), a timepoint prior to the laCL destruction. We took advantage of the mosaic nature of tamoxifen-induced Cre recombination to compare BrdU labeling in cells with and without YAP/TAZ deletion within the same laCL. While there was a significant reduction of proliferation in YAP-negative TA and SR cells, BrdU signals were still present in cells with intact YAP expression (Figures 1M-1P and 1T). We next marked apoptotic cells using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). We utilized the Cre-responsive reporter allele, R26mT/mG (Muzumdar et al., 2007), to identify cells that underwent Cre recombination and were permanently labeled with membrane GFP (mG) (Figures S1D–S1J’); cells lacking Cre activity continued to express membrane tdTomato (mT). Using this strategy, we noted an increase in apoptosis among Yap/TazcKO cells, primarily in SR and distal TA cells; apoptosis also occurred in the OEE after the loss of TA/SR cells at a later timepoint (Figures 1Q–1S and S1U-S1X). Lastly, we tested the role of YAP/TAZ in laCL cell expansion using in vitro colony formation assays. Dissociated cells from control K14CreER;R26mT/mG laCLs routinely formed spheroids in 3D culture, whereas GFP-positive Yap/TazcKO;R26mT/mG mutant cells remained as single cells (Figures 1U–1Y). In contrast, Yap/TazcKO;R26mT/mG cells that escaped Cre activation (tdTomato-positive) maintained their ability to form colonies. Together, these results demonstrate an absolute requirement for YAP/TAZ in sustaining cell proliferation and survival in the adult incisor laCL.
YAP/TAZ prevent precocious differentiation in the mouse incisor epithelium
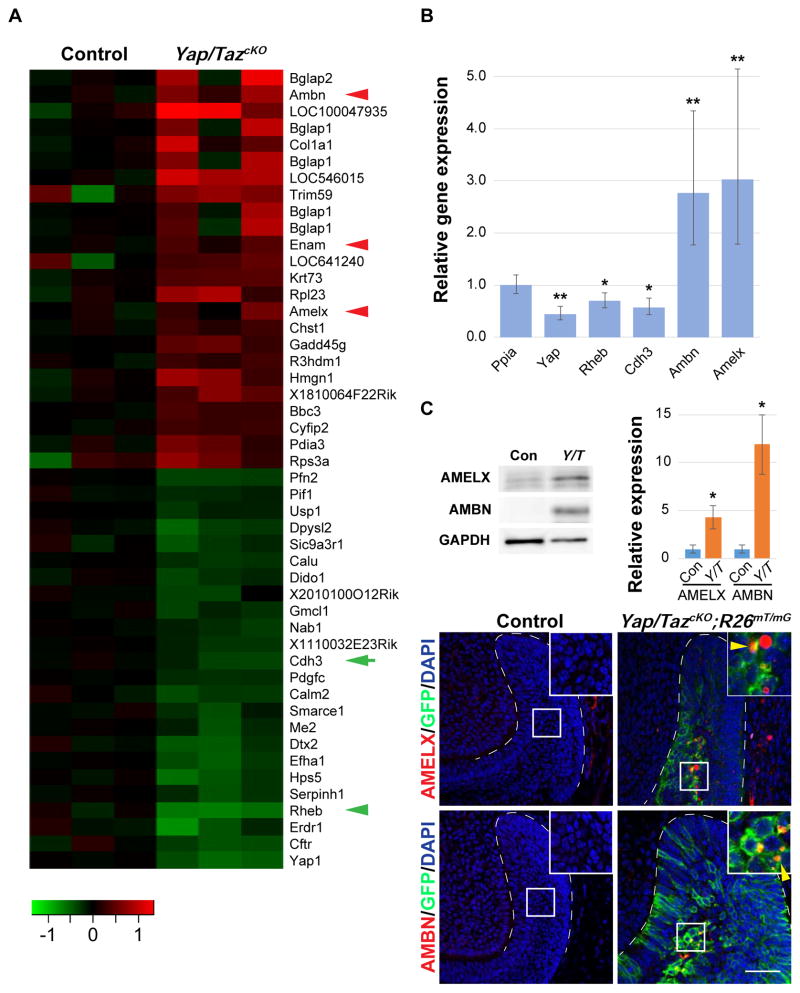
As YAP/TAZ are transcription cofactors, we next performed gene expression profiling using RNA from control and Yap/TazcKO laCLs 2 days after Cre induction, allowing us to detect early changes that occurred prior to tissue destruction. Among targets that were upregulated, we found several genes that mark differentiated ameloblasts, such as Amelogenin and Ameloblastin (Figure 2A). Increased expression of these genes was confirmed by qPCR analysis, immunoblotting, and in situ hybridization (Figures 2B, 2C, and S1Y–S1AB). Finally, we performed Amelogenin and Ameloblastin immunostaining and observed that, while these ameloblast markers were not expressed in control laCLs, they were readily detected in the Yap/TazcKO SR cells (Figures 2D–2G), indicating that, in the absence of YAP/TAZ, some laCL cells undergo precocious differentiation.
Figure 2. YAP/TAZ inhibit precocious differentiation in the laCL.
(A) Heat map of up- and down-regulated genes in control and Yap/TazcKO laCLs. Red arrowheads mark genes associated with ameloblast differentiation, green arrow and arrowhead mark Cdh3 and Rheb respectively.
(B) qPCR results comparing relative gene expression between control and Yap/TazcKO laCLs. Data are presented as mean ± SEM.
(C) Immunoblotting and relative expression (mean ± SD) of Amelogenin (AMELX) and Ameloblastin (AMBN) in control and Yap/TazcKO laCLs.
(D–G) Immunostaining of AMELX and AMBN in control and Yap/TazcKO laCLs. Yellow arrowheads mark ectopic AMELX and AMBN expression.
Dashed lines outline laCLs. Representative images, cropped blots and data are shown.
*p<0.05; **p<0.01.
Scale bar in G represents 50 μm in (D–G).
See also Figure S1
YAP/TAZ activate mTOR signaling by controlling Rheb expression
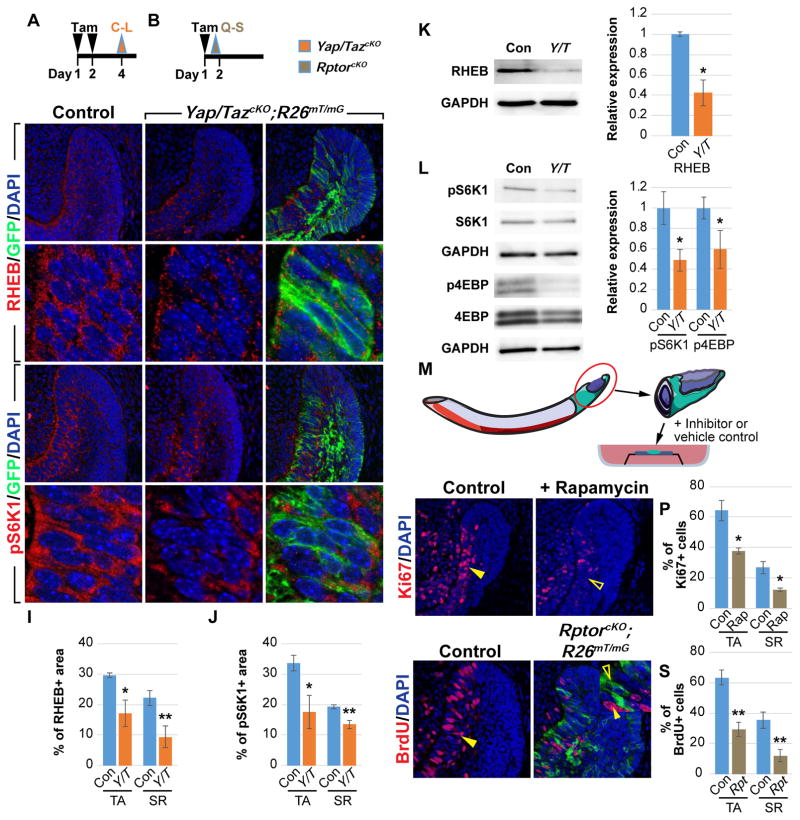
Our gene expression analysis identified a set of genes that were downregulated, and Gene Set Enrichment Analysis (GSEA) revealed that the mammalian target of rapamycin (mTOR) signaling pathway was one of the top modules affected (Table S1). In particular, expression of Rheb (Ras homolog enriched in brain), which encodes an activator of the mTOR Complex 1 (mTORC1), was reduced in the absence of YAP/TAZ (Figures 2A, 2B, and 3K). Furthermore, while immunostaining revealed that RHEB was expressed robustly throughout the entire TA and SR regions of control laCLs, its expression in Yap/TazcKO;R26mT/mG was downregulated in TA and SR cells that had undergone Cre-mediated recombination, as visualized by the presence of membrane GFP (Figures 3A, 3C–3E’, 3I, S2A and S2B). These results thus suggest that mTOR signaling is compromised in Yap/TazcKO. We tested this hypothesis by examining the expression of phospho-P70-S6 kinase (pS6K1) and phospho-translation initiation factor 4E-binding protein (p4EBP), two readouts of active mTOR signaling (Hay and Sonenberg, 2004). In line with RHEB expression, robust staining of pS6K1 and p4EBP was detected uniformly in control TA and underlying SR cells (Figures 3F, 3F’, S2E and S2E’), while their expression was significantly decreased in Yap/TazcKO;R26mT/mG laCLs (Figures 3G–3H’, 3J, and S2C–S2J). These results were confirmed by immunoblotting (Figure 3L).
Figure 3. YAP/TAZ are required for Rheb expression and mTOR signaling.
(A and B) Timelines indicating Cre induction (tamoxifen (Tam) injection, black arrowheads) and sample collection (orange and brown arrowheads).
(C–J) RHEB (C–E’) and pS6K1 (F–H’) immunostaining in control and Yap/TazcKO laCLs. The percentage of RHEB and pS6K1 positive area is quantified (I and J).
(K and L) Immunoblotting and relative expression of RHEB (K), pS6K1, and p4EBP (L).
(M) Schematic of the explant culture system.
(N–P) Ki67 expression in control and Rapamycin-treated explants. Closed and open arrowheads respectively mark normal and reduced proliferation. The percentage of Ki67-positive (+) cells per section is quantified (P).
(Q–S) BrdU labeling in control and RptorcKO laCLs. Closed and open arrowheads mark normal and reduced proliferation in wild type and mutant cells respectively. The percentage of BrdUpositive (+) cells per section is quantified (S).
Representative images, cropped blots and quantitative data are shown. Dashed lines outline laCLs. Scale bar in R represents 50 μm in (C–E, F–H, N, O, Q, and R) and 9.76 μm in (C’–E’ and F’–H’). All quantitative data are presented as mean ± SD. *p<0.05; **p<0.01.
See also Figure S2
Because mTOR signaling functions as a central regulator of cell proliferation and survival (Laplante and Sabatini, 2009), we reasoned that the decreased mTOR activity in Yap/TazcKO could explain some of the phenotypes we observed earlier, and thus that perturbation of the mTOR pathway may partially phenocopy Yap/TazcKO. We first took an explant approach, in which dissected wild type proximal incisors were cultured (Figure 3M) in the presence or absence of the mTORC1 inhibitor, Rapamycin. In control samples, cells continued to proliferate, while Rapamycin-treated incisors had reduced proliferation (Figures 3N–3P). To confirm the tissue-autonomous role of mTOR signaling in laCLs, we next perturbed mTOR signaling by using K14CreER to conditionally delete Rptor (Regulatory-associated protein of mTOR), which encodes a critical regulator of mTORC1 (Hara et al., 2002). The resultant RptorcKO mutants displayed a reduction in BrdU labelled cells 18 hours after Cre induction (Figures 3B, 3Q–3S, and S2N). The importance of Rptor for progenitor pool expansion became even more obvious in longer chased RptorcKO samples, as there was a near complete loss of GFP-positive Cre-recombined mutant TA cells and ameloblasts, which were replaced by proliferative GFP-negative wild type cells (Figures S2K-S2M’). Together, these results demonstrated that YAP/TAZ mediated-mTOR activation is critical for expanding the progenitor pool in laCLs.
ITGA3 and FAK signaling promotes nuclear YAP localization in laCLs
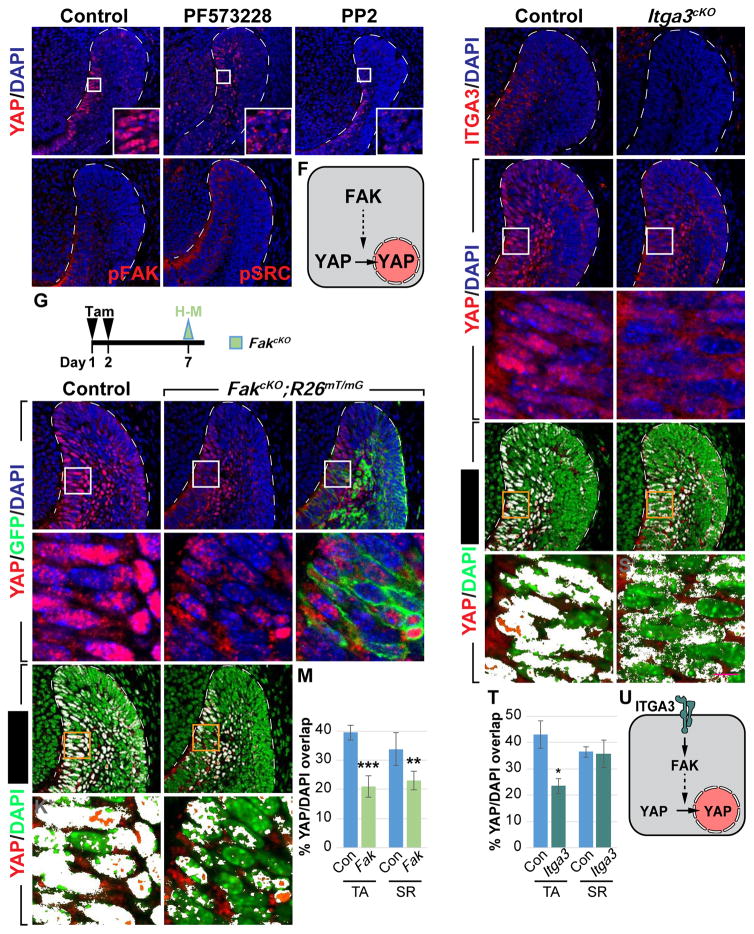
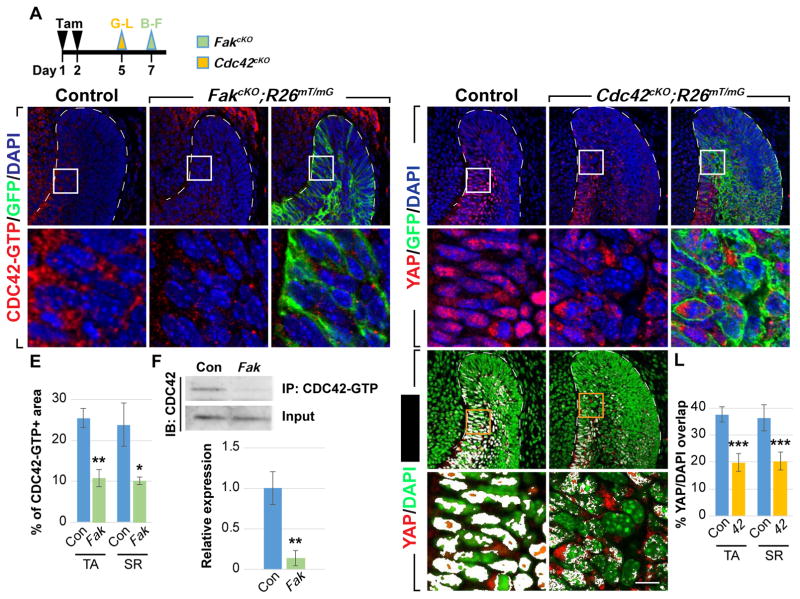
Given the critical roles that YAP/TAZ play in laCL maintenance, we set out to study the underlying mechanism that controls YAP nuclear localization in TA cells. We focused on YAP because our results above indicated that YAP is the primary regulator of TA proliferation and differentiation, with TAZ serving as a redundant alternate in the absence of YAP. To that end, we carried out a small-scale screen using the explant culture system in combination with inhibitors of several known YAP regulators (Table S2). While most of the drugs tested did not affect YAP nuclear localization in TA cells (data not shown), the FAK inhibitor PF573228 impeded YAP accumulation in the nucleus (n = 12/12, Figures 4A and 4B), suggesting that integrin/FAK signaling may play a role in YAP regulation in the laCL (Figure 4F). Similarly, incisors treated with PP2, an inhibitor of SRC kinase that functions downstream of FAK, also had reduced nuclear YAP (n = 4/6, Figure 4C). Consistent with these results, immunostaining of phospho-FAK and SRC (pFAK and pSRC) showed active FAK signaling in the TA region but not in the OEE, where there is low nuclear YAP (Figures 4D and 4E).
Figure 4. ITGA3/FAK signaling promotes YAP nuclear localization in TA cells.
(A–C) YAP immunostaining in control and drug-treated explants.
(D and E) pFAK and pSRC expression in laCLs.
(F) Schematic diagram of a TA cell, where FAK promotes YAP nuclear localization.
(G) Timeline depicting tamoxifen (Tam) treatment (black arrowheads) and sample collection (green arrowhead).
(H–M) YAP immunostaining in control and FakcKO laCLs. The percentage of YAP/DAPI overlap is quantified (K–M).
(N and O) ITGA3 expression in control and Itga3cKO laCLs.
(P–T) YAP immunostaining in control and Itga3cKO laCLs. The percentage of YAP/DAPI overlap is quantified (R–T).
(U) Schematic diagram showing ITGA3/FAK signaling promotes nuclear YAP accumulation in TA cells.
Representative images and quantitative data are shown. Dashed lines outline laCLs. Scale bar in S’ represents 50 μm in (A–E, H–J, K, L, N–Q, R, and S) and 9.76 μm in (H’–J’, K’, L’, P’, Q’, R’, and S’). All quantitative data are presented as mean ± SD. *p<0.05, **p<0.01; ***p<0.001.
See also Figures S3 and S4
To further study whether FAK is required in the adult laCL for YAP nuclear localization, we deleted Fak in the dental epithelium by generating K14CreER;Fak f/f;R26mT/mG (FakcKO) mice. Similar to our explant studies, deletion of Fak resulted in loss of nuclear YAP in both TA and adjacent SR cells (Figures 4G–4J’). The reduction in nuclear YAP was quantified by calculating the percentage of YAP/DAPI overlay in control and FakcKO TA/SR cells (Figures 4K–4M), as well as by comparing the average nuclear YAP signal intensity both between control and mutant laCLs and between Cre-recombined (GFP-positive) and non-recombined (GFP-negative) cells in FakcKO (Figures S3A and S3B).
Having established FAK as an upstream regulator of YAP, we sought to find the corresponding integrin receptor by first screening the spatial distribution of potential integrin subunits that were expressed in the laCL based on our microarray data. We found that integrin α3 (ITGA3) was specifically expressed in the TA region and in the neighboring SR cells (Figure 4N), and to investigate if ITGA3 functions upstream of YAP, we examined K14Cre;Itga3f/f mice (Itga3cKO), where Itga3 was deleted in the entire dental epithelium (Figure 4O). In these mutants, there was a significant loss of nuclear YAP in TA cells, although SR cells were not affected (Figures 4P–4T, and S3C). These data suggest that ITGA3 plays a dominant role in governing YAP localization in the TA region (Figure 4U), with other integrin subunits (such as ITGAV, which is present in the TA/SR regions as well, data not shown) performing similar functions in the SR. We also noted that, similar to YapcKO, both Itga3cKO and FakcKO laCLs appeared normal and had increased nuclear TAZ (Figures S4A–S4C’ and S4E), reinforcing the notion that compensation by TAZ ensues following disrupted YAP activities. To test this, we generated Fak/TazcKO double mutants and found that laCLs from these animals had reduced proliferation 2 days after Cre induction, and subsequently developed similar tissue loss as the Yap/TazcKO (n = 6/8, Figures S4F–S4O).
FAK functions through CDC42 to regulate YAP localization
To investigate the mechanism by which FAK regulates YAP, we first focused on CDC42, a member of the small Rho GTPase family that also includes RHOA and RAC, which are key mediators of FAK signaling and whose functions can be modulated by the same pathway (McLean et al., 2005). Using an antibody against the active, GTP-bound form of CDC42, we detected strong CDC42 activity in control TA and SR cells (Figures 5B and 5B’), while in FakcKO laCLs, only sparse CDC42-GTP staining was observed (Figures 5A, 5C–5E, S3D, and S3E). This was corroborated by immunoprecipitation, which pulled down more active CDC42 from control laCLs than from FakcKO samples (Figure 5F), confirming that FAK is required for robust CDC42 activation. These experiments also point to the possibility that FAK regulates YAP localization through CDC42 and predict that genetic ablation of CDC42 should phenocopy FakcKO and result in loss of nuclear YAP. To that end, we genetically removed Cdc42 in the dental epithelium using K14CreER;Cdc42f/f;R26mT/mG (Cdc42cKO) mice and probed the expression of YAP using immunostaining. Confirming our hypothesis, YAP failed to accumulate in the nucleus in the absence of CDC42 (Figures 5G–5L, S3F, and S3G). Deletion of Cdc42 also led to increased nuclear TAZ in the TA and inner SR regions (Figures S4D–S4E), consistent with what we observed in FakcKO and Itga3cKO. Lastly, we note that deletion of RhoA and Rac or overexpression of a dominant negative ROCK in laCLs did not affect YAP localization (data not shown). Collectively, these results reveal that FAK and CDC42 function within the same pathway to promote YAP nuclear localization in the laCL.
Figure 5. CDC42 acts downstream of FAK to regulate YAP nuclear localization.
(A) Timeline depicting tamoxifen (Tam) treatment (black arrowheads) and sample collection (green and yellow arrowheads).
(B–E) Immunostaining of active CDC42 in control and FakcKO laCLs. The percentage of area with positive CDC42-GTP staining is calculated (E).
(F) Immunoprecipitation of CDC42-GTP followed by CDC42 immunoblotting. Relative expression between control and FakcKO is quantified.
(G–L) YAP immunostaining in control and Cdc42cKO laCLs. The percentage of YAP/DAPI overlap is quantified (J–L).
Representative images, cropped blots and quantitative data are shown. Dashed lines outline laCLs. Scale bar in K’ represents 50 μm in (B–D, G–I, J, and K) and 9.76 μm in (B’–D’, G’–I’, J’, and K’). Data are presented as mean ± SD. *p<0.05; **p<0.01; ***p<0.001
See also Figure S4.
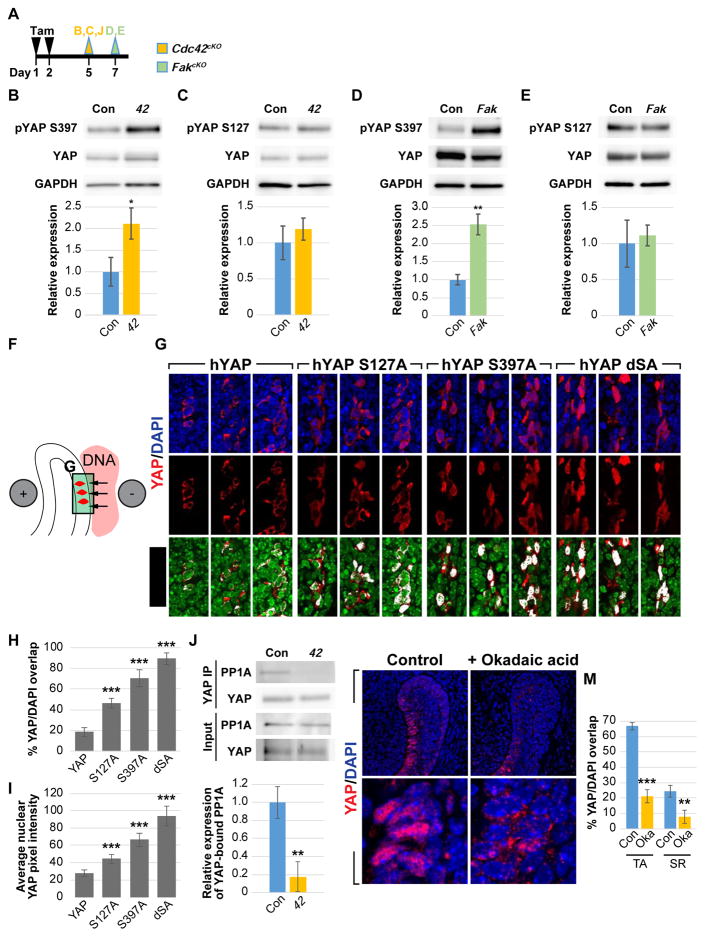
CDC42 regulates YAP phosphorylation at S397 through PP1A
To further understand the mechanism by which the FAK-CDC42 signaling axis regulates YAP localization, we examined the phosphorylation state of LATS, since the level of phospho-LATS (pLATS) reflects its ability to phosphorylate and inhibit YAP in many different systems (Zhao et al., 2007, 2010). Unexpectedly, we found that pLATS1 levels remained unchanged in the absence of CDC42 (Figure S5A), and we did not detect any alteration in the phosphorylation state of NDR1/2, which belong to the same NDR kinase family as LATS and have also been shown to phosphorylate YAP (Hergovich, 2016) (Figure S5B). Therefore, it is unlikely that CDC42 signals through LATS and NDR1/2 to regulate YAP in this context.
To more deeply investigate the mechanism underlying regulation of YAP localization by CDC42, we performed immunoblotting against pYAP-S127 and pYAP-S397. These phosphorylation sites are thought, based on cell culture experiments, to be critical for YAP cytoplasmic retention (S127) and protein stability (S397) respectively (Zhao et al., 2010). Interestingly, when compared to control laCLs, both Cdc42cKO and FakcKO laCLs showed an increase in pYAP-S397 but not pYAP-S127 (Figures 6A–6E), suggesting that signaling downstream of FAK and CDC42 preferentially controls YAP phosphorylation at S397. This raised two possibilities, the first being that changes in YAP localization seen in Cdc42cKO laCLs are indirect results of pYAP-S397-driven YAP degradation, and the second being that pYAP-S397 has a yet-to-be identified function in determining YAP localization. To address this, we utilized two mutant alleles of human Yap, hYapS127A and hYapS397A, which can no longer be phosphorylated at those sites and thus are able to translocate to the nucleus even in the presence of an inhibitory signal. We then electroporated these constructs in the OEE (Figure 6F), where YAP is usually restricted to the cytoplasm (Figure 1D). When we electroporated the control hYap, immunostaining using an antibody that only recognizes hYAP showed restriction to the OEE cytoplasm, as expected with wild-type YAP (Figure 6G). We next found that while expression of YapS127A resulted in increased nuclear YAP, the number of cells with nuclear YAP and the YAP signal intensity were both lower than hYapS397A-electroporated cells (Figures 6G–6I), pointing to YAP-S397 as the primary site for regulating YAP localization in the laCL. The outcome of YapS127A electroporation was also similar to genetic overexpression of YapS127A in laCLs, which was ineffective in driving YAP nuclear localization in the OEE (Figures S6A–S3C). Finally, hYapS127A,S397A electroporation produced the highest nuclear YAP (Figures 6G–6I), highlighting the importance of both phosphorylation sites in controlling YAP localization.
Figure 6. CDC42 signals through PP1A to regulate YAP S397 phosphorylation and localization.
(A) Timeline indicating tamoxifen (Tam) injection (black arrowheads) and sample collection (green and yellow arrowheads).
(B–E) Immunoblotting and relative expression of pYAP-S397 and pYAP-S127 in control, Cdc42cKO (B and C) and FakcKO (D and E) laCLs.
(F) Schematic diagram depicting delivery of YAP constructs to the OEE by electroporation.
(G–I) YAP immunostaining and YAP/DAPI overlap in explants electroporated with hYAP, hYAPS127A, hYAP-S397A, and hYAP-S127A,S397A (hYAP-dSA) in the OEE. 3 representative images are shown for each construct. The percentage of YAP/DAPI overlap (H) and average nuclear YAP pixel intensity (I) are quantified.
(J) YAP immunoprecipitation followed by PP1A detection. Relative expression of PP1A between control and Cdc42cKO is displayed.
(K–M) YAP immunostaining in control and okadaic acid-tread laCLs. The percentage of YAP/DAPI overlap is calculated (M).
Representative images, cropped blots and quantitative data are shown. Dashed lines outline laCLs. Scale bar in L’ represents 15 μm in (G), 50 μm in (K and L), and 9.76 μm in (K’–L’). All data are presented as mean ± SD. *p<0.05; **p<0.01; ***p<0.001.
See also Figures S3, S5 and S6
The preferential regulation at YAP-S397 also argues against a LATS-dependent mechanism downstream of FAK/CDC42, as LATS typically phosphorylates all YAP serine residues. Because PP1A may dephosphorylate YAP primarily on S397 (Qi et al., 2015), we tested whether PP1A binding to YAP was diminished upon Cdc42 deletion. To that end, we performed co-immunoprecipitation of YAP and PP1A and found a significant reduction in pulled-down PP1A (but not PP2A, data not shown) in Cdc42cKO laCL lysates (Figure 6J). This result then led to the prediction that PP1A is critical for activating YAP localization in the nucleus. Indeed, when cultured in the presence of okadaic acid, a PP1 inhibitor, incisor explants displayed a dramatic loss of nuclear YAP in TA cells (Figures 6K–M and S3H), thus establishing an FAK/CDC42/PP1A signaling axis that governs YAP localization in the incisor TA cells.
LATS1/2 function in parallel to regulate YAP localization
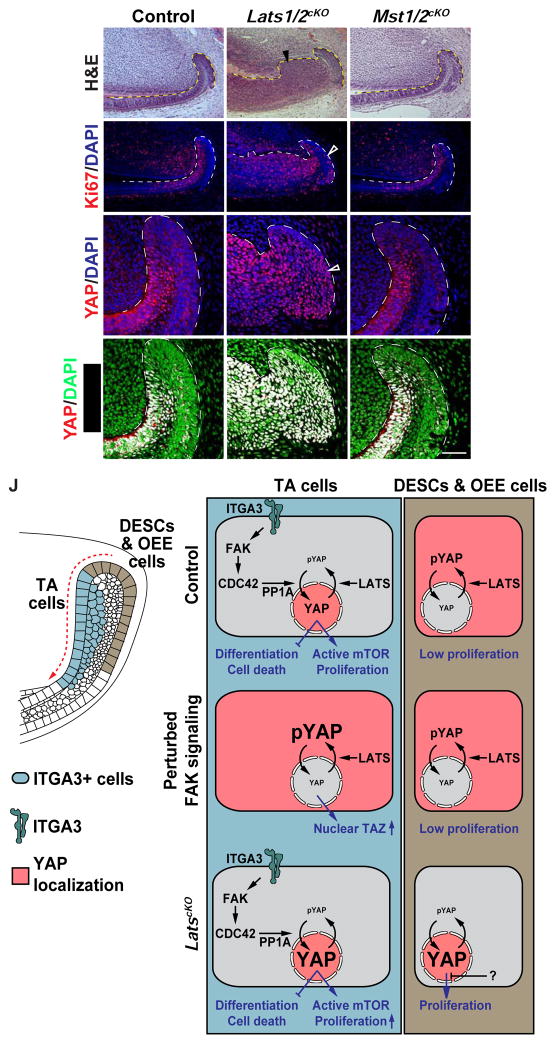
The results above, however, could not rule out the possibility that LATS1/2 function in parallel to modulate YAP phosphorylation and activity, and this hypothesis is supported by the presence of abundant pYAP-S127 staining throughout the entire laCL (Figure S5D). To test this, we generated mice with Lats1 and Lats2 (Lats1/2cKO) double deletions in the dental epithelium, and we observed a dramatic expansion of the dental epithelium in these mice one week after Cre activation (Figures 7A and 7B). Intriguingly, deletions of Mst1 and Mst2 did not result in any phenotype (Figures 7C, 7F, 7I, 7I’, and S5I-S5K), indicating that LATS1/2 activity is regulated by other kinases, which could include MAP4K (Meng et al., 2015; Zheng et al., 2015). The hyperplasia seen in Lats1/2cKO was limited to the TA region and the more distal ameloblasts, suggesting a differential response to loss of Lats1/2 in distinct cell types. This was corroborated by Ki67 staining, which showed only a marginal increase in the OEE but a striking upregulation in the more distal epithelium (Figures 7D and 7E). The expansion of proliferating cells was indicative of an enlarged TA region, and this was supported by the widespread expression of the TA marker, P-cadherin (Li et al., 2012), throughout the distal epithelium (Figures S5E and S5F). Surprisingly, even though Lats1/2cKO OEE was resistant to overproliferation, loss of Lats1/2 resulted in increased nuclear YAP and the corresponding RHEB expression in the entire laCL (Figures 7G–H’, S5G, and S5H), supporting the notion that nuclear accumulation of YAP is not always sufficient to drive cell proliferation (Chen et al., 2015). Thus, these experiments revealed that LATS1/2 are required in the laCL to restrain uncontrolled YAP activity and may do so in parallel to the FAK-CDC42 signaling axis described above.
Figure 7. LATS1/2 are required to prevent tissue hyperplasia in the laCL and a model for YAP-mediated incisor renewal.
(A–C) H&E staining of control, Lats1/2cKO and Mst1/2cKO proximal incisors. Arrowhead in (B) marks tissue hyperplasia.
(D–F) Ki67 expression in control, Lats1/2cKO and Mst1/2cKO dental epithelium. Open arrowhead in
(E) marks the few proliferating cells in Lats1/2cKO OEE.
(G–I’) YAP immunostaining and YAP/DAPI overlap in control, Lats1/2cKO and Mst1/2cKO laCLs. Arrowhead in (H) marks increased nuclear YAP in Lats1/2cKO.
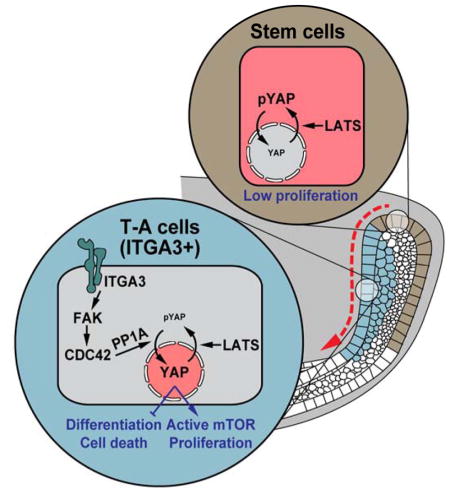
(J) Model for the regulation of incisor stem cell-based dental renewal. ITGA3 positive TA and inner SR cells are marked blue and DESCs/OEE cells are marked brown. Red shade represents YAP localization. In control TA cells, an ITGA3/FAK/CDC42/PP1A signaling axis drives YAP nuclear localization, which promotes proliferation by activating mTOR signaling and inhibits precocious differentiation and apoptosis. When FAK signaling is perturbed, loss of nuclear YAP is compensated by increased nuclear TAZ. In parallel, LATS1/2 fine-tune levels of nuclear YAP to prevent over-proliferation. In contrast to TA cells, DESCs/OEE cells are relatively inert and are resistant to YAP-driven proliferation.
Representative images and quantitative data are shown. Dashed lines outline the dental epithelium. Scale bar in I’ represents 130 μm in (A–F) and 50 μm in (G–I’).
See also Figure S5
Discussion
The homeostatic maintenance of self-renewing tissues depends on a continuous supply of differentiated cells from resident somatic stem cells. Using the adult mouse incisor as a model, we have uncovered a novel signaling network regulating TA cell proliferation and differentiation. Our data support a framework (Figure 7J) in which local induction of the Integrin-FAK-CDC42 signaling axis modulates YAP phosphorylation at S397 to control YAP localization and activity, which in turn govern progenitor cell proliferation and differentiation by means of transcriptional regulation of downstream effectors, such as RHEB. This pathway is counterbalanced by LATS activity and can be compensated by the functionally redundant TAZ. As a consequence, a robust system is in place that can be tuned to ensure adequate production of new cells in order to meet the homeostatic demand of the tissue and support continuous growth of the tooth, which is critical for the survival of the animal.
Maintenance of progenitor cells by YAP/TAZ
In this study, we identified YAP/TAZ as key regulators of mouse incisor renewal that promote TA cell proliferation, prevent apoptosis, inhibit precocious differentiation in dental progenitor cells, and maintain the overall structure of the tissue. This finding thus provides a mechanism for regulating the expansion of progenitor cells during continuous tissue renewal and resonates with a growing body of work on the roles of YAP/TAZ in stem/progenitor cells (Yu et al., 2015). Interestingly, the requirement for YAP/TAZ in tissue homeostasis differs between each organ. For instance, while YAP is indispensable for cell proliferation in the skin (Schlegelmilch et al., 2011), the mammary gland and intestine remain relatively normal after Yap deletion (Cai et al., 2010; Chen et al., 2014). Similarly, although Taz is essential for kidney and lung development (Makita et al., 2008; Reginensi et al., 2013), it is functionally redundant with YAP during heart and craniofacial development (Wang et al., 2016; Xin et al., 2011). Here, we found that YAP/TAZ have overlapping functions in the adult incisor, and ablation of Yap/Taz had a profound impact on the maintenance of laCLs, especially the TA and SR regions. The eventual loss of the entire laCL in the Yap/TazcKO is due to either an absolute dependence of OEE cells on TA/SR cells or on a yet-to-be identified role of YAP in the OEE cytoplasm. One potential cytoplasmic function of YAP/TAZ to be explored in the future is engagement in WNT signaling (Varelas et al., 2010), although the WNT pathway does not appear to be active in the laCL (Suomalainen and Thesleff, 2010).
Our analysis of LatscKO laCLs also revealed differences between TA cells and DESCs/OEE cells in response to increased nuclear YAP, as TA cells expanded into a multilayered structure upon Lats1/2 deletion, and DESCs/OEE cells were resistant to nuclear YAP-induced overproliferation. This thus points to the possibility that nuclear YAP acts as a permissive signal and that additional stimuli must be in place to drive proliferation. One candidate for such signals are the FGFs secreted from the mesenchyme overlying the TA cells. Attenuation of FGFR2b signaling in the dental epithelium impeded TA cell proliferation, and increased FGF signaling due to loss of Sprouty genes transformed the low proliferating lingual CL into a laCL-equivalent (Klein et al., 2008; Parsa et al., 2010). Indeed, FGF signaling has been shown to be required for YAP-induced proliferation in other contexts (Hua et al., 2016). Alternatively, the presence of nuclear YAP in LatscKO OEE cells is counterbalanced by a compensatory decrease in the overall YAP protein level (Chen et al., 2015). Finally, cells in the OEE are more densely clustered than TA cells and express cell adhesion molecules, such as E-cadherin and Claudin1, that are absent in TA cells (Li et al., 2012) and may add further control over cell proliferation.
Regulation of YAP by integrin/FAK signaling in progenitor cells
An important question in the field of Hippo signaling and stem cell biology is understanding how YAP activity is triggered to promote the expansion of tissue progenitors. We found that this is achieved in the incisor by restricted expression of ITGA3 and the corresponding activation of FAK signaling in the TA region, which subsequently promotes YAP nuclear localization through CDC42. Regulation of YAP by FAK signaling has been recently observed in other stem cell systems, including skeletal and epithelial stem cells (Elbediwy et al., 2016; Tang et al., 2013). However, in these cases RHOA was placed downstream of FAK, and CDC42 was instead an inhibitory signal through its role in apical polarity formation. The differences could be due to the use of distinct experimental models, as previous results were derived from cell culture studies. Along these lines, CDC42 is an essential regulator of YAP during kidney development and for podocyte survival, while deletion of RhoA and Rac had little effect (Huang et al., 2016; Reginensi et al., 2013), suggesting that CDC42 may be the predominant Rho GTPase for YAP regulation in vivo.
We also noted that YAP and TAZ are differentially regulated in the laCL, with TAZ being a compensatory effector when YAP or FAK activity is disrupted. This is similar to an earlier study, where hepatic or intestinal deletion of YAP resulted in TAZ nuclear localization (Moroishi et al., 2015), demonstrating that TAZ can function as a reserve pool in vivo that becomes activated in response to YAP loss. Indeed, deletion of both Fak and Taz phenocopies Yap/TazcKO, although with a milder phenotype, likely due to residual nuclear YAP in some cells.
Another critical aspect of the ITGA3/FAK/CDC42 signaling axis is that it is independent of LATS activity. Instead, we identified PP1A as an important modulator of YAP phosphorylation downstream of CDC42. PP1A itself could potentially be activated by the CDC42 effector, PAK2 (Zhang et al., 2013), and PAK2 activity was reduced in Cdc42cKO laCLs (Figure S5C). Interestingly, ITGA3/FAK/CDC42 signaling predominantly controls YAP phosphorylation at S397, but not S127, a phenomenon that has been previously shown in Netrin-1-induced PP1A dephosphorylation of YAP (Qi et al., 2015). As S397 phosphorylation affects YAP stability in vitro (Zhao et al., 2010), it is possible that, in the laCL, FAK signaling modulates YAP localization indirectly by maintaining YAP protein levels above a certain threshold. However, as YAP levels were comparable in both FakcKO and Cdc42cKO, an alternative explanation is that pYAP-S397 directly contributes to YAP localization. This is supported by two observations: first, overexpression of YapS127A did not result in an efficient upregulation of nuclear YAP in the laCL, and second, electroporation of a hYAPS127A,S397A construct resulted in higher nuclear YAP localization than YAPS127A. Taken together, our results indicate that the ITGA3/FAK/CDC42 signaling axis functions in parallel to LATS to promote nuclear YAP localization through dephosphorylation at YAP-S397.
The signaling axis described here likely functions in other stem cell settings as well, as Integrin/FAK signaling is prevalent in many different stem cell niches and is critical for maintaining cell proliferation, preserving the stem cell population and balancing renewal and differentiation (Prowse et al., 2011). For instance, conditional deletion of β1 integrin in the skin results in severe reduction of proliferation (Raghavan et al., 2000), whereas heightened integrin signaling potentiates cancer stem cell activities (Seguin et al., 2015). Indeed, α3β1 is crucial for promoting proliferation and tumor growth in skin cancers (Sachs et al., 2012), and it is plausible that YAP acts downstream of the aberrant signaling, as well as in other normal or pathological conditions where integrin signaling plays a role.
Transcriptional outputs of YAP/TAZ
The role of YAP/TAZ in transcriptional regulation has been well characterized (Yu et al., 2015), and in the incisor we found that YAP/TAZ facilitate TA cell expansion in part through their control of Rheb expression and thus mTOR activity. As a central effector of cell growth and proliferation, mTOR signaling has also been shown to mediate YAP function elsewhere (Hansen et al., 2015; Tumaneng et al., 2012), and our data add to the growing evidence that YAP is able to induce mTOR signaling through several different pathways.
Importantly, both Rapamycin treated and RptorcKO laCLs did not present any obvious loss of cell-cell adhesion analogous to what we observed in Yap/TazcKO, suggesting that additional downstream genes were responsible for the cell adhesion phenotype. One potential candidate is the cell adhesion molecule P-cadherin (encoded by Cdh3), which was downregulated both at the RNA and protein level (Figures 2B, and S2O-S2R) along with other genes, such as Serpinh1, Dpysl2, and Pfn2, that are also important for cytoskeletal regulation (Figure 2A). As a result, YAP/TAZ may maintain tissue integrity by controlling the expression of these genes to modulate cellular tension and ECM environment, in line with a recent finding in zebrafish (Porazinski et al., 2015).
Finally, YAP/TAZ are critical for inhibiting the expression of genes that are associated with differentiated cells. In Yap/TazcKO laCLs, activation of these genes primarily occurs in SR cells, likely because these cells are further along in the differentiation process and therefore more sensitive to loss of YAP/TAZ. It is currently unknown whether YAP/TAZ directly regulate the expression of these genes, and it will be important to address this in future experiments and in other tissues, which may shed light on how YAP/TAZ govern the balance between stem cell proliferation and differentiation. Taken together, these studies have uncovered a novel FAK-YAP-mTOR signaling pathway that governs proliferation and differentiation in tissue progenitor cells. This work helps to provide a framework for future research into the roles of Integrins and YAP in both normal and pathological conditions, as well as to help develop strategies for stem cell-based regeneration of dental and other mineralized tissues.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to and will be fulfilled by the corresponding author: ophir.klein@ucsf.edu
Experimental Model and Subject Details
Mouse lines and induction of alleles
K14CreER (Li et al., 2000), K14Cre (Dassule et al., 2000), YapS127A (Camargo et al., 2007), R26mT/mG (Muzumdar et al., 2007), and conditional alleles of Cdc42 (Wu et al., 2006), Fak (Beggs et al., 2003), Itga3 (Mitchell et al., 2009), Rac1 (Glogauer et al., 2003), RhoA (Jackson et al., 2011), dominant negative Rock2 (Kobayashi et al., 2004), Rptor (Sengupta et al., 2010), Lats1 and Lats2 (Heallen et al., 2011), Mst1 and Mst2 (Lu et al., 2010), Yap (Xin et al., 2011) and Taz (Wwtr1) (Xin et al., 2013) were group housed and genotyped as previously published (sequences provided in Table S3). The strains of these mice were the same as previously described in their respective references at the time of acquisition but were subsequently maintained on mixed backgrounds after breeding between different lines. All conditional lines were crossed to K14CreER, except Itga3f/f, which was bred to K14Cre. Adult mutant and Cre-negative littermate control mice, both males and females, at 8 weeks of age were selected randomly and used in all experiments. No criteria were employed to exclude the use of any mouse for experiments. For CreER activation, 5mg of tamoxifen dissolved in corn oil was delivered to both mutant and control mice through intraperitoneal injection. All experiments involving mice were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco (protocol #AN151723).
Sample sources for laCL cells and explants
For colony formation assays, dissected laCLs from K14CreER;R26mT/mG and Yap/TazcKO;R26mT/mG mice 48 hours after tamoxifen injection were used to generate dissociated cells. For explant culture studies, dissected proximal incisors from wild type C57BL/6 mice were used, either in conjunction with chemical inhibitors or for electroporation.
Method Details
Tissue preparation and histological analysis
Euthanized mice were first perfused using PBS and then 4% paraformaldehyde (PFA) in PBS. Mouse mandibles were dissected away from the rest of the cranium and fixed with 4% PFA in PBS overnight at 4°C. Mandibles were subsequently washed with PBS, dehydrated in 70% ethanol in water through serial ethanol washes, and equilibrated to paraffin using Leica ASP300S. Mandibles were then embedded in paraffin and sectioned at 6 μm. Hematoxylin & Eosin (H&E) staining was carried out using standard protocols.
Immunofluorescence staining
For immunofluorescence, paraffin sections were rehydrated, and antigen retrieval was performed by sub-boiling slides in pH 6.2 citrate buffer containing 10mM citric acid, 2mM EDTA, 0.05% Tween 20 using microwave for 15 minutes. For YAP immunostaining, samples were additionally washed with 2N HCl for 5 minutes. Primary and secondary antibodies used and corresponding dilutions were summarized in Table S4. Samples were blocked in 1X animal-free blocker (Vector Laboratories), supplemented with 2.5% heat inactivated goat serum, 0.02% SDS and 0.1% Triton-X. All antibodies were diluted in the same block without serum. For detection of CDC42-GTP, ITGA3 (Aggarwal et al., 2014), PCAD, p4EBP, pFAK, pMerlin, pS6K1, pSRC, pYAP, RHEB, SerpinH1, TAZ, and YAP, primary antibodies were first detected by biotinylated secondary antibodies (Table S4), and then sequentially amplified using VECTASTAIN Elite ABC HRP Kit (Vector Laboratories) and Tyramide Signal Amplification (PerkinElmer). DAPI (Invitrogen) was used for nuclear staining and all images were acquired using a Leica-TCS SP5 confocal microscope. TUNEL staining was performed according to manufacturer’s instructions (Sigma-Aldrich).
In situ hybridization
Section in situ hybridization was performed as previously described (Seidel et al., 2010) on tissue sections using digoxigenin (DIG)-labeled Yap, Taz, Amelx, and Ambn probes. Yap, Taz, Amelx, and Ambn fragments were subcloned using primers with T7 and T3 binding site-overhangs: Yap Fwd 5’-ATATTAATACGACTCACTATAGGGTCAATGCCGTCATGAACCCCAAG-3’, Yap Rev 5’-ATATAATTAACCCTCACTAAAGGGCCAGCCAGGATGTGGTCTTGTTC-3’, Taz Fwd 5’-ATATTAATACGACTCACTATAGGGCAAGTCGTGGCCACTAGCCTG-3’, Taz Rev 5’-ATATAATTAACCCTCACTAAAGGGTCCCGAGGTCAACATTTGTTCCTG-3’, Amelx Fwd 5’-ATATTAATACGACTCACTATAGGGCAGCCGTATCCTTCCTAT-3’, Amelx Rev 5’-ATATAATTAACCCTCACTAAAGATGCCCTGGTACCACTTCAT-3’, Ambn Fwd 5’-ATATTAATACGACTCACTATAGCACTCAGCAGCCACTGCTAC-3’, Ambn Rev 5’-ATATAATTAACCCTCACTAAAGCAGGGTTTTCCACCAATCAC-3’ from a mouse cDNA library that was reverse transcribed using RNA extracted from an E12.5 mouse brain or adult proximal incisors. For probe synthesis, PCR products were directly used in combination with T3 RNA polymerase (Promega) and generated probes were purified using QIAGEN RNeasy. Sections were hybridized with probes at 70°C overnight and then washed in SSC solutions. Bound probes were detected with an alkaline phosphatase conjugated anti-DIG antibody (Roche), followed by colorimetric development using BM Purple (Roche). Bright field images were obtained using a Leica DFC 500 camera with a Leica DM 5000B microscope. For double fluorescent in situ hybridization and immunostaining, a POD-conjugated anti-DIG antibody (Roche) was used, followed by signal detection using Tyramide Signal Amplification (PerkinElmer). Immunostaining for membrane GFP was then performed as described above. Fluorescent images were obtained using a Leica-TCS SP5 confocal microscope.
Microtomography
Mandibles were harvested and dehydrated through ethanol series to 70% ethanol in water. Samples were then soaked in phosphotungstic acid overnight to differentially stain soft tissues for μCT visualization using MicroXCT-200 (Xradia) with a spatial resolution of 0.5 μm. Images acquired were analyzed using Avizo (VSG).
Isolation of laCLs
laCLs were isolated from incisors as previously described (Biehs et al., 2013). In brief, proximal incisors (roughly 2 mm in length) were first collected by removing the surrounding jaw bones and being severed away with a pair of scissors. The dissected proximal incisors were then incubated in 0.8% EDTA in Ca2+/Mg2+ free PBS supplemented with 30 μg/ml DNase at 37°C for 30 minutes to separate the dental epithelium from the mesenchyme and periodontal tissues. laCLs that included both the bulbous portion, as well as the lateral wing-shaped epithelium, were subsequently dissected from the rest of the epithelium and collected in cold PBS for downstream applications.
Explant culture
Dissected proximal incisors were cultured on a 0.4 μm Millicell filter (Millipore) that was rested on a metal mesh (914 μm mesh opening, Spectrum Labs), such that the explants were grown at the liquid-air interface (refer to Figure 3M). The culture media used was composed of BGJB media (Thermo), 15% fetal calf serum, 200 μg/ml glutamine, 250 μg/ml ascorbic acid, 1% pen/strep, and with chemical inhibitors or DMSO control vehicle. Drugs used include Blebbistatin, Erlotinib, Ki16425, Latrunculin A, okadaic acid, PF573228, PP2, Rapamycin, and Y27632. These chemicals and their concentrations are listed in Table S2. In most cases, explants were cultured for 24 hours at 37°C before collection. Okadaic acid-treated explants were cultured for 12 hours and electroporated incisors were cultured for 8 hours.
Electroporation
Proximal incisors were first isolated as described above and incubated in 0.8% EDTA at 37°C for 10 minutes to separate the follicle layer from the dental epithelium. 2 μg/μl of DNA in PBS, intermixed with 0.66% carboxymethylcellulose, 1mM MgCL2, and 0.66% fast green, were mouth-pipetted into the space between the follicle layer and the outer enamel epithelium. 3 20 ms long 70V pulses were then applied using custom made platinum electrodes and a BTX-Harvard Apparatus ECM 830 electroporator to induce DNA uptake by OEE cells (Figure 6F). The electroporated samples were then cultured as explants for 8 hours. The following constructs were used for electroporation: pcDNA-Flag-Yap1 (a gift from Yosef Shaul, Addgene plasmid # 18881) (Levy et al., 2008), pCMV-flag S127A YAP, pCMV-Flag YAP S381A, and pQCXIH-Flag-YAP-S127/381A (gifts from Kunliang Guan, Addgene plasmid # 27370, 27377, and 33069) (Zhao et al., 2007, 2010).
Colony formation assay
To generate single laCL cell suspension, isolated laCLs were incubated with Accumax (Sigma) at 37°C for 30 minutes, spun down, and dissociated in 10 μl of media (DMEM/F12 (Gibco), 20 ng/ml EGF (R&D), 25 ng/ml bFGF (R&D), 1X B27 (Gibco), and 1% pen/strep). The cell suspension was passed through a 40 μm cell strainer (Fisher Scientific) and mixed with 80 μl of cold growth factor-reduced Matrigel (Corning), which was then sandwiched between an activated 18 mm circular cover glass (as prepared in Damljanović et al., 2005) and a Rain-X treated cover glass. Once the Matrigel was set, the Rain-X treated cover glass was removed and cells were cultured in media at 37°C for 72 hours before counting colonies.
Western blot and immunoprecipitation
Western blot and immunoprecipitation were performed based on manufacturer’s instructions (Thermo Fisher Scientific). For western blot, Isolated laCLs were pooled and lysed using RIPA buffer (Thermo Fisher Scientific) supplemented with 1X Halt Protease and Phosphatase Inhibitor (Thermo Fisher Scientific) and 0.2 Mm PMSF for 30 minutes at 4°C. Protein extracts were loaded in 4%–12% Bis-Tris gel (Thermo Fisher Scientific), transferred to nitrocellulose membrane, blocked with SuperBlock T20 (TBS) (Thermo Fisher Scientific), and immunobloted with primary antibodies (Table S4) at 4°C overnight. After incubation with appropriate HRP-conjugated secondary antibodies (Table S4) in room temperature for 1 hour, signals were detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and images were taken using Image Quant LAS 4000. For immunoprecipitation, laCLs were lysed using IP Lysis Buffer (Thermo Fisher Scientific) for 2 hours at 4°C. Lysates were incubated with antibodies against YAP (Santa Cruz) or CDC42-GTP (NewEast) that have been crosslinked with anti-mouse IgG Dynabeads (Thermo Fisher Scientific) for 2 hours at 4°C. Bound proteins were eluted in LDS Sample Buffer (Thermo Fisher Scientific) by heating at 90°C for 10 minutes and elutes were analyzed by western blot as described above. Quantification was performed using ImageJ (see below). AMELX, AMBN, and RHEB were normalized to GAPDH, p4EBP, pLATS1, pNDR1/2, pPAK, pS6K1, pYAP-S127, and pYAP-s397 were normalized to their respective total protein levels, and immunoprecipitated active CDC42 and PP1A were normalized to total CDC42 input and total YAP pulled-down respectively.
Expression profiling by microarray
Expression profiling of control and Yap/TazcKO laCLs by microarray was done in triplicates and the RNA used for each sample was extracted from 8 laCLs (2 males and 2 females) using the QIAGEN RNeasy micro kit. Total RNA quality was assessed using a Pico Chip on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Microarray was performed on Illumina Mouse Ref 8 v2.0 chips at the UCLA Neuroscience Genomics Core (UNGC). Raw bead-level microarray data were minimally processed by the UNGC (no normalization or background correction) using BeadStudio software (Illumina, San Diego, CA). Preprocessing of data was performed within the R statistical computing environment (R; http://cran.us.r-project.org). The SampleNetwork R function (Oldham et al., 2012) was used to determine outlying samples, assess technical batch effects, and perform data normalization. No outlying samples were removed. However, after quantile normalization a technical batch effect was found to be associated with “ArrayID” and was corrected using the ComBat R function (Johnson et al., 2007) with the type of sample (control or Yap/TazcKO) as a covariate. Following data preprocessing, differential expression analysis was performed to identify genes that were significantly up- or down-regulated in Yap/TazcKO laCLs (n=4) relative to controls (n=3). Probes on the microarray that were detected above background levels in more than one sample (n = 17174) were included in the analysis. Differential expression analysis was performed on log2-transformed expression data using the bayesT function from the Cyber-T R package (Baldi and Long, 2001; Kayala and Baldi, 2012). The p-values were adjusted to account for multiple testing using the false discovery rate (FDR) approach (Benjamini and Hochberg, 1995), and probes with FDR p < 0.05 were considered to be differentially expressed. However, as we took consideration that Cre recombination in Yap/TazcKO was not 100%, we also verified expression of genes that were above FDR p > 0.05 using immunostaining or qPCR. Gene enrichment analysis was performed using Gene Set Enrichment Analysis (GSEA) (http://software.broadinstitute.org/gsea/index.jsp).
qPCR analysis
For qPCR analysis, RNA from isolated Cre negative control and Yap/TazcKO laCLs was reverse-transcribed to cDNA using SensiFAST cDNA Synthesis Kit (Bioline). qPCR was performed using iTAQ CYBR green (Biorad) and the Eppendorf Realplex2 with the following conditions: 3 minutes at 95°C and 40 cycles of amplification (15 seconds at 95°C and 30 seconds at 60°C). The primer sets used are listed in Table S3. All measurements were normalized to Ppia (peptidylprolyl isomerase A) and the relative changes between control and Yap/TazcKO laCLs were determined using the 2−Δ ΔCT method (Livak and Schmittgen, 2001).
Statistical analysis
Statistics
All bar graphs display mean ± SD (standard deviation) with the exception of the qPCR result, which is shown as mean ± SEM (standard error of mean). Numbers of animals used for each experiment are detailed in Table S5. p values were derived from unpaired two tailed Student’s t tests, assuming unequal variance (*p<0.05; **p<0.01; ***p<0.001).
ImageJ image analysis
Colocalization of YAP or TAZ with DAPI was measured using the ImageJ plugin “Colocalization” (contributed by Pierre Bourdoncle). For determining percentage of immunostained area, positive immunofluorescence signals in TA or SR regions were first converted to 8 bit binary images and measured using the “Analyze Particles” function. The derived area was then divided by the total area of TA or SR regions to calculate the percentage of positive immunostaining. To determine average immunostaining pixel intensity, total pixel intensity in TA or SR regions was measured using the ImageJ “Measure” function and then divided by the total area of TA or SR regions. To compare average pixel intensity of immunostaining between Cre-recombined (GFP-positive) and non-recombined (GFP-negative) cells in mutant laCLs, membrane GFP signal was adjusted for thresholds and converted to a mask with holes filled. Immunostaining pixel intensity was then measured within and outside the masked regions, and divided by the total area of the masked and unmasked space respectively. When calculating nuclear YAP pixel intensity, an additional mask was created using DAPI signals. For western blot quantification, we used the inbuilt function for “Gels” to first convert band intensities into histograms, from which the area under the curve can be measured using the Wand tool and the relative expression between control and mutant samples were calculated.
Data availability
The microarray data were accessible through Gene Expression Omnibus (GEO): GSE87132
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Non-phospho-4E-BP1 (Thr46) (87D12) | Cell Signaling Technology | Cat# 4923S, RRID:AB_659944 |
| Rabbit polyclonal anti-Ameloblastin | Santa Cruz Biotechnology | Cat# sc-50534, RRID:AB_2226393 |
| Rabbit polyclonal anti-Amelogenin (FL-191) | Santa Cruz Biotechnology | Cat# sc-32892, RRID:AB_2226455 |
| Rat monoclonal anti-BrdU [BU1/75 (ICR1)] | Abcam | Cat# ab6326, RRID:AB_305426 |
| Rabbit polyclonal anti-CDC42 (P1) | Santa Cruz Biotechnology | Cat# sc-87, RRID:AB_631213 |
| Mouse monoclonal anti-CDC42-GTP | NewEast Biosciences | Cat# 26905, RRID:AB_1961759 |
| Mouse monoclonal anti-GAPDH | Acris Antibodies GmbH | Cat# ACR001P, RRID:AB_1616730 |
| Chicken polyclonal anti-GFP | Abcam | Cat# ab13970, RRID:AB_300798 |
| Rabbit polyclonal anti-ITGA3 | From: C Michael DiPersio; Aggarwal et al., 2014 | N/A |
| Rabbit monoclonal anti-Ki67 (SP6) | Thermo Fisher Scientific | Cat# RM-9106-S0, RRID:AB_2341197 |
| Rabbit monoclonal anti-Merlin (D3S3W) | Cell Signaling Technology | Cat# 12888S |
| Rabbit polyclonal anti-MST1-2/STK3-4 | Bethyl | Cat# A300-466A |
| Goat polyclonal anti-NDR1 (N-14) | Santa Cruz Biotechnology | Cat# sc-46184, RRID:AB_2196799 |
| Rabbit monoclonal anti-Phospho-4E-BP1 (Thr37/46) (236B4) | Cell Signaling Technology | Cat# 2855S, RRID:AB_560835 |
| Rabbit polyclonal anti-PAK1/2/3 | Cell Signaling Technology | Cat# 2604, RRID:AB_2160225 |
| Mouse monoclonal anti-P-cadherin (NCC-CAD-299) | Thermo Fisher Scientific | Cat# 13-5800, RRID:AB_2533023 |
| Rabbit polyclonal anti-pFAK (Phospho-Tyr397) | Assay Biotech | Cat# A0925, RRID:AB_10683791 |
| Rabbit polyclonal anti-pLATS1 (Thr1079) | Cell Signaling Technology | Cat# 9157S, RRID:AB_2133515 |
| Rabbit polyclonal anti-pMerlin (Ser518) | Rockland | Cat# 600-401-414, RRID:AB_2149813 |
| Rabbit monoclonal anti-pMerlin (Ser518) (D5A4I) | Cell Signaling Technology | Cat# 13281 |
| Rabbit polyclonal anti-pNDR1/2 (Phospho-Thr444/442) | Biorbyt | Cat# orb335842 |
| Mouse monoclonal anti-PP1 (E-9) | Santa Cruz Biotechnology | Cat# sc-7482, RRID:AB_628177 |
| Goat monoclonal anti-PP2A-Calpha/beta (C-20) | Santa Cruz Biotechnology | Cat# sc-6110, RRID:AB_216962 |
| Rabbit polyclonal anti-pPAK1 (Thr423)/pPAK2 (Thr402) | Cell Signaling Technology | Cat# 2601S, RRID:AB_330220 |
| Rabbit polyclonal anti-p70 S6 Kinase (Phospho-Thr389) | Assay Biotech | Cat# A0533, RRID:AB_10682683 |
| Rabbit polyclonal anti-phospho-p70 S6 Kinase (Thr389) | Cell Signaling Technology | Cat# 9205, |
| Rabbit polyclonal anti-pSRC (Tyr418) | Signalway | Cat# 11091 |
| Rabbit polyclonal anti-pYAP (Ser127) | Cell Signaling Technology | Cat# 4911S, RRID:AB_2218913 |
| Rabbit monoclonal anti-pYAP (Ser127) (D9W2I) | Cell Signaling Technology | Cat# 13008 |
| Rabbit monoclonal anti-pYAP (Ser397) (D1E7Y) | Cell Signaling Technology | Cat# 13619 |
| Rabbit polyclonal anti-RHEB | ProSci | Cat# 3501, RRID:AB_736009 |
| Rabbit monoclonal anti- p70 S6 Kinase (49D7) | Cell Signaling Technology | Cat# 2708, RRID:AB_390722 |
| Rabbit polyclonal anti-SerpinH1 | ABclonal | Cat# A-2517 |
| Rabbit polyclonal anti-WWTR1 (TAZ) | Sigma-Aldrich | Cat# HPA007415, RRID:AB_1080602 |
| Rabbit polyclonal anti-YAP | Cell Signaling Technology | Cat# 4912, RRID:AB_2218911 |
| Mouse monoclonal anti-YAP (63.7) | Santa Cruz Biotechnology | Cat# sc-101199, RRID:AB_1131430 |
| Rabbit monoclonal anti-YAP (human) [EP1674Y] | Abcam | Cat# ab52771, RRID:AB_2219141 |
| Goat polyclonal anti-rabbit IgG (H+L), Alexa Fluor 555 conjugated | Thermo Fisher Scientific | Cat# A21428, RRID:AB_10561552 |
| Goat polyclonal anti-rat IgG (H+L), Alexa Fluor 555 conjugated | Thermo Fisher Scientific | Cat# A-21434, RRID:AB_2535855 |
| Goat polyclonal anti-chicken IgY (H+L), Alexa Fluor 488 conjugate | Thermo Fisher Scientific | Cat# A-11039, RRID:AB_2534096 |
| Biotinylated goat polyclonal anti-rabbit IgG | Vector Laboratories | Cat# BA-1000, RRID:AB_2313606 |
| Biotinylated goat polyclonal anti-mouse IgG | Vector Laboratories | Cat# BA-9200, RRID:AB_2336171 |
| Goat polyclonal anti-rabbit IgG, HRP | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
| Horse polyclonal anti-mouse IgG, HRP | Cell Signaling Technology | Cat# 7076, RRID:AB_330924 |
| Mouse monoclonal TrueBlot anti-rabbit IgG, HRP | Rockland | Cat# 18-8816-31, RRID:AB_2610847 |
| Rat monoclonal TrueBlot anti-mouse IgG, HRP | Rockland | Cat# 18-8817-31, RRID:AB_2610850 |
| SmartBlot anti-goat IgG, HRP | Vicgene | Cat# va-6000-001 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| (−)-Blebbistatin | EMD Millipore | Cat# 203391; CAS: 856925-71-8 |
| Erlotinib HCl | Selleck Chemical | Cat# OSI-744; CAS: 183319-69-9 |
| Ki16425 | Selleck Chemical | Cat# S1315; CAS: 355025-24-0 |
| Latrunculin A, Latrunculia magnifica | EMD Millipore | Cat# 428021; CAS: 76343-93-6 |
| Okadaic acid, Prorocentrum sp. | EMD Millipore | Cat# 495609; CAS: 78111-17-8 |
| PF-573228 | Selleck Chemical | Cat# S2013; CAS: 869288-64-2 |
| PP2 | EMD Millipore | Cat# 529573; CAS: 172889-27-9 |
| Rapamycin | Selleck Chemical | Cat# S1039; CAS: 53123-88-9 |
| Recombinant Mouse EGF Protein | R&D Systems | Cat# 2028-EG; Accession# NP_034243 |
| Recombinant Human FGF basic | R&D Systems | Cat# 233-FB; Accession# P09038 |
| Y-27632 | Selleck Chemical | Cat# S1049; CAS: 129830-38-2 |
| Critical Commercial Assays | ||
| In Situ Cell Death Detection Kit, TMR red | Sigma-Aldrich | Cat# 12156792910 |
| SuperSignal West Pico Chemiluminescent Substrate | Thermo Fisher Scientific | Cat# 34080 |
| TSA Cyanine 3 Tyramide Reagent Pack | Perkin Elmer | Cat# SAT704B001EA |
| VECTASTAIN Elite ABC HRP Kit (Peroxidase, Standard) | Vector Laboratories | Cat# PK-6100 |
| Deposited Data | ||
| Raw and normalized microarray data | This paper | GEO: GSE87132 |
| Experimental Models: Cell Lines | ||
| N/A | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: CAT-Rho-K DN/3-1 | From: Jeffrey Bush, Kobayashi et al., 2004 | RBRC# RBRC01294 |
| Mouse: Cdc42tm1Brak | From: Cord Brakebusch; Wu et al., 2006 | MGI:3619134 |
| Mouse: Col1a1tm1(tetO-YAP1*)Fcam | From: Fernando | MGI:5316453 |
| Camargo; Camargo et al., 2007 | ||
| Mouse: Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo | The Jackson Laboratory; Muzumdar et al., 2007 | RRID:IMSR_JAX:00 7676; Cat# 007676; MGI:3716464 |
| Mouse: Itga3f/f | From: Michael DiPersio; Mitchell et al., 2009 | N/A |
| Mouse: Lats1tm1.1Jfm | From: Randy Johnson; Heallen et al., 2011 | RRID:IMSR_JAX:02 4941; MGI:5568586 |
| Mouse: Lats2tm1.1Jfm | From: Randy Johnson; Heallen et al., 2011 | RRID:IMSR_JAX:02 5428; MGI:5568589 |
| Mouse: Ptk2tm1Lfr | From: Valerie Weaver; Beggs et al., 2003 | MGI:2684666 |
| Mouse: Rac1tm1Djk | From: Jeffrey Bush; Glogauer et al., 2003 | RRID:IMSR_JAX:00 5550; MGI:2663662 |
| Mouse: Rptortm1.1Dmsa | From: Ajay Chawla; Sengupta et al., 2010 | RRID:IMSR_JAX:01 3188; MGI:4879103 |
| Mouse: RhoAf/f | From: Cord Brakebusch; Jackson et al., 2011 | N/A |
| Stk3tm1.1Rjo | From: Randy Johnson; Lu et al., 2010 | MGI:4430537 |
| Mouse: Stk4tm1.1Rjo | From: Randy Johnson; Lu et al., 2010 | RRID:IMSR_JAX:01 7635; MGI:4430536 |
| Mouse: tetO-Yap-eGFP | This paper | N/A |
| Mouse: Tg(KRT14-cre)1Amc | Dassule et al., 2000 | RRID:IMSR_JAX:00 4782; MGI:2445832 |
| Mouse: Tg(KRT14-cre/ERT2)1Ipc | Li et al., 2000 | MGI:2177426 |
| Mouse: Wwtr1tm1.1Eno | From: Randy Johnson; Xin et al., 2011 | MGI:5544289 |
| Mouse: Yap1tm1.1Eno | From: Randy Johnson; Xin et al., 2011 | MGI:5446483 |
| Recombinant DNA | ||
| pcDNA Flag Yap1 | Addgene | Cat# 18881 |
| pCMV-flag S127A YAP | Addgene | Cat# 27370 |
| pCMV-Flag YAP S381A | Addgene | Cat# 27377 |
| pQCXIH-Flag-YAP-S127/381A | Addgene | Cat# 33069 |
| Sequence-Based Reagents | ||
| Primer sequences | See Table S2 | N/A |
| Taz RNA probe | This paper | N/A |
| Yap RNA probe | This paper | N/A |
| Software and Algorithms | ||
| ImageJ | https://imagej.nih.gov/ij/ | N/A |
| ImageJ Colocalization plugin | https://imagej.nih.gov/ij/plugins/colocalization.html | N/A |
| SampleNetwork R function | Oldham et al., 2012 | N/A |
| ComBat R function | Johnson et al., 2007 | N/A |
| Other | ||
| N/A | ||
Supplementary Material
Acknowledgments
We thank Sarah Alto, Rebecca d’Urso, and Nicholas Wang for assistance with the mouse colony, Dr. Amnon Sharir for help with μCT imaging, Derek Power for technical assistance, Drs. Anandika Aggarwal, Aaditi Mujumdar, and Chandana Vundavalli for assistance with sample preparation, members of the Klein laboratory, Dr. Jeffrey Bush and Dr. Valerie Weaver for helpful discussions, Drs. Eric Olson, Randy Johnson, Louis Reichardt, Fernando Camargo, Cord Brakebusch, and RIKEN BRC for mouse lines. This work was funded by NIDCR R01-DE024988 and R35-DE026602 to O.D.K and F32-DE023705 and K99-DE025874 to J.K.H.
Footnotes
Author Contributions
Conceptualization, J.K.H. and O.D.K.; Methodology, J.K.H. and O.D.K.; Investigation, J.K.H., W.D., and C.M.D.; Formal analysis, S.S.; Writing–Original Draft, J.K.H. and O.D.K.; Writing–Review & Editing, J.K.H. and O.D.K.; Resources, C.M.D.; Funding Acquisition, J.K.H., M.C.O., C.M.D., and O.D.K.; Supervision, O.D.K.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal A, Al-Rohil RN, Batra A, Feustel PJ, Jones DM, DiPersio CM. Expression of integrin α3β1 and cyclooxygenase-2 (COX2) are positively correlated in human breast cancer. BMC Cancer. 2014;14:459. doi: 10.1186/1471-2407-14-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatol Baltim Md. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t -test and statistical inferences of gene changes. Bioinforma Oxf Engl. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- Biehs B, Hu JK, Strauli NB, Sangiorgi E, Jung H, Heber RP, Ho S, Goodwin AF, Dasen JS, Capecchi MR, et al. BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat Cell Biol. 2013;15:846–852. doi: 10.1038/ncb2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Heller E, Beronja S, Oshimori N, Stokes N, Fuchs E. An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature. 2012;485:104–108. doi: 10.1038/nature10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damljanović V, Lagerholm BC, Jacobson K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays. BioTechniques. 2005;39:847–851. doi: 10.2144/000112026. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Dev Camb Engl. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, Cordero J, Tan EH, Ridgway R, Brunton VG, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Dev Camb Engl. 2016;143:1674–1687. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol Baltim Md 1950. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- Hansen CG, Ng YLD, Lam WLM, Plouffe SW, Guan KL. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, Thesleff I. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A. The Roles of NDR Protein Kinases in Hippo Signalling. Genes. 2016;7 doi: 10.3390/genes7050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Lv X, He C, Remmenga SW, Rodabough KJ, Dong J, Yang L, Lele SM, Yang P, Zhou J, et al. YAP induces high-grade serous carcinoma in fallopian tube secretory epithelial cells. Oncogene. 2016;35:2247–2265. doi: 10.1038/onc.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhang L, Chen Y, Zhang H, Zhang Q, Li R, Ma J, Li Z, Yu C, Lai Y, et al. Cdc42 deficiency induces podocyte apoptosis by inhibiting the Nwasp/stress fibers/YAP pathway. Cell Death Dis. 2016;7:e2142. doi: 10.1038/cddis.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson B, Peyrollier K, Pedersen E, Basse A, Karlsson R, Wang Z, Lefever T, Ochsenbein AM, Schmidt G, Aktories K, et al. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell. 2011;22:593–605. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostat Oxf Engl. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Juuri E, Saito K, Ahtiainen L, Seidel K, Tummers M, Hochedlinger K, Klein OD, Thesleff I, Michon F. Sox2+ Stem Cells Contribute to All Epithelial Lineages of the Tooth via Sfrp5+ Progenitors. Dev Cell. 2012;23:317–328. doi: 10.1016/j.devcel.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ährlund-Richter L, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- Kayala MA, Baldi P. Cyber-T web server: differential analysis of high-throughput data. Nucleic Acids Res. 2012;40:W553–559. doi: 10.1093/nar/gks420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein OD, Lyons DB, Balooch G, Marshall GW, Basson MA, Peterka M, Boran T, Peterkova R, Martin GR. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci Off J Soc Neurosci. 2004;24:3480–3488. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol. 2015;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Adamovich Y, Reuven N, Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Li CY, Cha W, Luder HU, Charles RP, McMahon M, Mitsiadis TA, Klein OD. E-cadherin regulates the behavior and fate of epithelial stem cells and their progeny in the mouse incisor. Dev Biol. 2012;366:357–366. doi: 10.1016/j.ydbio.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Indra AK, Warot X, Brocard J, Messaddeq N, Kato S, Metzger D, Chambon P. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature. 2000;407:633–636. doi: 10.1038/35036595. [DOI] [PubMed] [Google Scholar]
- Li P, Silvis MR, Honaker Y, Lien WH, Arron ST, Vasioukhin V. αE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 2016;30:798–811. doi: 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer — a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, DiPersio CM. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778–1787. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015;29:1271–1284. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genes N Y N 2000. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Oldham MC, Langfelder P, Horvath S. Network methods for describing sample relationships in genomic datasets: application to Huntington’s disease. BMC Syst Biol. 2012;6:63. doi: 10.1186/1752-0509-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa S, Kuremoto KI, Seidel K, Tabatabai R, Mackenzie B, Yamaza T, Akiyama K, Branch J, Koh CJ, Al Alam D, et al. Signaling by FGFR2b controls the regenerative capacity of adult mouse incisors. Development. 2010;137:3743–3752. doi: 10.1242/dev.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, Hata S, Sasaki T, Krens SFG, Osada Y, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowse ABJ, Chong F, Gray PP, Munro TP. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011;6:1–12. doi: 10.1016/j.scr.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Qi Q, Li DY, Luo HR, Guan KL, Ye K. Netrin-1 exerts oncogenic activities through enhancing Yes-associated protein stability. Proc Natl Acad Sci U S A. 2015;112:7255–7260. doi: 10.1073/pnas.1505917112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Secades P, van Hulst L, Kreft M, Song JY, Sonnenberg A. Loss of integrin α3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proc Natl Acad Sci U S A. 2012;109:21468–21473. doi: 10.1073/pnas.1204614110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin L, Desgrosellier JS, Weis SM, Cheresh DA. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol. 2015;25:234–240. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, Cao T, Carano RAD, Curran T, Schober M, et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–3761. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Thesleff I. Patterns of Wnt pathway activity in the mouse incisor indicate absence of Wnt/beta-catenin signaling in the epithelial stem cells. Dev Dyn. 2010;239:364–372. doi: 10.1002/dvdy.22106. [DOI] [PubMed] [Google Scholar]
- Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Dev Cell. 2015;34:283–296. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, et al. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev Cell. 2013;25:402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al. The Hippo Pathway Regulates Wnt/β-Catenin Signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. EMBO J. 2015;34:1164–1179. doi: 10.15252/embj.201490386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiao Y, Hsu CW, Martinez-Traverso IM, Zhang M, Bai Y, Ishii M, Maxson RE, Olson EN, Dickinson ME, et al. Yap and Taz play a crucial role in neural crest-derived craniofacial development. Dev Camb Engl. 2016;143:504–515. doi: 10.1242/dev.126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AC, Khuu JK, Dang CY, Hu J, Tran KV, Liu A, Gomez S, Zhang Z, Yi R, Scumpia P, et al. Stem cell quiescence acts as a tumour suppressor in squamous tumours. Nat Cell Biol. 2014;16:99–107. doi: 10.1038/ncb2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Quondamatteo F, Lefever T, Czuchra A, Meyer H, Chrostek A, Paus R, Langbein L, Brakebusch C. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 2006;20:571–585. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Z, Zhang Z. PP1α, PP1β and Wip-1 regulate H4S47 phosphorylation and deposition of histone H3 variant H3.3. Nucleic Acids Res. 2013;41:8085–8093. doi: 10.1093/nar/gkt583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642–655. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray data were accessible through Gene Expression Omnibus (GEO): GSE87132
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Non-phospho-4E-BP1 (Thr46) (87D12) | Cell Signaling Technology | Cat# 4923S, RRID:AB_659944 |
| Rabbit polyclonal anti-Ameloblastin | Santa Cruz Biotechnology | Cat# sc-50534, RRID:AB_2226393 |
| Rabbit polyclonal anti-Amelogenin (FL-191) | Santa Cruz Biotechnology | Cat# sc-32892, RRID:AB_2226455 |
| Rat monoclonal anti-BrdU [BU1/75 (ICR1)] | Abcam | Cat# ab6326, RRID:AB_305426 |
| Rabbit polyclonal anti-CDC42 (P1) | Santa Cruz Biotechnology | Cat# sc-87, RRID:AB_631213 |
| Mouse monoclonal anti-CDC42-GTP | NewEast Biosciences | Cat# 26905, RRID:AB_1961759 |
| Mouse monoclonal anti-GAPDH | Acris Antibodies GmbH | Cat# ACR001P, RRID:AB_1616730 |
| Chicken polyclonal anti-GFP | Abcam | Cat# ab13970, RRID:AB_300798 |
| Rabbit polyclonal anti-ITGA3 | From: C Michael DiPersio; Aggarwal et al., 2014 | N/A |
| Rabbit monoclonal anti-Ki67 (SP6) | Thermo Fisher Scientific | Cat# RM-9106-S0, RRID:AB_2341197 |
| Rabbit monoclonal anti-Merlin (D3S3W) | Cell Signaling Technology | Cat# 12888S |
| Rabbit polyclonal anti-MST1-2/STK3-4 | Bethyl | Cat# A300-466A |
| Goat polyclonal anti-NDR1 (N-14) | Santa Cruz Biotechnology | Cat# sc-46184, RRID:AB_2196799 |
| Rabbit monoclonal anti-Phospho-4E-BP1 (Thr37/46) (236B4) | Cell Signaling Technology | Cat# 2855S, RRID:AB_560835 |
| Rabbit polyclonal anti-PAK1/2/3 | Cell Signaling Technology | Cat# 2604, RRID:AB_2160225 |
| Mouse monoclonal anti-P-cadherin (NCC-CAD-299) | Thermo Fisher Scientific | Cat# 13-5800, RRID:AB_2533023 |
| Rabbit polyclonal anti-pFAK (Phospho-Tyr397) | Assay Biotech | Cat# A0925, RRID:AB_10683791 |
| Rabbit polyclonal anti-pLATS1 (Thr1079) | Cell Signaling Technology | Cat# 9157S, RRID:AB_2133515 |
| Rabbit polyclonal anti-pMerlin (Ser518) | Rockland | Cat# 600-401-414, RRID:AB_2149813 |
| Rabbit monoclonal anti-pMerlin (Ser518) (D5A4I) | Cell Signaling Technology | Cat# 13281 |
| Rabbit polyclonal anti-pNDR1/2 (Phospho-Thr444/442) | Biorbyt | Cat# orb335842 |
| Mouse monoclonal anti-PP1 (E-9) | Santa Cruz Biotechnology | Cat# sc-7482, RRID:AB_628177 |
| Goat monoclonal anti-PP2A-Calpha/beta (C-20) | Santa Cruz Biotechnology | Cat# sc-6110, RRID:AB_216962 |
| Rabbit polyclonal anti-pPAK1 (Thr423)/pPAK2 (Thr402) | Cell Signaling Technology | Cat# 2601S, RRID:AB_330220 |
| Rabbit polyclonal anti-p70 S6 Kinase (Phospho-Thr389) | Assay Biotech | Cat# A0533, RRID:AB_10682683 |
| Rabbit polyclonal anti-phospho-p70 S6 Kinase (Thr389) | Cell Signaling Technology | Cat# 9205, |
| Rabbit polyclonal anti-pSRC (Tyr418) | Signalway | Cat# 11091 |
| Rabbit polyclonal anti-pYAP (Ser127) | Cell Signaling Technology | Cat# 4911S, RRID:AB_2218913 |
| Rabbit monoclonal anti-pYAP (Ser127) (D9W2I) | Cell Signaling Technology | Cat# 13008 |
| Rabbit monoclonal anti-pYAP (Ser397) (D1E7Y) | Cell Signaling Technology | Cat# 13619 |
| Rabbit polyclonal anti-RHEB | ProSci | Cat# 3501, RRID:AB_736009 |
| Rabbit monoclonal anti- p70 S6 Kinase (49D7) | Cell Signaling Technology | Cat# 2708, RRID:AB_390722 |
| Rabbit polyclonal anti-SerpinH1 | ABclonal | Cat# A-2517 |
| Rabbit polyclonal anti-WWTR1 (TAZ) | Sigma-Aldrich | Cat# HPA007415, RRID:AB_1080602 |
| Rabbit polyclonal anti-YAP | Cell Signaling Technology | Cat# 4912, RRID:AB_2218911 |
| Mouse monoclonal anti-YAP (63.7) | Santa Cruz Biotechnology | Cat# sc-101199, RRID:AB_1131430 |
| Rabbit monoclonal anti-YAP (human) [EP1674Y] | Abcam | Cat# ab52771, RRID:AB_2219141 |
| Goat polyclonal anti-rabbit IgG (H+L), Alexa Fluor 555 conjugated | Thermo Fisher Scientific | Cat# A21428, RRID:AB_10561552 |
| Goat polyclonal anti-rat IgG (H+L), Alexa Fluor 555 conjugated | Thermo Fisher Scientific | Cat# A-21434, RRID:AB_2535855 |
| Goat polyclonal anti-chicken IgY (H+L), Alexa Fluor 488 conjugate | Thermo Fisher Scientific | Cat# A-11039, RRID:AB_2534096 |
| Biotinylated goat polyclonal anti-rabbit IgG | Vector Laboratories | Cat# BA-1000, RRID:AB_2313606 |
| Biotinylated goat polyclonal anti-mouse IgG | Vector Laboratories | Cat# BA-9200, RRID:AB_2336171 |
| Goat polyclonal anti-rabbit IgG, HRP | Cell Signaling Technology | Cat# 7074, RRID:AB_2099233 |
| Horse polyclonal anti-mouse IgG, HRP | Cell Signaling Technology | Cat# 7076, RRID:AB_330924 |
| Mouse monoclonal TrueBlot anti-rabbit IgG, HRP | Rockland | Cat# 18-8816-31, RRID:AB_2610847 |
| Rat monoclonal TrueBlot anti-mouse IgG, HRP | Rockland | Cat# 18-8817-31, RRID:AB_2610850 |
| SmartBlot anti-goat IgG, HRP | Vicgene | Cat# va-6000-001 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| (−)-Blebbistatin | EMD Millipore | Cat# 203391; CAS: 856925-71-8 |
| Erlotinib HCl | Selleck Chemical | Cat# OSI-744; CAS: 183319-69-9 |
| Ki16425 | Selleck Chemical | Cat# S1315; CAS: 355025-24-0 |
| Latrunculin A, Latrunculia magnifica | EMD Millipore | Cat# 428021; CAS: 76343-93-6 |
| Okadaic acid, Prorocentrum sp. | EMD Millipore | Cat# 495609; CAS: 78111-17-8 |
| PF-573228 | Selleck Chemical | Cat# S2013; CAS: 869288-64-2 |
| PP2 | EMD Millipore | Cat# 529573; CAS: 172889-27-9 |
| Rapamycin | Selleck Chemical | Cat# S1039; CAS: 53123-88-9 |
| Recombinant Mouse EGF Protein | R&D Systems | Cat# 2028-EG; Accession# NP_034243 |
| Recombinant Human FGF basic | R&D Systems | Cat# 233-FB; Accession# P09038 |
| Y-27632 | Selleck Chemical | Cat# S1049; CAS: 129830-38-2 |
| Critical Commercial Assays | ||
| In Situ Cell Death Detection Kit, TMR red | Sigma-Aldrich | Cat# 12156792910 |
| SuperSignal West Pico Chemiluminescent Substrate | Thermo Fisher Scientific | Cat# 34080 |
| TSA Cyanine 3 Tyramide Reagent Pack | Perkin Elmer | Cat# SAT704B001EA |
| VECTASTAIN Elite ABC HRP Kit (Peroxidase, Standard) | Vector Laboratories | Cat# PK-6100 |
| Deposited Data | ||
| Raw and normalized microarray data | This paper | GEO: GSE87132 |
| Experimental Models: Cell Lines | ||
| N/A | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: CAT-Rho-K DN/3-1 | From: Jeffrey Bush, Kobayashi et al., 2004 | RBRC# RBRC01294 |
| Mouse: Cdc42tm1Brak | From: Cord Brakebusch; Wu et al., 2006 | MGI:3619134 |
| Mouse: Col1a1tm1(tetO-YAP1*)Fcam | From: Fernando | MGI:5316453 |
| Camargo; Camargo et al., 2007 | ||
| Mouse: Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo | The Jackson Laboratory; Muzumdar et al., 2007 | RRID:IMSR_JAX:00 7676; Cat# 007676; MGI:3716464 |
| Mouse: Itga3f/f | From: Michael DiPersio; Mitchell et al., 2009 | N/A |
| Mouse: Lats1tm1.1Jfm | From: Randy Johnson; Heallen et al., 2011 | RRID:IMSR_JAX:02 4941; MGI:5568586 |
| Mouse: Lats2tm1.1Jfm | From: Randy Johnson; Heallen et al., 2011 | RRID:IMSR_JAX:02 5428; MGI:5568589 |
| Mouse: Ptk2tm1Lfr | From: Valerie Weaver; Beggs et al., 2003 | MGI:2684666 |
| Mouse: Rac1tm1Djk | From: Jeffrey Bush; Glogauer et al., 2003 | RRID:IMSR_JAX:00 5550; MGI:2663662 |
| Mouse: Rptortm1.1Dmsa | From: Ajay Chawla; Sengupta et al., 2010 | RRID:IMSR_JAX:01 3188; MGI:4879103 |
| Mouse: RhoAf/f | From: Cord Brakebusch; Jackson et al., 2011 | N/A |
| Stk3tm1.1Rjo | From: Randy Johnson; Lu et al., 2010 | MGI:4430537 |
| Mouse: Stk4tm1.1Rjo | From: Randy Johnson; Lu et al., 2010 | RRID:IMSR_JAX:01 7635; MGI:4430536 |
| Mouse: tetO-Yap-eGFP | This paper | N/A |
| Mouse: Tg(KRT14-cre)1Amc | Dassule et al., 2000 | RRID:IMSR_JAX:00 4782; MGI:2445832 |
| Mouse: Tg(KRT14-cre/ERT2)1Ipc | Li et al., 2000 | MGI:2177426 |
| Mouse: Wwtr1tm1.1Eno | From: Randy Johnson; Xin et al., 2011 | MGI:5544289 |
| Mouse: Yap1tm1.1Eno | From: Randy Johnson; Xin et al., 2011 | MGI:5446483 |
| Recombinant DNA | ||
| pcDNA Flag Yap1 | Addgene | Cat# 18881 |
| pCMV-flag S127A YAP | Addgene | Cat# 27370 |
| pCMV-Flag YAP S381A | Addgene | Cat# 27377 |
| pQCXIH-Flag-YAP-S127/381A | Addgene | Cat# 33069 |
| Sequence-Based Reagents | ||
| Primer sequences | See Table S2 | N/A |
| Taz RNA probe | This paper | N/A |
| Yap RNA probe | This paper | N/A |
| Software and Algorithms | ||
| ImageJ | https://imagej.nih.gov/ij/ | N/A |
| ImageJ Colocalization plugin | https://imagej.nih.gov/ij/plugins/colocalization.html | N/A |
| SampleNetwork R function | Oldham et al., 2012 | N/A |
| ComBat R function | Johnson et al., 2007 | N/A |
| Other | ||
| N/A | ||