Abstract
In the context of an organism, epithelial cells by nature are designed to be the defining barrier between self and the outside world. This is especially true for the epithelial cells that form the lining of the digestive tract, which absorb nutrients and serve as a barrier against harmful substances. These cells are constantly bathed by a complex mixture of endogenous (bile acids, mucus, microbial metabolites) and exogenous (food, nutrients, drugs) bioactive compounds. From a cell biology perspective, this type of exposure would directly impact the plasma membrane, which consists of a myriad of complex lipids and proteins. The plasma membrane not only functions as a barrier but also as the medium in which cellular signaling complexes form and function. This property is mediated by the organization of the plasma membrane, which is exquisitely temporally (nanoseconds to minutes) and spatially (nanometers to micrometers) regulated. Since numerous bioactive compounds found in the intestinal lumen can directly interact with lipid membranes, we hypothesize that the dynamic reshaping of plasma membrane organization underlies the chemoprotective effect of select membrane targeted dietary bioactives (MTDBs).
Keywords: n-3 PUFA, Dietary bioactives, membrane order, membrane therapy, cancer prevention
Introduction
Chronic diseases and conditions such as heart disease, stroke, cancer, type 2 diabetes, obesity and arthritis account for the vast majority of health spending in the U.S. While today’s situation is grave, the chronic disease crisis looms even larger in the future. This is supported by claims that if the current trends continue, by 2025, chronic diseases will affect an estimated nearly half of the U.S. population[1]. In addition, the number of cancer cases diagnosed annually by 2050 is likely to double as a result of population aging. Therefore, if the healthcare community hopes to head off the coming storm, we need to expand efforts in chronic disease prevention soon[2]. Heading off this escalating burden of age-related illnesses requires an emphasis on primary (cancer) prevention research and training in cancer-related lifestyle decisions, including diet and exercise[2]. Unfortunately, less than 1.5% of total biomedical research funding is targeted to early detection and prevention of chronic disease[3]. As an example, colorectal cancer (CRC) is the third most common type of cancer in the U.S., accounting for roughly 8% of new cancer cases and 9% of cancer deaths in 2014[4]. Overall, CRC incidence and mortality rates have decreased in the past 20 years, attributed largely to use of CRC screening and polypectomy in adults over 50 years of age. However, among adults younger than 50 years, for whom screening is not recommended if at average risk, CRC incidence rates have been increasing by ~2% per year since 1994 in both men and women[4]. While genetic factors account for some of the CRC risk, environmental factors account for the majority of risk[5]. Here, we describe the future challenges to the cancer field regarding the identification of additional molecular mechanisms that can be targeted as part of novel prevention strategies.
1. Dietary chemoprevention and CRC risk
CRC risk could be greatly reduced through dietary modification, including increased dietary fiber intake and reduced fat intake[6]. With respect to dietary fat intake, in observational studies, the evidence is mixed for associations between total dietary fat, specific types of fat, and CRC[7]. Omega 3 (n-3; α-linolenic acid, ALA) and omega 6 (n-6; linoleic acid, LA) polyunsaturated fatty acids (PUFA) are essential nutrients that are incorporated into tissue membranes, and modulate a variety of physiologic roles, including production of eicosanoids[8] and pro-resolving lipid mediators[9,10]. Most noteworthy are the long-chain n-3 PUFA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are found in fish oils[11]. Although long chain n-3 PUFA can be metabolically generated from ALA, the process is not very efficient in humans[12]. Additionally, there is competition with long-chain n-6 PUFA synthesis, di-homo-gamma-linolenic acid, and arachidonic acid (AA), which are produced from LA, and are found in much greater abundance in a typical Western diet[13]. In general, n-6 PUFA are pro-inflammatory whereas mediators produced from n-3 PUFA tend to have opposing effects and exhibit anti-inflammatory properties[14,15]. However, cases of n-3 PUFA exhibiting immune enhancing properties do exist[16,17]. Given the strong association between inflammation and CRC[18], higher intakes of n-3 PUFA provide biological plausibility for a chemoprotective effect[8,19]. Indeed, experimental preclinical models consistently show reduced CRC risk with n-3 PUFA[20–24]; however, epidemiologic data are inconsistent and the majority of studies did not include PUFA intake from supplemental fish oil[25–29]. Two meta-analyses have concluded that fish intake is associated with decreased risk of CRC[30,31]; however, two systematic reviews of n-3 PUFA on cancer risk qualitatively concluded that there is inadequate[32] or limited[28] evidence to suggest an association between long-chain n-3 PUFA intake and CRC risk. In contrast to the epidemiologic literature, in an endoscopy-based case-control study on colorectal adenomas, serum n-3 PUFA levels were inversely associated with colorectal adenoma risk [33]. Recently, in the VITamins And Lifestyle (VITAL) cohort, it was noted that persons using fish oil supplements on 4+ days per week for 3+ years experienced 49% lower CRC risk than nonusers [34].
With respect to clinical studies, mounting evidence suggests that the consumption of fish oil may reduce colon cancer risk in humans[35–41]. EPA and DHA appear to be ideally suited to work either alone or in combination with chemoprotective drugs[42]. Recently, it was demonstrated that EPA reduced rectal polyp number and size in patients with familial adenomatous polyposis (FAP)[19,43]. Most impressive was the fact that fish oil derived n-3 PUFA suppressed FAP to a degree similar to the selective COX-2 inhibitor celecoxib. Ongoing clinical trials (Clinical Trials.gov) are currently examining the effects of EPA on subjects at high risk of CRC (NCT02069561); the combinatory role of EPA and DHA in reducing rectal cancer risk (NCT02534389), and the combinatory role of EPA and NSAIDs on polyp recurrence in the colon (NCT01070355; ISRCTN05926847)[44,45]. Collectively, these data indicate that n-3 PUFA hold promise as chemoprevention agents. Hence, establishing a causal role of n-3 PUFA in colon cancer prevention would have a major translational impact because these dietary nutrients are safe, well tolerated[46], relatively inexpensive and provide additional health benefits[47–49]. In addition, the ingestion of n-3 PUFA in combination with other agents with complementary anti-tumor action, e.g., curcumin[50–55] and drugs[56],may improve their efficacy in colon cancer prevention/therapy. However, before a drug-nutrient combination approach can be adopted, it is imperative that we fully elucidate the molecular mechanisms of action.
Polyphenolic and terpenoid phytochemicals have become increasingly popular with consumers in part because of their putative health benefits. Of these, turmeric (Curcuma longa Linn) extracts, including curcumin (diferuloylmethane), a yellow color pigment of turmeric, have been shown to suppress colitis and colon cancer development in experimental models and placebo controlled clinical trials[56–63]. Ongoing clinical trials (Clinical Trials.gov) are currently examining the role of curcumin with respect to aberrant crypt foci (NCT00365209), cell proliferation and apoptosis in colonic mucosa (NCT00118989), drug combination therapy (NCT00745134), and treatment of FAP (NCT00927485, NCT00641147)[64]. Recent data from one of these Phase IIa clinical trials indicates that consumption of curcumin at 4 g per day for 30 days significantly (by 40%) reduces aberrant crypt foci (ACF) number in men and women, >40 years of age with a history of smoking and 8 or more rectal ACF by magnification chromoendoscopy[65]. Importantly, several studies have confirmed that curcumin is well tolerated in humans and is directly incorporated into colonic mucosa[65,66].
n-3 PUFA and curcumin paradoxically increase injury scores in an inflamed animal model [52], while the same n-3 PUFA dose has beneficial effects in a colon cancer model [67]. Along these lines, it has been demonstrated that fish oil feeding increases colon inflammation in a genetically susceptible mouse model [68], while exhibiting a chemo-protective tumor suppressing effect in an inflamed colon cancer animal model [69]. These findings suggest that adverse effects associated with preclinical studies may depend on the animal model and treatment dose/duration. In contrast, the majority of n-3 PUFA and curcumin associated clinical studies have reported beneficial outcomes (Table 1). For example, n-3 PUFA reduce rectal polyp number and size in FAP [43] and curcumin reduces aberrant crypt foci formation [65]. No adverse effects were reported following high dose n-3 PUFA [70] and curcumin [71] treatment.
Table 1.
Adverse and beneficial effects of marine-derived n-3 PUFA and curcumin in animal and clinical studies.
| Bioactive compound | Daily dose (n, duration of feeding)-mouse | Treatment | Amount of bioactive found in colon | Adverse biological effect | Human Equivalent Dose | Reference (PMID number) |
|---|---|---|---|---|---|---|
| n-3 PUFA+ curcumin | 46 mg n-3 PUFA and 90 mg curcumin (n=15, 3 wks) | 5 days of 2.5 % DSS followed by 16 days recovery period+3 days of 1.5 % DSS followed by a 14 days recovery period | N.A. | n-3 PUFA±curcumin increase DSS-induced injury score | 8.6 g n-3 PUFA and 17 g curcumin | 21401974 |
| n-3 PUFA | Approximately ~80 mg (n=20–30, 5 wks) | SMAD3 / mice | N.A. | Increased colon inflammation by increasing numbers of local neutrophils and inflammatory cytokine gene expression in the colon | 8.6 g n-3 PUFA | 26297475 |
| Bioactive compound | Daily dose (n, duration of feeding)-mouse | Treatment | Amount of bioactive found in colon | Beneficial biological effect | Human Equivalent Dose | Reference (PMID number) |
| n-3 PUFA+ curcumin | 46 mg n-3 PUFA and 45 mg curcumin (n=7–8, 3 wks) | Azoxymethane (AOM) | 29–64 ug/g crypt wet weight | Remove DNA damaged Lgr5 stem cells and reduce nuclear β-catenin in ACF | 8.6 g n-3 PUFA and 8.5 g curcumin | 27831561 |
| 1-4 ug/g crypt wet weight | ||||||
| n-3 PUFA | 130 mg n-3 PUFA (n=22–25, 15 wks) | One week after the AOM injection, mice were exposed to 3 cycles of 1% dextran sulfate sodium (DSS) for 4 days followed by 17 days of recovery | N.A. | fish oil fed animals developed fewer tumors | 24.7 g n-3 PUFA | 22761867 |
| Bioactive compound | Treatment dose (in vivo/ex vivo, time) | Treatment | Amount of bioactive found in colon | Beneficial biological effect | Reference (PMID number) | |
| Curcumin | 5–20 uM (ex vivo, 0.5–24 h) | Inflammatory disease: Inflammatory bowel disease | N.A. | Suppressed p38 MAPK activation, reduced IL-1beta, and enhanced IL-10 levels in mucosal biopsies; suppressed MMP-3 in colonic myofibroblasts | Not appliable | 19878610 |
| Bioactive compound | Daily dose (n, duration of treatment)-Human | Patient type | Amount of bioactive found in tissue or plasma | Beneficial biological effect | Mouse Equivalent Dose | Reference (PMID number) |
| Curcumin | 4 g (n=5, 3 months) | bladder cancer, oral leucoplakia, patiesnts with intestinal metaplasia of the stomach, CIN or Bowen's disease | 0.51 uM in serum | Histologic improvement in 28% patients with various high-risk and pre-malignant lesions. No treatment-related toxicity up to 8 g/day | 21.2 mg curcumin | 11712783 |
| 6 g (n=4, 3 months) | 0.63 uM in serum | 31.8 mg curcumin | ||||
| 8 g (n=2, 3 months) | 1.77 uM in serum | 42.4 mg curcumin | ||||
| 2 g (n=22, 1 month) | Phase II colon cancer | not detactable in serum, 8.2 ug/g protein in rectal mucosa | no effect on ACF reduction | 10.6 mg curcumin | 21372035 | |
| 4 g (n=19, 1 month) | 0.01 nM in serum, 3.8 ug/g protein in rectal mucosa | 40% reduction in ACF number in smokers | 21.2 mg curcumin | |||
| EPA | 2 g (n=55, 6 months) | Familial adenomatous polyposis (FAP) | Significant increase in rectal mucosal EPA (2.6–fold) and DPA (1.8-fold) content after EPA treatment compared with placebo. No significant change in mucosal AA and DHA content. | 22.4% reduction in polyp number | 10.6 mg n-3 PUFA | 20348368 |
| LOVAZA | 3.36 g (n=180, 6months) | Acute myocardial infarction | N.A. | Reduction of adverse left ventricular remodeling, noninfarct myocardial fibrosis, and serum biomarkers of systemic inflammation | 17.8 mg n-3 PUFA | 27482002 |
| LOVAZA | 17.6 g (n=12, 3 wks) | Healthy volunteers administered LPS | Lipid ratios of EPA and DHA in red blood cells (RBC) membranes is increased whereas, AA is decreased by n-3 PUFA supplementation | Anti-inflammatory actions in vivo remains speculative | 93.3 mg n-3 PUFA | 26180051 |
N.A., not available / did not measure
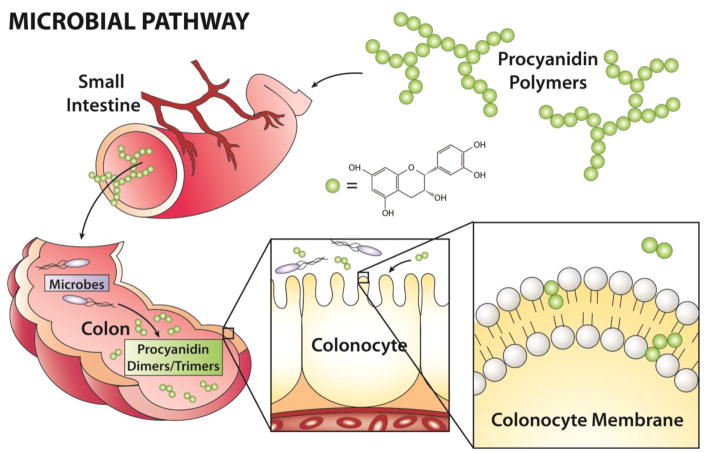
As a major component of the Mediterranean diet[72], walnuts (Juglans regia L.) contain a number of nutritional bioactive compounds, including PUFA, tocopherols, and membrane targeted dietary bioactive (MTDB)-like phenolic compounds, e.g., procyanidins[73–78]. Although intact procyanidins have some systemic biological activity, they are poorly absorbed and pass into the distal intestine (colon) where they are further metabolized by gut microbes to generate monomeric catechin and epicatechin compounds along with other dimers-hexamer species[79–81]. These microbial metabolites can interact with the apical membranes of colonic epithelial cells (Figure 1). Recent evidence from our lab and others suggest that the phenolic compound (+)-catechin, and its dimeric form procyanidin B2 can alter the biophysical properties of cell plasma membranes[82,83] (Figure 2), similar to what has been observed with other MTDB’s such as n-3 PUFA and curcumin[8,84–86]. Interestingly, walnuts have been shown to inhibit the growth of human cancer cell lines[87,88], and colon cancer in mouse models[89–91], similar to what has been demonstrated with other MTDB’s such as n-3 PUFA[8,23,69,92–95]. In addition, a meta-analysis concluded that nut consumption is associated with decreased risk of cancer mortality, further supporting the inclusion of nut consumption for cancer prevention[96]. However, the properties of procyanidins that contribute to a reduction in cancer risk have not been well characterized. In comparison, tocopherols exert beneficial properties by promoting plasma membrane repair[97], and attenuate oxidation of polyunsaturated phospholipids[98,99].
Figure 1. Putative role of microbial metabolites of procyanidin based dietary bioactives as modulators of colonocyte membrane-dependent oncogene signaling and cancer risk.
We hypothesize that select poorly digestible dietary-microbial derived bioactives can promote a chemoprotective cell membrane microenvironment in the colon.
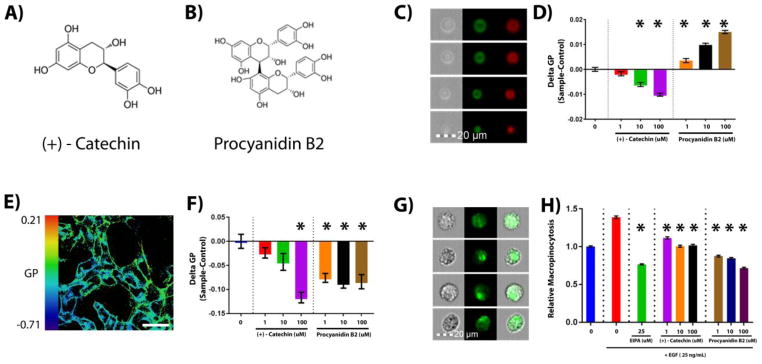
Figure 2. Procyanidins modulate colonocyte plasma membrane organization.
In order to determine if microbial metabolites of procyanidins directly modulate plasma membrane biophysical properties (membrane order), immortalized young adult mouse colonocyte (YAMC) cells expressing oncogenic HRasG12V were incubated with a membrane order sensitive dye Di-4-ANEPPDHQ (5 uM) for 30 min prior to generation of giant plasma membrane vesicles (GPMVs). GPMVs were incubated with, (A) (+)-Catechin, or (B) Procyanidin B2, for at least 30 min, followed by determination of GPMV generalized polarization (GP) by (C) imaged based flow cytometry using an Amnis FlowSight system. Emission wavelengths of 480-560 and 640–745 were used for ordered (Green) and disordered (Red) channels, respectively. (D) GP was defined as the integrated fluorescence intensity from the ordered channel minus that of the disordered channel normalized by the total intensity (sum of the two channels). Quantification of membrane order is represented as mean GP, normalized to the untreated control for at least 4000 individual vesicles from two separate experiments. Statistical significance between untreated control and treatments (*P<0.05) was determined using 1-way ANOVA and Dunnett’s multiple comparisons test. (E) To determine if microbe derived metabolites indirectly modify plasma membrane biophysical properties, membrane order was also determined in live YAMC-HRasG12V cells, where cytoskeletal influences contribute to membrane biophysical properties. Experiments were performed by confocal microscopy using a Zeiss 780 system, after incubation with compounds for at least 30 min. Emission wavelengths of 508–544 and 651–695 were used for ordered and disordered channels, respectively. Scale bar, 50 μM. (F) Quantification of membrane order is represented as mean GP normalized to the untreated control for at least 10 fields of view containing approximately 100 cells. Statistical significance between untreated control and treatments (*P<0.05) was determined using 1-way ANOVA and Dunnett’s multiple comparisons test. (G) To assess effects on cytoskeletal-membrane dependent macropinocytosis, YAMC-HRasG12V cells were serum starved (0.5% FBS) for 18 h, then incubated with a macropinocytosis inhibitor (EIPA) or procyanidin metabolites for 30 min prior to EGF (25 ng/mL) stimulation for 5 min in the presence of fluorescently (FITC) labeled dextran (70 kDa, 1 mg/ml). (H) Quantification of macropinocytosis, normalized to non-stimulated control, for at least 13,000 cells from two separate experiments. Statistical significance between EGF stimulated control and treatments (*P<0.05) was determined using 1-way ANOVA and Dunnett’s multiple comparisons test.
2. Putative mechanisms of MTDB action: Importance of nanoclustering as a dominant feature of plasma membrane organization
One of the criticisms facing the dietary chemoprevention field is the fact the dietary bioactives, i.e., constituents in foods or dietary supplements other than those needed to meet basic human nutritional needs [100], appear to be pleiotropic and affect diverse physiological processes including cell membrane structure/function, eicosanoid signaling, nuclear receptor activation, and inflammatory responses. Investigators are challenged to explain and unify these apparently disconnected signaling nodes. We propose a unifying mechanistic hypothesis to explain the function of these bioactives. Specifically, we postulate that n-3 PUFA and curcumin / curcuminoids / procyanidins fall into a unique class of MTDB’s which, because of their unique amphiphilic properties, are capable of modulating plasma membrane hierarchical organization, leading to the disruption of oncogenic signaling, and thereby ultimately reduce tumor growth.
With respect to membrane structure, the plasma membrane is composed of a heterogeneous mixture of lipids and proteins, whose distinct order maintains efficient signal transduction. Membrane lipids can undergo phase separations and interact selectively with membrane proteins and sub-membrane cytoskeletal elements[101]. Although, still controversial in nature, lipid rafts are believed to be dynamic and small (10–200 nm) membrane microdomains enriched in sphingolipids and/or cholesterol, which function as sorting platforms for many membrane-associated proteins[102–106]. Stabilization of these domains is generally thought to be maintained by lipid and cytoskeletal influences[107,108]. Recent evidence suggests that lipid rafts may modulate the malignant transformation process. For example, the levels of lipid rafts are increased in many types of cancer[109–111]. There is also evidence suggesting that disruption of lipid rafts in cancer can lead to increased responsiveness to anti-cancer therapies[112,113]. Additionally, some anti-cancer drugs have beneficial effects through alteration of the protein content of lipid rafts[114,115]. In colon cancer, lipid rafts have been shown to function in cell death-mediated signaling[116,117], cell entry/bioavailability of bioactive compounds[118], and localization of key proteins involved in immune response[119]. These findings indicate that lipids can no longer be ignored in the structures of membrane complexes, due to their ability to fine-tune and stabilize different signaling interfaces[120–122].
Highly relevant to the cancer biology field, it is now recognized that the geometry of biological membranes is tightly intertwined with signal processing capability[123]. According to this emerging picture, protein and lipid nanoclusters can be organized to form domains that are capable of facilitating signaling events[124–126]. The formation of dimers/nanoclusters is believed to be driven by cortical actin and/or proximal transmembrane proteins[124]. Currently, protein-protein, lipid-lipid and protein-lipid nanoclusters are considered a predominant feature of the plasma membrane and appear to mediate critical signaling processes[126], including signal integration and cross talk of the transduction of oncogenic Ras and the epidermal growth factor receptor (EGFR)[126–128] regulated pathways. This is noteworthy, because there is emerging evidence that drugs and MTDB’s can attenuate Ras and EGFR[126,129] activity by modulating nanocluster organization. In accordance with these findings, we hypothesize that MTDB’s are capable of disrupting clustering/dimerization of membrane associated proteins, leading to attenuation of downstream oncogenic signaling and the suppression of tumor growth (Figure 3).
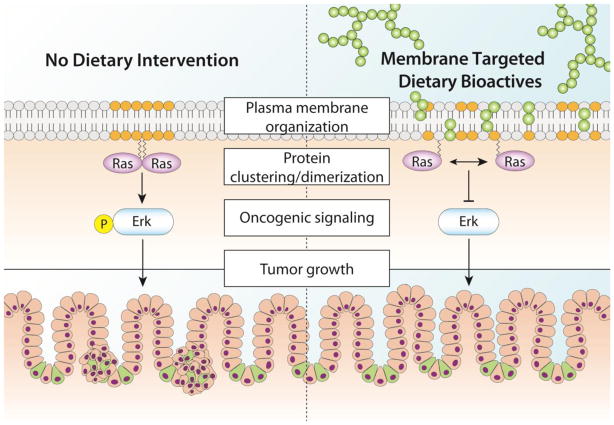
Figure 3.
Proposed mechanism describing the role of MTDB’s as modulators of colonocyte membrane-dependent oncogene signaling and cancer risk. 1) Procyanidins remodel plasma membrane organization. 2) Membrane remodeling disrupts Ras nanocluster/dimerization. 3) MTDB’s suppress Ras nanocluster/dimerization and attenuate oncogenic signaling. 4) This results in a reduction in tumor initiation/growth.
3. Effects of MTDB’s on membrane organization and signaling
There is a growing body of in vitro and in vivo evidence indicating that MTDB’s reshape plasma membrane domains. For example, EPA and DHA, whose levels are readily influenced by diet in general [130], affect diverse physiological processes including cell membrane structure/function and signaling[8,84]. n–3 PUFA are rapidly incorporated into cells, primarily into membrane phospholipids at the sn-2 position[131,132]. Specificaly, DHA is known to influence membrane fluidity, ion permeability, fatty acid exchange, and resident protein function [133,134], including the inhibition of EGFR signaling in tumor bearing mice by reducing localization of EGFR to lipid rafts [69]. The presence of long chain n-3 PUFA in membrane phospholipids imparts unique physicochemical properties which have been linked to alterations in plasma membrane structure and function and its pleiotropic chemoprotective effects[122,132,135–138]. Interestingly, other MTDB’s known to reshape membrane domains, e.g., curcumin, capsaicin, and glycyrrhizin, exhibit similar properties[139–145]. For example, curcumin inserts deep into the membrane in a trans-bilayer orientation, anchored by hydrogen bonding to the phosphate group of lipids in a manner analogous to cholesterol. Similar to cholesterol, curcumin induces segmental ordering in the membrane [139,146,147]. These properties may explain why curcumin can suppress EGFR localization to lipid rafts decreasing EGF stimulation in cells [148]. Although intact procyanidins have some systemic biological activity, they are poorly absorbed and pass into the distal intestine (colon) where they are further metabolized by gut microbes to generate monomeric, dimers-hexamer species[79–81]. These microbial metabolites interact with the apical membranes of colonic epithelial cells (Figure 1). Recent evidence from our lab and others suggest that the phenolic compound (+)-catechin, and its dimeric form procyanidin B2 can alter the biophysical properties of cell plasma membranes[82,83] (Figure 2), similar to what has been observed with other MTDB’s such as n-3 PUFA and curcumin[8,84–86]. These findings are consistent with previous observations that procyanidins have the ability to modulate membrane biophysical properties[82,83].
To further evaluate the effects of procyanidins compounds on plasma membrane organization, we utilized the polarity sensitive dye di-4-ANEPPDHQ (Di4). We utilized Di4 over the commonly used dye, laurdan, because Di4 exhibits slower internalization kinetics in live cells[149,150]. This provides a more representative measure of plasma membrane organization. In addition, although laurdan and Di4 are both used to quantify membrane order[151], recent findings highlight the fact that they probe different properties of the membrane, with Di4 being more sensitive to cholesterol status[152]. Di4 excites at 488 nm and its emission maximum emits at 565 nm or 605 nm in ordered and disordered membranes, respectively[149,151]. We chose the in vitro young adult mouse colonocyte (YAMC) cell model expressing oncogenic HRasG12V[153] as a representation of normal colonocytes on the route to malignancy[154]. These cells typically exhibit upregulated macropinocytosis[155], which is the cellular process of nonselective endocytic uptake of extracellular lipids and proteins driven by membrane-lipid and cytoskeletal remodeling[156,157]. Macropinocytosis provides the fuel for Ras-driven tumor growth resulting in altered metabolism[155,158], which creates a dependency that can be exploited as a pharmacological target[159,160]. To specifically further assess how exogenous treatments affect the plasma membrane, we generated giant plasma membrane vesicles (GPMVs) which retain most of the full diversity of native membrane components, but lack cytoskeletal attachment[161]. This type of reductionist approach allows us to probe specific questions regarding the interaction between diet-derived bioactives and lipid membranes without the complication of compensatory mechanisms imparted by the live cell such as membrane tension[162] and cytoskeletal remodeling[108]. GPMVs were isolated from YAMC-HRasG12V cells pre-labeled with Di4 and incubated with varying doses of (+)-catechin and procyanidin B2 (Figure 2C&D). We utilized a wide range of doses (1–10 uM) which have been associated with circulating levels in vivo (0.1–3 uM) [163–168], and higher doses (100 uM) that are present in the lumen of the colon[79,169–172]. A short incubation time frame (30 min) was used to mimic the passage of these compounds through the colon, and avoid large changes in gene or protein expression that may occur during longer incubation periods. Membrane order of GPMVs was then determined using imaging based flow-cytometry. Interestingly, (+)-catechin dose-dependently reduced membrane rigidity while procyanidin B2 increased rigidity. We subsequently used confocal microscopy to determine if the same effects would occur in live cells (Figures 2E&F), which are generally more refractive to plasma membrane changes[173,174]. Surprisingly, both compounds decreased membrane rigidity in intact cells. Internalization of fluorescently labeled dextran was used as an indicator of macropinocytosis[175], which was quantified by image based flow-cytometry. Incubation of cells with low doses of these compounds attenuated epidermal growth factor (EGF) stimulated macropinocytosis, with procyanidin B2 exhibiting greater inhibitory activity (Figure 2G&H). These proof of principle experiments demonstrate how procyanidin based MTDBs can modulate cellular processes including plasma membrane organization in both a cytoskeletal dependent and independent manner.
4. Potential effects of MTDB’s on cancer development and stem cells
It is noteworthy that many proteins involved in colon cancer cell signaling, including transmembrane receptors and G proteins, localize to lipid rafts[109] and nanocluster[176]. For example, the EGFR, a tyrosine kinase that plays a critical role in cell proliferation and resistance to cancer therapy[177], requires lipid raft localization and nanoclustering for efficient signaling[112,176,178]. n-3 PUFA in part through a reduction in lipid raft cholesterol composition, displace EGFR from rafts, leading to an altered phosphorylated state[179–181]. This in turn has been linked to the suppression of colonocyte downstream signaling events involving EGFR, such as phosphorylation of ERK1/2, STAT3, Akt and activation of H- and KRas[92,136,181].
As mentioned above, since changes in plasma membrane structure alter receptor-mediated cell signaling[182], there is mounting interest regarding the use MTDB’s to modulate membrane-mediated signaling pathways and their target genes. Consistent with the fact that DHA has been shown to significantly alter plasma membrane lipid raft composition [136,138], our lab recently demonstrated that n-3 PUFA alter EGFR lipid raft localization and HRAS, KRAS and NRAS activation [181]. These findings can be attributed to the fact that prolonged intake of dietary lipids modifies membrane order[183]. Interestingly, other MTDB’s, e.g., curcumin, have been shown to alter localization of α6β4/EGFR to lipid rafts[184]. It is noteworthy that the ordering effect of curcumin is strongest in the head group region of the phospholipid bilayer[139,185], whereas n-3 PUFA acyl chains impact the organization of the tail group region within rafts[186–188] implying that these bioactives may act synergistically at the membrane.
Wnt signaling is important for the maintenance of stem cells of various lineages. A classic example is in the digestive tract, where in the crypt of the colon the loss of transcription factor TCF4 leads to depletion of stem cells[189]. Dysregulation of Wnt/β-catenin signaling via genetic alterations of APC, β-catenin or Axin2 drives stem cell hyperproliferation which promotes colorectal cancer[190–192]. In contrast, neonatal mice lacking TCF4 exhibit reduced proliferation in the crypt [189]. These findings are consistent with the fact that chronic upregulation of Wnt signaling in Lgr5 positive stem cells drives colon cancer[193]. Interestingly, from a membrane perspective, Wnt signaling components, e.g., Lypd6 and CK1γ, have been shown to mainly localize to lipid rafts in the plasma membrane[194]. This is noteworthy, because lipid rafts play a fundamental role in mediating multiple cell functions, including signal transduction[195]. LRP6 is localized to both raft and non-raft fractions but its phosphorylation by GSK-3 and CK1γ, essential for the Wnt-dependent accumulation of β-catenin, resides primarily in lipid rafts, not in the non-lipid raft[196]. Thus, it has been proposed that the localization of these proteins to lipid rafts actively contributes to the stabilization of β-catenin[197,198]. Based on these findings, we propose that MTDB’s may modulate the Wnt signaling pathway.
5. Summary
With respect to all human malignancies, 35% are linked directly to diet and an additional 14-20% to obesity[5]. Consistent with these data, cancer risk can be lowered by 36% when humans adhere to healthy dietary principles, e.g., high intake of fruits, vegetables, and whole grains and low meat consumption[199]. Therefore, it is imperative that health professionals make sound dietary/lifestyle recommendations. However, even though there are many observational / epidemiological studies linking diet and cancer risk, the association cannot be easily explained mechanistically. Therefore, establishing a causal role for cancer dietary chemoprevention approaches that are generally free of safety problems intrinsic to drugs administered over long periods of time would have a major translational impact in cancer prevention and patient survivorship[46,199]. In view of this need, our long-term goal is to better understand the molecular mechanisms modulating cell responses to MTDB’s. Specifically, we propose that by altering cell membrane nanoscale assemblies, and possibly protein spatial localization and signaling, that select amphiphilic dietary agents, e.g., n-3 PUFA, curcumin, procyanidins, will reduce oncogenic signaling and cancer risk.
Highlights.
Diet dynamically shapes plasma membrane organization
Intrinsic adaptation of plasma membrane organization mediates cell signaling
Diet-derived polyphenolic compounds disrupt oncogenic Ras-driven dependencies
Acknowledgments
This work was supported by NIH grants R35CA197707, CA168312, and P30ES023512, the Cancer Prevention Research Institute of Texas (CPRIT) and the American Institute for Cancer Research (AICR). Natividad Fuentes is a recipient of a Predoctoral Fellowship in Pharmacology/Toxicology from the PhRMA Foundation. Natividad Fuentes and Michael Salinas are recipients of the National Science Foundation Texas A&M University System Louis Stokes Alliance for Minority Participation (TAMUS LSAMP) Bridge to the Doctorate Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boult C, Bringewatt R, Cooper B, Friedland R, Komisar H, Niefeld M, Powe N, Rice D, Rother J, Summer L, Vladeck B. [accessed March 15, 2017];Partnership for Solutions Partnership for Solutions. n.d http://www.partnershipforsolutions.org/DMS/files/chronicbook2004.pdf.
- 2.Vogelstein B, Kinzler KW. Winning the war: science parkour. Sci Transl Med. 2012;4:127ed2. doi: 10.1126/scitranslmed.3004019. [DOI] [PubMed] [Google Scholar]
- 3.Moses H, Dorsey ER, Matheson DHM, Thier SO. Financial anatomy of biomedical research. JAMA. 2005;294:1333–1342. doi: 10.1001/jama.294.11.1333. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2015. 2015 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index.
- 5.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 80- 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vargas AJ, Thompson PA. Diet and nutrient factors in colorectal cancer risk. Nutr Clin Pr. 2012;27:613–623. doi: 10.1177/0884533612454885. [DOI] [PubMed] [Google Scholar]
- 7.Song M, Garrett WS, Chan AT. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology. 2015;148:1244–1260.e16. doi: 10.1053/j.gastro.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN. Treating inflammation and infection in the 21st century: new hints from decoding resolution mediators and mechanisms. FASEB J. 2017 doi: 10.1096/fj.201601222R. fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strobel C, Jahreis G, Kuhnt K. Survey of n-3 and n-6 polyunsaturated fatty acids in fish and fish products. Lipids Health Dis. 2012;11:144. doi: 10.1186/1476-511X-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002;88:411–20. doi: 10.1079/BJN2002689. [DOI] [PubMed] [Google Scholar]
- 13.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. http://www.ncbi.nlm.nih.gov/pubmed/16841856. [DOI] [PubMed] [Google Scholar]
- 14.Spite M, Clària J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fat Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J Lipid Res. 2013;54:3130–3138. doi: 10.1194/jlr.M042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petursdottir DH, Hardardottir I. Dietary fish oil increases the number of splenic macrophages secreting TNF-alpha and IL-10 but decreases the secretion of these cytokines by splenic T cells from mice. [accessed March 13, 2017];J Nutr. 2007 137:665–70. doi: 10.1093/jn/137.3.665. http://www.ncbi.nlm.nih.gov/pubmed/17311957. [DOI] [PubMed] [Google Scholar]
- 18.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 19.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61:135–149. doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 20.Reddy BS, Burill C, Rigotty J. Effect of diets high in omega-3 and omega-6 fatty acids on initiation and postinitiation stages of colon carcinogenesis. Cancer Res. 1991;51:487–491. http://www.ncbi.nlm.nih.gov/pubmed/1821094. [PubMed] [Google Scholar]
- 21.Chang WL, Chapkin RS, Lupton JR. Fish Oil Blocks Azoxymethane-Induced Rat Colon Tumorigenesis by Increasing Cell Differentiation and Apoptosis Rather Than Decreasing Cell. J Nutrtion. 1997;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 22.Piazzi G, D’Argenio G, Prossomariti A, Lembo V, Mazzone G, Candela M, Biagi E, Brigidi P, Vitaglione P, Fogliano V, D’Angelo L, Fazio C, Munarini A, Belluzzi A, Ceccarelli C, Chieco P, Balbi T, Loadman PM, Hull MA, Romano M, Bazzoli F, Ricciardiello L. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int J Cancer. 2014;135:2004–13. doi: 10.1002/ijc.28853. [DOI] [PubMed] [Google Scholar]
- 23.Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem Phys Lipids. 2008;153:14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy BS, Patlolla JM, Simi B, Wang SH, Rao CV. Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022–8027. doi: 10.1158/0008-5472.CAN-05-0212. [DOI] [PubMed] [Google Scholar]
- 25.Watson AJM, Collins PD. Colon cancer: a civilization disorder. Dig Dis. 2011;29:222–8. doi: 10.1159/000323926. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Sheppard L. Dietary fat and cancer: consistency of the epidemiologic data, and disease prevention that may follow from a practical reduction in fat consumption. Cancer Causes Control. 1990;1:81–109. doi: 10.1007/BF00053187. http://www.ncbi.nlm.nih.gov/pubmed/2102280. [DOI] [PubMed] [Google Scholar]
- 27.Henderson MM. International differences in diet and cancer incidence. J Natl Cancer Inst Monogr. 1992:59–63. http://www.ncbi.nlm.nih.gov/pubmed/1616812. [PubMed]
- 28.Gerber M. Omega-3 fatty acids and cancers: a systematic update review of epidemiological studies. Br J Nutr. 2012;107(Suppl):S228-39. doi: 10.1017/S0007114512001614. [DOI] [PubMed] [Google Scholar]
- 29.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, Anderson GL, Assaf AR, Bassford T, Bowen D, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Harrigan RC, Hays J, Heber D, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Kotchen JM, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lewis CE, Manson JE, Margolis KL, Mossavar-Rahmani Y, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Stefanick ML, Van Horn L, Vitolins MZ, Wactawski-Wende J, Wallace RB, Whitlock E. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–654. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Feng B, Li K, Zhu X, Liang S, Liu X, Han S, Wang B, Wu K, Miao D, Liang J, Fan D. Fish Consumption and Colorectal Cancer Risk in Humans: A Systematic Review and Meta-analysis. Am J Med. 2012;125:551–559.e5. doi: 10.1016/j.amjmed.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Pot GK, Majsak-newman G, Geelen A, Harvey LJ, Nagengast FM, Witteman BJM, Van De Meeberg PC, Timmer R, Tan A, Wahab PJ, Hart AR, Williams MP, Przybylska-phillips K, Dainty JR, Schaafsma G, Kampman E, Lund EK. Fish consumption and markers of colorectal cancer risk : a multicenter randomized controlled trial. 2009;1–3:354–361. doi: 10.3945/ajcn.2009.27630.1. [DOI] [PubMed] [Google Scholar]
- 32.MacLean CH, Newberry SJ, Mojica Wa, Khanna P, Issa AM, Suttorp MJ, Lim Y-W, Traina SB, Hilton L, Garland R, Morton SC. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 33.Pot GK, Geelen A, van Heijningen EM, Siezen CL, van Kranen HJ, Kampman E. Opposing associations of serum n-3 and n-6 polyunsaturated fatty acids with colorectal adenoma risk: an endoscopy-based case-control study. Int J Cancer. 2008;123:1974–1977. doi: 10.1002/ijc.23729. [DOI] [PubMed] [Google Scholar]
- 34.Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer. 2014;66:716–27. doi: 10.1080/01635581.2013.804101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, Parrella P, Canetta C, Gentiloni N, De Vitis I, et al. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. 1994;107:1709–1718. doi: 10.1016/0016-5085(94)90811-7. http://www.ncbi.nlm.nih.gov/pubmed/7958682. [DOI] [PubMed] [Google Scholar]
- 36.Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103:883–891. doi: 10.1016/0016-5085(92)90021-p. http://www.ncbi.nlm.nih.gov/pubmed/1386825. [DOI] [PubMed] [Google Scholar]
- 37.Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-y. http://www.ncbi.nlm.nih.gov/pubmed/8224635. [DOI] [PubMed] [Google Scholar]
- 38.Caygill CP, Charlett A, Hill MJ. Fat, fish, fish oil and cancer. Br J Cancer. 1996;74:159–164. doi: 10.1038/bjc.1996.332. http://www.ncbi.nlm.nih.gov/pubmed/8679451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Wang J, Luo C, Fujii T, Ichikawa H, Shirai T, Tokudome S. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003;193:17–24. doi: 10.1016/s0304383502007176. http://www.ncbi.nlm.nih.gov/pubmed/12691819. [DOI] [PubMed] [Google Scholar]
- 40.Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Color Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 41.Hall MN, Chavarro JE, Lee I-M, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17:1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaughan VC, Hassing MR, Lewandowski PA. Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer. 2013;108:486–492. doi: 10.1038/bjc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West NJ, Clark SK, Phillips RKS, Hutchinson JM, Leicester RJ, Belluzzi A, Hull MA. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–25. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 44.Cockbain AJ, Volpato M, Race AD, Munarini A, Fazio C, Belluzzi A, Loadman PM, Toogood GJ, Hull Ma. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut. 2014;63:1760–8. doi: 10.1136/gutjnl-2013-306445. [DOI] [PubMed] [Google Scholar]
- 45.Hull Ma, Sandell AC, Montgomery Aa, Logan RF, Clifford GM, Rees CJ, Loadman PM, Whitham D. A randomized controlled trial of eicosapentaenoic acid and/or aspirin for colorectal adenoma prevention during colonoscopic surveillance in the NHS Bowel Cancer Screening Programme (The seAFOod Polyp Prevention Trial): study protocol for a randomized cont. Trials. 2013;14:237. doi: 10.1186/1745-6215-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:125–32. doi: 10.1016/j.plefa.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Di Minno MN, Tremoli E, Tufano A, Russolillo A, Lupoli R, Di Minno G. Exploring newer cardioprotective strategies: omega-3 fatty acids in perspective. Thromb Haemost. 2010;104:664–680. doi: 10.1160/TH10-01-0008. [DOI] [PubMed] [Google Scholar]
- 48.Harris WS. Are n-3 fatty acids still cardioprotective? Curr Opin Clin Nutr Metab Care. 2013;16:141–149. doi: 10.1097/MCO.0b013e32835bf380. [DOI] [PubMed] [Google Scholar]
- 49.Rizos EC, Elisaf MS. Current evidence and future perspectives of omega-3 polyunsaturated fatty acids for the prevention of cardiovascular disease. Eur J Pharmacol. 2013;706:1–3. doi: 10.1016/j.ejphar.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 50.Vasudevan A, Yu Y, Banerjee S, Woods J, Farhana L, Rajendra SG, Patel A, Dyson G, Levi E, Maddipati KR, Majumdar AP, Nangia-Makker P. Omega-3 fatty acid is a potential preventive agent for recurrent colon cancer. Cancer Prev Res. 2014;7:1138–1148. doi: 10.1158/1940-6207.CAPR-14-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altenburg JD, Bieberich AA, Terry C, Harvey KA, Vanhorn JF, Xu Z, Jo Davisson V, Siddiqui RA. A synergistic antiproliferation effect of curcumin and docosahexaenoic acid in SK-BR-3 breast cancer cells: unique signaling not explained by the effects of either compound alone. BMC Cancer. 2011;11:149. doi: 10.1186/1471-2407-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia Q, Ivanov I, Zlatev ZZ, Alaniz RC, Weeks BR, Callaway ES, Goldsby JS, Davidson LA, Fan Y-Y, Zhou L, Lupton JR, McMurray DN, Chapkin RS. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br J Nutr. 2011;106:519–29. doi: 10.1017/S0007114511000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saw CLL, Huang Y, Kong A-N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: docosahexaenoic acid or eicosapentaenoic acid. Biochem Pharmacol. 2010;79:421–30. doi: 10.1016/j.bcp.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui RA, Harvey KA, Walker C, Altenburg J, Xu Z, Terry C, Camarillo I, Jones-Hall Y, Mariash C. Characterization of synergistic anti-cancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer. 2013;13:418. doi: 10.1186/1471-2407-13-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swamy MV, Citineni B, Patlolla JMR, Mohammed A, Zhang Y, Rao CV. Prevention and treatment of pancreatic cancer by curcumin in combination with omega-3 fatty acids. Nutr Cancer 60 Suppl. 2008;1:81–9. doi: 10.1080/01635580802416703. [DOI] [PubMed] [Google Scholar]
- 56.Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597–601. http://www.ncbi.nlm.nih.gov/pubmed/9973206. [PubMed] [Google Scholar]
- 57.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 58.Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin Gastroenterol Hepatol. 2006;4:1502–1506. doi: 10.1016/j.cgh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, Wexner SD, Giardiello FM. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ, Steward WP. Phase I Clinical Trial of Oral Curcumin : Biomarkers of Systemic Activity and Compliance. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 62.Nautiyal J, Banerjee S, Kanwar SS, Yu Y, Patel BB, Sarkar FH, Majumdar AP. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer. 2011;128:951–961. doi: 10.1002/ijc.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park W, Amin ARMR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, Kakarala M, Carpenter PM, McLaren C, Meyskens FL, Brenner DE. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Irving GR, Howells LM, Sale S, Kralj-Hans I, Atkin WS, Clark SK, Britton RG, Jones DJ, Scott EN, Berry DP, Hemingway D, Miller AS, Brown K, Gescher AJ, Steward WP. Prolonged biologically active colonic tissue levels of curcumin achieved after oral administration--a clinical pilot study including assessment of patient acceptability. Cancer Prev Res. 2013;6:119–128. doi: 10.1158/1940-6207.CAPR-12-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim E, Davidson LA, Zoh RS, Hensel ME, Salinas ML, Patil BS, Jayaprakasha GK, Callaway ES, Allred CD, Turner ND, Weeks BR, Chapkin RS. Rapidly cycling Lgr5+ stem cells are exquisitely sensitive to extrinsic dietary factors that modulate colon cancer risk. Cell Death Dis. 2016;7:e2460. doi: 10.1038/cddis.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duriancik DM, Comstock SS, Langohr IM, Fenton JI. High levels of fish oil enhance neutrophil development and activation and influence colon mucus barrier function in a genetically susceptible mouse model. J Nutr Biochem. 2015;26:1261–1272. doi: 10.1016/j.jnutbio.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skarke C, Alamuddin N, Lawson JA, Ferguson JF, Reilly MP, FitzGerald GA. Bioactive products formed in humans from fish oils. J Lipid Res. 2015;56:1808–20. doi: 10.1194/jlr.M060392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. http://www.ncbi.nlm.nih.gov/pubmed/11712783. [PubMed] [Google Scholar]
- 72.Falasca M, Casari I, Maffucci T. Cancer chemoprevention with nuts. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju238. [DOI] [PubMed] [Google Scholar]
- 73.Han X, Shen T, Lou H. Dietary Polyphenols and Their Biological Significance. Int J Mol Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 74.Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–8. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- 75.Amaral JS, Casal S, Pereira JA, Seabra RM, Oliveira BPP. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J Agric Food Chem. 2003;51:7698–702. doi: 10.1021/jf030451d. [DOI] [PubMed] [Google Scholar]
- 76.Kornsteiner M, Wagner K-H, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–387. doi: 10.1016/j.foodchem.2005.07.033. [DOI] [Google Scholar]
- 77.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem. 2004;52:4026–37. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 78.Grace MH, Warlick CW, Neff SA, Lila MA. Efficient preparative isolation and identification of walnut bioactive components using high-speed counter-current chromatography and LC-ESI-IT-TOF-MS. Food Chem. 2014;158:229–38. doi: 10.1016/j.foodchem.2014.02.117. [DOI] [PubMed] [Google Scholar]
- 79.Choy YY, Jaggers GK, Oteiza PI, Waterhouse AL. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J Agric Food Chem. 2013;61:121–7. doi: 10.1021/jf301939e. [DOI] [PubMed] [Google Scholar]
- 80.Verstraeten SV, Fraga CG, Oteiza PI. Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance. Food Funct. 2015;6:32–41. doi: 10.1039/c4fo00647j. [DOI] [PubMed] [Google Scholar]
- 81.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. [accessed July 7, 2015];Am J Clin Nutr. 2005 81:243S–255S. doi: 10.1093/ajcn/81.1.243S. http://www.ncbi.nlm.nih.gov/pubmed/15640487. [DOI] [PubMed] [Google Scholar]
- 82.Verstraeten SV, Jaggers GK, Fraga CG, Oteiza PI. Procyanidins can interact with Caco-2 cell membrane lipid rafts: involvement of cholesterol. Biochim Biophys Acta. 2013;1828:2646–53. doi: 10.1016/j.bbamem.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Erlejman AG, Verstraeten SV, Fraga CG, Oteiza PI. The interaction of flavonoids with membranes: potential determinant of flavonoid antioxidant effects. Free Radic Res. 2004;38:1311–20. doi: 10.1080/10715760400016105. [DOI] [PubMed] [Google Scholar]
- 84.Chapkin RS, DeClercq V, Kim E, Fuentes NR, Fan YY. Mechanisms by Which Pleiotropic Amphiphilic 3 PUFA Reduce Colon Cancer Risk. Curr Color Cancer Rep. 2014;10:442–452. doi: 10.1007/s11888-014-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–6243. doi: 10.4049/jimmunol.181.9.6236. http://www.ncbi.nlm.nih.gov/pubmed/18941214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsukamoto M, Kuroda K, Ramamoorthy A, Yasuhara K. Modulation of raft domains in a lipid bilayer by boundary-active curcumin. Chem Commun. 2014;50:3427–3430. doi: 10.1039/c3cc47738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jerónimo C, Silva BM. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem Toxicol. 2010;48:441–7. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 88.Salimi M, Majd A, Sepahdar Z, Azadmanesh K, Irian S, Ardestaniyan MH, Hedayati MH, Rastkari N. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm Biol. 2012;50:1416–22. doi: 10.3109/13880209.2012.682118. [DOI] [PubMed] [Google Scholar]
- 89.Nagel JM, Brinkoetter M, Magkos F, Liu X, Chamberland JP, Shah S, Zhou J, Blackburn G, Mantzoros CS. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition. 2012;28:67–75. doi: 10.1016/j.nut.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tsoukas MA, Ko B-J, Witte TR, Dincer F, Hardman WE, Mantzoros CS. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J Nutr Biochem. 2015;26:776–83. doi: 10.1016/j.jnutbio.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Nakanishi M, Chen Y, Qendro V, Miyamoto S, Weinstock E, Weinstock GM, Rosenberg DW. Effects of Walnut Consumption on Colon Carcinogenesis and Microbial Community Structure. Cancer Prev Res (Phila) 2016;9:692–703. doi: 10.1158/1940-6207.CAPR-16-0026. [DOI] [PubMed] [Google Scholar]
- 92.Turk HF, Monk JM, Fan YY, Callaway ES, Weeks B, Chapkin RS. Inhibitory effects of omega-3 fatty acids on injury-induced epidermal growth factor receptor transactivation contribute to delayed wound healing. Am J Physiol Cell Physiol. 2013;304:C905-17. doi: 10.1152/ajpcell.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan YY, Ran Q, Toyokuni S, Okazaki Y, Callaway ES, Lupton JR, Chapkin RS. Dietary fish oil promotes colonic apoptosis and mitochondrial proton leak in oxidatively stressed mice. Cancer Prev Res. 2011;4:1267–1274. doi: 10.1158/1940-6207.CAPR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, Kang JX, McMurray DN, Chapkin RS. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–3991. doi: 10.1158/0008-5472.CAN-07-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kolar S, Barhoumi R, Jones CK, Wesley J, Lupton JR, Fan YY, Chapkin RS. Interactive effects of fatty acid and butyrate-induced mitochondrial Ca(2)(+) loading and apoptosis in colonocytes. Cancer. 2011;117:5294–5303. doi: 10.1002/cncr.26205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr. 2015;101:783–93. doi: 10.3945/ajcn.114.099515. [DOI] [PubMed] [Google Scholar]
- 97.Howard AC, McNeil AK, McNeil PL, Faulkner JA, Nguyen L, Reis S. Promotion of plasma membrane repair by vitamin E. Nat Commun. 2011;2:597. doi: 10.1038/ncomms1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leng X, Kinnun JJ, Marquardt D, Ghefli M, Kučerka N, Katsaras J, Atkinson J, Harroun TA, Feller SE, Wassall SR. α-Tocopherol Is Well Designed to Protect Polyunsaturated Phospholipids: MD Simulations. Biophys J. 2015;109:1608–1618. doi: 10.1016/j.bpj.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Atkinson J, Harroun T, Wassall SR, Stillwell W, Katsaras J. The location and behavior of α-tocopherol in membranes. Mol Nutr Food Res. 2010;54:641–651. doi: 10.1002/mnfr.200900439. [DOI] [PubMed] [Google Scholar]
- 100.Wallace TC, Blumberg JB, Johnson EJ, Shao A. Dietary Bioactives : Establishing a Scientific Framework for Recommended Intakes. Adv Nutr. 2015;6:1–4. doi: 10.3945/an.114.007294.evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horejsi V, Hrdinka M. Membrane microdomains in immunoreceptor signaling. FEBS Lett. 2014;588:2392–2397. doi: 10.1016/j.febslet.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 102.Frisz JF, Lou K, Klitzing HA, Hanafin WP, Lizunov V, Wilson RL, Carpenter KJ, Kim R, Hutcheon ID, Zimmerberg J, Weber PK, Kraft ML. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci U S A. 2013;110:E613-22. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kraft ML. Plasma membrane organization and function: moving past lipid rafts. Mol Biol Cell. 2013;24:2765–2768. doi: 10.1091/mbc.E13-03-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science (80- ) 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 106.Levental I, Veatch SL. The Continuing Mystery of Lipid Rafts. J Mol Biol. 2016;428:4749–4764. doi: 10.1016/j.jmb.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blanchard GJ, Busik JV. Interplay between Endothelial Cell Cytoskeletal Rigidity and Plasma Membrane Fluidity. Biophysj. 2017;112:831–833. doi: 10.1016/j.bpj.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jahn KA, Su Y, Braet F. Multifaceted nature of membrane microdomains in colorectal cancer. World J Gastroenterol. 2011;17:681–690. doi: 10.3748/wjg.v17.i6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1105–1107. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Irwin ME, Mueller KL, Bohin N, Ge Y, Boerner JL. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2011;226:2316–2328. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fedida-Metula S, Feldman B, Koshelev V, Levin-Gromiko U, Voronov E, Fishman D. Lipid rafts couple store-operated Ca2+ entry to constitutive activation of PKB/Akt in a Ca2+/calmodulin-, Src- and PP2A-mediated pathway and promote melanoma tumor growth. Carcinogenesis. 2012;33:740–750. doi: 10.1093/carcin/bgs021. [DOI] [PubMed] [Google Scholar]
- 114.George KS, Wu S. Lipid raft: A floating island of death or survival. Toxicol Appl Pharmacol. 2012;259:311–319. doi: 10.1016/j.taap.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hryniewicz-Jankowska A, Augoff K, Biernatowska A, Podkalicka J, Sikorski AF. Membrane rafts as a novel target in cancer therapy. Biochim Biophys Acta. 2014;1845:155–165. doi: 10.1016/j.bbcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- 117.Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B, Counillon L, Gulbins E, Lagadic-Gossmann D, Dimanche-Boitrel MT. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res. 2007;67:7865–7874. doi: 10.1158/0008-5472.CAN-07-0353. [DOI] [PubMed] [Google Scholar]
- 118.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, Weinstein IB. The inhibitory effect of (-)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–6501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 119.Bene L, Bodnar A, Damjanovich S, Vamosi G, Bacso Z, Aradi J, Berta A, Damjanovich J. Membrane topography of HLA I, HLA II, and ICAM-1 is affected by IFN-gamma in lipid rafts of uveal melanomas. Biochem Biophys Res Commun. 2004;322:678–683. doi: 10.1016/j.bbrc.2004.07.171. [DOI] [PubMed] [Google Scholar]
- 120.Barrera NP, Zhou M, Robinson CV. The role of lipids in defining membrane protein interactions: insights from mass spectrometry. Trends Cell Biol. 2013;23:1–8. doi: 10.1016/j.tcb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 121.Lin X, Lorent JH, Skinkle AD, Levental KR, Waxham MN, Gorfe AA, Levental I. Domain Stability in Biomimetic Membranes Driven by Lipid Polyunsaturation. J Phys Chem B. 2016 doi: 10.1021/acs.jpcb.6b06815. acs.jpcb.6b06815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Levental KR, Lorent JH, Lin X, Skinkle AD, Surma MA, Stockenbojer EA, Gorfe AA, Levental I. Polyunsaturated Lipids Regulate Membrane Domain Stability by Tuning Membrane Order. Biophys J. 2016;110:1800–1810. doi: 10.1016/j.bpj.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmick M, Bastiaens PI. The interdependence of membrane shape and cellular signal processing. Cell. 2014;156:1132–1138. doi: 10.1016/j.cell.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K. Nanoclustering as a dominant feature of plasma membrane organization. J Cell Sci. 2014;127:4995–5005. doi: 10.1242/jcs.146340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou Y, Hancock JF. Ras nanoclusters: Versatile lipid-based signaling platforms. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbamcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 126.Ariotti N, Fernandez-Rojo MA, Zhou Y, Hill MM, Rodkey TL, Inder KL, Tanner LB, Wenk MR, Hancock JF, Parton RG. Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. J Cell Biol. 2014;204:777–792. doi: 10.1083/jcb.201307055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou Y, Cho KJ, Plowman SJ, Hancock JF. Nonsteroidal anti-inflammatory drugs alter the spatiotemporal organization of Ras proteins on the plasma membrane. J Biol Chem. 2012;287:16586–16595. doi: 10.1074/jbc.M112.348490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Janosi L, Li Z, Hancock JF, Gorfe AA. Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proc Natl Acad Sci U S A. 2012;109:8097–8102. doi: 10.1073/pnas.1200773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nussinov R, Jang H, Tsai CJ. Oligomerization and nanocluster organization render specificity. Biol Rev Camb Philos Soc. 2014 doi: 10.1111/brv.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res. 1997;38:2012–2022. http://www.ncbi.nlm.nih.gov/pubmed/9374124. [PubMed] [Google Scholar]
- 131.Chapkin RS, Akoh CC, Miller CC. Influence of dietary n-3 fatty acids on macrophage glycerophospholipid molecular species and peptidoleukotriene synthesis. J Lipid Res. 1991;32:1205–1213. http://www.ncbi.nlm.nih.gov/pubmed/1940643. [PubMed] [Google Scholar]
- 132.Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, Wassall SR. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103:228–37. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stillwell W, Wassall SR. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/S0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 134.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: Emerging mediators of inflammation. Prostaglandins Leukot Essent Fat Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chapkin RS, Wang N, Fan YY, Lupton JR, Prior IA. Docosahexaenoic acid alters the size and distribution of cell surface microdomains. Biochim Biophys Acta. 2008;1778:466–471. doi: 10.1016/j.bbamem.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 137.Ma DW, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem. 2004;15:700–706. doi: 10.1016/j.jnutbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Seo J, Barhoumi R, Johnson AE, Lupton JR, Chapkin RS. Docosahexaenoic acid selectively inhibits plasma membrane targeting of lipidated proteins. FASEB J. 2006;20:770–772. doi: 10.1096/fj.05-4683fje. [DOI] [PubMed] [Google Scholar]
- 139.Barry J, Fritz M, Brender JR, Smith PES, Lee D. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy. The Case of the Antioxidant Curcumin. 2009:4490–4498. doi: 10.1021/ja809217u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Filippov AV, Kotenkov SA, Munavirov B, Antzutkin ON. Effect of Curcumin on Lateral Diffusion of Phosphatidylcholines in Saturated and Unsaturated Bilayers. Langmuir. 2014;30:10686–1690. doi: 10.1021/la502338c. [DOI] [PubMed] [Google Scholar]
- 141.Lin ML, Lu YC, Chen HY, Lee CC, Chung JG, Chen SS. Suppressing the formation of lipid raft-associated Rac1/PI3K/Akt signaling complexes by curcumin inhibits SDF-1alpha-induced invasion of human esophageal carcinoma cells. Mol Carcinog. 2014;53:360–379. doi: 10.1002/mc.21984. [DOI] [PubMed] [Google Scholar]
- 142.Tsukamoto M, Kuroda K, Ramamoorthy A, Yasuhara K. Modulation of raft domains in a lipid bilayer by boundary-active curcumin. Chem Commun (Camb) 2014;50:3427–30. doi: 10.1039/c3cc47738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barnoud J, Rossi G, Marrink SJ, Monticelli L. Hydrophobic Compounds Reshape Membrane Domains. PLoS Comput Biol. 2014;10:e1003873. doi: 10.1371/journal.pcbi.1003873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fu Y, Zhou E, Wei Z, Song X, Liu Z, Wang T, Wang W, Zhang N, Liu G, Yang Z. Glycyrrhizin inhibits lipopolysaccharide-induced inflammatory response by reducing TLR4 recruitment into lipid rafts in RAW264.7 cells. Biochim Biophys Acta. 2014;1840:1755–1764. doi: 10.1016/j.bbagen.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 145.Lundbaek JA, Birn P, Tape SE, Toombes GE, Sogaard R, Koeppe RE, 2nd, Gruner SM, Hansen AJ, Andersen OS. Capsaicin regulates voltage-dependent sodium channels by altering lipid bilayer elasticity. Mol Pharmacol. 2005;68:680–689. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 146.Hung W-C, Chen F-Y, Lee C-C, Sun Y, Lee M-T, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–4338. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ingolfsson HI, Koeppe RE, Andersen OS. Curcumin is a Modulator of Bilayer Material Properties†. Biochemistry. 2007;46:10384–10391. doi: 10.1021/bi701013n. [DOI] [PubMed] [Google Scholar]
- 148.Soung YH, Chung J. Curcumin inhibition of the functional interaction between integrin α6β4 and the epidermal growth factor receptor. Mol Cancer Ther. 2011;10:883–91. doi: 10.1158/1535-7163.MCT-10-1053. [DOI] [PubMed] [Google Scholar]
- 149.Sezgin E, Sadowski T, Simons K. Measuring Lipid Packing of Model and Cellular Membranes with Environment Sensitive Probes. Langmuir. 2014;30:8160–8166. doi: 10.1021/la501226v. [DOI] [PubMed] [Google Scholar]
- 150.Sezgin E, Waithe D, Bernardino de la Serna J, Eggeling C. Spectral Imaging to Measure Heterogeneity in Membrane Lipid Packing. ChemPhysChem. 2015;16:1387–1394. doi: 10.1002/cphc.201402794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc. 2012;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- 152.Amaro M, Reina F, Hof M, Eggeling C, Sezgin E. Laurdan and Di-4-ANEPPDHQ probe different properties of the membrane. J Phys D Appl Phys. 2017;50:134004. doi: 10.1088/1361-6463/aa5dbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.D’Abaco GM, Whitehead RH, Burgess AW. Synergy between Apc min and an activated ras mutation is sufficient to induce colon carcinomas. [accessed December 5, 2016];Mol Cell Biol. 1996 16:884–91. doi: 10.1128/mcb.16.3.884. http://www.ncbi.nlm.nih.gov/pubmed/8622690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith G, Carey FA, Beattie J, Wilkie MJV, Lightfoot TJ, Coxhead J, Garner RC, Steele RJC, Wolf CR. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci. 2002;99:9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bohdanowicz M, Grinstein S. Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev. 2013;93:69–106. doi: 10.1152/physrev.00002.2012. [DOI] [PubMed] [Google Scholar]
- 157.Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–43. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- 158.Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD. Human Pancreatic Cancer Tumors Are Nutrient Poor and Tumor Cells Actively Scavenge Extracellular Protein. Cancer Res. 2015;75:544–553. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: Mission Possible? Nat Rev Drug Discov. 2014;13:828–51. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zwartkruis FJT, Burgering BMT. Ras and macropinocytosis: trick and treat. Cell Res. 2013;23:982–983. doi: 10.1038/cr.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sezgin E, Kaiser H-J, Baumgart T, Schwille P, Simons K, Levental I. Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc. 2012;7:1042–51. doi: 10.1038/nprot.2012.059. [DOI] [PubMed] [Google Scholar]
- 162.Lee I-H, Saha S, Polley A, Huang H, Mayor S, Rao M, Groves JT. Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J Phys Chem B. 2015;119:4450–9. doi: 10.1021/jp512839q. [DOI] [PubMed] [Google Scholar]
- 163.Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. [accessed March 13, 2017];Am J Clin Nutr. 2002 76:798–804. doi: 10.1093/ajcn/76.4.798. http://www.ncbi.nlm.nih.gov/pubmed/12324293. [DOI] [PubMed] [Google Scholar]
- 164.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. [accessed March 13, 2017];J Nutr. 2000 130:2073S–85S. doi: 10.1093/jn/130.8.2073S. http://www.ncbi.nlm.nih.gov/pubmed/10917926. [DOI] [PubMed] [Google Scholar]
- 165.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. [accessed March 13, 2017];Am J Clin Nutr. 2005 81:230S–242S. doi: 10.1093/ajcn/81.1.230S. http://www.ncbi.nlm.nih.gov/pubmed/15640486. [DOI] [PubMed] [Google Scholar]
- 166.Suganuma M, Takahashi A, Watanabe T, Iida K, Matsuzaki T, Yoshikawa H, Fujiki H. Biophysical Approach to Mechanisms of Cancer Prevention and Treatment with Green Tea Catechins. Molecules. 2016;21:1566. doi: 10.3390/molecules21111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–439. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang C, Wang H. Cancer Preventive Activities of Tea Catechins. Molecules. 2016;21:1679. doi: 10.3390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Auger C, Mullen W, Hara Y, Crozier A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. [accessed March 13, 2017];J Nutr. 2008 138:1535S–1542S. doi: 10.1093/jn/138.8.1535S. http://www.ncbi.nlm.nih.gov/pubmed/18641203. [DOI] [PubMed] [Google Scholar]
- 170.Tsang C, Auger C, Mullen W, Bornet A, Rouanet J-M, Crozier A, Teissedre P-L. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. [accessed March 13, 2017];Br J Nutr. 2005 94:170–81. doi: 10.1079/bjn20051480. http://www.ncbi.nlm.nih.gov/pubmed/16115350. [DOI] [PubMed] [Google Scholar]
- 171.YANG C, LAMBERT J, JU J, LU G, SANG S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–273. doi: 10.1016/j.taap.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lambert JD, Lee M-J, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. DOSE-DEPENDENT LEVELS OF EPIGALLOCATECHIN-3-GALLATE IN HUMAN COLON CANCER CELLS AND MOUSE PLASMA AND TISSUES. Drug Metab Dispos. 2005;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 173.Podkalicka J, Biernatowska A, Majkowski M, Grzybek M, Sikorski AF. MPP1 as a Factor Regulating Phase Separation in Giant Plasma Membrane-Derived Vesicles. Biophys J. 2015;108:2201–11. doi: 10.1016/j.bpj.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Biernatowska A, Podkalicka J, Majkowski M, Hryniewicz-Jankowska A, Augoff K, Kozak K, Korzeniewski J, Sikorski AF. The role of MPP1/p55 and its palmitoylation in resting state raft organization in HEL cells. Biochim Biophys Acta - Mol Cell Res. 2013;1833:1876–1884. doi: 10.1016/j.bbamcr.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 175.Commisso C, Flinn RJ, Bar-Sagi D. Determining the macropinocytic index of cells through a quantitative image-based assay. Nat Protoc. 2014;9:182–192. doi: 10.1038/nprot.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang Y, Gao J, Guo X, Tong T, Shi X, Li L, Qi M, Wang Y, Cai M, Jiang J, Xu C, Ji H, Wang H. Regulation of EGFR nanocluster formation by ionic protein-lipid interaction. Cell Res. 2014;24:959–76. doi: 10.1038/cr.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Arteaga CL, Engelman JA. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell. 2014;25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 179.Rogers KR, Kikawa KD, Mouradian M, Hernandez K, McKinnon KM, Ahwah SM, Pardini RS. Docosahexaenoic acid alters epidermal growth factor receptor-related signaling by disrupting its lipid raft association. Carcinogenesis. 2010;31:1523–1530. doi: 10.1093/carcin/bgq111. [DOI] [PubMed] [Google Scholar]
- 180.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553. doi: 10.1093/jn/137.3.548. http://www.ncbi.nlm.nih.gov/pubmed/17311938. [DOI] [PubMed] [Google Scholar]
- 181.Turk HF, Barhoumi R, Chapkin RS. Alteration of EGFR spatiotemporal dynamics suppresses signal transduction. PLoS One. 2012;7:e39682. doi: 10.1371/journal.pone.0039682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gawrisch K, Soubias O, Mihailescu M. Insights from biophysical studies on the role of polyunsaturated fatty acids for function of G-protein coupled membrane receptors. Prostaglandins Leukot Essent Fat Acids. 2008;79:131–134. doi: 10.1016/j.plefa.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pollock AH, Tedla N, Hancock SE, Cornely R, Mitchell TW, Yang Z, Kockx M, Parton RG, Rossy J, Gaus K. Prolonged Intake of Dietary Lipids Alters Membrane Structure and T Cell Responses in LDLr-/- Mice. J Immunol. 2016;196:3993–4002. doi: 10.4049/jimmunol.1501261. [DOI] [PubMed] [Google Scholar]
- 184.Soung YH, Chung J. Curcumin inhibition of the functional interaction between integrin alpha6beta4 and the epidermal growth factor receptor. Mol Cancer Ther. 2011;10:883–891. doi: 10.1158/1535-7163.MCT-10-1053. [DOI] [PubMed] [Google Scholar]
- 185.Hung WC, Chen FY, Lee CC, Sun Y, Lee MT, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–4338. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shaikh SR, Kinnun JJ, Leng X, Williams Ja, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim Biophys Acta. 2014;1848:1–9. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 187.Shaikh SR, Locascio DS, Soni SP, Wassall SR, Stillwell W. Oleic- and docosahexaenoic acid-containing phosphatidylethanolamines differentially phase separate from sphingomyelin. Biochim Biophys Acta. 2009;1788:2421–2426. doi: 10.1016/j.bbamem.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shaikh SR, Dumaual AC, Castillo A, LoCascio D, Siddiqui RA, Stillwell W, Wassall SR. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study. Biophys J. 2004;87:1752–1766. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 190.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science (80- ) 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. http://www.ncbi.nlm.nih.gov/pubmed/9065402. [DOI] [PubMed] [Google Scholar]
- 191.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science (80- ) 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. http://www.ncbi.nlm.nih.gov/pubmed/9065401. [DOI] [PubMed] [Google Scholar]
- 192.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, Christensen E, Schmidt SS, Roche PC, Smith DI, Thibodeau SN. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 193.Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 194.Ozhan G, Sezgin E, Wehner D, Pfister AS, Kuhl SJ, Kagermeier-Schenk B, Kuhl M, Schwille P, Weidinger G. Lypd6 enhances Wnt/beta-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev Cell. 2013;26:331–345. doi: 10.1016/j.devcel.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 195.Escriba PV, Busquets X, Inokuchi J, Balogh G, Torok Z, Horvath I, Harwood JL, Vigh L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res. 2015;59:38–53. doi: 10.1016/j.plipres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 196.Sakane H, Yamamoto H, Kikuchi A. LRP6 is internalized by Dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J Cell Sci. 2010;123:360–368. doi: 10.1242/jcs.058008. [DOI] [PubMed] [Google Scholar]
- 197.Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 198.Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 199.Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169:1355–1362. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]