Abstract
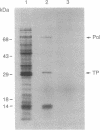
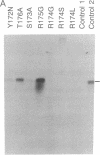
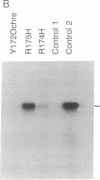
A group of proteins that act as primers for initiation of linear DNA replication are called DNA-terminal proteins (terminal proteins). We have found a short stretch of conserved amino acid sequence among the terminal proteins from six different sources. The location of this sequence motif is also similar among the different terminal-proteins. To determine the functional role of this terminal-protein domain in DNA replication, we have studied the bacteriophage PRD1 system. The PRD1 terminal protein and DNA polymerase genes were cloned into expression vectors, and the recombinant plasmids were used for constructing PRD1 terminal protein mutants. Site-directed mutagenesis and functional analysis showed that one of the two arginines (Arg-174) in the conserved sequence is critical for the initiation complex-forming activity of the PRD1 terminal protein. Replacement of Arg-174 by noncharged amino acids resulted in nonfunctional terminal protein. Phenylglyoxal, an alpha-dicarbonyl compound that reacts with the guanidino group of arginine, inhibits initiation complex formation between PRD1 terminal protein and dGMP. On the basis of these results, we propose that Arg-174 represents, at least in part, the binding site for phosphate groups of dGTP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Mindich L. Characterization of the DNA-protein complex at the termini of the bacteriophage PRD1 genome. J Virol. 1984 May;50(2):309–315. doi: 10.1128/jvi.50.2.309-315.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R., Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 1988 Dec 20;7(13):4185–4192. doi: 10.1002/j.1460-2075.1988.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghäuser J. A reactive arginine in adenylate kinase. Biochim Biophys Acta. 1975 Aug 26;397(2):370–376. doi: 10.1016/0005-2744(75)90126-6. [DOI] [PubMed] [Google Scholar]
- Berghäuser J., Schirmer R. H. Properties of adenylate kinase after modification of Arg-97 by phenylglyoxal. Biochim Biophys Acta. 1978 Dec 20;537(2):428–435. doi: 10.1016/0005-2795(78)90527-5. [DOI] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borders C. L., Jr, Riordan J. F. An essential arginyl residue at the nucleotide binding site of creatine kinase. Biochemistry. 1975 Oct 21;14(21):4699–4704. doi: 10.1021/bi00692a021. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman B. S., Chiu N. Y. Yeast inorganic pyrophosphatase. 3. Active-site mapping by electrophilic reagents and binding measurements. Biochemistry. 1973 Apr 24;12(9):1676–1682. doi: 10.1021/bi00733a003. [DOI] [PubMed] [Google Scholar]
- Daemen F. J., Riordan J. F. Essential arginyl residues in Escherichia coli alkaline phosphatase. Biochemistry. 1974 Jul 2;13(14):2865–2871. doi: 10.1021/bi00711a014. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- García P., Hermoso J. M., García J. A., García E., López R., Salas M. Formation of a covalent complex between the terminal protein of pneumococcal bacteriophage Cp-1 and 5'-dAMP. J Virol. 1986 Apr;58(1):31–35. doi: 10.1128/jvi.58.1.31-35.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Hanks S. K. Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family. Proc Natl Acad Sci U S A. 1987 Jan;84(2):388–392. doi: 10.1073/pnas.84.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso J. M., Salas M. Protein p3 is linked to the DNA of phage phi 29 through a phosphoester bond between serine and 5'-dAMP. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6425–6428. doi: 10.1073/pnas.77.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
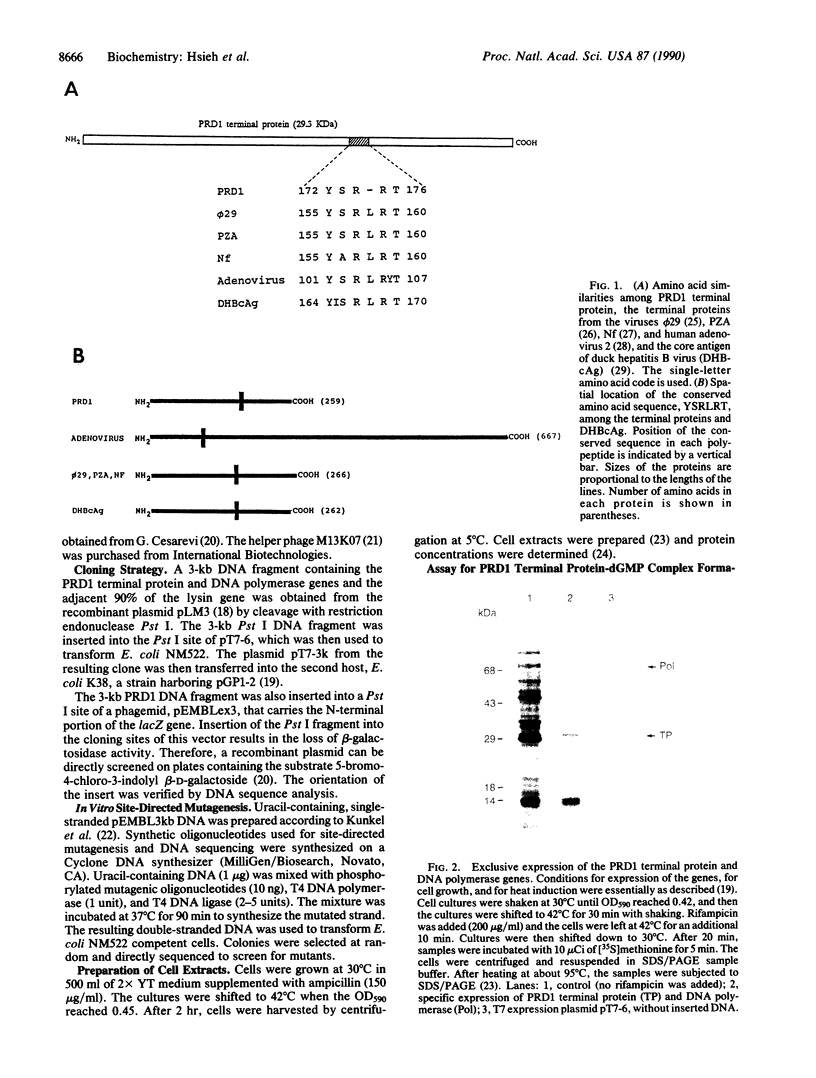
- Hsieh J. C., Jung G. H., Leavitt M. C., Ito J. Primary structure of the DNA terminal protein of bacteriophage PRD1. Nucleic Acids Res. 1987 Nov 11;15(21):8999–9009. doi: 10.1093/nar/15.21.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G. H., Leavitt M. C., Hsieh J. C., Ito J. Bacteriophage PRD1 DNA polymerase: evolution of DNA polymerases. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8287–8291. doi: 10.1073/pnas.84.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Leavitt M. C., Ito J. Nucleotide sequence of Bacillus phage Nf terminal protein gene. Nucleic Acids Res. 1987 Jul 10;15(13):5251–5259. doi: 10.1093/nar/15.13.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Mindich L., McGraw T. Molecular cloning of bacteriophage PRD1 genomic fragments. Mol Gen Genet. 1983;190(2):233–236. doi: 10.1007/BF00330645. [DOI] [PubMed] [Google Scholar]
- Paces V., Vlcek C., Urbánek P., Hostomský Z. Nucleotide sequence of the major early region of Bacillus subtilis phage PZA, a close relative of phi 29. Gene. 1985;38(1-3):45–56. doi: 10.1016/0378-1119(85)90202-1. [DOI] [PubMed] [Google Scholar]
- Powers S. G., Riordan J. F. Functional arginyl residues as ATP binding sites of glutamine synthetase and carbamyl phosphate synthetase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2616–2620. doi: 10.1073/pnas.72.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Conservation of short patches of amino acid sequence amongst proteins with a common function but evolutionarily distinct origins: implications for cloning genes and for structure-function analysis. Nucleic Acids Res. 1988 Sep 26;16(18):9017–9026. doi: 10.1093/nar/16.18.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstein J., Gilles A. M., Rose T., Wittinghofer A., Saint Girons I., Bârzu O., Surewicz W. K., Mantsch H. H. Structural and catalytic role of arginine 88 in Escherichia coli adenylate kinase as evidenced by chemical modification and site-directed mutagenesis. J Biol Chem. 1989 May 15;264(14):8107–8112. [PubMed] [Google Scholar]
- Savilahti H., Bamford D. H. The complete nucleotide sequence of the left very early region of Escherichia coli bacteriophage PRD1 coding for the terminal protein and the DNA polymerase. Gene. 1987;57(1):121–130. doi: 10.1016/0378-1119(87)90183-1. [DOI] [PubMed] [Google Scholar]
- Sollazzo M., Frank R., Cesareni G. High-level expression of RNAs and proteins: the use of oligonucleotides for the precise fusion of coding-to-regulatory sequences. Gene. 1985;37(1-3):199–206. doi: 10.1016/0378-1119(85)90273-2. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Kuhn C., Will H., Schaller H. Comparative sequence analysis of duck and human hepatitis B virus genomes. J Med Virol. 1985 Apr;15(4):323–333. doi: 10.1002/jmv.1890150402. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F. Adenoviral DNA replication: DNA sequences and enzymes required for initiation in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):741–750. doi: 10.1101/sqb.1983.047.01.085. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. The reaction of phenylglyoxal with arginine residues in proteins. J Biol Chem. 1968 Dec 10;243(23):6171–6179. [PubMed] [Google Scholar]
- Viale A. M., Vallejos R. H. Identification of an essential arginine residue in the beta subunit of the chloroplast ATPase. J Biol Chem. 1985 Apr 25;260(8):4958–4962. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Watabe K., Leusch M., Ito J. Replication of bacteriophage phi 29 DNA in vitro: the roles of terminal protein and DNA polymerase. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5374–5378. doi: 10.1073/pnas.81.17.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe K., Shin M., Ito J. Protein-primed initiation of phage phi 29 DNA replication. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4248–4252. doi: 10.1073/pnas.80.14.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H., Ito J. Nucleotide sequence of the major early region of bacteriophage phi 29. Gene. 1982 Mar;17(3):323–335. doi: 10.1016/0378-1119(82)90149-4. [DOI] [PubMed] [Google Scholar]
- Yudkin J. Sugar and disease. Nature. 1972 Sep 22;239(5369):197–199. doi: 10.1038/239197a0. [DOI] [PubMed] [Google Scholar]