Abstract
The recent global (re)emergence of arthropod-borne viruses (arboviruses), such as chikungunya and Zika virus, was widely reported in the media as though it was a new phenomenon. This is not the case. Arboviruses and other human microbial pathogens have been (re)emerging for centuries. The major difference today is that arbovirus emergence and dispersion are more rapid and geographically extensive, largely due to intensive growth of global transportation systems, arthropod adaptation to increasing urbanisation, our failure to contain mosquito population density increases and land perturbation. Here we select examples of (re)emerging pathogenic arboviruses and explain the reasons for their emergence and different patterns of dispersal, focusing particularly on the mosquito vectors which are important determinants of arbovirus emergence. We also attempt to identify arboviruses likely to (re)emerge in the future.
Keywords: Emerging arboviruses,; Arthropods,; Mosquitoes,; Evolution,; Anthropology,; Dispersal,; Global distribution
1. Introduction
Five human epidemic mosquito-borne arboviruses, yellow fever virus (YFV), dengue virus (DENV), West Nile virus (WNV), chikungunya virus (CHIKV) and Zika virus (ZIKV), have emerged in both hemispheres during recent centuries. Other mosquito-borne arboviruses have emerged in specific regions of the world but not, as yet, in both hemispheres [1], [2]. These include Japanese encephalitis virus (JEV), St Louis encephalitis virus (SLEV), Murray Valley encephalitis virus (MVEV), Usutu virus (USUV), Spondweni virus (SPOV), O'nyong nyong virus (ONNV) [3], [4] and Rift Valley fever virus (RVFV). Whilst many determinants of arbovirus emergence and dispersal have an anthropological basis, the role of the arthropod vectors and their feeding preferences (anthropophilic and/or ornithopophilic) in global or local geographic expansion, are of paramount importance (1, 5–7).
In August 1999, reports of fatal encephalitis in horses, birds and humans heralded the emergence of a well-known Old World (OW) arbovirus, West Nile virus, in New York [8], [9], [10]. Within five years, WNV was endemic throughout North America, southern Canada, the Caribbean and South America [11], [12]. All phylogenetic evidence suggests that WNV was introduced only once, implying that the introduction was anthropologically based rather than natural dispersal via migratory birds which would have almost certainly occurred more than once [1]. Nevertheless, once the virus had been introduced into the New York area of the US, migratory birds played a major role in the epidemiology and dispersal of WNV throughout North America [11], [13].
In 2005 CHIKV re-emerged on the southern Indian Ocean island of La Réunion and neighbouring islands and rapidly dispersed to India [14], [15] becoming known as the Indian Ocean lineage (IOL). Eight years later an Asian lineage emerged and rapidly dispersed across islands in the Pacific Ocean reaching St Martin in late 2013 [16], [17], [18]. Following its arrival in the Caribbean, epidemic chikungunya fever with associated long-term arthralgic sequelae, dispersed throughout tropical Latin America. This was not the first time CHIKV had visited the Americas [19]. As will be described in detail below clinical records of patients in some epidemics in North America, presumed to have been caused by dengue virus, are more consistent with infection by CHIKV introduced from Africa on the ships trading with the Americas.
In 2007 the African ZIKV that had dispersed to and circulated in Malaysia, ~ 60 years earlier, emerged in the Yap Federated States of Micronesia infecting ~ 73% of the population [20] but did not disperse beyond Micronesia. However, a subsequent Asian lineage proved to be the common ancestor of the French Polynesian (FP) 2013 isolates. By early 2015 the descendant lineage of these viruses was isolated in North East Brazil and rapidly dispersed throughout tropical Latin America [20].
These pandemics were not unprecedented. Many arboviruses are zoonotic, infecting a wide variety of arthropods, other animals including birds in their sylvatic habitats and humans as incidental hosts. Over many years, arboviruses have evolved balanced relationships with these sylvatic hosts. Thus, morbidity and mortality is rarely seen in these sylvatic animals when they are infected by arboviruses. For example YFV rarely kills non-human primates (NHP) in African forests [7], [21]. In contrast, because human infections by sylvan arboviruses are generally rare, these balanced relationships have not been established in humans and consequently they show significant morbidity and mortality following infection by sylvan arboviruses [22]. However, with the exception of epidemic arboviruses such as DENV, ZIKV and CHIKV, human infections are generally not essential to maintain the arbovirus life cycle as they are usually dead-end hosts for arboviruses [23].
As a result of the impact of increases in population density through urbanisation and the development of global transportation systems the exposure frequency of humans to mosquitoes and the global mobility of humans have significantly increased. Accordingly, the patterns of virus-vector-host interactions have changed during recent centuries. In parallel, agricultural capacity, animal husbandry and widespread deforestation have intensified in response to the demands of industrial development, urbanisation and rising human population densities [11], [24], [25], [26]. In fact, humans have been increasingly encroaching on wild habitats which previously were a sanctuary for arthropods to interact and evolve with wild animals and plants. Consequently, contemporary arthropods are frequently exposed to the modern human environment and domestic animals and livestock to which they rapidly adapt. This adaptive process is defined as domestication [27] and is discussed below.
In 2001 phylogenetic and phylogeographic analyses of flaviviruses illustrated how tree topology and virus-vector-host association reflect the feeding preferences of different arthropod species [5]. Here, these types of investigations are used to compare and contrast the emergence characteristics of arboviruses with different vector preferences to illustrate the role of arthropods and anthropological activities in arbovirus emergence and geographic dispersal.
2. Emergence and global dispersal of selected Aedes-associated arboviruses
Aedes species mosquitoes may breed in tree holes or other forms of vegetation, and also in man-made environments including houses, plant pots, discarded car tyres, bottles, cans, etc. Depending on the local habitat, they feed primarily on available non-human mammals [27], [28], [29]. Ae aegypti evolved from the sylvan African Ae formosus becoming an anthropophilic species that breeds in domestic (urban) and peridomestic environments and feeds primarily on humans [30]. These urban/peridomestic mosquitoes are recognised as the domestic version of Ae aegypti. They have shaped the epidemiological history of some emergent arboviruses during recent centuries.
2.1. Yellow fever virus
The human disease yellow fever has been known in Africa for centuries. In the Central and West African forests and the surrounding savannah YFV is transmitted between NHP and a range of arboreal mosquito species such as Ae formosus, Ae africanus, Ae simpsoni where they co-habit the forest canopy and/or overlap in the surrounding savannah. The NHP involved in the sylvatic cycle rarely develop fatal disease [21] [1], [31]. Human yellow fever epidemics in Africa arise irregularly when the chain of virus transmission from the forest involves peridomestic Aedes species (Ae simpsoni, Ae aegypti) on the fringes of the forest. These mosquitoes, biting both humans and NHP, can sustain small-scale epidemics in rural and peridomestic regions which may overlap with anthropophilic and fully domesticated Ae aegypti that prefer densely populated urban areas. Thus, the transmission pattern changes from a forest or jungle cycle to an urban cycle characterised by rapid human to mosquito to human transmission [7], [21], [32]. Yellow fever epidemics may spread quickly in densely populated urban areas, as reported recently in Angola and the Republic of Congo [33].
In the New World (NW), yellow fever was first reported on the Caribbean islands of Barbados and Guadeloupe in 1647. Initially, it was known as Yellow Jack fever [34]. After decimating the crews of sailing ships and civilian populations in various ports on the east coast of North America, the disease appeared in New York in 1668. Throughout the 17th to 19th centuries, deaths due to yellow fever played a major role in determining the fate of geopolitical conflicts in the Americas, with larger numbers of fatalities amongst British and French troops, than numbers killed in battle. Subsequently, in 1889, the impact on the construction of the Panama canal was so severe that the project was halted pending the implementation of mosquito control programmes [35], [36], [37].
With the knowledge of hindsight, the cause of these epidemics had remained unknown until in 1848 the physician Josiah Nott observed that places not visited by steamboats had been uniformly exempt from the disease [38] and this was the first record that implicated ships as the potential source of yellow fever [39]. Nott also suggested mosquitoes as the possible source of the disease and subsequently Carlos Finlay convinced the American authorities that eradication of the disease might be achievable by eliminating Ae aegypti. Supporting evidence for this theory was obtained in experiments on human volunteers who allowed YFV-infected mosquitoes to feed on them [40]. Based on this evidence mosquito control programmes, led by William Gorgas, were carried out in Cuba and the region where the Panama Canal was being developed. These programmes resulted in reduction of disease incidence in Cuba and along the Panama Canal.
The slave ships also carried fare-paying passengers returning to Europe from the Americas and many of them died from yellow fever during the journey to Europe. In 1819, 2200 human deaths due to YFV infection were reported in Cadiz, Spain [41]. This epidemic was followed by outbreaks in Swansea in 1865 (South Wales, United Kingdom) and St Nazaire (France), [42], [43]. There are many other such records [44].
Thus, the historical records implied that yellow fever virus was introduced into the NW primarily via the slave trade which occurred from the 15th to the early 20th century. These assumptions were tested using molecular epidemiological studies in the 21st century. The results indicate that the ancestral lineage of YFV originated in Africa between 2000 and 4000 years ago. Moreover, South American YFV separated from African YFV ~ 200–400 years ago. Thus, the molecular epidemiological data support the original concept that YFV was introduced to the Americas during the slave trading period [1], [31], [45], [46].
Recent evidence based on DNA sequencing and large-scale single nucleotide polymorphisms (SNP) of Ae aegypti supports the above evidence that populations of these mosquitoes in the NW are derived directly from African populations. Thus, African Ae aegypti species accompanied humans on the ships and were the primary vector in the NW of YFV and, as will be discussed below, DENV and CHIKV. Following their introduction from Africa the genetic data illustrate that Ae aegypti-gradually continued to disperse from the Americas, on ships trading across the Pacific Ocean to Asia and Australia [18], [27], [47], [48]. Ae. aegypti colonisation of Asia was estimated to have occurred in the late 19th century when dengue fever was first reported in Asia. In urban settings this coincided with the arrival of the only urban dengue vector Ae. aegypti [49]. Nowadays, the evidence that Ae aegypti was carried on the slave ships to the Americas and onwards across the Pacific Ocean appears indisputable. However, the suggestion that Ae aegypti did not expand eastwards Out of Africa [27] seems inconsistent with the records of the eastward spread of African DENV and CHIKV in Asia during the early 19th century (see further comments below).
In summary, Ae aegypti originated in Africa and then colonized the Americas, Oceania and the Asian tropics via trading during the 17th to 19th centuries [18], [27], [47], [48], [50]. This redistribution of Ae aegypti coincided with the appearance of yellow fever and dengue fever in the Americas and dengue fever in Asia [1], [32], [49], [51]. More recently Ae aegypti has played a major role in the emergence of the global pandemics caused by CHIKV and ZIKV [52], [53], [54]. Recently, the adaptation of Ae. aegypti to breeding in peri-domestic and domestic environments where they have the tendency to enter houses, attracted by human odour [55], feeding primarily on humans [30], [56] and laying eggs that survive in nutrient poor water, has been referred to as human commensalism [27]. Thus, adaptation to the urban environment has been paramount in the emergence of Ae aegypti-associated arboviruses.
2.2. Dengue virus
The first disease outbreaks in the Americas likely to have been caused by DENV were recorded in the French West Indies in 1635 and Panama in 1699 [57], [58]. Subsequently, dengue fever reached epidemic proportions in cities on the east coast of North America such as Philadelphia [59] always coinciding with the arrival of slave ships, carrying the domesticated form of Ae aegypti [27], [32]. Thus, as with YFV, the first DENV that emerged in the Americas was of African origin. However, DENV and YFV exhibit important differences in the Americas. Although neotropical wild mammals can be infected with DENV the virus does not appear to have established a sylvatic existence in the NW forests. Moreover there is no evidence of spillover to humans [60] and infectious DENV has only been demonstrated to circulate amongst humans and Aedes species mosquitoes. In contrast YFV established a sylvatic forest existence which involves infection of the local NHP and sylvan mosquito species Haemagogus and Sabethes. In the case of YFV the sylvatic form may spill over to humans as is currently being witnessed in Brazil and neighbouring countries [61]. This explains why, unlike DENV, YFV is not dependent on Ae aegypti for long-term survival in South America.
The primary arthropod vector of DENV in urban environments is Ae aegypti. However, DENV is also transmissible by Ae albopictus which attains high densities in suburban, rural, and sylvatic/forest areas and is now present in all tropical/sub-tropical and warmer temperate regions of the world. However, Ae. albopictus tends to be absent from densely crowded cities which are low in vegetation and outdoor breeding sites [62], [63]. Dengue virus causes between 60 and 140 million clinically apparent cases of dengue fever/haemorrhagic fever/shock syndrome annually [64] throughout all tropical and warmer temperate regions. This contrasts with YFV which is endemic solely in Africa and Latin America and since the cessation of the slave trade causes urban epidemics only sporadically in the Americas. Thus Ae albopictus appears to be an important ancilliary vector of DENV and a major contributor to its wider global distribution and greater epidemiological importance when compared with YFV. Indeed, it could be argued that the poorer competence of Ae albopictus for YFV when compared with DENV could be an important reason why YFV has never managed to invade Asia.
2.3. Chikungunya virus
Although CHIKV is an alphavirus, it shares epidemiological, ecological and biogeographical features with the Ae aegypti-associated flaviviruses YFV, DENV and ZIKV, including dependence on sylvan Aedes in the forest cycle and domestic Aedes in the human epidemic transmission cycle [11], [17]. During the 17th to 19th centuries, dengue and chikungunya fever were often mis-diagnosed [19], [65], [66] despite the fact that the clinical symptoms are distinguishable on the basis of differences in disease onset and sequelae following recovery from the acute infection as summarised in detail [65], [66]. Chikungunya infection does not result in premonitory symptoms. Onset is sudden, often including pain in the palm of the hands and soles of the feet associated with movement and accompanied by stiffness in the muscles. Following recovery from acute infection, painful and debilitating polyarthritic-type sequelae that may last for months are experienced. In contrast dengue fever presents with a variety of premonitory symptoms, including some or all of the following, fever, sore throat, severe pains in the back of the head or eyeballs, nausea, vomiting, disagreeable taste in the mouth followed by rash on day 3 or 4 post-onset. Recovery from acute dengue fever usually occurs 7 to 10 days after onset with no long-term articular sequelae. Consequently, some of the epidemics that occurred in the Americas and in Africa during the 17th to 19th centuries clinically diagnosed as dengue fever were probably caused by CHIKV [19], [65], [66], [67]. Halstead states “Christie explicitly linked the 1827–1828 epidemic of “kidinga pepo” in the Americas to the 1823 epidemic in Zanzibar”. The original evidence that CHIKV was probably causing epidemics in the Americas at this time was based on the clinical picture which as illustrated above differs significantly from that of dengue fever. Secondly, epidemics clinically identified as “kidinga pepo”, now recognised as chikungunya fever, originating in eastern Africa and Zanzibar had crossed the Indian Ocean at roughly 40 to 50-year intervals from 1770 through to 2005–2014. The earliest outbreaks coincided with the appearance of CHIKV-like epidemics in the Americas [66]. Halstead noted that the two most recent periodic outbreaks of chikungunya fever in 1963–1964 and 2005–2014 were confirmed by the isolation and identification of CHIKV [19]. Thus, the 1963–1964 epidemic confirmed the pronounced clinical differences between syndromes caused by DENV and CHIKV supporting the original diagnostic criteria. Based on this evidence and the coincidence of epidemics of YFV and DENV/CHIKV appearing in New World cities each time a slave ship arrived, it is clear that CHIKV was also introduced into the Americas via ships trading from Africa across the Atlantic Ocean. It is also important to note that during this period DENV and CHIKV epidemics originating on the east coast of Africa also dispersed to India and Australasia [66].
These observations imply that Ae. aegypti and possibly also Ae. albopictus, in Asia, must have been associated with DENV and CHIKV epidemics that dispersed to Asia out of east Africa. The coincident appearance in the Americas of DENV and CHIKV epidemics confirms that they were also transported to that region of the world at the same time [66]. However, the concept of an eastward dispersal of Ae aegypti from Africa to Australasia appears to contradict the contention [27] that there was no evidence of Ae aegypti dispersing eastwards out of Africa. It is currently believed that Ae. aegypti which appeared for the first time in Asia towards the end of the 19th century [49] were derived from the NW having crossed the Pacific Ocean via the ships that traded across Oceania [18], [27], [47], [48], [50]. Thus, the possibility of bi-directional dispersal of Ae aegypti, i.e. out of Africa both westwards and eastwards and westwards out of the Americas, and consequent geographic overlap in Asia should not be overruled without further analysis.
Following the isolation and characterisation of CHIKV in Africa [68], [69], sporadic spillover infections of human outbreaks of sylvan chikungunya fever continued to be recorded in Africa but when CHIKV was introduced into south east Asia the outbreaks were transmitted by domestic Ae aegypti and Ae albopictus in urban and peri-domestic areas respectively. As more CHIKV isolates were studied they were assigned to three geographically distinct lineages, enzootic West African, enzootic East/Central/South African (ECSA), and endemic/epidemic Asian lineages [70].
In 2004–2005, chikungunya fever was reported on the east coast of Africa and the east African islands of Lamu and Madagascar. Outbreaks of chikungunya fever, involving ~ 300,000 cases, were reported on islands in the Southern Indian Ocean including Comoros, Mauritius and La Réunion [54], [71]. This was probably not the first time CHIKV had caused an epidemic on La Réunion [19]. Nevertheless, its appearance was surprising because Ae aegypti, the normal mosquito vector of human infections, was not common on this island. However, Ae albopictus was identified as the predominant vector species and an E226V amino acid substitution in the E1 glycoprotein of epidemic CHIKV was shown to enhance transmissibility of this CHIKV by Ae albopictus [14], [71], [72]. This Indian Ocean lineage (IOL) which dispersed to India and Southeast Asia, caused millions of cases [73] many of which were inadvertently carried by infected humans to other countries in the OW [74], [75]. Viruses in the ECSA lineage continued to cause sporadic outbreaks of fever in Africa. The Asian lineage emerged out of Africa and was first recognised in Southeast Asia in the early 1950s where it diverged to produce two lineages. It subsequently appeared in Indonesia in the 1980s and continued to cause small outbreaks. In 2006, two divergent clades of this Asian lineage were circulating in Southeast Asia and on the western Pacific islands. The lineage that finally reached St Martin in the Caribbean in 2013 originated from the clade comprising viruses from Indonesia, the South Pacific islands and the Philippines. Significantly, these Asian lineage strains which reached the Americas, did not possess the E1-A226V mutation which enhances transmissibility of CHIKV in Ae albopictus but they retained the E1-A98T amino acid substitution which suppresses the activity of the E1-A226V substitution thus restricting the American lineage viruses to Ae aegypti [76].
2.4. Zika virus
Zika virus (ZIKV) is a close relative of DENV and YFV (Fig. 1). It was first identified in the African forests in the late 1940s [77] where, by analogy with YFV, it circulates between non-human primates and sylvatic mosquitoes that include Aedes africanus [52]. Until recently, ZIKV had been considered a relatively innocuous human arboviral pathogen [52]. However, it has now been associated with microcephaly in Oceania, the Americas, Asia and Africa and also Guillain Barré syndrome. Additionally, non-vector-borne transmission in the form of materno-fetal, sexual, and posttransfusion have also been associated with ZIKV infection [52], [78], [79]..
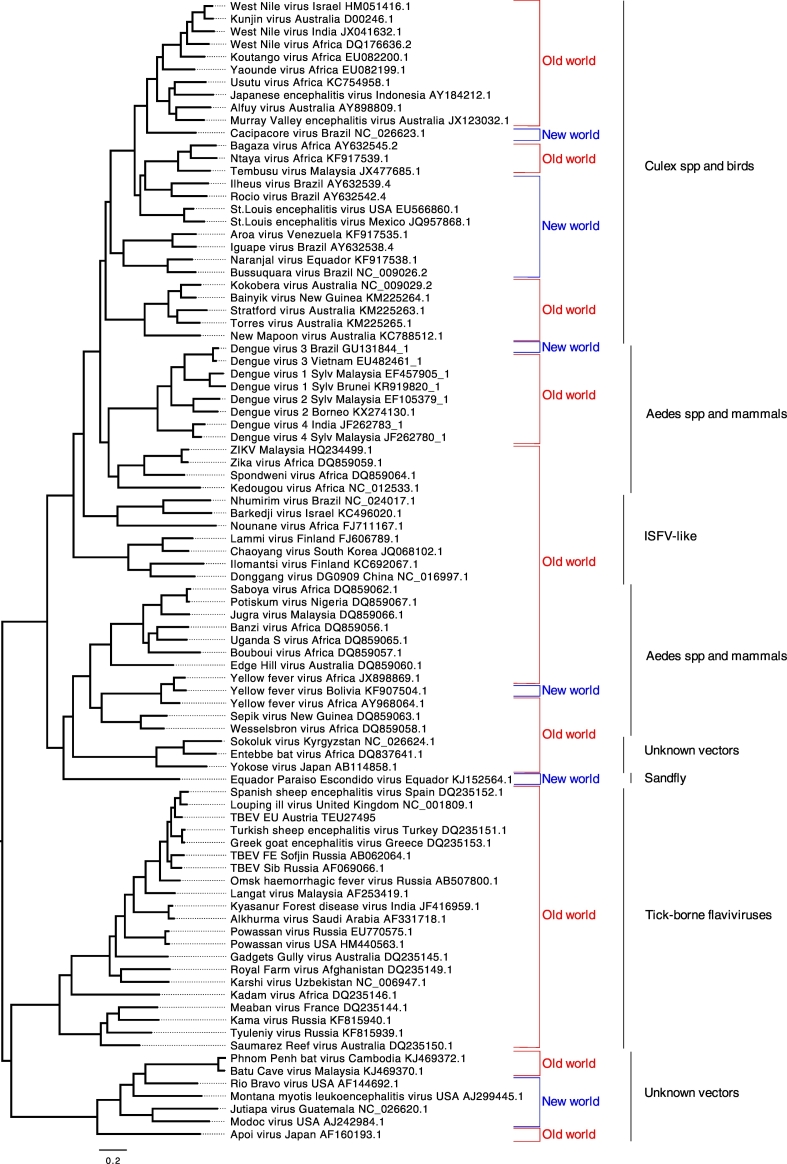
Fig. 1.
Phylogenetic analysis of representative arthropod-borne (and no-known vector) flaviviruses based on the entire open-reading frames. An amino acid alignment was constructed using Mafft v.7.266. Following alignment and model-testing, a maximum likelihood tree was computed using PhyML v.3.1 employing the GTR + gamma model of nucleotide substitution with 1000 bootstraps and using the Subtree Pruning and Regrafting branch-swapping algorithm. All bootstraps exceeded 80%. The tree was edited and visualised with FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree). The tree was mid-point rooted for visual purposes only. New World and Old World refers to the most likely geographic origin of the viruses. “ISFV-like” refers to insect-specific flaviviruses which show close relationships with conventional arboviruses. The primary arthropod (or no-known vector) with which the viruses are associated is shown for each group of viruses identified by the vertical lines on the right hand side of the Figure.
Two geographically overlapping lineages of ZIKV, West African and East African are now recognised [80] and a descendant Asian lineage was first identified in 1969 in Malaysia [81]. This Asian lineage was the most recent common ancestor (MRCA) of all Asian, Oceanic, Caribbean and Latin American viruses that ultimately emerged to cause epidemic outbreaks throughout the tropical Western Hemisphere [20]. In 2007, ZIKV unexpectedly caused an epidemic involving ∼ 73% of the population on Yap Island in Micronesia [82]. Prior to this epidemic only fourteen clinically identified cases of Zika fever had previously been reported. Interestingly, an epidemic of chikungunya fever had arisen in Micronesia in 2006 [54] and in common with the 2007 ZIKV epidemic all phylogenetic data imply that Micronesia was a dead-end for both of these viruses quite likely due to the geographic and commercial isolation of the Micronesian state (north of the equator) from other Oceanic states including French Polynesia (south of the equator). In other words the epidemiological dispersion patterns of CHIKV and ZIKV from Asia, across the Pacific to the Americas are strikingly similar. Moreover, DENV which is also transmitted by Ae aegypti was also circulating on these islands at the same time. These overlapping dispersion patterns for three epidemic arboviruses clearly emphasise the importance of the Ae aegypti vector for transmission and emergence. The additional impact of increasing global commercial transportation has contributed immensely to these almost identical epidemic events in Micronesia and subsequently in other regions of Oceania [6], [83].
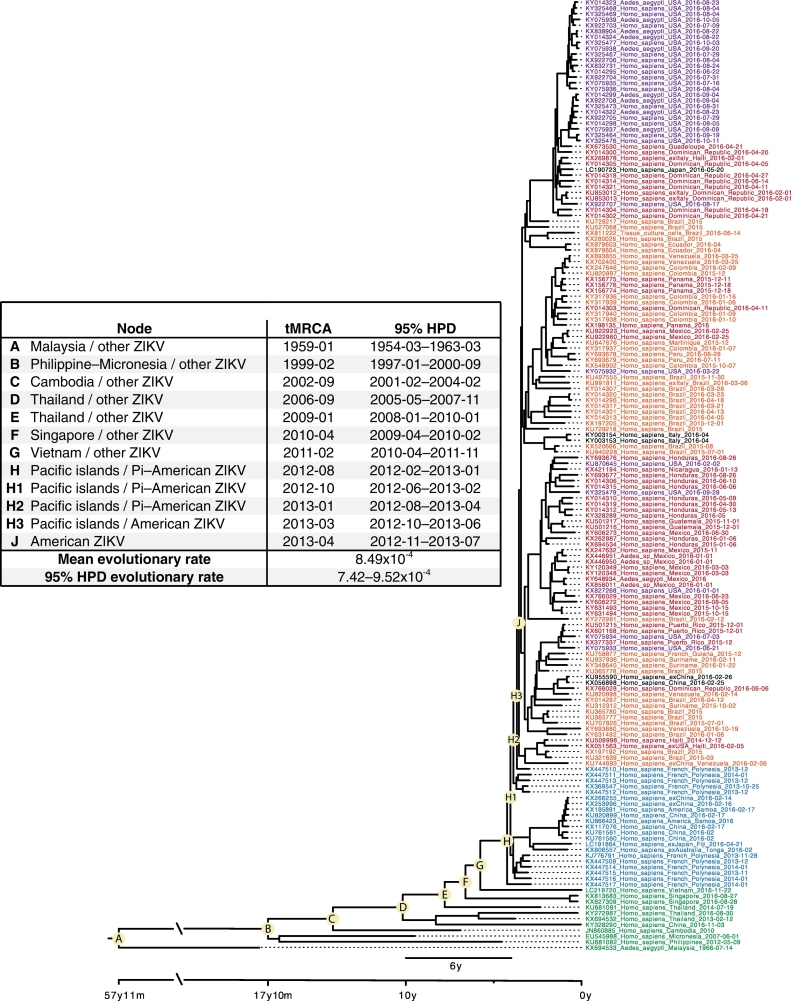
Following the 2007 epidemic in Micronesia which was caused by the introduction of ZIKV from Malaysia, no further epidemics were recorded on the Pacific Ocean islands until 2013 on French Polynesia (FP). The ancestral lineage that initiated the outbreak in FP appears to have been circulating in Thailand, Singapore and Vietnam prior to its introduction into FP (Fig. 2) whence increased microcephaly, subsequently defined more broadly as congenital Zika virus syndrome (CZVS), Guillain-Barre syndrome (GBS) and non-vector-borne transmission of ZIKV (materno-fetal, sexual and post-transfusion) first emerged [52]. Zika virus then continued to disperse across the Pacific Ocean reaching and spreading throughout the tropical and sub-tropical regions of the Americas during 2015–2016 [20]. As the number of cases of CZVS in newborns increased, particularly in Brazil, the World Health Organization declared ZIKV a “Public Health Emergency of International Concern.” Concurrently, increasing numbers of infected visitors returning from the Pacific islands and Latin America to their homelands in North America, Europe, Asia, Africa, Australasia, and New Zealand were recorded and accumulating numbers of autochthonous and/or non-vectored cases are still being reported in these countries.
Fig. 2.
Phylogenetic tree for Asian, Oceanic and American ZIKV isolates. The accompanying Table indicates the estimated time in years from the present (22/11/2016) to the most recent common ancestor (MRCA) for each of the nodes identified as A to J. The base of the tree is represented by the Malaysian isolate which is the first recognised descendant of the African lineages (not shown in this tree). To reconstruct the temporal evolution of the Asian lineage of ZIKV, 165 unique complete or near complete open reading frame genomes were retrieved on 27/03/2017 from NCBI GenBank (www.ncbi.nlm.nih.gov/genbank) and aligned using Mafft v.7.266 keeping the reading frame intact. Evolutionary rates and time to most recent common ancestor were estimated using BEAST 1.8.3, employing the GTR nucleotide substitution model with gamma distribution, a strict molecular clock with a CTMC prior, and a Bayesian skyline coalescent tree prior with a piecewise-constant demographic model. The dataset was run twice for 100 million generations each, sampling every 10,000 generations to ensure sufficient mixing of chains (ESS > 1000). After burn-in for each run, a consensus tree was produced using LogCombiner and TreeAnnotator (BEAST package). The consensus tree was then viewed and annotated in FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/). All computations were performed at the CIPRES web portal (www.phylo.org).
With the discovery of these associated diseases in humans, increasing surveillance raised the awareness of ZIKV infections in south east Asian countries. Inclusion of the Thai, Singapore and Vietnam isolates in a global phylogenetic analysis confirmed that ZIKV had circulated for many years in this region of Asia and throughout the period of global emergence and spread of ZIKV to the NW (Fig. 2). Moreover, the three isolates shared common ancestry and unique amino acid substitutions with the Caribbean, French Polynesian and American clades.
2.5. Summary
In summary human pathogenic Aedes species-associated flaviviruses, which include YFV, DENV1, 2, 3, 4 and ZIKV, together with the alphavirus CHIKV, exhibit similar ecological, evolutionary and epidemiological patterns. They all have forest origins in the OW which involve transmission cycles between sylvatic forms of Ae aegypti and other Ae species. In common with Ae aegypti, they have all been inadvertently shipped across the Atlantic and/or Pacific Ocean. They have all exploited the domestic form of Ae aegypti which has adapted to urban environments with a preference for living inside houses [84]. Consequently, they can sustain transmission between humans without having to depend on their natural reservoir forest cycles. Ae albopictus and other anthropophilic Aedes species provide a supporting role for virus transmission in semi-urban/rural environments and sylvan environments. For years, YFV was considered to be an exception. It did not appear to sustain epidemics at distances from the forest transmission cycle. However, with the domestication of Ae aegypti, expanding transportation systems and vast increases in urbanisation, this may no longer be the case. Thus yellow fever could potentially emerge and disperse in a similar manner to DENV, CHIKV and ZIKV. Indeed, the recent epidemic of yellow fever in Angola and the Republic of Congo involved thousands of humans with the constant threat of further spread.
South American outbreaks of yellow fever rarely spill over from the sylvatic forest Hemagogus/Sabethes cycle into suburban and urban areas. However, there is currently serious concern in Brazil that an outbreak primarily involving sylvatic and surrounding environments appears to be spreading further afield. Despite the administration of millions of vaccine doses, this outbreak had caused at least 215 human deaths and > 600 simian fatalities by mid-March 2017. Moreover, multiple reports of cases being exported between states highlight the possibility of urban yellow fever arising. Thus, whilst transportation has played a major role in the dispersal of Ae aegypti-and their associated arboviruses, the primary factor for the global emergence of these epidemic viruses, in each case, is the adaptation to and domestication of the arthropod vector Ae aegypti to urban environments with a preference for feeding on humans.
It is also becoming clear that other OW arboviruses have also been introduced into the NW during the past few centuries. For example, Mayaro virus (MAYV) and the related Una virus (UNAV) were both isolated in the NW but they are closely related to viruses in the OW Semliki Forest virus (SFV) sero-complex [2], [85] supporting the assumption that they were introduced relatively recently. Furthermore, MAYV and UNAV cause poly-arthritis in humans which is typical of many OW alphaviruses, whereas the NW alphaviruses EEEV, VEEV, WEEV and related sub-species typically cause encephalitis [2].
3. Emergence of Culex-species associated flaviviruses
3.1. Background
Emerging or re-emerging human pathogenic Culex-species-associated flaviviruses include Japanese encephalitis virus (JEV), West Nile virus (WNV), St Louis encephalitis virus (SLEV), Usutu virus (USUV) and Murray Valley encephalitis virus (MVEV). Fig. 1 illustrates their close phylogenetic relationships. However, these viruses were first distinguished from the Aedes-species associated flaviviruses when it was demonstrated that they formed distinct clades in phylogenetic trees (5). Whilst they all have their own particular geographic distributions, they are primarily associated with ornithophilic Culex species mosquitoes, which will also feed on other animals including humans. In contrast with the Aedes-species associated flaviviruses described earlier, the association of these viruses with ornithophilic Culex species means that their geographic dispersion is strongly influenced by the birds that they infect. For example, migratory birds can carry these viruses long distances. Moreover, most of the Culex-associated viruses typically produce encephalitic infections [5] whereas Aedes species-associated human pathogenic viruses tend to cause febrile, flu-like illnesses and/or haemorrhagic disease in humans and animals. The molecular basis of these different pathogenetic characteristics has not yet been defined.
3.2. Japanese encephalitis virus
In terms of morbidity and mortality, JEV is the most important arbovirus in this Culex-associated group. This virus is widely distributed throughout Asia and Australasia [86]. The principle vertebrate amplification hosts of JEV are waterbirds (Ardeidae family), pigs, horses, poultry and possibly bats. Humans are incidental hosts. This virus has been isolated from a wide range of arthropod species including Cx. tritaeniorhynchus (the principle vector), Cx. bitaeniorhynchus, Cx. vishnui, Cx. pseudovishnui, Cx. gelidus, Cx. fuscocephala, Cx. quinquefasciatus, Cx. pipiens pallens, Cx. bitaeniorhynchus, Cx. annulirostris, Cx. whitomorei, Cx. pseudovishnui, Cx. fuscocephala, Cx. annulus, Cx. gelidus, Cx. orientalis, Ae. albopictus, lineatopennis, and Ae. Assamensis, Aedes togoi, Ae. japonicus, Ae. vexans nipponii, Anopheles annularis, Armigeres subalbatus, and An. vagus [87], [88]. It has also been isolated from ticks but their role in JEV epidemiology is not known. Importantly, JEV was recently shown to be orally transmissible between pigs [89] and whilst oral transmission is not unprecedented amongst arboviruses [90], [91], [92], it could have epidemiological significance since pigs are important amplification hosts for JEV. Moreover, the principle vector of JE, Cx. tritaeniorhynchus, feeds preferentially on pigs [93], [94].
Estimates of human morbidity and mortality vary but figures of 50 to 175,000 clinical cases and 10,000 deaths per year [95], [96], [97] are probably underestimates. Japanese encephalitis was first recognised in Japan in 1935 [98]. Historically, epidemics were believed to have dispersed in a south easterly direction [99]. However, based on phylogenetic and geographic knowledge of the different JEV genotypes it is believed that JEV emerged in south east Asia and was dispersed via three recognised bird migratory routes, to India and Pakistan, north through central China, and north east through eastern China including Japan [100]. Moreover, since the African virus USUV is the closest relative of JEV, the data support the hypothesis of dispersal of an ancestral lineage out of Africa with subsequent emergence of JEV in southern Asia [1], [101].
Five JEV genotypes [102], [103] which probably evolved in the order GV, GIV, GIII, GII, and GI [104], [105] are recognised. Phylogenetic, molecular and seroprevalence studies show changes in the genotypes that have prevailed at different times during the past 50 or more years. GIII was previously dominant, but gradual replacement of GI led to the latter being dominant or cocirculating with GIII in many JEV-active regions [106], [107], [108], [109]. However, GV now appears to be emerging as the predominant genotype [110]. This has epidemiological significance because GV is antigenically the most diverse genotype. It also has important implications for epidemic control strategies since the current JEV vaccine shows limited efficacy against the emerging GV [110]. Moreover, Cx. tritaeniorhynchous the primary vector of JEV was recently identified in north western Greece [111] thus potentially increasing the risk of JEV emergence in Europe. Indeed, the even more recent detection of autochthonous co-infections of a human by both YFV and JEV in Angola [112], lends support to the idea that JEV may already have broken free of its apparent Asian boundaries.
Whilst the determinants of emergence of these different genotypes have not been identified, they may be the result of a combination of factors including (i) natural genetic variability, (ii) adaptation to the specific variants of mosquito species prevalent in different regions of Asia, (iii) the introduction of modernised farming practices including management of rice paddy fields and isolation of pig farms from urban environments, (iv) the implementation of concerted JEV immunisation programmes across many Asian countries and (v) the possible impact of climate change on migratory bird patterns and mosquito distribution [113]. Thus, JEV appears highly adaptable to changing environmental conditions and the fact that the virus has been identified over such a wide geographic range, tempts one to speculate that JEV could evolve another genotype (GVI?) and/or emerge beyond the boundaries of Asia into Europe, central/eastern Oceania and the NW.
3.3. West Nile virus
West Nile virus, known as Kunjin virus in Australia, is closely related to JEV, USUV and MVEV (Fig. 1) and within the genus Flavivirus, is second to DENV in the extent of its global distribution. Although WNV and JEV share similar vectors, i.e., Culex spp., and vertebrate hosts, including birds, horses, and pigs, WNV is more widely distributed across the world than JEV. One possible explanation for this difference in geographic distribution is that the ancestral WNV lineage emerged in Africa and was dispersed by birds taking north and north east migratory routes into Europe [1], whereas the presumed ancestral lineage of JEV and other related Asian viruses were dispersed along more easterly bird migratory routes that took them into Asia. Clearly, many other factors such as commercial shipping movements, with accompanying mosquitoes, large-scale pig-farming in Asia, overlapping bird migratory routes and availability of appropriately competent mosquito species would have contributed to the distinct geographic regions in which WNV and JEV finally became established as human epidemic viruses.. The phylogenetic relationships of these viruses (including Usutu virus, Murray Valley encephalitis virus, Kunjin virus and several other less well studied flaviviruses) are consistent with this interpretation (Fig. 1). The closest relatives of JEV, i.e., USUV, an African virus, MVEV, and ALFV, which are Australian viruses, are discussed below. However, in evolutionary terms, these lineages would have also emerged as human pathogens during recent centuries from the common African ancestral lineage. Therefore, because JEV is not found in Africa and is closely related to the Australian viruses, it is likely that JEV also emerged recently in Asia and this is supported by recent studies [105].
Based on serological evidence [114], [115], [116], WNV circulates in the absence of clinical disease, in the majority of humans and a wide variety of different animal species but at times of the year when ornthophilic Culex species mosquitoes are abundant WNV may also cause zooepidemics with disease symptoms ranging from sub-clinical/mild/febrile, to encephalitic with or without flaccid paralysis and fatality [7]. The zooepidemiological success of WNV is largely attributable to its capacity to infect and be transmitted, respectively, by an extensive variety of birds and mosquito species [7], [117], [118]. For example in the OW, WNV has been isolated from 43 mosquito species including Cx. pipiens, Cx. modestus and Coquillettidia richiardii in Europe, Cx. tritaeniorhynchus, Cx. vishnui and Cx. Quinquefasciatus in Asia and in Africa and the Middle East Cx. univittatus, Cx. poicilipes, Cx. neavei, Cx. decens, Aedes albocephalus and Mimomyia spp. In the United States, the virus had been isolated from 65 different mosquito species by 2012 [118], [119] but Cx. pipiens, Cx. restuans, Cx. quinquefasciatus and Cx. tarsalis are considered to be the major maintenance vectors. However, association of the virus with specific mosquito species does not guarantee that they are active in the transmission cycle. West Nile virus has also been recovered from soft ticks, hard ticks [5], [117] and sandflies (unpublished observations), but their role in WNV epidemiology is unknown. The virus infects a wide range of avian species [120] which contribute significantly to its success as a major pathogenic arbovirus and although it has been isolated from many different mammalian and reptilian species, most appear to be dead-end hosts [117].
The emergence of West Nile encephalitis in humans, horses and birds in New York in 1999 [9], [10], followed by its rapid and extensive dispersal throughout mainland USA, neighbouring Canada and Mexico [121], raised the awareness of WNV, to new heights. Despite this it was already a known important zooepidemic arbovirus in Africa, the Middle East, Europe and Asia/Australasia where spasmodic and sometimes explosive epidemics had been recorded since the first reported human cases of WNV fever in Israel [122], [123], [124]. Noticeably, however, early reports of outbreaks in the OW were not associated with human or avian mortality. The reasons for the increased global interest in WNV after it appeared in North America, were the surprisingly high numbers of deaths in birds, horses and even humans. However ~ 80% of human WNV infections are asymptomatic and deaths associated with infection represent < 1% of humans presenting with encephalitis many of whom are elderly and immunocompromised [7], [125]. Nevertheless, nearly 44,000 human cases of clinically apparent WNV infections were recorded in the US by August 2016 [126] and as the vast majority of milder cases are not diagnosed, the estimates of infections will be much higher than this. One of the questions arising from the morbidity/mortality data in the USA that has not yet been adequately answered is what is happening in South America? Why, for example even though there have been one or two reports of WNV activity, are there few clinically apparent cases of WNV infection in humans? One plausible explanation is that several antigenically related flaviviruses circulate in South America including SLEV, Iheus (ILHV), Rocio (ROCV), Cacicapore virus (CPCV), DENV, YFV, Aroa (AROAV), Naranjal (NJLV), Bussuquara (BSQV), and Iguape virus (IGUV). Thus, cross-reactive immunity arising from sub-clinical infections with related flaviviruses might explain this enigma. Other factors, such as specific bird migratory routes, distribution of vector competent ornithophilic/anthropophilic mosquitoes in relation to human population distribution, etc., presumably also contribute to the apparent failure of WNV to establish as a serious pathogen in Central America, South America and on the Caribbean islands [121].
3.4. St Louis encephalitis virus
West Nile virus was not the first human encephalitic Culex species-associated flavivirus to appear in the Americas. St Louis encephalitis virus (SLEV) was first identified in 1933 following a human epidemic in St Louis, Missouri [127]. During subsequent years, SLEV emerged throughout the Americas from Canada to Argentina and the Caribbean and since 1933, there have been > 40 epidemics in North America [128] most of which involved only a few cases of encephalitis. Records show that most human infections by SLEV are clinically inapparent and that cases of encephalitis have mostly occurred in the east and central states of the US. Importantly, very few human cases of encephalitis due to SLEV have been reported from South America or the Caribbean possibly, as was suggested for WNV, reflecting the presence of other antigenically related flaviviruses. Unlike WNV which is widespread and found in both urban and rural areas of mainland US, outbreaks of SLEV are less widespread and occur mostly in rural areas where the patients are geographically widely dispersed. This reflects the higher preference of SLEV for peri-domestic and/or sylvan Culex species and is likely to be a major factor in distinguishing the lower level epidemicity of SLEV when compared with WNV.
In considering the origin of SLEV, a recent publication identified an ancestral variant in the rainforests located in the Palenque National Park of Mexico and it was estimated that the most recent common ancestor of this lineage and epidemic SLEV strains was about 330 years ago [129]. It was proposed that subsequent epidemic lineage expansion occurred in two separate waves, representing emergence near the Palenque region and in the lower Mississippi and Amazon delta. However, independent recent studies show that a variant of SLEV which emerged in Arizona and California in 2015, closely resembled Argentinian isolates made in 2005 [130]. Such south to north dispersion of SLEV, via migratory birds is well known [131], [132] and implies the possibility of a South/Central American origin for SLEV in the Americas. However, it also prompts the question, what is the ancestral origin of SLEV? This virus shares common ancestry with many OW flaviviruses including Tembusu virus (TMUV), Israel Turkey meningoencephalitis virus (ITV), Bagaza virus (BAGV), JEV, WNV, USUV, MVEV and Koutangou virus (KOUV).
Estimates for the time of appearance of Palenque strains in the Americas are compatible with those of YFV, DENV and CHIKV, i.e., during the early slave-trading period from Africa [129]. Thus, it is not inconceivable that an OW ancestral lineage of the Palenque strain could have been introduced to Central or South America via the slave ships that carried many other viruses, bacterial pathogens and mosquito vectors to and from the Americas as described earlier. For this to happen, the ancestral lineage of SLEV and appropriate Culex species would need to have circulated on the ships in the manner described for Ae aegypti. Unlike Aedes species, Culex species eggs do not withstand dessication. Nevertheless, on the ships, the required supply of humans and water for breeding of the mosquitoes and maintenance of the virus transmission cycle would provide the conditions necessary for virus and vector survival during the voyage. Moreover, in common with Aedes species, sylvatic Culex species are known to adapt rapidly to the domestic form [133] which would be a necessary requirement on the long journey from the OW to the NW. Additionally, it is believed that North American Cx. p. pipiens shares hybrid ancestry with Old World Cx. p. pipiens and Cx. pipiens f. molestus, implying the introduction of OW Culex species to the NW [134].
3.5. Murray Valley encephalitis virus
The aetiological agent of Australian encephalitis, MVEV shares common ancestry with JEV, USUV and WNV (Fig. 1). Between the early and mid-1900s six outbreaks of Australian encephalitis were recorded in south-eastern Australia [135] and the disease was also sporadically associated with neurological infections of horses. Subsequently, MVEV caused small outbreaks until 2011 when, following an unusually wet period, high level MVEV activity occurred in many areas of Australia. Since then clinical cases have been largely confined to the western and central parts of northern Australia [135]. The virus was first isolated in 1951 from a patient who died with acute encephalitis during an epidemic in Victoria, Australia. The primary mosquito vector is Cx. annulirostris although other species including Cx. bitaeniorynchus and Ae normanensis are also competent vectors of MVEV transmission [136]. There is also evidence of the presence of MVEV in New Guinea and possibly Indonesia. Water and land-birds are potential reservoirs for MVEV and seropositivity has been detected in domestic animals including, horses, dogs, foxes and opposums [137]. In view of the fact that migratory birds are potential reservoirs of MVEV it is surprising that the disease is not reported in more countries bordering with Australia. However, this could reflect the relative lack of pathogenicity for the birds with which the virus is associated resulting in a lack of reported sick or dead birds.
3.6. Usutu virus
Usutu virus, shares common ancestry with WNV, JEV and MVEV (Fig. 1). It was first isolated in 1959, from Cx. neavei collected near the river Usutu, in Ndumu, South Africa [136]. Although human infections with USUV are characterised by fever and rash this African virus attracted little attention until it emerged in and around Vienna, Austria in August 2001, causing an outbreak of bird deaths [138]. Subsequently it was retrospectively discovered that USUV had circulated in northern Italy in 1996–1998 [139]. This supported earlier serological evidence of the sub-clinical circulation of USUV in birds in the UK [114], [115] and Italy [116] which was presumably occurring throughout Europe but was not being investigated at that time. Thus, it appears that the virus gradually dispersed, via migratory birds, from Africa through southern Mediterranean countries, eventually reaching Italy and neighbouring countries, including Austria, Switzerland, Czech Republic, Hungary, Italy, France, Spain, Germany, Belgium and the Netherlands [140]. This recent report of multi-lineage circulation of USUV in birds in northern Europe adds support to the interpretation of USUV emergence in Europe but as yet it is not clear whether the different lineages arose in Africa or Europe. Regardless of their evolutionary source, current evidence from Italy indicates that USUV has been circulating sub-clinically for some years. Moreover, a total of 10 cases of encephalitis due to infection with USUV have now been identified in the Modena region of Italy [141]. Indeed, there are significant analogies between the recent USUV outbreaks in birds and those of WNV lineage 2 in central Europe in 2008–2009. After a few years of limited local circulation, WNV subsequently spread to the Balkan states and northern Greece [123], [124]. Usutu virus was also identified in the brain of dead bats (Pipistrellus pipistrellus) collected in southwest Germany in 2013 [142]. Bats are considered to be natural reservoir hosts of a wide diversity of viruses. Thus, the fact that they appear to have died following infection suggests that they may not be natural reservoirs for USUV. Whether USUV will continue to expand geographically in Europe and/or elsewhere causing human epidemics and epizootics in birds, remains to be seen. Nevertheless, when one considers the increasing numbers of bird deaths and the recent observations of human USUV infections in northern Italy [141] and the acute human infection in a German blood donor [143] this continuous geographic expansion of USUV in Europe is a clear indication that USUV appears to be emerging as a significant avian and human pathogen in both northern and southern Europe. This could have widespread implications for European public and veterinary health agencies.
3.7. Summary
In order to illustrate how vectors can influence patterns of emergence and dispersal, we selected examples of arboviruses with preference for either anthropophilic Ae. aegypti or ornithophilic Culex species. Below, we describe three more epizootic viruses, for which different vector preferences from those described above have evolved, to illustrate further how the specific vector can impact on dispersal patterns. We recognise that other factors such as inadvertent re-dispersion of mosquitoes via transportation can also impact on arbovirus evolution and dispersal. However, here we have considered only reproducible patterns associated with specific vectors.
3.8. Spondweni virus
Spondweni virus (SPOV) is currently the closest known relative of ZIKV (Fig. 1) and therefore one might expect it to resemble ZIKV in its life cycle but this is not the case. SPOV is not known to have a forest sylvatic cycle that involves NHP. In fact its natural life cycle has not been adequately identified. This virus causes outbreaks of fever and rash in humans but has never been identified outside Africa. However, it has been isolated from a variety of African mosquito species, including Mansonia uniformis, Mansonia africana, A.e cuminsii, Cx. neavei but the principle vector appears to be the sylvatic African mosquito Ae. circumluteolus [136] i.e., not Ae aegypti or Ae albopictus, both of which are efficient vectors of ZIKV transmission. Indeed, under experimental laboratory conditions, these latter two mosquito species are poorly competent for SPOV amplification and transmission [144]. In contrast with ZIKV, SPOV is vectored by mosquitoes that are currently confined to Africa. Consequently, without a significant change in the vector competence of Ae aegypti and/or Ae albopictus for SPOV, perhaps by mutation of the virus, the likelihood of SPOV mimicking the global emergence of ZIKV, appears to be low.
3.9. O'nyong nyong virus
O'nyong nyong virus (ONNV) and CHIKV are closely related and co-circulate in the African forests and also in urban environments where dengue fever and malaria are also present [145]. In 1959, ONNV caused a major epidemic in East Africa involving over 2 million cases [146] and a second major epidemic emerged in southern Uganda nearly 40 years later [4]. This periodicity is reminiscent of the 40–50 year periods between chikungunya fever epidemics. However, unlike CHIKV, ONNV has only ever been recognised in sub-Saharan Africa. The most likely explanation for the failure of ONNV to disperse Out of Africa is that the primary vectors of ONNV are anopheline mosquitoes, typically Anopheles funestus and An. gambiae. These mosquito species are widespread throughout sub-Saharan Africa and are known vectors of the malaria parasite Plasmodium falciparum. They are closely associated with human habitats but apart from An. gambiae being detected in Natal (Brazil) during the 1930s for a relatively brief period, they have not otherwise been associated with human epidemics outside the African continent. Thus, whilst ONNV has a wide distribution within the African continent, it is limited in its global dispersal capability due to the current unavailability of competent ONNV vectors that circulate both within and outside Africa.
3.10. Rift Valley fever virus
Rift Valley fever virus (RVFV), family Bunyaviridae, genus Phlebovirus, is an epizootic arbovirus which, based on serological evidence and localised outbreaks, is associated with disease in Central West, East and South Africa and the Arabian Peninsula, including Yemen, where large numbers of ruminants are imported from South Africa to feed the masses during religious festivals [147]. The virus is transmissible by a wide variety of mosquitoes including Aedes, Culex, Anopheles, Mansonia, Eretmapodites species some of which have a preference for feeding on specific wild animal species but will also feed opportunistically on humans. The virus-vector-host relationships of RVFV are complex, and depend on the variability of the ecoclimatic zones, biogeographical domains, habitats, vectorial capacity, breeding sites and distribution, and vertebrate hosts in which the virus circulates [148], [149], [150], [151], [152], [153]. For example, in regions of Africa where cattle and sheep are farmed, Aedes and Culex species become predominant during the rainy season, particularly when the wetlands become flooded. Anthropological influences such as irrigation and development of rice fields are also associated with epizootic episodes [154], [155]. The El Niño–Southern Oscillation also has an impact particularly in east Africa when wetlands become flooded. In contrast, in areas of Africa with limited rainfall, RVF emergence may be initiated via vertical transmission of the virus in eggs of Aedes spp. Following heavy rainfall the newly arising mosquitoes feed on ruminants causing epizootics [156]. In arid areas such as the Arabian Peninsula, outbreaks are associated with above average rainfall when the water forms temporary pools or floods [153].
Intermittent epizootic outbreaks of acute Rift Valley fever may result in large numbers of abortions, deaths and other disease syndromes in cattle, sheep, goats and camels. Most commonly, RVFV- incidentally-infected humans have either no symptoms or a mild illness associated with fever and liver abnormalities. Patients usually experience fever, generalized weakness, back pain, and dizziness at the onset of the illness. They typically, recover within two days to one week after onset of illness. However, during RVFV epizootics up to 10% of humans infected by contact with infectious blood or bitten by an infected mosquito may develop more severe symptoms, including ocular disease, encephalitis or inflammation of the brain, seizures (< 1% of patients) or haemorrhagic fever [157]. In the Arabian Peninsula and Yemen where RVFV has caused epidemics, localised outbreaks might be triggered by importation from Africa of RVFV-infected ruminants.
Rift Valley fever virus is generally considered to be a candidate for emergence and global dispersion but thus far, others considered less likely, such as ZIKV and CHIKV have emerged out of Africa and dispersed globally largely because they are primarily transmissible by the domestic form of Ae. aegypti throughout the tropics and sub-tropics in the human urban environment. In contrast, RVFV is transmissible by a wide variety of mosquitoes including Aedes species, most of which are adapted to the local habitats in Africa, as described above. Ae. aegypti is not a recognised primary vector of RVFV and based on its history, the likelihood of expansion outside Africa and Saudi Arabia appears to be low. However, RVFV could be inadvertently introduced via infected mosquitoes into a tropical region where competent domestic Ae. aegypti predominate in the urban environment. Under such circumstances one shudders to think what the consequences might be!
4. Summary and conclusions
For this review we selected emerging human pathogenic arboviruses that dispersed from their ancestral OW origins to the NW (YFV, DENV, ZIKV, CHIKV, WNV, SLEV, MAYV and UNAV). We contrasted these with others that originated in the OW but currently have not emerged in the NW (JEV, USUV, MVEV, SPOV, ONNV, RVFV). With the exception of WNV and SLEV, the primary arthropod vectors of the globally dispersed viruses are either the domesticated variant of the African sylvatic Ae. formosus, i.e., Ae. aegypti or in the case of YFV and MAYV Haemagogus and Sabethes species in the Brazilian forests. Apart from ZIKV which was introduced very recently into the NW, the others were probably introduced to the NW two to four centuries ago via slave trading ships. At least two of these viruses, i.e., DENV and CHIKV, have continued to cross the Oceans at relatively frequent intervals, either via infected vectors on Ocean-going commercial vessels or via infected humans. It is worth pointing out that although Ae. albopictus has clearly contributed to the emergence of CHIKV and is an important ancilliary vector of DENV in the peri-domestic rural environment, this mosquito species does not appear to be making the impact on the global epidemiology of arboviruses that was being predicted. Nevertheless, taking into account the fact that Ae albopictus has already adapted to temperate environments, and is susceptible to infection by several arboviruses [158] it will be interesting to see whether or not this situation changes in the future.
In contrast with the Aedes species-associated viruses, WNV, SLEV and other viruses in the JEV-related serocomplex are primarily vectored by ornithophilic/anthropophilic Culex species and in general are dispersed via infected migratory birds. Thus, their natural dispersion patterns are largely dependent on the migratory routes of the infected birds acting as hosts and reservoirs. West Nile virus broke away from this constraint when it was introduced into North America possibly via a commercial flight into New York carrying one or more infected mosquitoes, from the Middle East or North Africa. The alternative possibility that a stray migratory bird introduced the virus from Africa/Israel is unlikely during the summer when birds do not migrate. We argued earlier that SLEV might represent a descendant lineage of an African/Asian ancestral virus that was introduced to the Americas via the slave ships and subsequently became extinct at the source of origin, and we have also cited viruses such as Aedes species-associated MAYV and UNAV which presumably crossed the oceans via ships. However, none of the other Culex species-associated flaviviruses have thus far achieved global distribution status.
Why was WNV successful in achieving this? This virus has been isolated from an extremely wide range of arthropods and infects many animal and bird species. Moreover, it displays wide genetic variability [159]. Thus, with increasing numbers of aircraft daily filling the skies, the likelihood of WNV crossing the oceans was high. However, none of the Culex species-associated flaviviruses has become established across the islands in the Pacific although JEV and MVEV have been reported in the western Pacific region of Australasia. Birds tend to stay close to land masses flying northerly and southerly routes. Thus, as shipping and/or aircraft movements continue to increase, the risk of introduction of one or more of these Culex species-associated flaviviruses into Oceania, Europe or the Americas will increase. If one had to speculate which arbovirus is likely to be the first to escape its localised geographic region then Japanese encephalitis virus must be a prime candidate since it is genetically adaptable and its primary vector has now been detected in Greece, i.e., a Southern European region. One could argue that without pigs as an amplification host, JEV is unlikely to be a major threat outside Asia. However, the global pig industry has grown enormously in recent decades and wild pig populations are expanding in Europe, Scandinavia and the US. This increases the risk of JEV global emergence despite the fact that there is a JE vaccine. It remains to be seen whether or not the first detection of JEV genomic sequence in a human in Africa [112] reflects the early stages of JEV emergence in this country.
Finally, is there a perceived risk that YFV, RVFV, ONNV or SPOV could emerge and become globalised in the manner observed for DENV, CHIKV and ZIKV? Increasing urbanisation, transportation (particularly aircraft) and mosquito adaptation to the urban environment are major driving forces behind arbovirus emergence. Therefore, despite the current failure of these arboviruses to expand beyond their historical boundaries, it would be foolish to assume that one or more of these proven epidemic viruses will not “break out” in the future. Indeed, the recent outbreaks of yellow fever in Africa and South America look ominously threatening, particularly with the increasing domesticity of Ae aegypti and the global distribution of the likely secondary vector Ae albopictus.
We confined this review to mosquito-borne human pathogenic arboviruses that have generated the largest attention in terms of public health and research. We avoided discussing the possible impact of climate change which could have a long-term impact. Nevertheless, the driving forces to which we have referred virtually guarantee that arboviruses will continue to emerge and disperse globally. We must learn from history and be prepared to expect the unexpected!
Contribution of authors
EG drafted the manuscript and JP generated the phylogenetic trees and dating estimates. All co-authors provided input to the literature research, manuscript preparation, proofreading, illustrations and critical analysis of the submitted review.
Funding information
This study was partly funded under the EU FP7 Project PREDEMICS (grant agreement no. 278433) and the EU Horizon 2020 Project EVAg (grant agreement no. 653316). EG, JP, RC, and XDL are members of the EU programme of the European Union, ZIKAlliance.
Declaration of no conflict of interest
All authors have signed a declaration that they have read the manuscript and have no conflict of interest relating to the review.
Ethical statement
The historical and scientific contents of this review are based entirely on published scientific literature and have no implications relating to human or ethical standards.
Acknowledgements
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. J.H.-O.P., R.C., E.A.G., and X.D.L. are members of the ZIKAlliance programme of the European Union.
References
- 1.Gould E.A., de Lamballerie X., Zanotto P.M., Holmes E.C. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv. Virus Res. 2003;59:277–314. doi: 10.1016/s0065-3527(03)59008-x. [DOI] [PubMed] [Google Scholar]
- 2.Gould E.A., Coutard B., Malet H., Morin B., Jamal S., Weaver S.C. Understanding the alphaviruses: Recent research on important emerging pathogens and progress towards their control. Antivir. Res. 2009 doi: 10.1016/j.antiviral.2009.07.007. Jul 16 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams M.C., Woodall J.P., Corbet P.S., Gillett J.D. O'nyong-nyong fever: an epidemic virus disease in East Africa. VIII. Virus isolations from anopheles mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1965;59:300–306. doi: 10.1016/0035-9203(65)90012-x. [DOI] [PubMed] [Google Scholar]
- 4.Lanciotti R.S., Ludwig M.L., Rwaguma E.B., Lutwama J.J., Kram T., Karabatsos N. Emergence of O'nyong-nyong fever in Uganda after a 35-year absence: genetic characterization of the virus. Virology. 1998;252:258–268. doi: 10.1006/viro.1998.9437. [DOI] [PubMed] [Google Scholar]
- 5.Gaunt M.W., Sall A.A., de Lamballerie X., Falconar A.K., Dzhivanian T.I., Gould E.A. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J. Gen. Virol. 2001;82(Pt 8):1867–1876. doi: 10.1099/0022-1317-82-8-1867. [DOI] [PubMed] [Google Scholar]
- 6.Lounibos L.P. Invasions by insect vectors of human disease. Annu. Rev. Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 7.Gould E.A., Solomon T. Pathogenic flaviviruses. Lancet. 2008;371(9611):500–509. doi: 10.1016/S0140-6736(08)60238-X. [DOI] [PubMed] [Google Scholar]
- 8.Steele K.E., Linn M.J., Schoepp R.J., Komar N., Geisbert T.W., Manduca R.M. Pathology of fatal West Nile virus infections in native and exotic birds during the 1999 outbreak in New York City. N. Y. Vet. Pathol. 2000;37(3):208–224. doi: 10.1354/vp.37-3-208. [DOI] [PubMed] [Google Scholar]
- 9.Lanciotti R.S., Roehrig J.T., Deubel V., Smith J., Parker M., Steele K. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science N. Y. 1999;286(5448):2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 10.Briese T., Jia X.Y., Huang C., Grady L.J., Lipkin W.I. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- 11.Gould E.A., Higgs S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009;103:109–121. doi: 10.1016/j.trstmh.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehrig J.T. West Nile virus in the United States - a historical perspective. Viruses. 2013;5(12):3088–3108. doi: 10.3390/v5123088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen J., Moore F., Panella N., Edwards E., Bru R., Hughes M. Migrating birds as dispersal vehicles for West Nile virus. EcoHealth. 2006;3:79–85. [Google Scholar]
- 14.Schuffenecker I., Iteman I., Michault A., Murri S., Frangeul L., Vaney M.C. Genome microevolution of chikungunya viruses causing the Indian ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charrel R.N., de Lamballerie X., Raoult D. Chikungunya outbreaks--the globalization of vector-borne diseases. N. Engl. J. Med. 2007;356(8):769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 16.Thiberville S.D., Boisson V., Gaudart J., Simon F., Flahault A., de Lamballerie X. Chikungunya fever: a clinical and virological investigation of outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl Trop. Dis. 2013;7(1):e2004. doi: 10.1371/journal.pntd.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen R., Puri V., Fedorova N., Lin D., Hari K.L., Jain R. Comprehensive genome-scale phylogenetic study provides new insights on the global expansion of chikungunya virus. J. Virol. 2016;90(23):10600–10611. doi: 10.1128/JVI.01166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett K.L., Shija F., Linton Y.M., Misinzo G., Kaddumukasa M., Djouaka R. Historical environmental change in Africa drives divergence and admixture of Aedes aegypti mosquitoes: a precursor to successful worldwide colonization? Mol. Ecol. 2016;25(17):4337–4354. doi: 10.1111/mec.13762. [DOI] [PubMed] [Google Scholar]
- 19.Halstead S.B. Reappearance of Chikungunya, Formerly Called Dengue, in the Americas. Emerg. Infect. Dis. 2015;21(4):557–561. doi: 10.3201/eid2104.141723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersson J.H., Eldholm V., Seligman S.J., Lundkvist A., Falconar A.K., Gaunt M.W. How Did Zika Virus Emerge in the Pacific Islands and Latin America? MBio. 2016;7(5) doi: 10.1128/mBio.01239-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strode G.K. McGraw-Hill; New York: 1951. Yellow Fever. [Google Scholar]
- 22.Jentes E.S., Poumerol G., Gershman M.D., Hill D.R., Lemarchand J., Lewis R.F. The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the informal WHO working group on geographic risk for yellow fever. Lancet Infect. Dis. 2011;11(8):622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- 23.Kenney J.L., Brault A.C. The role of environmental, Virological and vector interactions in dictating biological transmission of arthropod-borne viruses by mosquitoes. Adv. Virus Res. 2014;89:39–83. doi: 10.1016/B978-0-12-800172-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 24.Kilpatrick A.M., Randolph S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380(12):61151–61159. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubler D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 2002;33(4):330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 26.Liang G., Gao X., Gould E.A. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg. Microb. Infect. 2015;4:1–5. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell J.R., Tabachnick W.J. History of domestication and spread of Aedes aegypti—a review. Mem. Inst. Oswaldo Cruz. 2013;108(Suppl. 1):11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattingly P.F. Genetical aspects of the Aedes aegypti problem. I. Taxonom: and bionomics. Ann. Trop. Med. Parasitol. 1957;51(4):392–408. [PubMed] [Google Scholar]
- 29.Mattingly P.F. Taxonomy of Aedes aegypti and related species. Bull. World Health Org. 1967;36:552–554. [PMC free article] [PubMed] [Google Scholar]
- 30.Harrington L.C., Edman J.D., Scott T.W. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001;38(3):411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 31.Moureau G., Cook S., Lemey P., Nougairede A., Forrester N.L., Khasnatinov M. New insights into flavivirus evolution, taxonomy and biogeographic history, extended by analysis of canonical and alternative coding sequences. PLoS One. 2015;10(2):e0117849. doi: 10.1371/journal.pone.0117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabachnick W.J. Evolutionary genetics and arthropod-borne diseases. The yellow fever mosquito, Aedes aegypti. Am. J. Entomol. 1991;37:14–24. [Google Scholar]
- 33.Wilder-Smith A., Monath T.P. Responding to the threat of urban yellow fever outbreaks. Lancet Infect. Dis. 2017;17(3):248–250. doi: 10.1016/S1473-3099(16)30588-6. [DOI] [PubMed] [Google Scholar]
- 34.McNeill J.R. Yellow Jack and Geopolitics: environment, epidemics, and the struggles for empire in the American tropics. OAH Mag. Hist. 2004;18(3):9–13. [PubMed] [Google Scholar]
- 35.Bres P.L. A century of progress in combating yellow fever. Bull. World Health Organ. 1986;64(6):775–786. [PMC free article] [PubMed] [Google Scholar]
- 36.Seligman S.J., Casanova J.L. Yellow fever vaccine: worthy friend or stealthy foe? Expert Rev. Vaccines. 2016;15(6):681–691. doi: 10.1080/14760584.2016.1180250. [DOI] [PubMed] [Google Scholar]
- 37.Nogueira P, editor The early history of yellow fever. Yellow Fever, a Symposium in Commemoration of Carlos Juan Finlay, 1955; 2009; http://jdc. jefferson.edu/yellow_fever_symposium/10.
- 38.Bloom K.J. Louisiana State University Press; Baton Rouge, Louisiana: 1993. The Mississippi Valley's Great Yellow Fever Epidemic of 1878; pp. 1–290. [Google Scholar]
- 39.Calisher C.H., Gould E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003;59:1–17. doi: 10.1016/s0065-3527(03)59001-7. [DOI] [PubMed] [Google Scholar]
- 40.Reed W. Propagation of yellow fever: observations based on recent researches. Med. Rec. 1901;60:210-09. [Google Scholar]
- 41.London K.C. 2017. Yellow fever in Cadiz. [Google Scholar]
- 42.Smith C.E., Gibson M.E. Yellow fever in South Wales. Med. Hist. 1986;30:322–340. doi: 10.1017/s0025727300045737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillemand B. The yellow fever epidemic in Saint-Nazaire in 1861. Hist. Sci. Med. 2006;40(1):23–36. [PubMed] [Google Scholar]
- 44.Morillon M., Mafart B., Matton T. Ecological Aspects of Past Settlement in Europe. European Anthropological Association; Budapest: 2002. Yellow fever in Europe in 19th century; pp. 1–23. [Google Scholar]
- 45.Zanotto P.M., Gould E.A., Gao G.F., Harvey P.H., Holmes E.C. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc. Natl. Acad. Sci. 1996;93(2):548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryant J.E., Holmes E.C., Barrett A.D.T. Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog. 2007;3(5):e75. doi: 10.1371/journal.ppat.0030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown J.E., Evans B.R., Zheng W., Obas V., Barrera-Martinez L., Egizi A. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution, Lancaster, Pa. 2013;68(2):514–525. doi: 10.1111/evo.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crawford J.E., Alves J.M., Palmer W.J., Day J.P., Sylla M., Ramasamy R. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol. 2017;15(1):16. doi: 10.1186/s12915-017-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith C.E. The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. Hyg. 1956;59(10):243–251. [PubMed] [Google Scholar]
- 50.Brown J.E., McBride C.S., Johnson P., Ritchie S., Paupy C., Bossin H. Worldwide patterns of genetic differentiation imply multiple 'domestications' of Aedes aegypti, a major vector of human diseases. Proc. Biol. Sci. 2011;278(1717):2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urdaneta-Marquez L., Failloux A.B. Population genetic structure of Aedes aegypti, the principal vector of dengue viruses. Infect. Genet. Evol. 2011;11(2):253–261. doi: 10.1016/j.meegid.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Musso D., Gubler D.J. Zika virus. Clin. Microbiol. Rev. 2016;29(3):487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weaver S.C., Forrester N.L. Chikungunya: evolutionary history and recent epidemic spread. Antivir. Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Weaver S.C., Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015;372(13):1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 55.McBride C.S., Baier F., Omondi A.B., Spitzer S.A., Lutomiah J., Sang R. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515(7526):222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gouck H.K. Host preferences of various strains of Aedes aegypti and A. Simpsoni as determined by an olfactometer. Bull. World Health Organ. 1972;47(5):680–683. [PMC free article] [PubMed] [Google Scholar]
- 57.Halstead S.B., editor. Dengue: Overview and History. Imperial College Press; London: 2008. [Google Scholar]
- 58.Gubler D.J. In: Dengue and Dengue Haemorrhagic Fever: Its History and Resurgence as a Global Public Health Problem. Gubler DJaK G., editor. CAB International; New York: 1997. pp. 1–22. [Google Scholar]
- 59.Rush A.B., editor. An Account of the Bilious Remitting Fever, as It Appeared in Philadelphia in the Summer and Autumn of the Year 1780. Prichard and Hall; Philadelphia: 1789. [Google Scholar]
- 60.Lavergne A., Lacoste V., Germain A., Matheus S., Dussart P., Deparis X. Dengue virus infection in neotropical forest mammals: incidental hosts or potential reservoirs? Med. Trop. (Mars) 2009;69(4):345–350. [PubMed] [Google Scholar]
- 61.Organization PAH, Organization WH . Washington, D.C; PAHO/WHO: 2017. Epidemiological Update: Yellow Fever. 3 April. [Google Scholar]
- 62.Gratz Critical review of the vector status of Aedes albopictus. Med. Vet. Ent. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 63.Rudnick A., Hammon W.M.D. Newly recognized Aedes aegypti problems in Manila and Bangkok. Mosq. News. 1960;20:247–249. [Google Scholar]
- 64.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christie J. Remarks on "Kidinga Pepo": a peculiar form of exanthematous disease. Br. Med. J. 1872;1(596):577–579. doi: 10.1136/bmj.1.596.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carey D.E. Chikungunya and dengue: a case of mistaken identity? J. Hist. Med. Allied Sci. 1971;26:243–262. doi: 10.1093/jhmas/xxvi.3.243. [DOI] [PubMed] [Google Scholar]
- 67.Morens D.M., Fauci A.S. Chikungunya at the Door - Déjà Vu All Over Again? N. Engl. J. Med. 2014;371:885–887. doi: 10.1056/NEJMp1408509. [DOI] [PubMed] [Google Scholar]
- 68.Lumsden W.H.R. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53. II. General description and epidemiology. Trans. R. Soc. Trop. Med. Hyg. 1955;49:33–57. doi: 10.1016/0035-9203(55)90081-x. [DOI] [PubMed] [Google Scholar]
- 69.Ross R.W. The Newala Epidemic. III. The virus: isolation pathogenic properties and relationship to the epidemic. J. Hyg. (Lond.) 1956;54:177–191. doi: 10.1017/s0022172400044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of Chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000;81(Pt 2):471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 71.de Lamballerie X., Leroy E., Charrel R.N., Ttsetsarkin K., Higgs S., Gould E.A. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol. J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vanlandingham D.L., Tsetsarkin K., Klingler K.A., Hong C., McElroy K.L., Lehane M.J. Determinants of vector specificity of o'nyong nyong and chikungunya viruses in Anopheles and Aedes mosquitoes. Am. J. Trop. Med. Hyg. 2006;74(4):663–669. [PubMed] [Google Scholar]
- 73.Arankalle V.A., Shrivastava S., Cherian S., Gunjikar R.S., Walimbe A.M., Jadhav S.M. Genetic divergence of Chikungunya viruses in India (1963-2006) with special reference to the 2005-2006 explosive epidemic. J. Gen. Virol. 2007;88(Pt 7):1967–1976. doi: 10.1099/vir.0.82714-0. [DOI] [PubMed] [Google Scholar]
- 74.Panning M., Grywna K., van Esbroeck M., Emmerich P., Drosten C. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerg. Infect. Dis. 2008;14:416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lanciotti R.S., Kosoy O.L., Laven J.J., Panella A.J., Velez J.O., Lambert A.J. Chikungunya virus in US travelers returning from India, 2006. Emerg. Infect. Dis. 2007;13(5):764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen R., Puri V., Fedorova N., Lin D., Hari K.L., Jain R. Comprehensive genome scale phylogenetic study provides new insights on the global expansion of chikungunya virus. J. Virol. 2016;90(23):10600–10611. doi: 10.1128/JVI.01166-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dick G. Zika virus. II. Pathogenicity and physical properties. Trans. R. Soc. Trop. Med. Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 78.Nutt C., Adams P. Zika in Africa—the invisible epidemic? Lancet. 2017;389:1595–1596. doi: 10.1016/S0140-6736(17)31051-6. [DOI] [PubMed] [Google Scholar]
- 79.Musso D., Roche C., Robin E., Nhan T., Teissier A., Cao-Lormeau V.M. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015;21(2):359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faye O., Freire C.C., Iamarino A., de Oliveira J.V., Diallo M., Zanotto P.M. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl. Trop. Dis. 2014;8(1):e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marchette N.J., Garcia R., Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969;18(3):411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 82.Duffy M.R., Chen T.H., Hancock W.T., Powers A.M., Kool J.L., Lanciotti R.S. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 83.Tatem A.J., Hay S.I., Rogers D.J. Global traffic and disease vector dispersal. Proc. Natl. Acad. Sci. 2006;103:6242–6247. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trpis M., Hausermann W. Genetics of house-entering behavior in East African populations of Aedes aegypti (L) (Diptera: Culicidae) and its relevance to speciation. Bull. Entomol. Res. 1978;68:521–532. [Google Scholar]
- 85.Forrester N.L., Palacios G., Tesh R.B., Savji N., Guzman H., Sherman M. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J. Virol. 2012;86(5):2729–2738. doi: 10.1128/JVI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prevention CfDCa . CDC Features; 2015. Japanese Encephalitis. [Google Scholar]
- 87.Sucharit S., Surathin K., Shrestha S.R. Vectors of Japanese encephalitis virus (JEV): species complexes of the vectors. Southeast Asian J. Trop. Med. Public Health. 1989;20(4):611–621. [PubMed] [Google Scholar]
- 88.Kim H., Cha G.W., Jeong Y.E., Lee W.G., Chang K.S., Roh J.Y. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS One. 2015;10(2):e0116547. doi: 10.1371/journal.pone.0116547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ricklin M.E., Garcia-Nicolas O., Brechbuhl D., Python S., Zumkehr B., Nougairede A. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat. Commun. 2016;7:10832. doi: 10.1038/ncomms10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caini S., Szomor K., Ferenczi E., Székelyné Gaspar Á., Csohán Á., Krisztalovics K. Tick-borne encephalitis transmitted by unpasteurised cow milk in western Hungary. Eur. Surveill. 2011;17(12) [PubMed] [Google Scholar]
- 91.Kuno G. Transmission of arboviruses without involvement of arthropod vectors. Acta Virol. 2001;45(3):139–150. [PubMed] [Google Scholar]
- 92.Komar N., Lanciotti R., Bowen R., Langevin S., Bunning M. Detection of West Nile virus in oral and cloacal swabs collected from bird carcasses. Emerg. Infect. Dis. 2002;8(7):741–742. doi: 10.3201/eid0807.020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gresser I., Hardy J.L., Hu S.M., Scherer W.F. Factors influencing transmission of Japanese B encephalitis virus by a colonized strain of Culex tritaeniorhynchus Giles, from infected pigs and chicks to susceptible pigs and birds. Am. J. Trop. Med. Hyg. 1958;7(4):365–373. doi: 10.4269/ajtmh.1958.7.365. [DOI] [PubMed] [Google Scholar]
- 94.Simpson D.I., Smith C.E., Marshall T.F., Platt G.S., Way H.J., Bowen E.T. Arbovirus infections in Sarawak: the role of the domestic pig. Trans. R. Soc. Trop. Med. Hyg. 1976;70(1):66–72. doi: 10.1016/0035-9203(76)90010-9. [DOI] [PubMed] [Google Scholar]
- 95.Impoinvil D.E., Baylis M., Solomon T. Japanese encephalitis: on the One Health agenda. Curr. Top. Microbiol. Immunol. 2013;365:205–247. doi: 10.1007/82_2012_243. [DOI] [PubMed] [Google Scholar]
- 96.van den Hurk A.F., Ritchie S.A., Mackenzie J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 97.Campbell G.L., Hills S.L., Fischer M., Jacobson J.A., Hoke C.H., Hombach J.M. Estimated global incidence of Japanese encephalitis: a systematic review. Bull. World Health Organ. 2011;89(10):766–774. doi: 10.2471/BLT.10.085233. (74A-74E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mitamura T.K., Mori M., Okubo K. Kansai Iji, 260–261:1-5. Isolation of Japanese encephalitis virus from the brain of a human. Kansai Iji. 1935;260–261:1–5. [Google Scholar]
- 99.Mackenzie J.S., Gubler D.J., Petersen L.R. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004;10(12 Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 100.Gao X., Liu H., Wang H., Fu S., Guo Z., Liang G. Southernmost Asia is the source of Japanese encephalitis virus (genotype 1) diversity from which the viruses disperse and evolve throughout Asia. PLoS Negl. Trop. Dis. 2013;7(9):e2459. doi: 10.1371/journal.pntd.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solomon T., Ni H., Beasley D.W., Ekkelenkamp M., Cardosa M.J., Barrett A.D. Origin and evolution of Japanese encephalitis virus in southeast Asia. J. Virol. 2003;77(5):3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mohammed M.A., Galbraith S.E., Radford A.D., Dove W., Takasaki T., Kurane I. Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype. Infect. Genet. Evol. 2011;11(5):855–862. doi: 10.1016/j.meegid.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 103.Li M.H., Fu S.H., Chen W.X., Wang H.Y., Guo Y.H., Liu Q.Y. Genotype v Japanese encephalitis virus is emerging. PLoS Negl. Trop. Dis. 2011;5(7):e1231. doi: 10.1371/journal.pntd.0001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schuh A.J., Guzman H., Tesh R.B., Barrett A.D. Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987. Vector Borne Zoonotic Dis. 2013;13(7):479–488. doi: 10.1089/vbz.2011.0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gao X., Liu H., Li M., Fu S., Liang G. Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol. J. 2015;12(43) doi: 10.1186/s12985-015-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yun S.M., Cho J.E., Ju Y.R., Kim S.Y., Ryou J., Han M.G. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983–2005. Virol. J. 2010;7:127. doi: 10.1186/1743-422X-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nitatpattana N., Dubot-Peres A., Gouilh M.A., Souris M., Barbazan P., Yoksan S. Change in Japanese encephalitis virus distribution, Thailand. Emerg. Infect. Dis. 2008;14(11):1762–1765. doi: 10.3201/eid1411.080542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nga P.T., del Carmen Parquet M., Cuong V.D., Ma S.P., Hasebe F., Inoue S. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J. Gen. Virol. 2004;85(Pt 6):1625–1631. doi: 10.1099/vir.0.79797-0. [DOI] [PubMed] [Google Scholar]
- 109.Wang H.Y., Takasaki T., Fu S.H., Sun X.H., Zhang H.L., Wang Z.X. Molecular epidemiological analysis of Japanese encephalitis virus in China. J. Gen. Virol. 2007;88(Pt 3):885–894. doi: 10.1099/vir.0.82185-0. [DOI] [PubMed] [Google Scholar]
- 110.Cao L., Fu S., Gao X., Li M., Cui S., Li X. Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PLoS Negl. Trop. Dis. 2016;10(5):e0004686. doi: 10.1371/journal.pntd.0004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patsoula E., Beleri S., Vakali A., Pervanidou D., Tegos N., Nearchou A. Records of Aedes albopictus (Skuse, 1894) (Diptera; Culicidae) and Culex tritaeniorhynchus (Diptera; Culicidae) Expansion in Areas in Mainland Greece and Islands. Vector Borne Zoonotic Dis. 2017;17(3):217–223. doi: 10.1089/vbz.2016.1974. [DOI] [PubMed] [Google Scholar]
- 112.Simon-Loriere E., Faye O., Prot M., Casademont I., Fall G., Fernandez-Garcia M.D. Autochthonous Japanese encephalitis with yellow fever coinfection in Africa. N. Engl. J. Med. 2017;376(15):1483–1485. doi: 10.1056/NEJMc1701600. [DOI] [PubMed] [Google Scholar]
- 113.Gao X., Li X., Li M., Fu S., Wang H., Lu Z. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951–2011. PLoS Negl. Trop. Dis. 2014;8(8):e3015. doi: 10.1371/journal.pntd.0003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buckley A., Dawson A., Moss S.R., Hinsley S.A., Bellamy P.E., Gould E.A. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J. Gen. Virol. 2003;84:2807–2817. doi: 10.1099/vir.0.19341-0. [DOI] [PubMed] [Google Scholar]
- 115.Buckley A., Dawson A., Gould E.A. Detection of seroconversion to West Nile virus, Usutu virus and Sindbis virus in UK sentinel chickens. Virol. J. 2006;3:71. doi: 10.1186/1743-422X-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rizzoli A., Rosa R., Rosso F., Buckley A., Gould E. West Nile virus circulation detected in northern Italy in sentinel chickens. Vector Borne and Zoonotic Dis. 2007;7:411–417. doi: 10.1089/vbz.2006.0626. [DOI] [PubMed] [Google Scholar]
- 117.Hubalek Z., Halouzka J. West Nile fever--a reemerging mosquito-borne viral disease in Europe. Emerg. Infect. Dis. 1999;5(5):643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gould E.A. Evolution of the Japanese encephalitis serocomplex viruses. Curr. Top. Microbiol. Immunol. 2002;267:391–404. doi: 10.1007/978-3-642-59403-8_19. [DOI] [PubMed] [Google Scholar]
- 119.ArboNET C. Mosquito Species in Which West Nile Virus Has Been Detected, United States. 1999–2012. https://wwwcdcgov/westnile/resources/pdfs/mosquitospecies1999-2012pdf
- 120.Perez-Ramirez E., Llorente F., Jimenez-Clavero M.A. Experimental infections of wild birds with West Nile virus. Viruses. 2014;6(2):752–781. doi: 10.3390/v6020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gubler D.J. The continuing spread of West Nile virus in the western hemisphere. Clin. Infect. Dis. 2007;45(8):1039–1046. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 122.Bernkopf H., Levine S., Nerson R. Isolation of West Nile virus in Israel. J Infect Dis. 1953;93:207–218. doi: 10.1093/infdis/93.3.207. [DOI] [PubMed] [Google Scholar]
- 123.Bakonyi T., Ferenczi E., Erdelyi K., Kutasi O., Csorgo T., Seidel B. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2017;165(1–2):61–70. doi: 10.1016/j.vetmic.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Danis K., Papa A., Theocharopoulos G., Dougas G., Athanasiou M., Detsis M. Outbreak of West Nile virus infection in Greece, 2010. Emerg. Infect. Dis. 2010;17(10):1868–1872. doi: 10.3201/eid1710.110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: review of the literature. JAMA. 2013;310(3):308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prevention CfDCa . 2016. CDC Features West Nile Virus. [Google Scholar]
- 127.Muckenfuss R.S., Armstrong C., Webster L.T. Etiology of the 1933 epidemic of encephalitis. JAMA. 1934;103(10):731–733. [Google Scholar]
- 128.Reisen W.K. Epidemiology of St Louis encephalitis virus. Adv. Virus Res. 2003;61:139–183. doi: 10.1016/s0065-3527(03)61004-3. [DOI] [PubMed] [Google Scholar]
- 129.Kopp A., Gillespie T.R., Hobelsberger D., Estrada A., Harper J.M., Miller R.A. Provenance and geographic spread of St. Louis encephalitis virus. MBio. 2013;4(3):e00322-13. doi: 10.1128/mBio.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.White G.S., Symmes K., Sun P., Fang Y., Garcia S., Steiner C. Reemergence of St. Louis encephalitis virus, California, 2015. Emerg. Infect. Dis. 2016;22(12):2185–2188. doi: 10.3201/eid2212.160805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gould E.A., Moss S.R., Turner S.L. Evolution and dispersal of encephalitic flaviviruses. Archs Virol. 2004;(18):65–84. doi: 10.1007/978-3-7091-0572-6_6. Supplementum. [DOI] [PubMed] [Google Scholar]
- 132.Auguste A.J., Pybus O.G., Carrington C.V. Evolution and dispersal of St. Louis encephalitis virus in the Americas. Infect. Genet. Evol. 2009;9(4):709–715. doi: 10.1016/j.meegid.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 133.Townroe S., Callaghan A. British container breeding mosquitoes: the impact of urbanisation and climate change on community composition and phenology. PLoS One. 2014;9(4):e95325. doi: 10.1371/journal.pone.0095325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fonseca D.M., Keyghobadi N., Malcolm C.A., Mehmet C., Schaffner F., Mogi M. Emerging vectors in the Culex Pipiens Complex. Science, N. Y. 2004;303(5663):1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- 135.Selvey L.A., Dailey L., Lindsay M., Armstrong P., Tobin S., Koehler A.P. The changing epidemiology of Murray Valley encephalitis in Australia: the 2011 outbreak and a review of the literature. PLoS Negl. Trop. Dis. 2014;8(1):e2656. doi: 10.1371/journal.pntd.0002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karabatsos N. 3rd ed. American Society for Tropical Medicine and Hygiene; San Antonio, Texas: 1985. International Catalogue of Arthropod-borne Viruses; pp. 137–152. Suppl. 1. [Google Scholar]
- 137.Mackenzie J.S., Williams D.T. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56(6–7):338–356. doi: 10.1111/j.1863-2378.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 138.Weissenbock H., Kolodziejek J., Url A., Lussy H., Rebel-Bauder B., Nowotny N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, Central Europe. Emerg. Infect. Dis. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manarolla G., Bakonyi T., Gallazzi D., Crosta L., Weissenbock H., Dorrestein G.M. Usutu virus in wild birds in northern Italy. Vet. Microbiol. 2010;141(1–2):159–163. doi: 10.1016/j.vetmic.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 140.Cadar D., Luhken R., van der Jeugd H., Garigliany M., Ziegler U., Keller M. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Eur. Surveill. 2017;22(4) doi: 10.2807/1560-7917.ES.2017.22.4.30452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grottola A., Marcacci M., Tagliazucchi S., Gennari W., Di Gennaro A., Orsini M. Usutu virus infections in humans: a retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017;23(1):33–37. doi: 10.1016/j.cmi.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 142.Cadar D., Becker N., Campos Rde M., Borstler J., Jost H., Schmidt-Chanasit J. Usutu virus in bats, Germany, 2013. Emerg. Infect. Dis. 2014;20(10):1771–1773. doi: 10.3201/eid2010.140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cadar D., Maier P., Muller S., Kress J., Chudy M., Bialonski A. Blood donor screening for West Nile virus (WNV) revealed acute Usutu virus (USUV) infection, Germany, September 2016. Eur. Surveill. 2016;22(14) doi: 10.2807/1560-7917.ES.2017.22.14.30501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Haddow A.D., Nasar F., Guzman H., Ponlawat A., Jarman R.G., Tesh R.B. Genetic Characterization of Spondweni and Zika Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl. Trop. Dis. 2016;10(10):e0005083. doi: 10.1371/journal.pntd.0005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.LaBeaud A.D., Banda T., Brichard J., Muchiri E.M., Mungai P.L., Mutuku F.M. High rates of o'nyong nyong and chikungunya virus transmission in coastal Kenya. PLoS Negl. Trop. Dis. 2015;9(2):e0003436. doi: 10.1371/journal.pntd.0003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson B.K., editor. O'nyong-nyong Virus Disease. CRC Press; Boca Raton, F. L.: 1988. [Google Scholar]
- 147.Abdo-Salem S., Tran A., Grosbois V., Gerbier G., Al-Qadasi M., Saeed K. Can environmental and socioeconomic factors explain the recent emergence of Rift Valley fever in Yemen, 2000–2001? Vector Borne Zoonotic Dis. 2011;11(6):773–779. doi: 10.1089/vbz.2010.0084. [DOI] [PubMed] [Google Scholar]
- 148.Jeanmaire E.M., Rabenarivahiny R., Biarmann M., Rabibisoa L., Ravaomanana F., Randriamparany T. Prevalence of Rift Valley fever infection in ruminants in Madagascar after the 2008 outbreak. Vector Borne Zoonotic Dis. 2011;11(4):395–402. doi: 10.1089/vbz.2009.0249. [DOI] [PubMed] [Google Scholar]
- 149.Andriamandimby S.F., Randrianarivo-Solofoniaina A.E., Jeanmaire E.M., Ravololomanana L., Razafimanantsoa L.T., Rakotojoelinandrasana T. Rift Valley fever during rainy seasons, Madagascar, 2008 and 2009. Emerg. Infect. Dis. 2010;16(6):963–970. doi: 10.3201/eid1606.091266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fontenille D. Arbovirus transmission cycles in Madagascar. Arch. Inst. Pasteur Madagascar. 1989;55(1):1–317. [PubMed] [Google Scholar]
- 151.Tantely M.L., Rakotoniaina J.C., Tata E., Andrianaivolambo L., Razafindrasata F., Fontenille D. Biology of mosquitoes that are potential vectors of Rift Valley fever virus in different biotopes of the central highlands of Madagascar. J. Med. Entomol. 2013;50(3):603–610. doi: 10.1603/me12069. [DOI] [PubMed] [Google Scholar]
- 152.Tantely L.M., Boyer S., Fontenille D. A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 2014;92(4):722–729. doi: 10.4269/ajtmh.14-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tantely L.M., Boyer S., Fontenille D. A review of mosquitoes associated with Rift Valley fever virus in Madagascar. Am. J. Trop. Med. Hyg. 2015;92(4):722–729. doi: 10.4269/ajtmh.14-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Diallo M., Lochouarn L., Ba K., Sall A.A., Mondo M., Girault L. First isolation of the Rift Valley fever virus from Culex poicilipes (Diptera: Culicidae) in nature. Am. J. Trop. Med. Hyg. 2000;62(6):702–704. doi: 10.4269/ajtmh.2000.62.702. [DOI] [PubMed] [Google Scholar]
- 155.Turell M.J., Faran M.E., Cornet M., Bailey C.L. Vector competence of Senegalese Aedes fowleri (Diptera: Culicidae) for Rift Valley fever virus. J. Med. Entomol. 1988;25(4):262–266. doi: 10.1093/jmedent/25.4.262. [DOI] [PubMed] [Google Scholar]
- 156.Chamchod F., Cosner C., Cantrell R.S., Beier J.C., Ruan S. Transmission dynamics of Rift Valley fever virus: effects of live and killed vaccines on epizootic outbreaks and enzootic maintenance. Front. Microbiol. 2016;6:1568. doi: 10.3389/fmicb.2015.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Preventions CfDCa . 2013. Rift Valley Fever. [Google Scholar]
- 158.Vanlandingham D.L., Higgs S., Huang Y.J. Aedes Albopictus (Diptera: Culicidae) and mosquito-borne viruses in the United States. J. Med. Entomol. 2016;53(5):1024–1028. doi: 10.1093/jme/tjw025. [DOI] [PubMed] [Google Scholar]
- 159.Rizzoli A., Jimenez-Clavero M.A., Barzon L., Cordioli P., Figuerola J., Koraka P. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Eur. Surveill. 2015;20(20) doi: 10.2807/1560-7917.es2015.20.20.21135. [DOI] [PubMed] [Google Scholar]