Differences in the chloroplast lipid importer subunits of Arabidopsis and Brachypodium, 16:3 and 18:3 plants, respectively, explain the unique phenotypes of the respective reduced function mutants.
Abstract
The import of lipids into the chloroplast is essential for photosynthetic membrane biogenesis. This process requires an ABC transporter in the inner envelope membrane with three subunits, TRIGALACTOSYLDIACYLGLYCEROL (TGD) 1, 2, and 3, named after the oligogalactolipids that accumulate in the respective Arabidopsis thaliana mutants. Unlike Arabidopsis, in the model grass Brachypodium distachyon, chloroplast lipid biosynthesis is largely dependent on imported precursors, resulting in a characteristic difference in chloroplast lipid acyl composition between the two plants. Accordingly, Arabidopsis is designated as a 16:3 (acyl carbons:double bounds) plant and Brachypodium as an 18:3 plant. Repression of TGD1 (BdTGD1) in Brachypodium affected growth without triggering oligogalactolipid biosynthesis. Moreover, expressing BdTGD1 in the Arabidopsis tgd1-1 mutant restored some phenotypes but did not reverse oligogalactolipid biosynthesis. A 27-amino acid loop (L45) is solely responsible for the incomplete functioning of BdTGD1 in Arabidopsis tgd1-1. Coevolutionary analysis and coimmunoprecipitation assays showed that the TGD1 L45 loop interacts with the mycobacterial cell entry domain of TGD2. To explain the observed differences in oligogalactolipid biosynthesis between the two species, we suggest that excess monogalactosyldiacylglycerol derived from chloroplast-derived precursors in Arabidopsis tgd1-1 is converted into oligogalactolipids, a process absent from Brachypodium with reduced TGD1 levels, which assembles monogalactosyldiacylglycerol exclusively from imported precursors.
INTRODUCTION
Photosynthetic membranes are organized into thylakoids, which are some of the most complex membrane systems found in nature (Hurlock et al., 2014). The galactoglycerolipids monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) are the predominant lipids in thylakoid membranes and are indispensable for photosynthesis (Bastien et al., 2016). Diacylglycerol (DAG) is the precursor for glycerolipid biosynthesis in the chloroplast envelope. DAG precursors can be synthesized either by the prokaryotic pathway in the chloroplast inner envelope membrane or by the eukaryotic pathway that provides precursors assembled at the endoplasmic reticulum (ER) for chloroplast membrane lipids (Hurlock et al., 2014; Bastien et al., 2016). As a consequence of distinct substrate specificities of the involved acyltransferases (Ohlrogge and Browse, 1995), galactoglycerolipids from the prokaryotic pathway contain a C16 fatty acids (FAs) attached to the sn-2 position of the glycerol moiety, while those from the eukaryotic pathway contain a C18 FA at the sn-2 position (Frentzen et al., 1983; Joyard et al., 1991). The relative contribution of the prokaryotic and eukaryotic pathways to galactoglycerolipid biosynthesis is not conserved across different plant species. In 16:3 (acyl carbon:double bonds) plants including Arabidopsis thaliana, both pathways are utilized, while 18:3 plants, such as the model grass Brachypodium distachyon, rely exclusively on the eukaryotic pathway for galactoglycerolipid biosynthesis (Mongrand et al., 1998). Although precursor import from the ER is the major route for chloroplast glycerolipid biosynthesis in 18:3 plants, our current understanding of chloroplast lipid import is mostly based on 16:3 plants.
Bacteria and chloroplasts, descendants of a photosynthetic bacterial progenitor, have conserved mechanisms for lipid transport across their membranes. In bacteria, ABC transporters in the MKL family provide a mechanism of lipid transfer, which contributes to the maintenance of membrane integrity (Dassa and Bouige, 2001; Dassa, 2011; Li et al., 2012). In plants, TRIGALACTOSYLDIACYLGLYCEROL (TGD) 1, 2, and 3 form a bacterial-type ABC lipid importer in the chloroplast inner envelope membrane (Roston et al., 2012). These subunits are closely related to the MKL family of transporters (Dassa and Bouige, 2001; Li et al., 2012), and presumed orthologs are common to microalgae and land plants (Hori et al., 2016). In the Arabidopsis TGD1, 2, 3 complex, TGD1 is predicted to be the permease protein, forming a transmembrane channel of an ABC transporter with multiple membrane-spanning domains (Xu et al., 2003). TGD2 encodes a phosphatidic acid (PtdOH) binding protein tethered to the chloroplast inner envelope membrane facing the outer envelope membrane and resembling the membrane-tethered substrate binding protein of some bacterial ABC transporters (Awai et al., 2006; Lu and Benning, 2009). TGD3 encodes a small ATPase (Lu et al., 2007). Mutations in any of these three TGD proteins in Arabidopsis cause a reduction in ER-derived galactoglycerolipids in the chloroplasts. Beyond this, the Arabidopsis tgd mutants show a complex lipid phenotype, including the accumulation of triacylglycerol (TAG) in leaves, a decrease of C18:3 FAs in the galactoglycerolipids, and the accumulation of oligogalactoglycerolipids, such as trigalactosyldiacylglycerol (TGDG). The latter phenotype led to the naming of these proteins. Oligogalactoglycerolipids are synthesized as a result of the activation of a galactolipid:galactolipid galactosyltransferase encoded by SENSITIVE TO FREEZING2 (SFR2) (Xu et al., 2003; Moellering et al., 2010).
As ABC lipid transporters evolve new functionalities in different organisms, coevolution of each component of the transporter is required. Coevolution in this case refers to coordinated changes that occur in pairs of biomolecules, i.e., protein complex subunits, to maintain or to refine functional interactions between these pairs (Pazos and Valencia, 2008; de Juan et al., 2013). At the molecular level, coevolution of a protein pair directly affects physical contact or any other direct interaction, such as the transfer of energy or substrates between them. Within a given protein complex, the components are expected to coevolve and share similarities in their evolutionary histories, which can be quantified through the correlation of the respective phylogenetic trees (Pazos and Valencia, 2008). Coevolution-based computational methods have been developed to predict the interaction domains in proteins that are known to interact (Jothi et al., 2006; Ovchinnikov et al., 2015).
Here, we set out to explore whether the TGD1, 2, 3 complexes of 16:3 and 18:3 plants have diverged in their function, as thylakoid lipid assembly entirely relies on lipid precursor import in the latter. Toward this end, we identified the TGD1 ortholog of Brachypodium, an 18:3 plant, and showed that it is involved in chloroplast lipid import in this grass. Employment of a computational approach integrated with extensive heterologous complementation analysis in an Arabidopsis tgd1-1 mutant background identified a species-specific, coevolving, and functionally relevant motif pair within two TGD components.
RESULTS
Prediction of the Coevolution of TGD1, 2, and 3 in Different Organisms
TGD1, 2, and 3 orthologous proteins are commonly found in both microalgae and land plants (Hori et al., 2016), and they tend to form a stable complex in vivo (Roston et al., 2012). Therefore, we hypothesized that each component interacts in intricate and functionally essential ways with each other and that this interaction requires coevolution of the interacting proteins in the contact domains as plants diverge during evolution. Conducting an in silico interprotein coevolutionary analysis using the MirrorTree server (http://csbg.cnb.csic.es/mtserver) and STRING database (http://www.string-db.org), we were able to predict with high confidence functional and/or physical interaction between TGD1 and TGD2 or TGD3, and between TGD2 and TGD3 (Supplemental Table 1). We will explore these interactions and their species-specific coevolution in the Brachypodium and Arabidopsis TGD1, 2, 3 complexes. We chose these two model plants because we questioned whether TGD-dependent lipid transport is conserved between a 16:3 and 18:3 plant, Arabidopsis and Brachypodium, respectively.
Repression of BdTGD1 Impaired Growth and Development in Brachypodium
In the TGD complex, the TGD2 and TGD3 proteins are located on opposite leaflets of the chloroplast inner envelope membrane, with TGD1 being predicted to cross the membrane multiple times, making contact with both TGD2 and TGD3. TGD1 was chosen as the initial target subunit to disturb the complex in an 18:3 plant. We first identified a possible TGD1 ortholog in Brachypodium by BLASTP analysis in Phytozome (http://www.phytozome.net/) using the Arabidopsis TGD1 sequence as the query. Only one protein encoded by Bradi5g17820 was identified as a possible ortholog. Alignment of AtTGD1 and Bradi5g17820 showed 73% sequence identity and 87% sequence similarity over 350 amino acids. Therefore, we tentatively designated Bradi5g17820 as BdTGD1.
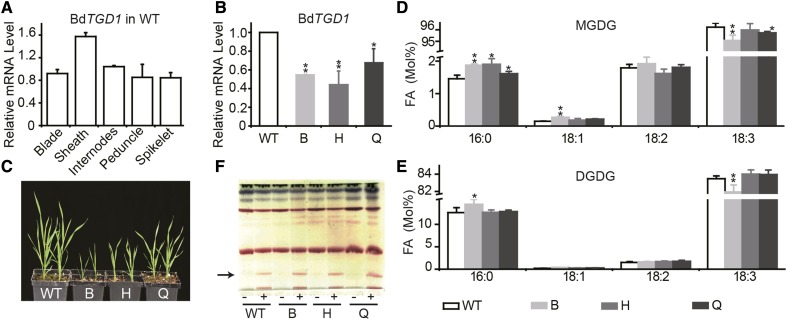
To explore the function of BdTGD1 in Brachypodium, we measured the abundance of BdTGD1 mRNA in different tissues (Supplemental Figures 1A to 1F) by quantitative RT-PCR using UBIQUITIN-CONJUGATING ENZYME18 (BdUBC18, Bradi4g00660) as an internal control (Hong et al., 2008). Similar to AtTGD1 (http://jsp.weigelworld.org/expviz/expviz.jsp?experiment=developmentandnormalization=absoluteandprobesetcsv=At1g19800andaction=Run), BdTGD1 showed substantial expression in both vegetative and reproductive tissues (Figure 1A; Supplemental Figures 1A to 1F). We next used an RNA interference (RNAi) approach to reduce BdTGD1 expression in Brachypodium using the cauliflower mosaic 35S promoter (Pro35S) to drive the expression of the RNAi construct. Three representative lines, B, H, and Q, were selected for further analysis. BdTGD1 transcript levels in leaf blades of 4-week-old transgenic plants were 50, 60, and 33% lower than in wild-type Bd21-3 (Figure 1B). In Arabidopsis, reduced functioning of AtTGD1 causes a growth deficiency, including reduced plant height (Xu et al., 2005) and reduced chlorophyll content in leaves (Xu et al., 2003), while complete loss of function appears to be embryo-lethal (Xu et al., 2005). Similarly, all Brachypodium Pro35S:BdTGD1 RNAi lines had stunted growth (Figure 1C), with a shorter stature (Supplemental Figure 1G), fewer primary tillers (Supplemental Figure 1H), and fewer internodes in the main stem than wild type (Supplemental Figure 1I). The chlorophyll content was also significantly reduced in these lines (Supplemental Figure 1J).
Figure 1.
Plant Growth and Development of Brachypodium Pro35S:BdTGD1 RNAi Lines.
(A) and (B) Relative BdTGD1 mRNA levels in different tissues of wild-type Bd21-3 (A) and 4-week-old Pro35S:BdTGD1RNAi lines (B). Different Brachypodium tissues were harvested as described in Supplemental Figures 1A to 1F. Total RNA was isolated and relative mRNA levels were determined by quantitative RT-PCR using BdUBC18 as an internal control. Different tissues from one plant were pooled together for one sample. The error bars represent sd; n = three independent plants. Significant differences are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(C) Developmental phenotypes of Pro35S:BdTGD1 RNAi lines. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(D) FA composition of MGDG of 4-week-old Brachypodium leaf blades. The molar percentages of individual FAs relative to total FAs in MGDG were quantified using GC-FID of FAMEs. The error bars represent sd; n = three independent plants. Significant differences are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(E) FA composition of DGDG of 4-week-old Brachypodium leaf blades. Molar percentages of individual FAs relative to total FAs in DGDG were quantified by GC-FID of FAMEs. The error bars represent sd; n = three independent plants. Significant differences are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(F) Analysis of glycolipids. A thin-layer chromatogram of polar lipids is shown. Total lipids were extracted form 4-week-old Brachypodium leaf blades. Glycolipids were visualized by α-naphthol staining. The arrow indicates TGDG; + indicates lipid preparation 24 h after 1M MgCl2 infiltration; − indicates lipid samples from leaf blades without Mg2+ treatment. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
The Arabidopsis tgd1-1 mutant has a diagnostically altered FA composition of MGDG and DGDG (Xu et al., 2003, 2005) with a reduced 18:3/16:3 (carbon:double bonds) ratio in MGDG and DGDG. For Brachypodium, an 18:3 plant lacking 16:3-containing thylakoid lipids, we observed a subtler change in the FA composition of each lipid in the BdTGD1 RNAi line, as expected. However, these subtle changes were statistically significant. The relative amounts of 16:0 and 18:1 were increased at the expense of 18:3 in MGDG (Figure 1D) and DGDG (Figure 1E) in Pro35S:BdTGD1 RNAi leaf blades without altering the relative lipid levels (Supplemental Figure 2). Aside from the strong decrease in the 18:3/16:3 ratio observed for Arabidopsis, the changes in other FAs were similar to those observed for the Arabidopsis tgd1-1 mutant. It should be noted that transgenic line B, with the most pronounced lipid phenotype, which can be taken as an indicator for reduced abundance or functionality of the TGD1 protein, also displayed the most severe growth phenotype among the three independent lines (Figure 1C), even though TGD1 transcript levels were not the most strongly reduced in this line (Figure 1B).
In addition to changes in the acyl compositions of MGDG and DGDG, reduced-function mutations in any of the TGD proteins of Arabidopsis lead to the accumulation of oligogalactoglycerolipids such as TGDG due to the activation of SFR2 in leaves (Xu et al., 2003; Awai et al., 2006; Lu et al., 2007). Surprisingly, no TGDG was observed in leaf blade lipid extracts of the BdTGD1 RNAi lines, although Brachypodium has a functional SFR2, which can be activated by the infiltration of leaf blades with Mg2+ (Figure 1F) as in Arabidopsis (Barnes et al., 2016). In addition, the Brachypodium genome contains one possible SFR2 ortholog (XP003577231.1). Alignment of AtSFR2 and XP003577231.1 showed 59% sequence identity over 656 amino acids. Thus, the lack of TGDG accumulation in the leaf blades of Brachypodium Pro35S:BdTGD1 RNAi lines was not caused by the absence of SFR2. Instead, it seemed possible that SFR2 activation in the Arabidopsis tgd1-1 mutant depends on the presence of an active prokaryotic pathway. To test this possibility, we examined Arabidopsis tgd1-1 mutant embryos for the presence of TGDG because it was previously shown that the flux through the prokaryotic pathway is strongly reduced in these tissues, while similar to 18:3 plants, the eukaryotic pathway is predominant in embryos (Xu et al., 2005). Indeed, TGDG was also not detected in Arabidopsis tgd1-1 developing embryos (Supplemental Figure 3).
Effects of TGD1 Repression on Seed Development and Seed Lipid Metabolism
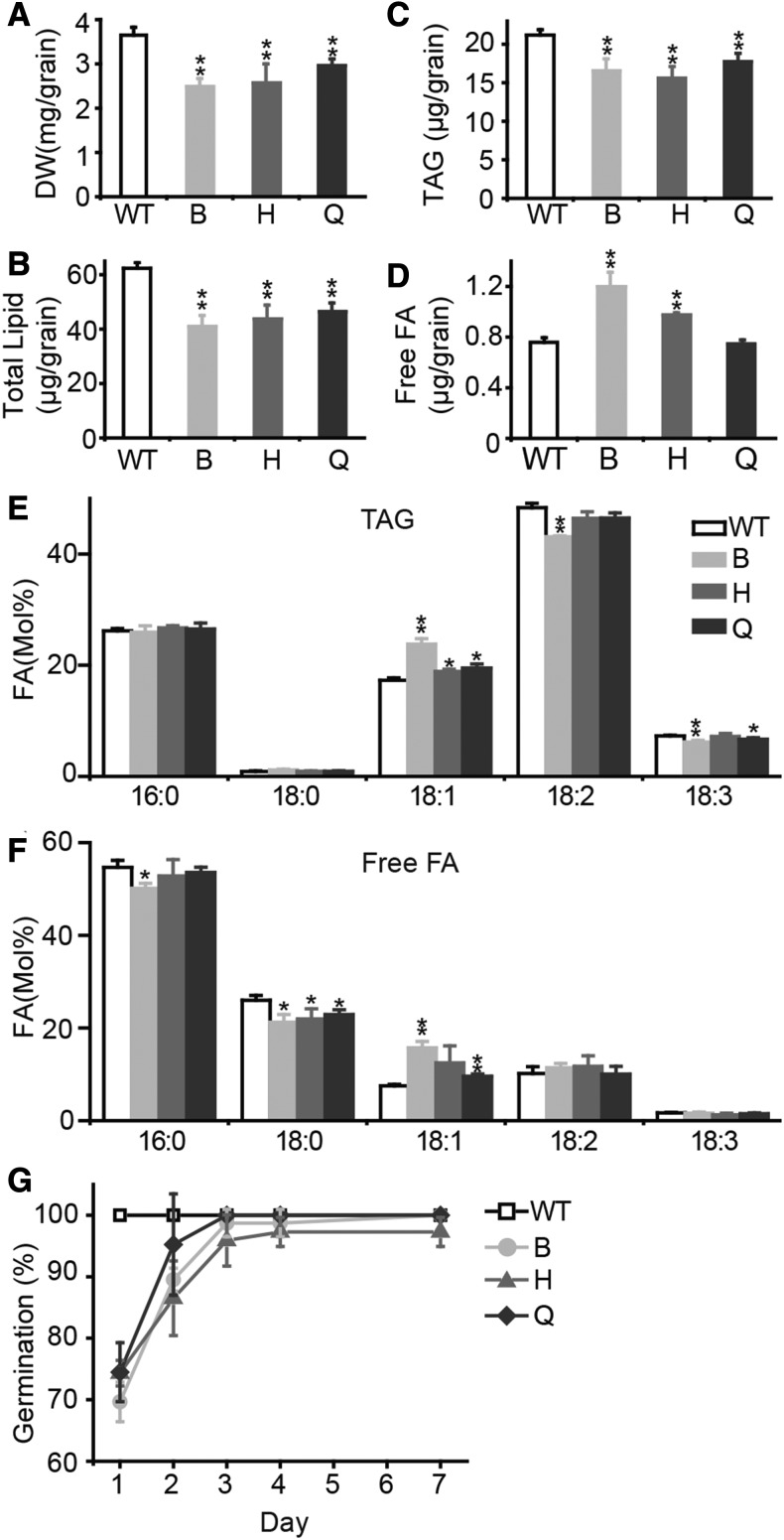
Because BdTGD1 is expressed in vegetative and reproductive tissues (Figure 1A), we expected that TGD1 might also be important for embryo lipid metabolism and embryo development. Indeed, reduction of BdTGD1 expression in Brachypodium led to a 32% reduction in grain dry weight (Figure 2A). Total FA contents (Figure 2B) and TAG contents (Figure 2C) were reduced, while the levels of free FAs were up to ∼1.7-fold higher in the strongest BdTGD1 RNAi line (Figure 2D). The acyl group profiles of TAG (Figure 2E) and free FAs (Figure 2F) were altered as well, with slight decreases in 18:3 and an increase in 18:1. Germination of Pro35S:BdTGD1 RNAi grains was delayed. Only 70% of the Pro35S:BdTGD1 RNAi grains germinated after 24 h in the light, compared with 100% germination of wild-type grains (Figure 2G). In the Arabidopsis tgd1-1 mutant carrying a leaky allele, ∼50% of the seeds are aborted (Xu et al., 2005). Interestingly, similar lipid phenotypes were also observed in the Arabidopsis tgd1-1 aborted seeds (Supplemental Figure 4) compared with Pro35S:BdTGD1 RNAi grains. Thus, TGD1 may have a function in embryo metabolism and development that is conserved between Arabidopsis and Brachypodium.
Figure 2.
Reduction of BdTGD1 Expression Affects Grain Development and Grain Lipid Metabolism.
(A) Dry weights (DW) of Pro35S:BdTGD1 RNAi grains. Three sets of 15 grains were averaged for each line; the error bars represent sd. Significant differences are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(B) to (F) FA content (B), TAG content (C), free FA content (D), and acyl composition of TAG (E) and free FA (F) in dry grains of Pro35S:BdTGD1 RNAi lines and wild-type Bd21-3. Lipids from three independent plant extracts were averaged. Five Brachypodium grains were used in each extraction. The total amount of each lipid species per grain and the molar percentage of individual FA relative to total FAs in each lipid were quantified by GC-FID of FAMEs. The error bars represent sd. Significant differences are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
(G) Germination rates of Pro35S:BdTGD1 RNAi lines and Bd21-3 dry grains sown on Murashige and Skoog medium with no sucrose. Three sets of 15 seeds per line were observed throughout the experiment and averaged each day; error bars represent sd. B, H, and Q represent three independent Pro35S:BdTGD1 RNAi lines.
Pro35S:BdTGD1 Partially Restored Arabidopsis tgd1-1 Phenotypes
To experimentally determine in planta whether BdTGD1 is functionally equivalent to AtTGD1, we expressed BdTGD1 in the Arabidopsis tgd1-1 mutant under the control of the 35S promoter (Pro35S:BdTGD1) and compared it to a tgd1-1 line expressing Pro35S:AtTGD1 and the tgd1-1 mutant itself. Of the 19 transgenic Pro35S:BdTGD1 plants obtained, eight were subjected to lipid analysis. The relative amount of 18:3 in MGDG correlated with BdTGD1 mRNA levels in different lines (Supplemental Figures 5A and 5B), suggesting BdTGD1 is functional in Arabidopsis. Three independent T3 homozygous lines, 6, 14, and 19, were chosen for further experiments. Similar to the broad native expression of AtTGD1 (Supplemental Figure 5C) in the wild type, the three lines showed broad expression of BdTGD1 in vegetative and reproductive tissues, with the highest transcript levels in leaves (Supplemental Figure 5C), as expected for expression driven by the 35S promoter.
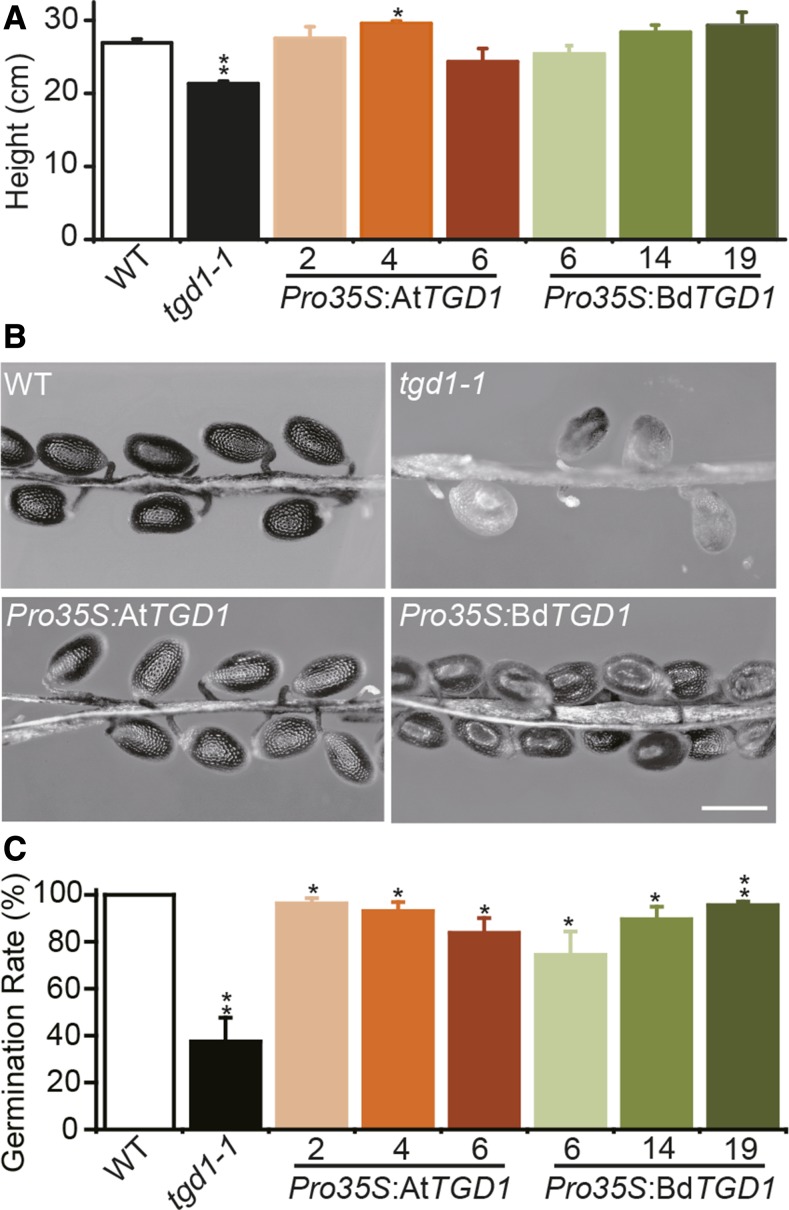
The plant heights of both Pro35S:BdTGD1- and Pro35S:AtTGD1-expressing lines were not significantly different from that of wild-type Arabidopsis (Col2) (Figure 3A), indicating rescue. As previously reported for Arabidopsis tgd1-1 developing siliques, a considerable number of embryos were aborted (Figure 3B) approximately at the heart stage (Xu et al., 2005), which is consistent with an overall 50% reduction in seed germination (Figure 3C). These phenotypes were restored by Pro35S:BdTGD1 and Pro35S:AtTGD1 expression (Figures 3B and 3C). The aborted seeds of the Arabidopsis tgd1-1 mutant had no lipid droplets in the embryo, unlike the surrounding tissue (Supplemental Figures 6E to 6G). The ultra-structures of the developing embryos in the tgd1-1 lines expressing Pro35S:AtTGD1 (Supplemental Figures 6H and 6I) or Pro35S:BdTGD1 (Supplemental Figures 6J and 6K) were very similar to the wild type. However, some irregular, large lipid droplets were observed in Pro35S:BdTGD1 cotyledon cells (Supplemental Figure 6L), possibly indicating incomplete restoration of the tgd1-1 phenotype by Pro35S:BdTGD1.
Figure 3.
BdTGD1 Restores Developmental Phenotypes of the Arabidopsis tgd1-1 Mutant.
(A) Heights of 6-week-old plants. The wild type and three independent Pro35S:AtTGD1- or Pro35S:BdTGD1-expressing lines each (designated by numbers) in the Arabidopsis tgd1-1 mutant background and the tgd1-1 mutant were compared. Three plants for each of the three independent transgenic lines were averaged; the error bars represent sd. Significant differences compared with the wild type are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01.
(B) Seed phenotypes. Representative siliques are shown opened 10 d after flowering. The wild type and three independent Pro35S:AtTGD1- or Pro35S:BdTGD1-expressing lines each (designated by numbers) in the Arabidopsis tgd1-1 mutant background and the tgd1-1 mutant were compared. Bar = 500 μm.
(C) Germination (radicle emergence) of seeds sown on Murashige and Skoog medium containing 1% sucrose kept under standard Arabidopsis growth conditions for 7 d. The wild type and three independent Pro35S:AtTGD1- or Pro35S:BdTGD1-expressing lines each (designated by numbers) in the Arabidopsis tgd1-1 mutant background and the tgd1-1 mutant were compared. Results from three agar plates containing 100 seeds each were averaged for each line; the error bars represent sd. Significant differences compared with the wild type are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01.
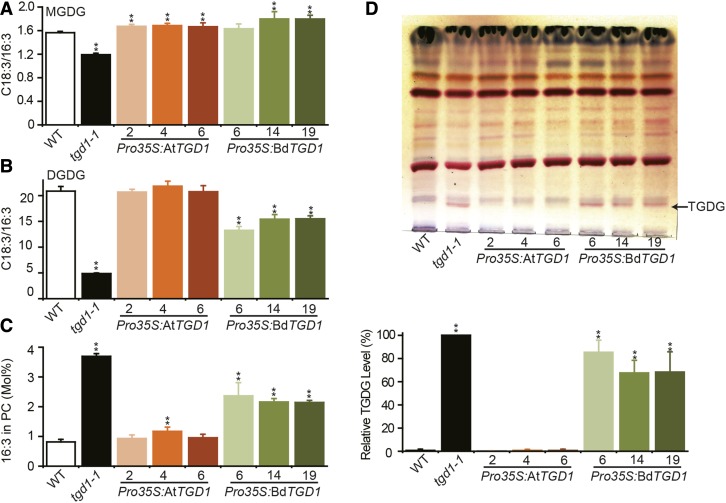
We examined lipid extracts from 4-week-old leaf tissues from Pro35S:AtTGD1, Pro35S:BdTGD1, wild-type, and tgd1-1 lines. The FA profiles of total lipids (Supplemental Figure 7A), relative FA composition (Supplemental Figures 7B to 7D), and lipid levels (Supplemental Figures 8A to 8C) of MGDG, DGDG, and phosphatidylcholine (PC) were almost fully restored by expression of Pro35S:BdTGD1 and Pro35S:AtTGD1. The extent of restoration of ER-to-chloroplast lipid trafficking by Pro35S:BdTGD1 can be estimated based on the C18:3/C16:3 FA ratio in MGDG and DGDG, which is diagnostic for the relative contribution of eukaryotic and prokaryotic pathways for chloroplast membrane lipid biosynthesis because 16:3 species are exclusively produced by the prokaryotic pathway. Accordingly, in Arabidopsis tgd1-1 leaves, the C18:3/16:3 ratios for MGDG (Figure 4A) and DGDG (Figure 4B) were reduced to 76% and 23% of wild-type levels, respectively, as expected for the reduction in molecular species derived from the eukaryotic pathway in the mutant. Expression of either Pro35S:AtTGD1 or Pro35S:BdTGD1 in Arabidopsis tgd1-1 led to a restoration of the C18:3/16:3 ratio in MGDG (Figure 4A). However, only Pro35S:AtTGD1 expression fully reversed the C18:3/16:3 ratio in DGDG (Figure 4B). Because DGDG biosynthesis is more dependent on the eukaryotic pathway than MGDG biosynthesis (Heinz and Roughan, 1983), this result suggests that ER-to-chloroplast lipid transfer was still impaired in the Arabidopsis tgd1-1 mutant expressing Pro35S:BdTGD1. Furthermore, the presence of 16:3 in PC, which is characteristic of the tgd1-1 mutant (Xu et al., 2005, 2008), was also only partially reversed in the Pro35S:BdTGD1-expressing tgd1-1 lines (Figure 4C). The presence of 16:3 in PC in tgd1-1 appears to be a consequence of increased flux of the prokaryotic pathway into extraplastidic lipids in this mutant. Most interestingly, the accumulation of TGDG, which is diagnostic for the Arabidopsis tgd1-1 mutant (Xu et al., 2003), was not reversed by the expression of Pro35S:BdTGD1 (Figure 4D; Supplemental Figures 8D to 8F). Notably, although BdTGD1 transcript levels in leaf tissues differed among the three Pro35S:BdTGD1 lines (Supplemental Figure 5C), the extent of restoration of ER-to-chloroplast lipid trafficking in the three lines was similar (Figure 4), suggesting that incomplete restoration of the Arabidopsis tgd1-1 lipid phenotype by BdTGD1 is the result of functional differences between BdTGD1 and AtTGD1 rather than transgene expression levels.
Figure 4.
Expression of Pro35S:BdTGD1 in the Arabidopsis tgd1-1 Mutant Only Partially Restored Leaf Lipid Phenotypes.
(A) to (C) Ratios of C18:3/16:3 in MGDG (A) and DGDG (B) and molar fraction of 16:3 in PC (C). The wild type and three independent Pro35S:AtTGD1- or Pro35S:BdTGD1-expressing lines each (designated by numbers) in the Arabidopsis tgd1-1 mutant background and the tgd1-1 mutant were compared. Total lipids were extracted from 4-week-old Arabidopsis leaves. The molar percentages of individual FAs relative to total FAs in each lipid species were quantified by GC-FID of FAMEs. The error bars represent sd from three independent plants. Significant differences compared with the wild type are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01.
(D) Expression of Pro35S:BdTGD1 did not rescue TGDG accumulation in the Arabidopsis tgd1-1 mutant. The upper panel shows thin-layer chromatography of polar lipids. The wild type and three independent Pro35S:AtTGD1- or Pro35S:BdTGD1-expressing lines each (designated by numbers) in the Arabidopsis tgd1-1 mutant background and the tgd1-1 mutant were compared. Glycolipids were visualized by α-naphthol staining. The arrow indicates the diagnostic TGDG (pink band). The lower panel shows the relative quantification of TGDG in both Pro35S:BdTGD1 and Pro35S:AtTGD1 lines based on the TLC results in Figure 4D and Supplemental Figures 8D and 8E. The TGDG content in tgd1-1 was treated as 100%. The relative amount of TGDG in individual transgenic lines was compared with that in tgd1-1 using ImageJ. The error bars represent sd from three independent plants. Significant difference compared with the wild type is indicated (**P ≤ 0.01, Student’s t test).
Differences in a 27-Amino Acid Loop Are Responsible for the Reduced Functionality of BdTGD1 in the Arabidopsis tgd1-1 Mutant
TGD1 encodes the predicted membrane-spanning permease of the ABC transporter (Xu et al., 2003, 2005; Roston et al., 2012). Because the permease activity of TGD1 has not been ascertainable in vitro, and ABC transporters lack sequence conservation in their transmembrane domain components (Verrier et al., 2008), functional domains of the plant TGD1 proteins have not been experimentally established. Given that AtTGD1 and BdTGD1 show 73% sequence identity and 87% sequence similarity (Supplemental Figure 9), specific differences between the two proteins may account for the observed reduction in functionality of BdTGD1 in Arabidopsis tgd1-1.
ABC transporter permeases typically contain five or six transmembrane spanning helices (Locher, 2016). Employing five different prediction servers, we found that the consensus predicted structure between AtTGD1 and BdTGD1 showed hydrophobic domains equivalent to six transmembrane helices (TM1-6; Supplemental Figures 9 and 10), with the C terminus facing the chloroplast outer envelope membrane (Supplemental Table 2). Further protein sequence alignment indicated that TM1-6 are highly conserved among predicted TGD1 orthologs in 34 different plant species (Supplemental Figure 11), whereas the N-terminal sequences (TGDN) up to the first transmembrane helix showed low similarity (Supplemental Figure 12).
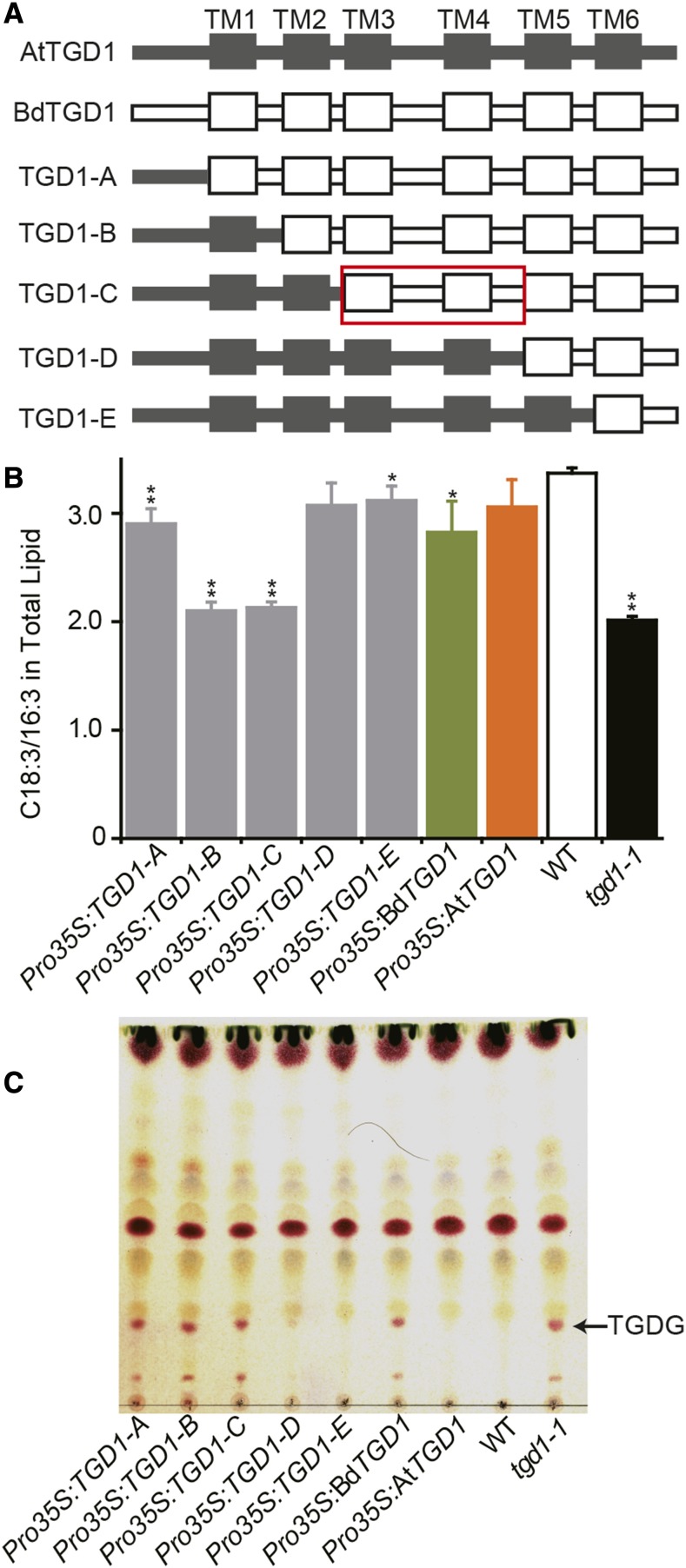
Hypothesizing that a specific sequence domain may be responsible for the different lipid phenotypes and functionality of AtTGD1 and BdTGD1 in the Arabidopsis tgd1-1 mutant, we generated a set of chimeric coding sequences by systematically swapping the equivalent sequences encoding specific domains from AtTGD1 to BdTGD1. The chimeric coding sequences were expressed in the Arabidopsis tgd1-1 mutant under the control of the 35S promoter (Pro35S:TGD1-A through Pro35S:TGD1-E; Figure 5A) to determine their functionality. Three independent lines for each construct were chosen (Supplemental Figure 13). Phenotypic analysis of the transformants included assaying C18:3/16:3 in total lipid extracts as a measure for restoration of lipid import into chloroplasts and the formation of TGDG as a measure of SFR2 activity. Systematically extending AtTGD1 from the N terminus in the chimeric construct showed that N-terminal Arabidopsis sequences up to the beginning of TM5 were required to fully restore the wild-type lipid phenotype based on both criteria mentioned above (TGD1-D; Figure 5; Supplemental Figure 13). However, the most N-terminal sequence of AtTGD1 (TGD1-A) seemed to partially restore the fraction of C18:3/16:3 in total lipids, but not the TGDG phenotype.
Figure 5.
The TGD1 TM3-TM5 Region Is Important for the Different Functionality of BdTGD1 in Arabidopsis tgd1-1.
(A) Series of chimeric proteins generated for identification of sequence regions causing functional-divergence between AtTGD1 and BdTGD1. AtTGD1 sequences are gray and BdTGD1 sequences white. The red box indicates the TM3-TM5 region relevant for functional diversity of AtTGD1 and BdTGD1.
(B) Ratios of C18:3/16:3 in total lipids of Arabidopsis leaf tissues. Total lipids were extracted from 4-week-old Arabidopsis leaves. The molar percentages of individual FAs relative to total FAs in each lipid species were quantified using GC-FID of FAMEs. Representative individual plants from three independent lines for each construct (as shown in Supplemental Figure 13) were averaged, and the error bars represent sd. Significant differences compared with the wild type are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01.
(C) Thin-layer chromatography analysis of polar lipids of a single representative plant for each construct is shown. Glycolipids were visualized by α-naphthol staining. TGDG is indicated.
To exclude the possibility that the differential restoration of the lipid phenotypes for each construct was caused by differences in the expression of the chimeric TGD1 cDNAs, we designed a primer pair located in the coding sequence common to all TGD1-chimeric proteins. Although the relative transcript levels of the biological repeats (leaf tissues from three independent plants per line) of TGD1 chimeric lines Pro35S:TGD1-B and Pro35S:TGD1-C were similar, or even higher than that of the Pro35S:TGD1-D and Pro35S:TGD1-E lines (Supplemental Figure 13A), they could not fully rescue the lipid phenotypes (Supplemental Figures 13B and 13C), suggesting that functional differences in the TGD1 chimeric proteins and not transgene expression levels caused all of the different lipid phenotypes. Considering all data, the likely BdTGD1 sequence primarily responsible for the phenotypic differences seems to reside within the sequence from the N terminus of the TM3 domain up to the N terminus of the TM5 domain (Figure 5A, red box).
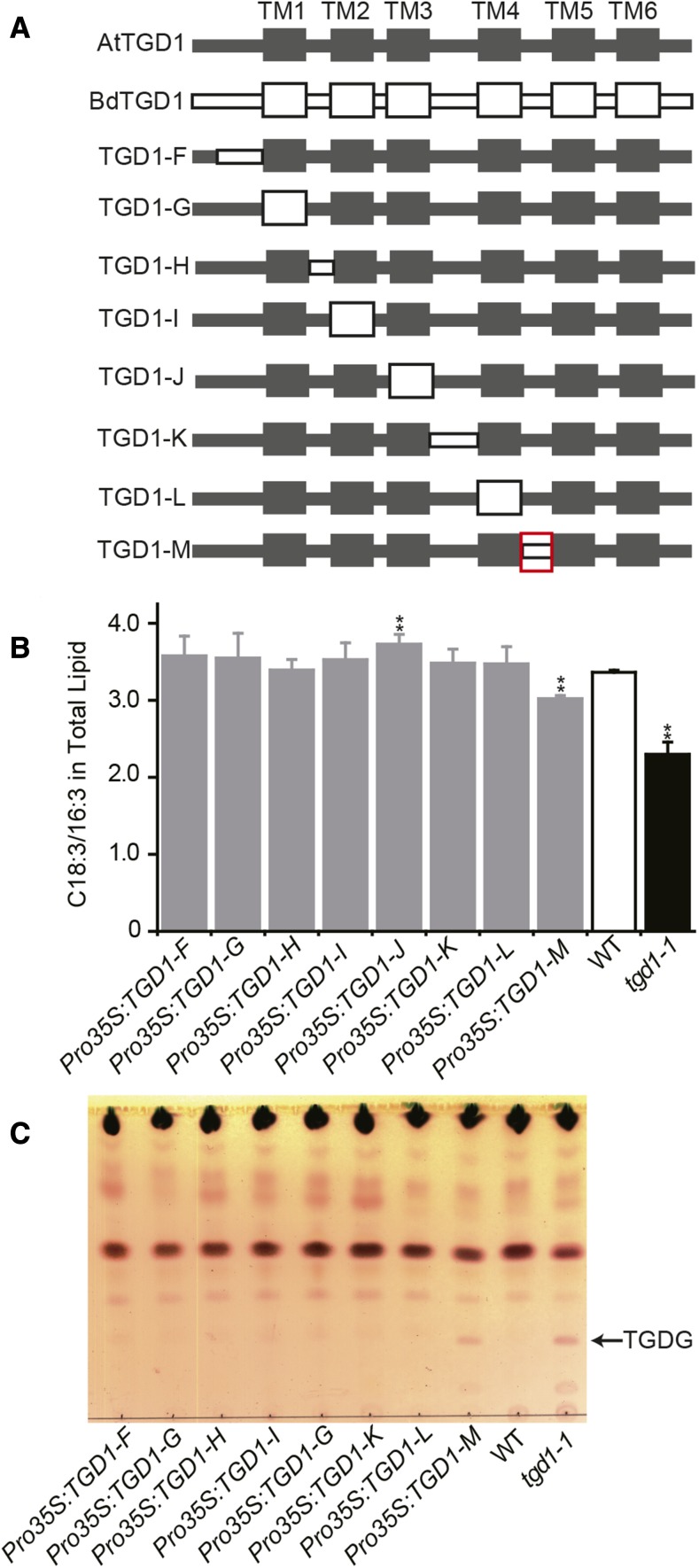
To further narrow down the sequence responsible for these differences, we generated a second set of chimeric proteins by exchanging only one transmembrane helix or one loop at a time from BdTGD1 into AtTGD1 (through Pro35S:TGD1-F to Pro35S:TGD1-M; Figure 6A). Three independent lines for each construct were chosen (Supplemental Figure 14), and the relative expression levels of the transgenes were similar for most of the transgenic lines (Supplemental Figure 14A). Remarkably, swapping only a single 27-amino acid loop between TM4 and TM5 (L45) from BdTGD1 into AtTGD1 (Pro35S:TGD1-M; Figure 6A) led to the same reduced function phenotype as for the entire BdTGD1 protein in this complementation assay. The C18:3/16:3 ratio in total lipids of Arabidopsis tgd1-1 expressing Pro35S:TGD1-M (3.0% ± 0.0%) was intermediate between the wild type (3.4% ± 0.0%) and tgd1-1 (2.3% ± 0.2%) (Figure 6B; Supplemental Figure 14B); TGDG was observed in the leaves of plants expressing this construct (Figure 6C; Supplemental Figure 14C). The loop L45 is located within the TM3-5 region, which we identified as being most likely causal to the functional difference in the two proteins in the complementation assay (red box in Figure 5A).
Figure 6.
A Small Loop between TM4 and TM5 Is Responsible for the Difference in Functionality of BdTGD1 in Arabidopsis tgd1-1.
(A) Series of chimeric proteins generated for identification of functional-divergence between AtTGD1 and BdTGD1. AtTGD1 sequences are gray; BdTGD1 sequences are white. The red box indicates L45, which gives rise to the functional differences between AtTGD1 and BdTGD1.
(B) Ratios of C18:3/16:3 in total lipids of Arabidopsis leaf tissues. Total lipids were extracted from 4-week-old Arabidopsis leaves. The molar percentages of individual FAs relative to total FAs in each lipid species were quantified using GC-FID of FAMEs. Representative individual plants from three independent lines for each construct (as shown in Supplemental Figure 14) were averaged, and the error bars represent sd. Significant differences compared with the wild type are indicated (Student’s t test): *P < 0.05 and **P ≤ 0.01.
(C) Thin-layer chromatography analysis of polar lipids of a single representative plant for each construct is shown. Glycolipids were visualized by α-naphthol staining. TGDG is indicated.
Coevolution and Direct Interaction of TGD1-L45 and the TGD2-MCE Domains
The discovery of L45 as the mostly divergent domain affecting the functionality of BdTGD1 in Arabidopsis tgd1-1 raised the question of its specific function. Since TGD1, 2, and 3 coevolved with each other in different organisms (Supplemental Table 1), it seemed possible that TGD1-L45 is required for the direct interaction between TGD1 and one of the two other components of the complex, which is specific for the BdTGD1 or AtTGD1 proteins, respectively, explaining the incomplete heterologous complementation results. Since topological prediction indicates that TGD1-L45 faces the chloroplast intermembrane space (Supplemental Table 2), just like the bulk of TGD2, but not TGD3, which is located on the inside of the inner envelope membranes, we hypothesized that TGD1-L45 provides direct contact with its respective TGD2 partner.
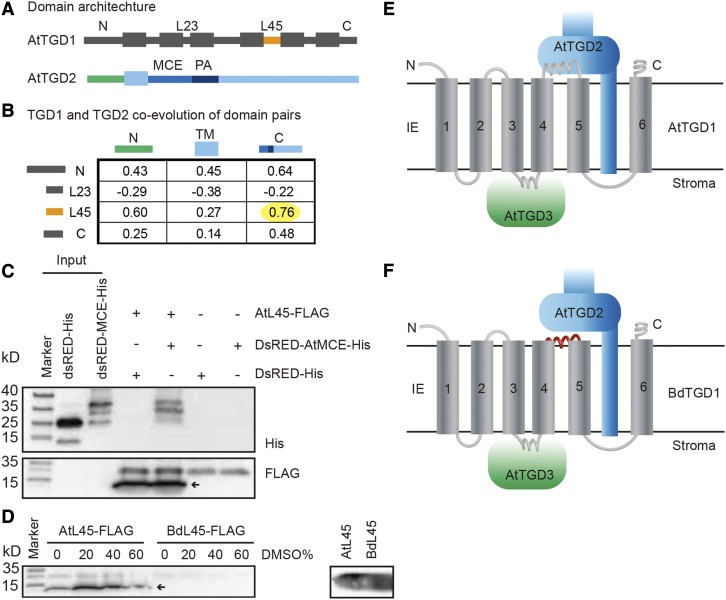
To test this hypothesis, we performed a coevolutionary analysis of domains in TGD1 and TGD2 from 28 plant species, including both 16:3 plants and 18:3 plants (Supplemental Table 3), using Tol-Mirrortree (Pazos et al., 2005; Jothi et al., 2006). Ribosomal 18s rRNA sequences were used to take into account speciation (Pazos et al., 2005; Gong et al., 2014). We inferred three domains for the Arabidopsis TGD2 protein (Figure 7A): (1) a short N-terminal domain containing the transit peptide with a short remnant in the mature protein located on the inner face of the chloroplast inner envelope membrane (TGD2N); (2) a transmembrane-spanning domain (TGD2TM); and (3) a long C-terminal domain that faces the chloroplast intermembrane space (TGD2C; Figure 7A) (Lu and Benning, 2009). For TGD1, we focused on the domains that share the same topology as L45 (loops L45 and L23, the loop between TM2 and TM3) and the N and C termini (Figure 7A), i.e., facing the intermembrane space. Tol-Mirrortree coevolution analysis clearly showed that the TGD2C domain and L45 of TGD1 had the highest correlation score among all domain pairs (Figure 7B), as would be expected for two interacting domains in two coevolving proteins (Jothi et al., 2006). Repeating the analysis with domains of TGD1 facing the inside of the inner envelope membrane and TGD3, a small TLRV-motif within the loop between the third and fourth membrane spanning helices of TGD1 showed a positive correlation with the TGD3 Q-loop (Supplemental Figure 15). Figure 7E shows these predicted interactions in the topological structure model for the TGD complex.
Figure 7.
L45 in TGD1 Coevolved with the MCE Domain in TGD2.
(A) Domain architecture of AtTGD1 and AtTGD2.
(B) Correlation scores by Tol-Mirrortree to assess the coevolution between all possible domains in TGD2 and loops in TGD1 facing the chloroplast intermembrane space. The yellow highlight indicates the highest score among all the analyzed pairs, showing the correlation between the TGD1-L45 and the TGD2-MCE domain.
(C) Coimmunoprecipitation detecting interaction of TGD1-L45 and TGD2-MCE domain. The AtTGD1-L45-FLAG was bound to anti-FLAG magnetic beads and incubated with DsRED-AtTGD2-MCE-His or DsRED-His. Bound proteins were detected by immunoblot using anti-His and anti-FLAG antibodies. The arrow indicates the specific band.
(D) Effect of DMSO on the binding of AtTGD1-L45 or BdTDG1-L45 with anti- FLAG magnetic beads. The left panel shows the binding in different concentrations of DMSO. Bound proteins were detected by immunoblot using anti-FLAG antibody. The right panel shows the amount of AtL45 and BdL45 used in the assay on the left. The arrow indicates the size of the specific band.
(E) Topology of the interaction between components of the Arabidopsis TGD1, 2, 3 complex in the inner envelope membrane (IE). N and C termini are indicated.
(F) Topology of the interaction between components of the heterologous complex containing BdTGD1 and AtTGD2 and AtTGD3 in the Arabidopsis tgd1-1 mutant expressing BdTGD1. The conserved interaction between TGD1 and TGD3 is shown as mentioned in the text. AtTGD1-L45, which faces the intermembrane space of the chloroplast, interacts with the AtTGD2 MCE domain. The L45 motif in BdTGD1, which is unable to interact with the AtTGD2-MCE domain, is shown in red.
The C-terminal portion of TGD2, TGD2C, can be further divided into three functional domains: (1) a mammalian cell entry (MCE) domain, as found in mycobacterial surface proteins, (2) a core PtdOH binding domain, and (3) a helical region, which forms a needle-like projection with a hollow core for lipid transport (Ekiert et al., 2017) and contributes to lipid binding outside the core segment (Lu and Benning, 2009). The most likely domain for mediating the interaction with TGD1-L45 is the MCE domain because four residues in TGD1-L45 appear to coevolve with residues in the TGD2 MCE domain (Supplemental Figure 16). To obtain corroborating evidence for a direct interaction between TGD1-L45 and TGD2-MCE, we performed coimmunoprecipitation analysis. The AtTGD1-L45 C terminus was fused to a FLAG tag and the N terminus of AtTGD2-MCE was fused to a Discosoma sp red fluorescent protein (DsRED) to increase the solubility, while the AtTGD2-MCEC terminus was fused to a HIS-tag for detection (Lu and Benning, 2009). As shown in Figure 7C, AtTGD1-L45-FLAG showed an interaction with DsRED-AtTGD2-MCE-His, as both proteins coimmunoprecipitated (Figure 7C; Supplemental Figures 17A and 17B).
To address whether BdTGD1-L45 cannot interact with AtTGD2, as predicted by the heterologous complementation experiments described above, we synthesized BdTGD1-L45-FLAG. However, the BdTGD1-L45-FLAG peptide had poor solubility in water (<0.1 mg/mL), while AtTGD1-L45-FLAG was soluble in water at a concentration as high as 10 mg/mL (Supplemental Table 4). Using mixtures of DMSO, in which both peptides are soluble, and water, AtTGD1-L45-FLAG bound at all concentrations of DMSO tested (up to 60%) to the anti-FLAG beads, but BdTGD1-L45-FLAG did not (Figure 7D; Supplemental Figure 17C), preventing us from performing the reciprocal experiment. We calculated the hydrophobicity of the coevolving TGD1-L45 and TGD2-MCE peptides in different Brassicaceae and Poaceae species using GRAVY (Kyte and Doolittle, 1982; (http://www.bioinformatics.org/sms2/protein_gravy.html), as shown in Table 1. All TDG1-L45 peptides of the Poaceae family including Brachypodium had higher hydrophobicity than those of the Brassicaceae family. Correspondingly, the hydrophobicity of the TGD2-MCE domain of Poaceae increased compared with that of Brassicaceae, another indication of coevolution and direct interaction of the TGD1-L45 and TGD2-MCE peptides in both plant families.
Table 1. Hydrophobicity of TDG1-L45 and TGD2-MCE.
| Species | TDG1-L45 | TGD2-MCE | |
|---|---|---|---|
| Brassicaceae | Arabidopsis lyrata | 0.311 | 0.087 |
| Arabidopsis thaliana | 0.311 | 0.097 | |
| Brassica rapa | 0.311 | 0.054 | |
| Camelina sativa | 0.333 | 0.083 | |
| Poaceae | Sorghum bicolor | 0.548 | 0.165 |
| Setaria italica | 0.548 | 0.218 | |
| Zea mays | 0.548 | 0.136 | |
| Brachypodium distachyon | 0.474 | 0.235 | |
| Panicum virgatum | 0.548 | 0.223 | |
| Oryza sativa | 0.378 | 0.259 |
Protein sequences of putative TGD1 and TGD2 orthologs from both the Brassicaceae and Poaceae families were used to calculate their hydrophobicity (GRAVY) using http://www.bioinformatics.org/sms2/protein_gravy.html.
Taking all data into account, we propose a model for the interaction between the components of the TGD1, 2, 3 complex in plants (Figures 7E and 7F). The differences in the sequences and physical properties of TGD1-L45 between the two species explain why interaction of BdTGD1-L45 and AtTGD2-MCE may be disturbed, leading to incomplete restoration of the phenotypes of the Arabidopsis tgd1-1 mutant by the introduction of BdTGD1 in a heterologous complementation experiment. Compared with AtTGD1 L45, which appears to be stable in the chloroplast intermembrane space (Figure 7E), BdL45 may be buried in the outer envelope membrane (Figure 7F) due to its hydrophobic nature and therefore cannot interact with AtTGD2 in vivo.
DISCUSSION
Impairment of Chloroplast Lipid Import in 18:3 Plants Causes More Subtle Lipid Phenotypes Than in 16:3 Plants, but Similar Growth Defects
Based on our computational analysis, TGD1, 2, and 3 coevolved in all plant species examined (Supplemental Table 1), suggesting that TGD1, 2, and 3 tend to maintain their interaction in different species. Accordingly, a reduction in BdTGD1 expression in Brachypodium caused similar developmental phenotypes (Figures 1, 2A, and 2G; Supplemental Figures 1G to 1J) to those of the Arabidopsis tgd1-1 mutant (Xu et al., 2005), suggesting that the TGD complex has conserved physiological functions in both species. However, their lipid phenotypes differed.
Brachypodium is an 18:3 plant, in which chloroplast membrane lipid biosynthesis is primarily dependent on the eukaryotic pathway and the import of precursors from the ER (Heinz and Roughan, 1983; Ohlrogge and Browse, 1995). Hence, ER-to-chloroplast lipid transport should be essential in Brachypodium, and impairment of BdTGD1 should cause a severe growth phenotype, as observed (Figure 1C; Supplemental Figures 1G to 1J). However, because 16:3 MGDG is absent in 18:3 plants, changes in the acyl composition of MGDG are expected to be more subtle in 18:3 plants, and the 18:3/16:3 ratio is not diagnostic for the impairment of lipid import as in 16:3 plants. Indeed, the lipid phenotypes of Brachypodium Pro35S:BdTGD1 RNAi lines were mild in leaf blades (Figures 1D and 1E; Supplemental Figure 2). It should also be noted that the repression of BdTGD1 in the three lines was only partial. Because strong impairment of ER-to-chloroplast lipid transport in Brachypodium may cause lethality, we suspect that more severe lines were not recoverable using the chosen approach. Based on this reasoning, we also did not pursue a gene editing approach that would fully disrupt BdTGD1.
In principle, the reciprocal experiment, in which the Brachypodium Pro35S:BdTGD1 RNAi lines are transformed with Pro35S:AtTGD1 or the chimeric constructs (Supplemental Table 5), should have led to similar conclusions, but this experiment was impractical to conduct. Aside from the technical challenges of Brachypodium transformation with two selection markers, the lipid phenotypes were much more subtle for the Pro35S:BdTGD1 RNAi lines than for the Arabidopsis tgd1-1 mutant, making the interpretation of the data that much more difficult.
It should also be noted that the Poaceae have specific differences in lipid metabolism in addition to those observed for other 18:3 plants. One example is the presence of a homomeric acetyl-CoA carboxylase for FA biosynthesis, whereas almost all other plants have a heteromeric acetyl-CoA carboxylase (Mekhedov et al., 2000). Another is the absence of FATTY ACID DESATURASE5 (Mekhedov et al., 2000). Thus, further studies of the TGD orthologs from other 18:3 plant families will be needed for a more generalized understanding of the differences in ER-to-chloroplast lipid trafficking between 16:3 and 18:3 plants.
ER-to-Chloroplast Lipid Transport Is Important for Embryo Development
The TGD complex also plays an important role in embryo development. Functional reduction of TGD1 led to reduced seed lipid content in Arabidopsis (Supplemental Figure 4B) and Brachypodium (Figure 2B), as well as an increase in the abundance of free FAs (Supplemental Figure 4D; Figure 2D). Free FAs are detrimental to plant development, and Brachypodium is highly sensitive to the accumulation of free FAs (Yang et al., 2015). Exogenous and endogenous FAs are known to inhibit germination in lettuce (Lactuca sativa), oat (Avena sativa), and mustard (Sinapis alba) (Le Poidevin, 1965; Berrie, 1979; Stewart and Berrie, 1979; Metzger and Sebesta, 1982). Thus, the delayed germination in the Pro35S:BdTGD1 RNAi lines (Figure 2G) was conceivably caused by the increased level of free FAs in dry grains. As a side note, the tgd1-1 mutation in Arabidopsis not only leads to aborted embryos (Xu et al., 2005), but it also affects endosperm development, as we discovered here (Supplemental Figures 6E to 6G). In mature Arabidopsis seeds, endosperm persists only as a thin peripheral layer (Lafon-Placette and Köhler, 2014), which contains numerous discrete lipid droplets (Eastmond et al., 2002; Penfield et al., 2006). In contrast, most of the lipid droplets tended to fuse with each other in the endosperm of the aborted tgd1-1 seeds (Supplemental Figures 6E to 6G).
What Causes the Differential Activation of SFR2 in 16:3 and 18:3 Plants with Reduced TGD1 Function?
In Arabidopsis, the bulk of MGDG is assembled by MGD1 at the chloroplast inner envelope (Jarvis et al., 2000), while the bulk of DGDG is synthesized by DGD1 at the outer chloroplast envelope membrane (Froehlich et al., 2001). Notably, the expression of BdTGD1 in the Arabidopsis tgd1-1 mutant restored the acyl profile of MGDG (Figure 4A), but not that of DGDG (Figure 4B). Furthermore, the acyl composition of PC (Figure 4C) present in the outer envelope membrane and the ER was not restored, and the accumulation of TGDG (Figure 4D) due to the induction of SFR2 activity associated with the outer envelope membrane was not reversed. In essence, incomplete restoration of outer envelope membrane-related lipid phenotypes by BdTGD1 in the Arabidopsis tgd1-1 mutant was observed.
To understand this effect, one must consider the accumulation of prokaryotic, 16:3-containing MGDG in the Arabidopsis tgd1-1 mutant and the apparent preference of the DGDG synthase, DGD1, for eukaryotic, 18:3-containing MGDG. In Arabidopsis, the main MGDG synthase, MGD1, uses prokaryotic and eukaryotic DAG with the same affinity for MGDG biosynthesis (Awai et al., 2001), while DGD1 appears to prefer eukaryotic MGDG for DGDG biosynthesis. This conclusion is based on multiple lines of direct or indirect evidence: (1) the apparent DGDG acyl composition in Arabidopsis (Xu et al., 2003); (2) C14-acetate labeling of both intact leaves and isolated chloroplasts of spinach (Spinacia oleracea) to measure the de novo biosynthesis of galactoglycerolipids showed that DGD1 discriminates against plastid-derived MGDG (Heemskerk et al., 1991); (3) complementation analysis of the Arabidopsis dgd1-1 mutant using DGDG-biosynthetic enzymes other than DGD1, which do not discriminate against 16:3 MGDG as substrate (Kelly et al., 2016); and (4) C14-acetate labeling of Arabidopsis tgd1-1 leaves showing that the incorporation of label into MGDG was increased, but incorporation into DGDG did not change (Fan et al., 2013). Therefore, more prokaryotic substrate for MGDG biosynthesis by MGD1 is available in tgd1-1, and the resulting excess of prokaryotic MGDG can only be converted to prokaryotic DGDG by DGD1 at a reduced rate. However, SFR2, which is activated in the Arabidopsis tgd1-1 mutant, is able to convert the excess prokaryotic MGDG to DGDG and other oligogalactolipids, thereby maintaining a proper ratio of MGDG to DGDG in the thylakoids needed for stable membranes (Dörmann and Benning, 2002). Indeed, the major molecular species of TGDG in Arabidopsis tgd mutants contains 16:3 in the sn-2 position, indicating a prokaryotic origin (Xu et al., 2003; Okazaki et al., 2013). Moreover, when sfr2 is introduced into the tgd1-1 background, the DGDG content is increased compared with tgd1-1. Pulse-chase labeling of detached leaves with C14-acetate also showed an increased amount of radiolabeled DGDG after a 3-d chase. However, the radioactivity in PC, the eukaryotic precursor for galactoglycerolipid biosynthesis, remained similar to that in tgd1-1 (Fan et al., 2014). These results suggest that DGDG biosynthesis is increased in sfr2 tgd1-1 compared with the tgd1-1 single mutant and that the additional DGDG derives from prokaryotic MGDG. Although prokaryotic MGDG is not a preferred substrate for DGD1, the increased biosynthesis of DGDG in the double mutant may help to maintain a constant ratio of MGDG to DGDG important for lipid homeostasis and integrity of membranes in the chloroplast (Dörmann and Benning, 2002). The finding that prokaryotic PC and DGDG are still present in the tgd1-1 Arabidopsis mutant expressing BdTGD1 (Figures 4B and 4C) and that SFR2 remains induced (Figure 4D) suggests that excess prokaryotic MGDG is still being converted into outer membrane lipids due to incomplete restoration of the import of eukaryotic lipid precursors in these lines.
On the contrary, in Brachypodium leaf tissues, the prokaryotic pathway is inactive. Reduction in TGD1 activity in Brachypodium leaves in the Pro35S:BdTGD1-RNAi lines only caused a reduction in eukaryotic precursors, and the resulting eukaryotic MGDG can be used by DGD1. Thus, there is no need for the induction of SFR2 in Brachypodium leaves to maintain a balanced MGDG-to-DGDG ratio, as observed.
Unique Differences in the TGD1, 2, 3 Transporters of the Two Species Account for the Limited Functioning of the Heterologous Complex
To understand why restoration of lipid phenotypes in the tgd1-1 Arabidopsis mutant expressing BdTGD1 was incomplete, we took a closer look at the TGD1 protein and its interacting partners in the two species. The permease components of MKL lipid transporters share a similar core transmembrane domain topology with other bacterial type I small molecule importers, namely, five to six membrane-spanning helices (Bordignon et al., 2010; Locher, 2016). A typical permease component in type I ABC importers has its C terminus on the cytoplasmic side of the membrane (Casali and Riley, 2007). However, the C terminus of the permease domain in MKL lipid importers, such as Mce4 in Mycobaterium tuberculosis (Casali and Riley, 2007) and Lin in Candidatus Liberibacter asiaticus (Li et al., 2012), are located on the periplasmic face of the membrane. We propose that TGD1 has the equivalent topology, with the C terminus facing the intermembrane space of the chloroplast, based on two lines of evidence: (1) four of the five different online servers gave us the same predicted orientation of TGD1 across the membrane (Supplemental Table 2), and (2) the TLRV-motif in TGD1 L34 appears to coevolve with the Q loop in TGD3 with a high degree of correlation (Supplemental Figure 15), indicating that this loop likely provides the contact face for TGD1 and TGD3. The latter is known to be on the inner face of the inner envelope membrane (Lu et al., 2007). Furthermore, examining the recently solved structure of the Escherichia coli Mla phospholipid transporter (Ekiert et al., 2017), which is the closest homolog of the plant TGD complex, gives us confidence that L34 is facing the stroma side of the chloroplast, where TGD3 is located.
We have previously shown that TGD1, 2, 3 form a stable complex (Roston et al., 2011). Thus, in order for TGD1 and TGD2 to interact, coevolution of the interacting amino acids in both proteins should be observable. Indeed, one strongly coevolving residue pair suggestive of being present in potential contact sites between TGD1 and TGD2 is present in their transmembrane domains (Supplemental Figure 16A). The probability of AtTGD1239F in TM4 and of AtTGD2 106G in its respective single TM is 0.92 (Supplemental Figure 16A). These residues are conserved among all 28 plant species we analyzed (Supplemental Figure 11).
In addition to stable interaction, transient contacts between the permease component and the substrate binding protein are also required during the transport cycle. In the E. coli maltose transporter MalGFK2, docking of MBP (maltose binding protein), the substrate binding component of the complex, onto the periplasmic surface of MalG and MalF (forming the permease of the complex) induces a conformational change in the transporter and thereby stimulates ATP hydrolysis (Chen et al., 2001; Oldham et al., 2007). Here, L45 in AtTGD1 is located at the intermembrane face of the inner envelope membrane (Figure 7E) and interacts with the MCE domain of AtTGD2 (Figure 7C). Coevolutionary analysis suggested four potentially interacting residue pairs within the L45-MCE contact site (Supplemental Figure 16). It should be noted that the relatively low probability of the predicted residue pairs (0.46–0.36) might be indicative of a transient interaction, but the accuracy of the prediction is not diminished by this finding (http://gremlin.bakerlab.org/cplx_faq.php). Thus, the possibly transient interaction between AtTGD1-L45 and AtTGD2-MCE may be required for a conformational change in the TGD complex during the transport cycle. Therefore, changes in the topology of BdL45 may interfere with docking to the Arabidopsis TGD2 MCE domain, thereby causing inefficient lipid transport.
In summary, the TGD proteins provide a striking example of the predictive power of coevolution analysis for protein-protein interaction sites. However, in the absence of crystal structures of the different conformations of the TGD1, 2, 3 complex and a direct transport assay, the current analysis of TGD1, 2, 3 function remains indirect, although it allows us to deduce a reasonable picture for the function of the two different complexes in 16:3 and 18:3 plants.
METHODS
Plant Growth
Arabidopsis thaliana (ecotype Col-2) and Brachypodium distachyon (ecotype Bd21-3) were plated and grown as previously described (Yang et al., 2015). Arabidopsis plants were grown in 100 to 120 μE m−2 s−1 under 16 h light/8 h dark and at 22/18°C (day/night). Brachypodium plants were grown in 155 μE m−2 s−1 under 20 h light/4 h dark and at 24/18°C (day/night). For the screening of transgenic Arabidopsis and Brachypodium transgenic plants, 40 mg/L hygromycin B (Invitrogen) was added to the growth medium. Brachypodium ecotype (Bd21-3) was obtained from Curtis Wilkerson, Michigan State University.
Chlorophyll Quantification
Chlorophyll concentrations were calculated as described (Dörmann et al., 1995).
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously described (Yang et al., 2015) using the primers listed in Supplemental Table 6. Different Brachypodium tissues were harvested as shown in Supplemental Figures 1A to 1F. To synthesize cDNA, 1 µg total RNA was incubated with SuperScript II reverse transcriptase (Invitrogen). The synthesized cDNAs were 8-fold diluted with double distilled water for use as the template. Quantitative RT-PCR was performed using SYBR Green PCR Master mix (Life Technologies) following the manufacturer’s instructions.
Plasmid Construction and Plant Transformation
For BdTGD1 RNAi plasmid construction, total RNA was isolated from 4-week-old wild-type Brachypodium leaf blades using an RNeasy Plant Mini Kit (Qiagen). To synthesize cDNA, 1 µg total RNA was incubated with SuperScript II reverse transcriptase (Invitrogen). The Pro35S:BdTGD1 RNAi construct was built by first amplifying the 695-bp (359–1053 bp) cDNA of BdTGD1 using primers as shown in Supplemental Table 6. PCR products were purified and recombined into the pHELLSGATE12 vector as described (Wang et al., 2016). The plant expression cassette Pro35S -BdTGD1 hairpin-NOS terminator was digested with EcoRI and ScaI followed by blunting and was then inserted into the SfoI-digested pJJ247 vector. Transgenic Brachypodium plants were regenerated at the GLBRC plant transformation facility at MSU (http://glbrc.bch.msu.edu) as described (Vogel and Hill, 2008). Twenty-six independent transgenic Brachypodium lines were generated with reduced BdTGD1 transcript levels. Three lines (B, H, and Q) were selected for further lipid analyses and developmental phenotype observation in the T3 generation.
For TGD1 complementation and chimeric TGD1 lines, AtTGD1 and BdTGD1 full-length cDNAs were first amplified from 4-week-old Arabidopsis and Brachypodium wild-type leaf tissues, respectively. PCR products were purified and inserted into the pENTR/D-TOPO vector as described (Wang et al., 2016). AtTGD1-pENTR and BdTGD1-pENTR were then used as the templates to generate TGD1-A to TGD1-E DNA fragments by site-directed mutagenesis PCR as described (Reikofski and Tao, 1992). TGD1-F to TGD1-M were synthesized by IDT (http://www.idtdna.com/site). A description of the construct details is provided in Supplemental Table 5. Subsequently, TGD1-A to TGD1-M were inserted into the pENRT/D vector. All TGD1 fragments were finally recombined into the pMDC32 vector by the LR reaction using Gateway LR Clonase II Enzyme Mix (Life Technologies). Primers used to build these plasmids are shown in Supplemental Table 6.
For Pro35S:BdTGD1 expression in the Arabidopsis tgd1-1 background, 19 transgenic lines were obtained in the T1 generation, and eight of them were analyzed for RNA levels and lipid phenotypes. All eight lines showed restoration of reduced 18:3 in MGDG to different extents (Supplemental Figure 5B). Lines 6, 14, and 19 were carried forward to obtain T3 homozygous lines. For Pro35S:AtTGD1 expression in the Arabidopsis tgd1-1 background, 12 lines were obtained at the T1 generation, and five of these lines were analyzed for RNA and lipid phenotypes. All of these lines fully rescued the tgd1-1 lipid phenotypes. Lines 2, 4, and 6 were carried forward to obtain T3 homozygous lines. For all chimeric constructs, 5 to 10 T1 lines were screened, and three independent T3 lines for each construct were analyzed for lipids.
Developmental Phenotypes
For germination measurements, 15 Brachypodium grains or 100 Arabidopsis seeds were examined in each experiment. Seed germination was determined as radicle emergence from the seed coat. Data from 10 (4 weeks old) Brachypodium plants were averaged for measurements of plant height, primary tiller number, and internode number in the main stem.
Microscopy
For light and transmission electron microscopy, Arabidopsis dry seeds were imbibed at 22°C for 2 h and the seed coat was removed. Embryos were fixed in 4% (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.2, overnight at 4°C. After rinsing three times in the same buffer, the embryos were post fixed with 1% osmium tetroxide for 2 h at room temperature. After dehydration using an ethanol series, the material was embedded in Araldite-Epon resin mixture (Araldite 502/Embed 812 kit; Electron Microscopy Sciences). Sections were obtained using an RMC ultramicrotome (RMC). Semithin sections (1 μm) were stained with 0.05% (w/v) methylene blue and observed under a Leica MZ125 microscope. Ultrathin sections (70 nm) were examined in a JEOL100 CXII (Japan Electron Optics Laboratories) operated at 80 kV.
Lipid Analysis
For seed TAG and free FA methyl ester (FAME) analyses, 20 dry Arabidopsis seeds or five dry Brachypodium grains were processed as described (Yang et al., 2015). For total seed FAMEs analyses, 20 dry Arabidopsis seeds or five dry Brachypodium grains were directly used for FAME preparation at 80°C for 90 min and FAMEs were quantified by gas liquid chromatography using a flame ionization detector (GC-FID) as described (Wang and Benning, 2011).
For complex lipid analyses of vegetative tissues, total lipids were extracted from ∼100 mg fresh leaf tissues and processed as described (Awai et al., 2006). Lipid extracts were analyzed by thin-layer chromatography (TLC) and the acyl composition of individual lipids was determined following the preparation of FAMEs and their analysis by GC-FID as previously described (Wang and Benning, 2011). The FA composition of individual lipids was presented as the relative mol% ratio. The relative content of individual lipids was presented as mol% of each lipid species relative to total lipid levels. For example, the relative MGDG content in total lipids (MGDG mol%) = ∑(FAMEs in MGDG)/∑(FAMEs in total lipid)×100%).
For oligogalactoglycerolipid detection, total lipids were loaded onto silica gel TLC plates (Silica 60, DC-Fertigplatten SILGUR-25 plate; Macherey Nagel) and separated using a solvent system consisting of chloroform:methanol:acetic acid:water (85:20:10:4, v/v/v/v) (Barnes et al., 2016). Oligogalactoglycerolipids were visualized by spraying the plate with α-naphthol as described (Wang and Benning, 2011).
Coimmunoprecipitation Assay
The Arabidopsis TGD2 MCE domain (AtTGD2 protein sequence 119-204) was inserted into the DsRed-plw01-His plasmid [DsRed-AtTGD2 (119-204)-plw01-His] as described (Lu and Benning, 2009). His-MCE-DsRED protein expression and purification were performed as described (Lu and Benning, 2009). AtTGD1 L45-FLAG (AtTGD1 protein sequence 256-282: SDAVYGISINIIMDSAHRALRPWDIVS) and BdTGD1 L45-FLAG (BdTGD1 protein sequence 255-281: ADAIFGVSTSIILESARRALRPWDLIS) were synthesized by Genscript (http://www.genscript.com/). The solubility tests for AtL45 and BdL45 were also performed by Genscript. For immunoprecipitation assays, 2 μg of synthesized AtL45 or BdL45 was incubated with 4 μL of Anti-FLAG M2 Magnetic Beads (Sigma-Aldrich) in 1 mL TBS buffer at room temperature for 1 h with gentle rotation. The beads were then incubated in blocking buffer (1% BSA in TBS) at room temperature for 1 h and washed three times with TBS buffer. One microgram of purified His-MCE-DsRED or His-DsRED was added to the mixture, followed by incubation overnight at 4°C. After washing five times with TBS buffer containing 0.025% Triton X-100, the bound proteins were eluted with SDS loading buffer lacking β-mercaptoethanol and boiled for 5 min at 100°C. Interaction was visualized by immunoblot using polyclonal anti-His G18 (Santa Cruz Biotechnology; catalog no. sc-804; 1:2000 dilution) or monoclonal anti-FLAG M2 (Sigma-Aldrich; catalog no. F3165; 1:2000 dilution) antibodies.
Phylogenetic Analysis
The multiple sequence alignments (MSAs) between BdTGD1 and AtTGD1, and the putative orthologs of the TGD1 from 28 different plant species (Supplemental Table 3) were done with Jalview (Waterhouse et al., 2009), using the Mafft server, L-INS-i preset. The MSA results are provided in Supplemental File 1 (MSA between AtTGD1 and BdTGD1; Supplemental Figure 9), Supplemental File 2 (MSA of C-terminal sequence of the TGD1 from 28 different plant species; Supplemental Figure 11), and Supplemental File 3 (MSA of C-terminal sequence of the TGD1 from 28 different plant species; Supplemental Figure 12) as FASTA.
Coevolution Analysis
For multiple sequence alignments, a BLASTP analysis was performed with an e-value threshold of 1.0E−10 in both Phytozome (http://www.phytozome.net/) and NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastpandPAGE_TYPE=BlastSearchandLINK_LOC=blasthome). Putative orthologs of the TGD1, TGD2, and TGD3 proteins from 28 different plant species were included (Supplemental Table 3 and Supplemental Files 4 to 6). The multiple sequence alignments were performed by Clustal W with the following parameters: weight matrix, Gonnet; gap opening penalty, 10.0; and gap extension penalty, 0.2. For Tol-Mirrortree analysis, genetic distances were generated from the MSA by MEGA6. The 18s rRNA genomic sequences were used to correct the speciation influences. Pearson’s correlation coefficients were calculated as described (Jothi et al., 2006). The interprotein residue-residue coevolution pairs between TGD1 and TGD2 were predicted by GREMLIN (Ovchinnikov et al., 2014). Residue pairs with predicted probability higher than 75%, together with the residue pairs that were located within the L45-MCE region, are shown in Supplemental Figure 16 and were mapped onto the model structures.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers (https://www.arabidopsis.org/) used in this study are as follows: At1g19800 (AtTGD1), At3g20320 (AtTGD2), At1g65410 (AtTGD3), At3g06510 (AtSFR2), and At2g37620 (AtACTIN1). Brachypodium locus identifiers (http://www.phytozome.net/) are as follows: Bradi5g17820 (BdTGD1) and Bradi4g00660 (BdUBC18). Accession numbers for all putative TGD1, TGD2, and TGD3 orthologs were obtained from Phytozome (http://www.phytozome.net/) and NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastpandPAGE_TYPE=BlastSearchandLINK_LOC=blasthome) and are shown in Supplemental Table 3.
Supplemental Data
Supplemental Figure 1. Phenotypes of representative Pro35S:BdTGD1 RNAi lines.
Supplemental Figure 2. Relative lipid contents in total lipids did not change significantly in Pro35S:BdTGD1 RNAi leaf blades.
Supplemental Figure 3. TGDG did not accumulate in developing Arabidopsis embryos.
Supplemental Figure 4. Lipid phenotypes of Arabidopsis tgd1-1 mutant seeds.
Supplemental Figure 5. Correlation of transcript levels and the rescue of lipid phenotypes in different Pro35S:BdTGD1 lines.
Supplemental Figure 6. Expression of Pro35S:AtTGD1 and Pro35S:BdTGD1 restored the morphology and ultrastructure of Arabidopsis tgd1-1 mutant seeds.
Supplemental Figure 7. Expression of BdTGD1 and AtTGD1 restored the FA composition in the Arabidopsis tgd1-1 mutant.
Supplemental Figure 8. Expression of BdTGD1 and AtTGD1 restored lipid contents in the Arabidopsis tgd1-1 mutant.
Supplemental Figure 9. Alignment of TGD1 amino acid sequences from Brachypodium and Arabidopsis.
Supplemental Figure 10. Hydrophobic domain prediction in AtTGD1 and BdTGD1 by TMHMM.
Supplemental Figure 11. The predicted transmembrane helices located in the C terminus are conserved among different plant TGD1 orthologs.
Supplemental Figure 12. The N terminus is not conserved among different plant TGD1 orthologs.
Supplemental Figure 13. Lipid phenotype of Arabidopsis tgd1-1 mutants producing chimeric proteins TGD1-A to TGD1-E.
Supplemental Figure 14. Lipid phenotype of Arabidopsis tgd1-1 mutants producing chimeric proteins TGD1-F to TGD1-M.
Supplemental Figure 15. Coevolution analysis of plant TGD1 and TGD3.
Supplemental Figure 16. Coevolution analysis of plant TGD1 and TGD2.
Supplemental Figure 17. Interaction of the TGD1-L45 and TGD2-MCE domains.
Supplemental Table 1. Coevolution analysis of TGD1, 2, and 3.
Supplemental Table 2. Transmembrane domain prediction in AtTGD1 and BdTGD1.
Supplemental Table 3. Accession numbers of putative TGD1 and TGD2 orthologs.
Supplemental Table 4. Solubility test of BdL45 and AtL45.
Supplemental Table 5. Description of AtTGD1 and AtTGD2 constructs.
Supplemental Table 6. Primers used in this article.
Supplemental File 1. Sequence alignment of AtTGD1 and BdTGD1.
Supplemental File 2. Sequence alignment of C termini of TGD1s.
Supplemental File 3. Sequence alignment of N termini of TGD1s
Supplemental File 4. Sequence alignment of TGD1s for coevolution analysis.
Supplemental File 5. Sequence alignment of TGD2s for coevolution analysis.
Supplemental File 6. Sequence alignment of TGD3s for coevolution analysis.
Acknowledgments
We thank Shin-Han Shiu and Danny J. Schnell (Michigan State University) for valuable discussions. We thank Barbara B. Sears, Anna Hurlock, and Kun Wang (Michigan State University) for critically reading the manuscript. We thank Linda Danhof from the Great Lakes Bioenergy Research Center Plant Transformation Facility at Michigan State University for conducting the Brachypodium transformation. This work was funded in part by the U.S. Department of Energy Great Lakes Bioenergy Research Center Cooperative Agreement DE-FC02-07ER64494 and MSU AgBioResearch. In addition, this work was partially supported by a fellowship to A.L. from Michigan State University under the Training Program in Plant Biotechnology for Health and Sustainability (T32-GM110523).
AUTHOR CONTRIBUTIONS
Y.Y. designed and performed experiments, analyzed data, and wrote the first draft of the article. A.Z. performed the microscopy experiments and analyzed data. A.L. performed the screening and genotyping of Brachypodium Pro35S:BdTGD1 RNAi lines. C.B. supervised the study, designed experiments, analyzed data, coordinated the writing of the manuscript, and edited drafts of the manuscript.
Glossary
- DGDG
digalactosyldiacylglycerol
- MGDG
monogalactosyldiacylglycerol
- DAG
diacylglycerol
- ER
endoplasmic reticulum
- FA
fatty acid
- TAG
triacylglycerol
- TGDG
trigalactosyldiacylglycerol
- MCE
mammalian cell entry
- FAME
FA methyl ester
- GC-FID
gas liquid chromatography using a flame ionization detector
- TLC
thin-layer chromatography
- PC
phosphatidylcholine
References
- Awai K., Maréchal E., Block M.A., Brun D., Masuda T., Shimada H., Takamiya K., Ohta H., Joyard J. (2001). Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 98: 10960–10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awai K., Xu C., Tamot B., Benning C. (2006). A phosphatidic acid-binding protein of the chloroplast inner envelope membrane involved in lipid trafficking. Proc. Natl. Acad. Sci. USA 103: 10817–10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A.C., Benning C., Roston R.L. (2016). Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING2. Plant Physiol. 171: 2140–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien O., Botella C., Chevalier F., Block M.A., Jouhet J., Breton C., Girard-Egrot A., Maréchal E. (2016). New insights on thylakoid biogenesis in plant cells. Int. Rev. Cell Mol. Biol. 323: 1–30. [DOI] [PubMed] [Google Scholar]
- Berrie A.M. (1979). Possible role of volatile fatty acids and abscisic acid in the dormancy of oats. Plant Physiol. 63: 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordignon E., Grote M., Schneider E. (2010). The maltose ATP-binding cassette transporter in the 21st century--towards a structural dynamic perspective on its mode of action. Mol. Microbiol. 77: 1354–1366. [DOI] [PubMed] [Google Scholar]
- Casali N., Riley L.W. (2007). A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Sharma S., Quiocho F.A., Davidson A.L. (2001). Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA 98: 1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E. (2011). Natural history of ABC systems: not only transporters. Essays Biochem. 50: 19–42. [DOI] [PubMed] [Google Scholar]
- Dassa E., Bouige P. (2001). The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152: 211–229. [DOI] [PubMed] [Google Scholar]
- de Juan D., Pazos F., Valencia A. (2013). Emerging methods in protein co-evolution. Nat. Rev. Genet. 14: 249–261. [DOI] [PubMed] [Google Scholar]
- Dörmann P., Benning C. (2002). Galactolipids rule in seed plants. Trends Plant Sci. 7: 112–118. [DOI] [PubMed] [Google Scholar]
- Dörmann P., Hoffmann-Benning S., Balbo I., Benning C. (1995). Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7: 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond P.J., van Dijken A.J., Spielman M., Kerr A., Tissier A.F., Dickinson H.G., Jones J.D., Smeekens S.C., Graham I.A. (2002). Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 29: 225–235. [DOI] [PubMed] [Google Scholar]
- Ekiert D.C., Bhabha G., Isom G.L., Greenan G., Ovchinnikov S., Henderson I.R., Cox J.S., Vale R.D. (2017). Architectures of lipid transport systems for the bacterial outer membrane. Cell 169: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Yan C., Xu C. (2013). Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J. 76: 930–942. [DOI] [PubMed] [Google Scholar]
- Fan J., Yan C., Roston R., Shanklin J., Xu C. (2014). Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 26: 4119–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T.A., Stumpf P.K. (1983). Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur. J. Biochem. 129: 629–636. [DOI] [PubMed] [Google Scholar]
- Froehlich J.E., Benning C., Dörmann P. (2001). The digalactosyldiacylglycerol (DGDG) synthase DGD1 is inserted into the outer envelope membrane of chloroplasts in a manner independent of the general import pathway and does not depend on direct interaction with monogalactosyldiacylglycerol synthase for DGDG biosynthesis. J. Biol. Chem. 276: 31806–31812. [DOI] [PubMed] [Google Scholar]
- Gong P., Zhao M., He C. (2014). Slow co-evolution of the MAGO and Y14 protein families is required for the maintenance of their obligate heterodimerization mode. PLoS One 9: e84842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk J.W.M., Schmidt H., Hammer U., Heinz E. (1991). Biosynthesis and desaturation of prokaryotic galactolipids in leaves and isolated chloroplasts from spinach. Plant Physiol. 96: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Roughan P.G. (1983). Similarities and differences in lipid metabolism of chloroplasts isolated from 18:3 and 16:3 plants. Plant Physiol. 72: 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.Y., Seo P.J., Yang M.S., Xiang F., Park C.M. (2008). Exploring valid reference genes for gene expression studies in Brachypodium distachyon by real-time PCR. BMC Plant Biol. 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Nobusawa T., Watanabe T., Madoka Y., Suzuki H., Shibata D., Shimojima M., Ohta H. (2016). Tangled evolutionary processes with commonality and diversity in plastidial glycolipid synthesis in photosynthetic organisms. Biochim. Biophys. Acta 1861: 1294–1308. [DOI] [PubMed] [Google Scholar]
- Hurlock A.K., Roston R.L., Wang K., Benning C. (2014). Lipid trafficking in plant cells. Traffic 15: 915–932. [DOI] [PubMed] [Google Scholar]
- Jarvis P., Dörmann P., Peto C.A., Lutes J., Benning C., Chory J. (2000). Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc. Natl. Acad. Sci. USA 97: 8175–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jothi R., Cherukuri P.F., Tasneem A., Przytycka T.M. (2006). Co-evolutionary analysis of domains in interacting proteins reveals insights into domain-domain interactions mediating protein-protein interactions. J. Mol. Biol. 362: 861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Block M.A., Douce R. (1991). Molecular aspects of plastid envelope biochemistry. Eur. J. Biochem. 199: 489–509. [DOI] [PubMed] [Google Scholar]
- Kelly A.A., Kalisch B., Hölzl G., Schulze S., Thiele J., Melzer M., Roston R.L., Benning C., Dörmann P. (2016). Synthesis and transfer of galactolipids in the chloroplast envelope membranes of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 113: 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157: 105–132. [DOI] [PubMed] [Google Scholar]
- Lafon-Placette C., Köhler C. (2014). Embryo and endosperm, partners in seed development. Curr. Opin. Plant Biol. 17: 64–69. [DOI] [PubMed] [Google Scholar]
- Le Poidevin N. (1965). Inhibition of the germination of mustard seeds by saturated fatty acids. Phytochemistry 4: 525–526. [Google Scholar]
- Li W., Cong Q., Pei J., Kinch L.N., Grishin N.V. (2012). The ABC transporters in Candidatus Liberibacter asiaticus. Proteins 80: 2614–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher K.P. (2016). Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 23: 487–493. [DOI] [PubMed] [Google Scholar]
- Lu B., Benning C. (2009). A 25-amino acid sequence of the Arabidopsis TGD2 protein is sufficient for specific binding of phosphatidic acid. J. Biol. Chem. 284: 17420–17427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Xu C., Awai K., Jones A.D., Benning C. (2007). A small ATPase protein of Arabidopsis, TGD3, involved in chloroplast lipid import. J. Biol. Chem. 282: 35945–35953. [DOI] [PubMed] [Google Scholar]
- Mekhedov S., de Ilárduya O.M., Ohlrogge J. (2000). Toward a functional catalog of the plant genome. A survey of genes for lipid biosynthesis. Plant Physiol. 122: 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J.D., Sebesta D.K. (1982). Role of endogenous growth regulators in seed dormancy of Avena fatua: I. Short chain fatty acids. Plant Physiol. 70: 1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering E.R., Muthan B., Benning C. (2010). Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228. [DOI] [PubMed] [Google Scholar]
- Mongrand S., Bessoule J., Cabantous F., Cassagne C. (1998). The C16:3\C18:3 fatty acid balance in photosynthetic tissues from 468 plant species. Phytochemistry 49: 1049–1064. [Google Scholar]
- Ohlrogge J., Browse J. (1995). Lipid biosynthesis. Plant Cell 7: 957–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y., Kamide Y., Hirai M.Y., Saito K. (2013). Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 9 (suppl. 1): 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham M.L., Khare D., Quiocho F.A., Davidson A.L., Chen J. (2007). Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450: 515–521. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov S., Kamisetty H., Baker D. (2014). Robust and accurate prediction of residue-residue interactions across protein interfaces using evolutionary information. eLife 3: e02030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov S., Kinch L., Park H., Liao Y., Pei J., Kim D.E., Kamisetty H., Grishin N.V., Baker D. (2015). Large-scale determination of previously unsolved protein structures using evolutionary information. eLife 4: e09248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos F., Valencia A. (2008). Protein co-evolution, co-adaptation and interactions. EMBO J. 27: 2648–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos F., Ranea J.A., Juan D., Sternberg M.J. (2005). Assessing protein co-evolution in the context of the tree of life assists in the prediction of the interactome. J. Mol. Biol. 352: 1002–1015. [DOI] [PubMed] [Google Scholar]
- Penfield S., Li Y., Gilday A.D., Graham S., Graham I.A. (2006). Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikofski J., Tao B.Y. (1992). Polymerase chain reaction (PCR) techniques for site-directed mutagenesis. Biotechnol. Adv. 10: 535–547. [DOI] [PubMed] [Google Scholar]
- Roston R., Gao J., Xu C., Benning C. (2011). Arabidopsis chloroplast lipid transport protein TGD2 disrupts membranes and is part of a large complex. Plant J. 66: 759–769. [DOI] [PubMed] [Google Scholar]
- Roston R.L., Gao J., Murcha M.W., Whelan J., Benning C. (2012). TGD1, -2, and -3 proteins involved in lipid trafficking form ATP-binding cassette (ABC) transporter with multiple substrate-binding proteins. J. Biol. Chem. 287: 21406–21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R.R., Berrie A.M. (1979). Effect of temperature on the short chain fatty acid-induced inhibition of lettuce seed germination. Plant Physiol. 63: 61–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrier P.J., et al. (2008). Plant ABC proteins--a unified nomenclature and updated inventory. Trends Plant Sci. 13: 151–159. [DOI] [PubMed] [Google Scholar]
- Vogel J., Hill T. (2008). High-efficiency Agrobacterium-mediated transformation of Brachypodium distachyon inbred line Bd21-3. Plant Cell Rep. 27: 471–478. [DOI] [PubMed] [Google Scholar]
- Wang K., Hersh H.L., Benning C. (2016). SENSITIVE TO FREEZING2 aides in resilience to salt and drought in freezing-sensitive tomato. Plant Physiol. 172: 1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Benning C. (2011). Arabidopsis thaliana polar glycerolipid profiling by thin layer chromatography (TLC) coupled with gas-liquid chromatography (GLC). J. Vis. Exp. 49: 2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.M., Procter J.B., Martin D.M., Clamp M., Barton G.J. (2009). Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Fan J., Cornish A.J., Benning C. (2008). Lipid trafficking between the endoplasmic reticulum and the plastid in Arabidopsis requires the extraplastidic TGD4 protein. Plant Cell 20: 2190–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Fan J., Riekhof W., Froehlich J.E., Benning C. (2003). A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J. 22: 2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Fan J., Froehlich J.E., Awai K., Benning C. (2005). Mutation of the TGD1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell 17: 3094–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Munz J., Cass C., Zienkiewicz A., Kong Q., Ma W., Sanjaya J., Benning C. (2015). Ectopic expression of WRINKLED1 affects fatty acid homeostasis in Brachypodium distachyon vegetative tissues. Plant Physiol. 169: 1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]