Abstract
Background and Purpose
Prior reports from the Framingham Heart Study have identified cross-sectional associations of arterial stiffness, as reflected by carotid-femoral pulse wave velocity (CFPWV), and systolic blood pressure (SBP) with vascular brain injury. The purpose of this study is to examine free water (FW), fractional anisotropy (FA) and white matter hyperintensities (WMH) in relation to arterial stiffness among subjects of the Framingham Offspring and 3rd generation cohorts.
Methods
In 2422 participants aged 51.3±11.6 years, FA, FW and WMH were related to CFPWV using voxel-based linear and generalized linear regressions, adjusting for relevant covariables. Mean FW, mean FA and WMH burden (log-transformed) were computed within white matter (WM) region and related to SBP and CFPWV using multiple mediation analyses.
Results
CFPWV was found to be associated with higher FW, lower FA and higher WMH incidence in WM areas covering respectively 356.1, 211.8 and 10.9cc of the WM mask. Mediation analyses revealed that the effect of SBP on FW was mediated by CFPWV (direct and indirect effects: a=0.040, p<0.001 and a′=0.020, p>0.05). Moreover, the effect of CFPWV on FA was mediated by FW (direct and indirect effects: b=−0.092, p<0.001 and b′=0.012, p>0.05), whose effect on WMH was in turn mediated by FA (direct and indirect effects: c=0.246, p<0.001 and c′=0.116, p>0.05).
Conclusions
From these data, we propose a biomechanical hypothesis designed for future research experiments to explain how hemodynamic alteration may lead to WM injury by impacting cerebral water content and more subtly WM integrity, to finally lead to WMH development.
Introduction
Hypertension and arterial stiffness are common medical conditions among individuals 60 years of age and older.1 Besides being major risk factors for cardiovascular disease and mortality,2, 3 vascular alterations, including increased aortic stiffness as assessed by carotid-femoral pulse wave velocity, are also risk factors for subclinical cerebral vascular brain injury such as white matter hyperintensities (WMH), MRI infarcts, and accelerated brain atrophy.4–6 These imaging markers of brain aging also are associated with reduced cognitive ability6 and increased likelihood of incident stroke, mild cognitive impairment and dementia.7 Recent analyses from the Framingham Heart Study (FHS) Third Generation (average age 43) cohort suggested that hemodynamic alterations may play a central role in the early development of white matter (WM) degeneration which leads to symptomatic disease in later life8, 9.
Diffusion Tensor Imaging (DTI) measures the displacement of water molecules, which in a typical brain imaging experiment displace a few tens of microns10. DTI-derived fractional anisotropy (FA) reflects how water directionality is constrained along axons and characterizes the microstructural properties and integrity of WM. Nonetheless, standard single shell DTI algorithms are contaminated by partial volume of extracellular water, reducing the sensitivity and specificity of most metrics derived from DTI, including FA10. Consequently, efforts have been recently deployed to quantify this amount of extracellular water, also referred as free water (FW). FW reflects the amount of water molecules that do not experience flow and are not restricted by their surroundings, and therefore is independent to water directionality, as opposed to FA. While interest in this new imaging metric has been increasing in the context of sensitivity improvement of DTI in the vicinity of the ventricles and for the delineation of fibers such as the fornix11, 12, recent studies have also found that FW content constitutes a strong biomarker of subtle cerebral injury in individuals with various pathologies including Parkinson’s disease and schizophrenia13, 14.
The present study, therefore, had two objectives. First, we aimed to evaluate the sensitivity of FW as a marker of WM injury in association with elevated blood pressure and arterial stiffness among normal adult individuals. Second, despite our cross-sectional design that does not permit conclusions based on the chronology of mechanisms that arise with hemodynamic alteration, we used mediation analysis to propose a potential scenario of how hemodynamic alterations may first lead to increased FW content that subtly contributes to WM degeneration, as identified by reduced FA, to finally result in WMH formation. The study sample includes 2422 individuals from the Third Generation and Offspring community based cohorts of the FHS aged from 25 to 89 years old.
Methods
Study design and participants
The design of the FHS Third Generation (G3) and Offspring cohort studies has been detailed previously.15 A total of 2606 subjects underwent both brain MRI and arterial tonometry between 2009 and 2013. All subjects at assessment number 8 for the Offspring cohort and assessment number 2 for the 3rd Generation cohort were given the option to receive an MRI. As previously described16, all subjects who agreed to have MRI and were not excluded for health reasons were included in this analysis. Participants with prevalent stroke or dementia at the MRI evaluation, however, were excluded from the present analysis. Briefly, stroke was defined as an acute onset focal neurologic deficit of presumed vascular etiology, lasting ≥24 hours or resulting in death. Dementia was diagnosed according to the criteria of the DSM-IV.17 Participants were also excluded from the present analysis for the following reasons: other neurologic disorders that might confound the assessment of brain volumes, bad quality or incomplete tonometry data or with poor brain MRI quality, resulting in a sample of 2422 individuals. Demographic characteristics are summarized in Table 1.
Table 1.
Subject’s characteristics
| Tonometry only N=2272 |
Tonometry with MRI N=2422 |
P-value | |
|---|---|---|---|
| Age, years | 56.38 ± 13.64 | 51.28 ± 11.67 | <0.001 |
| Male | 1043 (45.91) | 1102 (45.50) | 0.78 |
| Systolic blood pressure, mmHG | 122.73 ± 17.37 | 118.25 ± 14.75 | <0.001 |
| Total Cholesterol, mg/dL | 186.66 ± 36.91 | 187.02 ± 34.71 | 0.73 |
| Carotid-femoral pulse wave, m/s, median (Q1, Q3) | 7.90 (6.70, 10.00) | 7.20 (6.30, 8.60) | <0.001 |
| History of diabetes | 238 (10.76) | 150 (6.22) | <0.001 |
| Current smoker | 259 (11.41) | 196 (8.10) | <0.001 |
| Treatment for hypertension | 836 (36.86) | 620 (25.60) | <0.001 |
Data presented as mean (SD) or n (%), unless otherwise stated
The Institutional Review Boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Procedures
Noninvasive hemodynamic data acquisition is described in the Supplement file (please see Supplemental Data). Carotid-femoral pulse wave velocity (CFPWV) was calculated from tonometry waveforms and body surface transit distance, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch with the use of the suprasternal notch as a fiducial point.18 The carotid-femoral transit path spans the descending aorta, making CFPWV a measure of descending thoracic and abdominal aortic stiffness. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured as the average of two physician recorded BPs in a seated position.
We used DTI measures of free water content (FW), of fractional anisotropy (FA), which is a sensitive indicator of WM integrity, and of WMH. FW and FA were computed from DTI using previously described algorithms.12, 19 Segmentation of WMH and total cranial volume (TCV) were performed from FLAIR and T1-weighted images by automated procedures previously described.20, 21 FW, FA and WMH maps were finally coregistered to a minimum deformation template for group statistical analyses. Each voxel in these co-registered multimodal volumetric images corresponds to the same location in the brain across all individuals. This allows corresponding imaging measures (FW, FA or WMH occurrence) to be related to a host of independent variables. WMH volumes were log-transformed to normalize population variance.
Statistical analyses
Voxel-based regressions
The objective of the voxel-based analysis was to determine if, at the voxel level, water content as indicated by FW, subtle WM degeneration, as indicated by lower FA and most advanced stage of WM injury, as indicated with WMH occurrence, were associated with individual CFPWV. To achieve this goal, we used separate linear and generalized linear regressions with either FW (continuous measures), FA (continuous measures) or WMH (occurrence) as the dependent variables (separate models for each), and CFPWV as independent variable, adjusting for a set of standard risk factors including age, sex, use of antihypertensive therapy, total cholesterol, presence of diabetes mellitus, TCV and time between clinical and MRI exams.
Mediation analyses
Mean FW, mean FA and log-transformed WMH volume were computed for each subject by superimposing the WM mask to each individual’s FW, FA or WMH images. Because FW appeared to be more sensitive to arterial stiffness (see Figure 1) than FA and WMH, in terms of both spatial extent and association’s strength, we then conducted mediation analyses to investigate whether the reported association between abnormal hemodynamic measures and WMH9, 22 may be mediated by DTI-derived measures, including FW and FA. Briefly, mediation exists when a predictor affects a dependent variable indirectly through a third variable (the mediator).23 Mediation analyses investigate the effect of SBP on FW with CFPWV as a mediator (Model 1), the effect of CFPWV on FA with FW as a mediator (Model 2), the effect of FW on WMH with FA as a mediator (Model 3). Each model included age, sex, use of antihypertensive therapy, total cholesterol, presence of diabetes mellitus, TCV and time between tonometry and MRI exams as covariates. In addition, Model 2 was also adjusted for SBP, and Model 3 for SBP and CFPWV. Indirect effects were examined using a 95% bootstrapped bias-corrected confidence interval established via bootstrapping with 1000 bootstrap samples.
Figure 1.
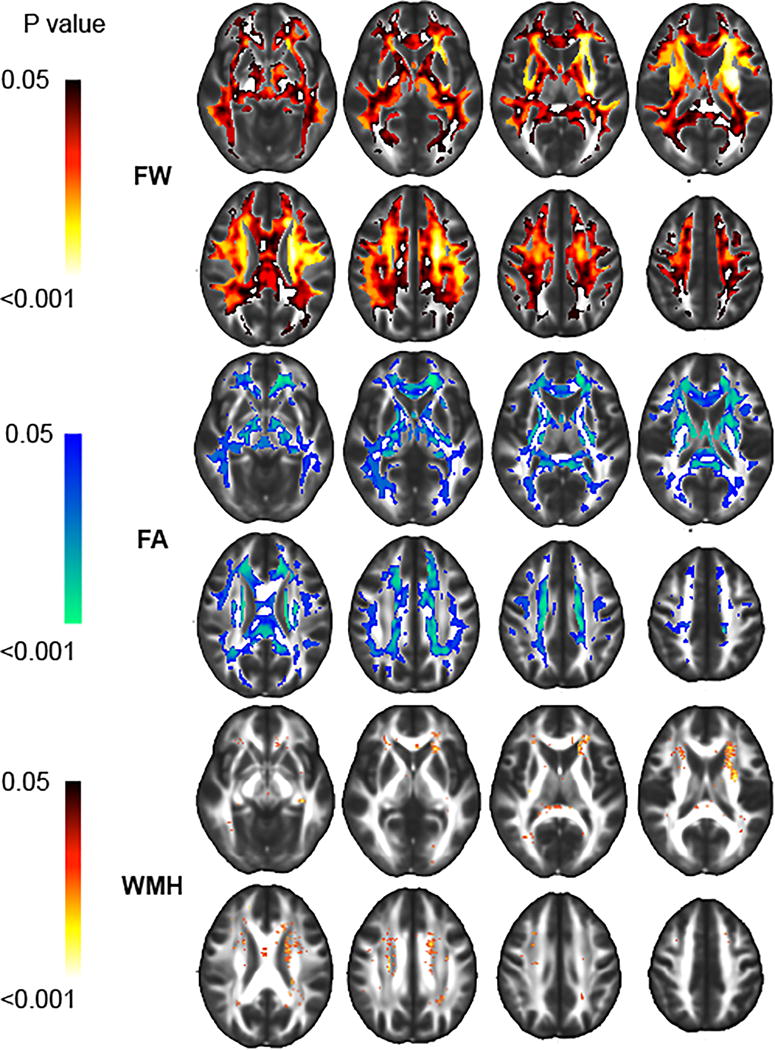
Significant associations between increasing carotid-femoral pulse wave velocity (CFPWV) with higher free water (FW), lower fractional anisotropy (FA) and higher white matter hyperintensities (WMH) occurrence. Color intensity indicates strength of association.
The details of the study methods, including subject recruitment and evaluation, image acquisition and processing, and statistical analysis, are described in the Supplemental file (please see http://stroke.ahajournals.org).
Results
Demographics
Compared with the remainder of the G3 and Offspring cohorts (see Table 1) without MRI, individuals included in this study were on average significantly younger (p<0.001), with significantly lower SBP (p<0.001) and CFPWV (p<0.001) and less likely to smoke, to receive treatment for hypertension or have diabetes (p values <0.001).
Voxel-based regressions
Higher CFPWV was associated with larger FW within voxels (referred to as RFW) that covered 356.1cc of the WM (see Table I in the Supplement file, please see http://stroke.ahajournals.org). WM tracts most implicated as a function of volume included the superior, anterior and posterior part of the corona radiata (13.50, 12.32 and 7.03cc respectively), the body, splenium and genu of corpus callosum region (12.03, 9.30 and 6.49cc respectively) and the superior longitudinal fasciculus (11.89cc, see Figure 1).
Higher CFPWV was associated with lower FA within voxels (referred to as RFA) that covered 211.8cc of the WM (see Table I in the Supplement file, please see http://stroke.ahajournals.org). WM tracts heavily involved, as a function of volume, also included the superior, anterior and posterior part of the corona radiata (6.85, 10.07 and 3.43cc respectively) and the body, splenium and genu of corpus callosum region (9.64, 8.65 and 6.02cc respectively, see Figure 1).
Higher CFPWV was also associated with higher WMH occurrence within voxels (referred to as RWMH) that covered 10.9cc of the WM (see Table I in the Supplement file, please see http://stroke.ahajournals.org) implicating mostly, in term of volume, the superior and anterior part of the corona radiata (1.49 and 2.31cc respectively) and the superior longitudinal fasciculus (0.41cc, see Figure 1).
Interestingly, the overlap between RFW and RFA regions covered 52% of RFW and 89% of RFA, indicating that almost half of the voxels with a significant association between CFPWV and FW were not significant for FA while almost all voxels showing significant associations between CFPWV and FA were significant for FW.
Similarly, the overlap between RFA and RWMH regions covered 3% of RFA and 66% of RWMH, indicating that most of the voxels with a significant association between CFPWV and FA were not significant for WMH while two thirds of the voxels showing significant associations between CFPWV and WMH were significant for FA.
The overlap between RFW and RWMH regions covered 3% of RFW and 95% of RWMH, indicating that most of the voxels with a significant association between CFPWV and FW were not significant for WMH while almost all of the voxels showing significant associations between CFPWV and WMH were significant for FW.
Mediation analyses
Figure 2 shows the mediation analyses results relating SBP, CFPWV, FW, FA and WMH. In Model 1, the direct association between SBP and FW was found to be significant (a=0.040, p<0.001) but mediated by CFPWV (indirect effect: a′=0.020, p>0.05, mediation effect: β=0.020, p<0.001). The significant mediation effect in this model reflects that fact that the influence of SBP on CFPWV was highly significant (β=0.165, p<0.001), as well as the influence of CFPWV on FW (β=0.122, p<0.001). Similarly, Model 2 revealed that CFPWV had a direct effect on FA (b=−0.092, p<0.001), that no longer remained significant when FW was included as a mediator in the model (indirect effect: b′=0.012, p>0.05, mediation effect: β=−0.104, p<0.001), and whose effect on FA was found to be highly significant (β=−0.851, p<0.001). Finally, Model 3 revealed that FW content had a direct effect on WMH burden (c=0.246, p<0.001), but this effect was found to be mediated by FA (indirect effect: c′=0.13, p>0.05, mediation effect: β=0.116, p<0.001), whose effect on WMH was found to be significant (β=−0.136, p<0.001).
Figure 2.
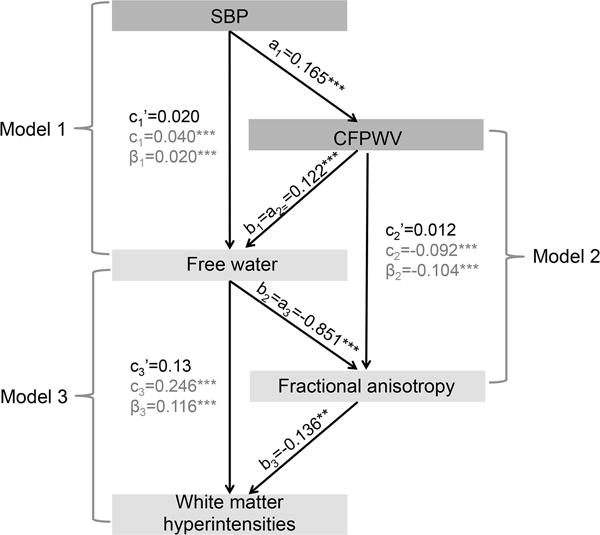
For each mediation Model i, (e.g. ai, bi, ci) indicate the direct effects, whereas ci′ indicates the indirect effect and βi the mediation effect. Mediation analyses investigate the effect of systolic blood pressure (SBP) on free water, with carotid-femoral pulse wave velocity (CFPWV) as mediator (Model 1), the effect of CFPWV on fractional anisotropy with free water as mediator (Model 2), the effect of free water on white matter hyperintensities burden with fractional anisotropy as mediator (Model 3). **: p<0.01; ***: p<0.001
Discussion
The present study suggests that, among a community-based sample aged 25 to 90 years, elevated blood pressure and arterial stiffness appears to trigger a pathophysiological cascade that includes an increase in free water content and subtle WM degeneration eventually leading to the development of WMH. From these data, we propose a biomechanical model whereby cerebral microvascular damage is initiated by hemodynamic alterations that have been shown to manifest in subclinical cognitive dysfunction within the FHS Offspring cohort24.
Hemodynamic alteration
While there is now substantial evidence that elevated arterial stiffness is the earliest manifestation of systolic hypertension, controversy exists regarding the direction and nature of this relationship, with evidence implicating arterial stiffness in the manifestation and progression of hypertension as well as vice versa.25 For example, the Framingham Heart Study, using data from Exam 7 to predict SBP and PWV in Exam 8 (7 years later on average), found that elevated PWV in Exam 7 was associated with higher SBP in Exam 8, but SBP at Exam 7 was not associated with higher PWV in Exam 8.26 Conversely, findings from the Baltimore Longitudinal Study of Aging indicate that higher PWV was associated with larger increases in SBP over time but also that higher SBP was associated with accelerated increase of PWV over the same observational period. Recently, however, the SardiNIA study brought new insights in the understanding of the cross-talk trajectory of PWV and SBP over time by showing evidence of a striking dissociation in the trajectories PWV versus various measures of blood pressure with advancing age.27 This finding challenges the notion of a tight coupling between blood pressure and PWV, particularly in later life. Therefore, while our mediation analyses suggest that the effect of SBP on free water content is modulated by CFPWV, and not the reverse, this cross-sectional study cannot resolve the question as to whether vascular mechanical or hemodynamic alterations initiate this process. Longitudinal assessments, emphasizing the earliest changes in SBP and CFPWV, particularly in younger individuals, are likely necessary to further examine this question.
Extracellular water
The main finding of this work is that arterial stiffness is strongly associated with the content of FW within most of the cerebral WM, particularly in the area of the middle cerebral artery WM vascular territory, suggesting that FW constitutes a more sensitive biomarker of WM injury in association with hemodynamic alteration than FA and WMH (see Figure 1). Increasing numbers of DTI studies have reported that correcting for extracellular water content improves the sensitivity of DTI in the vicinity of the ventricles and for the delineation of fibers such as the fornix.12 However, only a few studies have examined FW content, as estimated from DTI images, specifically as a biomarker of cerebral injury, in individuals with pathologies including Parkinson’s disease or schizophrenia.13, 14 To our knowledge, this study is the first to investigate differences in FW content at voxel-based level among participants in a large community-based study. Using this approach, we found evidence of strong and spatially distributed associations between increased arterial stiffness and the content of FW, identifying a potentially new stage in the pathological process leading to WMH (and possibly small vessel cerebral infarction). It also raises the question of how the biological mechanisms that lead to stiffening of the arterial wall promote an increase in extracellular water content.
One hypothesis is that stiffening of the aorta increases transmission of pulsatile energy into small vessels that are not accustomed to high levels of pulsatility. High levels of pulsatility could lead to changes in pre-capillary pressure gradients thereby resulting in greater flow of solute into the interstitium until interstitial pressure equilibrates with intravascular pressure and a new equilibrium is achieved, albeit with an expanded extravascular FW content. A second hypothesis is that arterial stiffening and elevated blood pressure may result in subtle blood-brain barrier dysfunction, shifting the equilibrium point between arterial and osmotic pressure, which in turn may allow extra water, toxins, proteases, or other substances in the blood to enter the brain interstitial space and disturb the composition of the interstitial fluid within the WM milieu. Additional studies relating arterial stiffness to measures derived from additional MRI sequences such as dynamic contrast-enhanced imaging28 could be used to directly test this hypothesis.
Free water excess and white matter integrity
A second important finding is that while, by their nature, WMH are expected to be associated with the amount of FW content, in our analysis this association was mediated by differences is FA. The underlying process by which this occurs, however, is unclear. One hypothesis could be that alterations in the composition of interstitial fluids, through the increase of FW, may contribute to subtle demyelination and axonal destruction over time. Indeed, previous studies have reported associations between reduced FA and higher level of interstitial proteins, including beta-amyloid protein.29 The design of the present study, however, does not enable identification of the components within FW that may be implicated in the degeneration process of demyelination or axon loss in neural fiber tracts.
A second hypothesis is that, rather than demyelination, an increase in FW content may result in axonal dispersion, lessening the constraint of water directionality along axons, as reflected by reduced FA. Again, our cross sectional design does not allow us to conclude that FW increase precedes or is concurrent to a decrease in FA. Longitudinal studies using probabilistic tractography, which measures voxel-based connectivity index along WM pathways that reflects fiber organization such as tract geometry and length, may help to answer this question.
White matter hyperintensities as the peak of WM injury
The present study showed, for the first time, voxel-based associations between arterial stiffness and occurrence of WMH, extending findings of previous studies reporting such associations, but at the regional level9, 22. WMH burden was also found to be associated with FA. These results confirm, within a larger cohort, previous DTI studies which show that WM degeneration is an insidious and continuous process, with WMH constituting the most advanced stage of injury.30 Recent studies have extended this finding using different quantitative imaging techniques. For example, larger burden of WMH was found to be associated with reduced cerebral blood flow, cerebrovascular reactivity and with increased blood-brain barrier permeability.31, 32 Importantly, WMH constitute the end-stage of demyelination and axonal damage, as reported by pathology studies,33 and while the excess of FW content might be reversible, much of WMH, as they appear on FLAIR images, likely reflect permanent damage, as supported by longitudinal MRI studies that tracked individual lesions over time.30 This evidence, in addition to the evidence that WMH burden is associated with trajectories of significant cognitive decline7 emphasizes the importance of early detection of change in WM properties as a preventive strategy against white matter injury and subsequent cognitive decline. In the present study, the association between elevated arterial stiffness and excess of FW content involved almost the entire cerebral WM, with associations between CFPWV and FW being strongest in regions where WMH occur (Figure 1). This finding suggests that FW may constitute a sensitive biomarker to assess and monitor therapies aimed at achieving the goal of reduced vascular brain injury, although the potential reversibility of increased interstitial FW has yet to be determined.
Strengths and limitations
The strengths of this study are the large, population-based setting with quantitative and sensitive measurements of WM integrity. Original features of this study are the wide age range of the study cohort as compared to previous publications. There, however, are limitations including the cross-sectional design that limits our ability to establish causal relations between arterial stiffness and brain aging measures. Additional longitudinal studies are needed to support the proposed order of cerebral mechanisms arising from differences in CFPWV. The present study also did not aim to decipher the conundrum of SBP and CFPWV as briefly described above and more fully addressed in other publications25 but instead proposes a hypothesis to explain how their combination contributes to WM degeneration. Finally, the FHS cohort is mostly of white descent and therefore does not fully represent the general population of the USA for whom the impact of hypertension and vascular stiffness may be even more relevant.
Conclusion
The present study finds that FW is a sensitive measure of subtle brain injury in association with increased CFPWV and proposes a biomechanical hypothesis to explain how hemodynamic alteration may lead to late clinically relevant WM degeneration (i.e. WMH). Our findings suggest that elevated carotid-femoral pulse wave velocity impacts the content of cerebral FW and WM integrity, as reflected by FA, finally leading to WMH development. Longitudinal studies are needed to assess the exact chronological order of these mechanisms that arise with hemodynamic alteration.
Supplementary Material
Acknowledgments
Source of Funding: The study was supported by NIH/NHLBI Contracts P30-AG010129, R01-AG033040, R01-AG08122, K23-LH118529, N01-HC-25195, HHSN268201500001I, HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, 2-K24-HL04334.
Footnotes
Disclosures
Dr. Maillard, Dr. Fletcher, Dr. Seshadri, Dr. Beiser, Dr. Satizabal, Dr. Himali and Dr. Vasan report no disclosures.
Dr. Pase is funded by an Australian National Health and Medical Research Council Early Career Fellowship (APP1089698).
Dr. Tsao is partially supported by an award from the American Heart Association (11CRP4930004)
Dr. DeCarli is a consultant to Novartis, Pharmaceuticals
Dr. Mitchell is owner of Cardiovascular Engineering Inc (a company that develops and manufactures devices to measure vascular stiffness) and is a consultant to Novartis, Merck and Servier.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander CM, Landsman PB, Teutsch SM, Haffner SM, Third National H, Nutrition Examination S et al. Ncep-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among nhanes iii participants age 50 years and older. Diabetes. 2003;52:1210–1214. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D’Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: The framingham study. Stroke. 2004;35:1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 5.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, et al. Prevalence and correlates of silent cerebral infarcts in the framingham offspring study. Stroke. 2008;39:2929–2935. doi: 10.1161/STROKEAHA.108.516575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: The framingham offspring study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 7.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of mri markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The framingham offspring study. Stroke. 2010;41:600–606. doi: 10.1161/STROKEAHA.109.570044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, et al. Effects of arterial stiffness on brain integrity in young adults from the framingham heart study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, et al. Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The framingham third generation cohort study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasternak O, Shenton ME, Westin CF. Estimation of extracellular volume from regularized multi-shell diffusion mri. Med Image Comput Comput Assist Interv. 2012;15:305–312. doi: 10.1007/978-3-642-33418-4_38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher E, Carmichael O, Pasternak O, Maier-Hein KH, DeCarli C. Early brain loss in circuits affected by alzheimer’s disease is predicted by fornix microstructure but may be independent of gray matter. Frontiers in aging neuroscience. 2014;6:106. doi: 10.3389/fnagi.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion mri. Magn Reson Med. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- 13.Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, et al. Longitudinal changes in free-water within the substantia nigra of parkinson’s disease. Brain. 2015;138:2322–2331. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burciu RG, Ofori E, Shukla P, Pasternak O, Chung JW, McFarland NR, et al. Free-water and bold imaging changes in parkinson’s disease patients chronically treated with a mao-b inhibitor. Hum Brain Mapp. 2016;37:2894–2903. doi: 10.1002/hbm.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The third generation cohort of the national heart, lung, and blood institute’s framingham heart study: Design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 16.Massaro JM, D’Agostino RB, Sr, Sullivan LM, Beiser A, DeCarli C, Au R, et al. Managing and analysing data from a large-scale study on framingham offspring relating brain structure to cognitive function. Stat Med. 2004;23:351–367. doi: 10.1002/sim.1743. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for dsm-iv axis i disorders (scid-i), clinical version. Washington, DC: American Psychiatric Press, Inc.; 1997. [Google Scholar]
- 18.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (wmh): Exploring the relationships between periventricular wmh, deep wmh, and total wmh burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher E, Singh B, Harvey D, Carmichael O, Decarli C. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Conference. 2012;2012:5319–5322. doi: 10.1109/EMBC.2012.6347195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustavsson AM, Stomrud E, Abul-Kasim K, Minthon L, Nilsson PM, Hansson O, et al. Cerebral microbleeds and white matter hyperintensities in cognitively healthy elderly: A cross-sectional cohort study evaluating the effect of arterial stiffness. Cerebrovasc Dis Extra. 2015;5:41–51. doi: 10.1159/000377710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychological methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 24.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, Decarli C, Au R, et al. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.AlGhatrif M, Lakatta EG. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr Hypertens Rep. 2015;17:12. doi: 10.1007/s11906-014-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scuteri A, Morrell CH, Orru M, Strait JB, Tarasov KV, Ferreli LA, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension. 2014;64:1219–1227. doi: 10.1161/HYPERTENSIONAHA.114.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huisa BN, Caprihan A, Thompson J, Prestopnik J, Qualls CR, Rosenberg GA. Long-term blood-brain barrier permeability changes in binswanger disease. Stroke. 2015;46:2413–2418. doi: 10.1161/STROKEAHA.115.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. The association between biomarkers in cerebrospinal fluid and structural changes in the brain in patients with alzheimer’s disease. J Intern Med. 2014;275:418–427. doi: 10.1111/joim.12164. [DOI] [PubMed] [Google Scholar]
- 30.Maillard P, Fletcher E, Lockhart SN, Roach AE, Reed B, Mungas D, et al. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721–1726. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Promjunyakul NO, Lahna DL, Kaye JA, Dodge HH, Erten-Lyons D, Rooney WD, et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. J Cereb Blood Flow Metab. 2016;36:1528–1536. doi: 10.1177/0271678X16651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sam K, Crawley AP, Conklin J, Poublanc J, Sobczyk O, Mandell DM, et al. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann Neurol. 2016;80:277–285. doi: 10.1002/ana.24712. [DOI] [PubMed] [Google Scholar]
- 33.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


