Abstract
Evidence from epidemiologic studies suggests a relation between the Mediterranean diet (MeDi) and cognitive function, but results are inconsistent. Prior reviews have not provided pooled data from meta-analysis of longitudinal studies and randomized controlled trials (RCTs), or they included younger adult participants. This systematic review and meta-analysis examines the impact of the MeDi on the cognitive functioning of healthy older adults. Fifteen cohort studies with 41,492 participants and 2 RCTs with 309 and 162 participants in intervention and control groups, respectively, were included. The primary outcome of interest was cognitive function, divided into domains of memory and executive function. Meta-analysis of cohort studies revealed a significant association between MeDi and older adults’ episodic memory (n = 25,369, r = 0.01, P = 0.03) and global cognition (n = 41,492, r = 0.05, P ≤ 0.001), but not working memory (n = 1487, r = 0.007, P = 0.93) or semantic memory (n = 1487, r = 0.08, P = 0.28). Meta-analysis of RCTs revealed that compared with controls, the MeDi improved delayed recall (n = 429, P = 0.01), working memory (n = 566, P = 0.03), and global cognition (n = 429, P = 0.047), but not episodic memory (n = 566, P = 0.15), immediate recall (n = 566, P = 0.17), paired associates (n = 429, P = 0.20), attention (n = 566, P = 0.69), processing speed (n = 566, P = 0.35), or verbal fluency (n = 566, P = 0.12). The strongest evidence suggests a beneficial effect of the MeDi on older adults’ global cognition. This article discusses the influence of study design and components of the MeDi on cognitive function and considers possible mechanisms.
Keywords: systematic review, meta-analysis, Mediterranean diet, cognitive functioning, healthy older adults
Introduction
As a result of longer lifespans, there has been a substantial increase in the prevalence of age-related cognitive decline (1), commonly observed as a steady decline in episodic memory and executive function (2). As decline approaches impairment, it is associated with a concomitant impairment in daily functioning (3) and an increased risk of incident dementia (4). Interventions focused on lifestyle factors such as a healthy diet could provide a cost-effective and practical approach to reducing or slowing age-related cognitive decline (5). The relative ease with which dietary interventions could be implemented may therefore have profound implications on public health policy (6).
The Mediterranean diet (MeDi), reflecting the food patterns of Greece and Southern Italy in the early 1960s (7), was first described in the Seven Countries Study by Keys et al. (8). Keys et al. reported that the MeDi was associated with a reduced risk of mortality from ischemic heart disease in particular, as well as cancers and other chronic diseases. Subsequent research provided supportive evidence linking the MeDi to a lower risk of mortality and improved health outcomes (9, 10). The MeDi is characterized by a high intake of vegetables, legumes, fruits, nuts, cereals, and olive oil but a low intake of saturated lipids and meat, moderate intake of fish, low to moderate intake of dairy products, and regular but moderate intake of alcohol (usually wine). This dietary pattern provides essential micronutrients, fibers, and other plant foods believed to promote good health (7).
Numerous epidemiological studies have investigated the relation between the MeDi and cognitive function. Systematic reviews of epidemiological studies suggest that the MeDi is associated with a reduced risk of mild cognitive impairment and dementia, including Alzheimer disease (AD) (9, 11–15), and a reduced decline in cognitive function, including episodic memory and executive function (13–17). However, findings regarding the impact of the MeDi on the cognitive health of older adults without cognitive impairment remain inconsistent across reviews, which may be attributable to a number of reasons. Previous reviews included data from younger and older adults (16), included older adults with cognitive impairment (14), considered dementia and AD outcomes alongside cognitive function (13, 15), included duplicate randomized controlled trial (RCT) data (16), or did not include RCT data (12, 17). Although 3 meta-analyses examined the relation between the MeDi and the risk of mild cognitive impairment (9, 11), cognitive impairment (18), and AD (11), there is currently no published meta-analysis to our knowledge that provides a quantitative measure of the association between the MeDi and specific cognitive functions of healthy older adults.
Our review updates the extant literature by examining the association between the MeDi and the cognitive performance of older adults without known cognitive impairment. Specifically, a systematic review and meta-analysis was conducted that included data from both RCTs and longitudinal cohort studies to investigate the relation between the MeDi and cognitive domains of memory (including recognition, immediate recall, delayed recall, face-name recall, paired associates, and semantic memory), executive function (including working memory, verbal fluency, reasoning, attention, and processing speed), and global cognitive function.
Methods
Search strategy
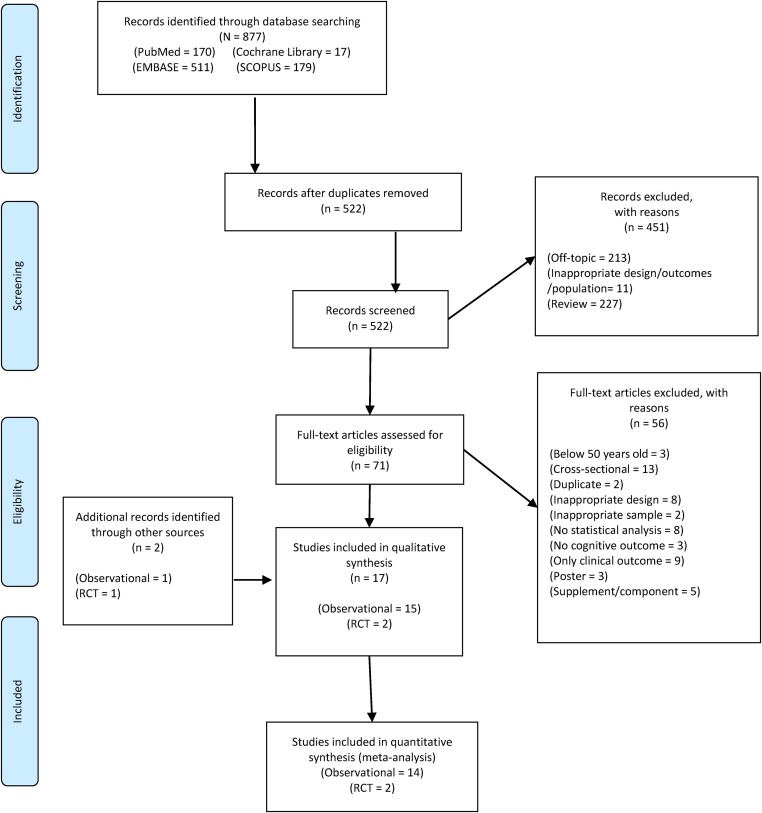
We searched the PubMed, Cochrane Library, EMBASE, and Scopus databases to identify RCTs and cohort studies written in English. Search terms “Mediterranean diet” and “cognition” were used (search strategy with results, Supplemental Table 1). We supplemented database searches with reference lists in review art, authors’ own files, and Google Scholar. We screened titles and abstracts to exclude articles that did not meet inclusion criteria. Full texts of remaining studies were then screened for eligibility by 2 independent reviewers. Disagreements were resolved through discussions with our expert authors (study selection flowchart, Figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. RCT, randomized controlled trial.
Selection criteria
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Cohort studies and RCTs that investigated the effects of the MeDi on the cognitive function of community-dwelling older adults (aged ≥50 y) with no known cognitive impairment were included. We excluded studies if participants had a diagnosis of any cardiovascular disease or other serious medical, psychiatric, or neurological problems (Supplemental Table 2). RCTs required ≥10 participants/condition to be included in the review. Two independent reviewers assessed the risk of bias in individual studies; the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) instrument was used to assess risk of bias in cohort studies (Supplemental Table 3), whereas guidelines outlined in Section 8 of the Cochrane Handbook for Systematic Reviews of Interventions were used to assess risk of bias in RCTs (Supplemental Table 4).
The primary outcome of interest was cognitive function, divided into the domains of memory and executive function. As in prior reviews (19, 20), subcategories were created within each domain, depending on measures used across studies. Memory subcategory tests included recognition, immediate recall, delayed recall, face-name recall, paired associates, and semantic memory. Executive function subcategory tests included working memory, verbal fluency, reasoning, attention, and processing speed. Composite measures of episodic memory (e.g., immediate and delayed recall) and executive function were also included. Global cognition was measured using composite measures of cognitive function.
Statistical analysis
Data extraction was conducted by 2 independent reviewers and cross-checked by a member of the expert panel. We used Comprehensive Meta-Analysis (CMA) software version 3.0 (Biostat) to conduct the analysis. For trials, the summary measure of treatment effect was the standardized mean difference, which is the absolute mean difference divided by the SD. The summary statistics required for each outcome were the number of participants in the intervention and control groups at baseline and post-test, the mean change from baseline, and the SD of the mean change. If change-from-baseline scores were not provided, they were calculated using baseline and posttest means and SDs. Change SDs were calculated assuming zero correlation between the measures at baseline and follow-up. Although this method may overestimate the SD of the change from baseline, it is a conservative approach, which is preferable in a meta-analysis (21). For cohort studies, Pearson’s r correlation coefficient was chosen, with a positive result indicating that the MeDi was associated with reduced cognitive decline. All effect sizes were first converted to Fisher’s Z and then to r. Where the predictor variable was continuous, unstandardized β values were standardized by dividing them by the SE where available. Where the SE was not available, the P value was used to estimate correlation. Standardized β values were converted to r by dividing them by the square root of the sample size. Where the predictor variable was categorical, β values were entered into CMA as either raw mean differences or as Cohen’s d as appropriate and converted to Fisher’s Z. ORs were converted to Fisher’s Z in CMA and then to r. Subgroups in cohort studies (e.g., tertiles of MeDi scores) were combined.
Individual effect sizes were combined using the inverse variance random-effects method (22). This was used to allow the incorporation of heterogeneity among studies. Heterogeneity was examined using Q, and any P value ≤0–10 was considered statistically significant (23). Inconsistency was examined using I2 and the following grades were applied: <25% (very low), 25 to <50% (low), 50 to <75% (moderate), and ≥75% (large) (23). Multiple-publication bias was avoided by using data from the most recently published study. Priority was given to outcomes that were adjusted for covariates and that removed cognitively impaired participants. For both meta-analyses of cognitive function, multiple tests of the same cognitive domain from the same study were collapsed into one effect size and subgroups were analyzed independently as separate effect sizes. Small-study effects (publication bias, etc.) were examined using funnel plots and the regression-intercept approach of Egger et al. (24), provided that there were ≥10 effect sizes (24, 25). To examine the effects of each result on the overall findings, outcomes were analyzed having deleted each study from the model once. Cumulative meta-analysis, ranked by year, was used to examine the accumulation of evidence over time (26). Sensitivity analyses were performed to investigate the robustness of the results with regard to 1) study factors (country or region, study length, age of sample, sex ratio, education, and risk of bias), 2) test factors (MeDi assessment, cognitive test used), and 3) analysis factors (whether covariates included age, sex, education, race, vascular risk factors, physical activity, energy intake, and removal of participants with dementia from baseline or analysis). Categorical variables were examined using moderator analysis, provided that there were ≥3 outcomes/category; and continuous variables were examined using meta-regression, provided that there were ≥4 outcomes for analysis.
Results
Study characteristics
Fifteen cohort studies were eligible for inclusion (Table 1). One study examined the same cohort as a later study and was not included in quantitative analysis (41). The remaining studies had 41,492 participants, with study lengths ranging from 2 to 10.6 y (mean: 5.7 y). The MeDi was scored using the methods of Trichopoulou et al. (28) in 8 studies, Panagiotakos et al. (34) in 2 studies, and the Mediterranean–Dietary Approaches to Stop Hypertension Trial diet intervention for neurodegenerative delay (35) in 1 study and alternate methods in 2 studies.
TABLE 1.
Characteristics of included cohort studies1
| Study characteristics |
Baseline demographics |
|||||||||
| Study, year (ref) | Population | Mean length, y | n | Mean age, y | Sex, % women | STROBE | Food intake assessment/MeDi score | Covariates | Outcome of interest | Additional notes |
| Cherbuin and Anstey, 2012 (27) | PATH Through Life study: population-based sample of community-dwelling adults aged 60–64 y randomly selected from electoral roll in Australia | 5 | 1491 | 62.5 ± 1.5 | 51.2 | 21 | CSIROFFQ plus Australian food composition tables | Age, sex, education, apoE ε4 status, BMI, physical activity, stroke, diabetes, hypertension, and energy intake | Global cognition2 | Participants with MCI and dementia were removed from analysis. |
| Trichopoulou et al. (28) | Among MeDi components, higher intake of fish was associated with less cognitive decline (P = 0.011) and a higher ratio of monounsaturated to saturated fats was associated with cognitive decline (P = 0.008) | |||||||||
| Féart et al., 2009 (29) | Three-City Study: population-based sample of community-dwelling adults aged ≥65 y from Bordeaux | 5 | 1410 | 75.9 ± 4.8 | 62.6 | 22 | Validated FFQ plus 24-h dietary recall assessed by dietitian | Age, sex, education, marital status, energy intake, physical activity, BMI, diabetes, hypercholesterolemia, hypertension, smoking status, stroke, depression, ≥5 medicines/d, apoE ε4 status, and their interaction with time | Global cognition3, immediate recall (BVRT2, FCSRT3), verbal fluency2 | Participants with dementia were removed from analysis; when participants with dementia were included, FCSRT score was nonsignificant (P = 0.08) |
| Trichopoulou et al. (28) | When MeDi was assessed as a categorical variable, the highest, but not middle, category was significantly associated with global cognition and FCSRT | |||||||||
| Galbete et al., 2015 (30) | SUN project: sample of university graduates aged ≥65 y in Spain | 2 | 823 | 61.9 ± 6 | 27.1 | 22 | Validated FFQ sent by mail | Age, sex, college education, follow-up time between baseline and cognitive evaluation, energy intake, physical activity, BMI, CVD, diabetes, hypercholesterolemia, hypertension, smoking status, and apoE ε4 status | Global cognition3 | In multivariate analysis, participants with higher adherence to the MeDi had significantly better cognitive function compared to the moderate and lowest categories |
| Trichopoulou et al. (28) | ||||||||||
| Gallucci et al., 2013 (31) | TRELONG study: population-based sample aged 70–79 y selected from registry office and stratified for age and sex | 7 | 309 | 79.1 ± 9.7 | 61.2 | 18 | Assessed intake of cereals, fish, vegetables, and fruit | None | Global cognition2 | The majority adhered to the MeDi (92%). Only univariate analysis was conducted with the MeDi variable, which found a nonsignificant association; the MeDi was not included in multivariate analysis |
| MeDi status (yes/no) based on food intake | ||||||||||
| Gardener et al., 2015 (32) | AIBL study: cohort study of healthy controls aged ≥60 y in Australia | 3 | 527 | 69.3 ± 6.4 | 60.2 | 19 | CCVFFQ assessed food intake over past year | Age, sex, years of education, country of birth (Australia vs. other), energy intake, angina, BMI, diabetes, heart attack, hypertension, smoking status, stroke, and apoE ε4 status | Attention2, episodic memory (composite2, RCFT2), executive function3, global cognition2, semantic memory2, and working memory2 | Those with MCI and AD were excluded from analysis; subgroup analysis found that MeDi was associated with better executive function in apoE ε4 allele carriers (P < 0.01) but not noncarriers |
| Trichopoulou et al. (28) | ||||||||||
| Koyama et al., 2015 (33) | HABC study: population sample of Medicare-eligible, community-dwelling adults aged 70–79 y in the United States | 8 | 2326 | 74.6 ± 2.9 | 51.3 | 21 | Block FFQ (Berkeley, CA) administered by trained examiners | Age, sex, education, BMI, smoking status, physical activity, depression, diabetes, energy intake, and SES | Global cognition (black subgroup3, white subgroup2) | Those with dementia included in analysis; MeDi was significantly associated with global cognition among black but not white subgroups when assessed both as tertiles (P = 0.01) and per increase in points (P = 0.02) |
| Panagiotakos et al. (34) | ||||||||||
| Morris et al., 2015 (35) | Rush MAP: sample of residents >40 y of retirement communities and senior public housing units in the United States | 4.7 | 960 | 81.4 ± 7.2 | 75 | 22 | FFQ administered during clinical evaluations | Age, sex, education, cognitive activities, apoE ε4 status, smoking status, physical activity, energy intake, stroke, myocardial infarction, diabetes, hypertension, time, and time interactions with each covariate | Episodic memory3, global cognition3, processing speed3, reasoning3, semantic memory3, working memory3 | Participants with dementia were excluded |
| MIND score | The difference in decline rates for highest vs. lowest tertile of MeDi score was equivalent to being 7.5 y younger in age | |||||||||
| Excluding those with MCI at baseline increased the association by 9.5%; removing those whose MeDi score changed significantly also increased the association (30–78%) with all cognitive functions (except reasoning) | ||||||||||
| Qin et al., 2015 (36) | CHNS study: population-based sample of community-dwelling adults aged ≥55 y in China | 5.3 | 1650 | 63.5 (NA) | 50.3 | 22 | Validated in-person 24-h dietary recalls over 3 consecutive days administered by trained interviewers | Age, sex, education, region, urbanization index, annual household income per capita, energy intake, physical activity, smoking status, BMI, hypertension, time, and time interactions with each covariate | Episodic memory2, global cognition (composite2, TICS-mod2) | Participants aged ≥65 y in the highest, but not medium, tertile had a significantly slower rate of cognitive decline in all domains compared with lowest tertile; outcomes in this age group were also significant when MeDi was assessed as a continuous variable |
| Trichopoulou et al. (28) adapted for China | No significant associations with cognitive function were found for adults aged <65 y or when age groups were combined | |||||||||
| No significant differences reported when excluding subjects with the lowest 10% baseline cognitive scores | ||||||||||
| Samieri et al., 2013 (37) | WHS: substudy of WHS participants aged ≥65 y from an RCT of low-dose aspirin and vitamin E supplements for primary prevention of CVD and cancer in women in the United States | 5 | 6174 | 71.9 ± 4.1 | 100 | 21 | Validated self-administered FFQ | Age, race, education, income, energy intake, physical activity, BMI, smoking, diabetes, hypertension, hypercholesterolemia, hormone use, depression, and treatment arm | Episodic memory2, global cognition2 | Results were nonsignificant for cognitive outcomes for all quintiles compared to the lowest quintile |
| Trichopoulou et al. (28) adapted for the United States | Among MeDi components, a higher ratio of monounsaturated to saturated fats was associated with more favorable episodic memory (P = 0.05) and global cognition (P = 0.03) | |||||||||
| Whole grain intake was associated with better global cognition (P = 0.02) | ||||||||||
| Samieri et al., 2013a (38) | NHS: substudy in participants from female registered nurses in the United States who were aged ≥70 y and free of stroke | 6 | 16,058 | 74.3 ± 2.3 | 100 | 20 | Self-administered FFQ | Age, education, physical activity, energy intake, BMI, smoking status, multivitamin use, depression, diabetes, hypertension, hypercholesterolemia, and myocardial infarction | Episodic memory2, global cognition (composite2, TICS2) | MeDi was significantly associated with mean cognitive score across assessments (P < 0.005 all domains) but not rate of change in cognitive score |
| Trichopoulou et al. (28) adapted for the United States | Among MeDi components, vegetable intake was associated with less decline in global cognition (P-trend = 0.04), and a higher ratio of monounsaturated to saturated fats was associated with less decline in episodic memory and global cognition (P-trend = 0.001) | |||||||||
| Scarmeas et al., 2006 (39) | WHICAP study: sample of 2 related cohorts who were Medicare beneficiaries aged ≥65 y stratified for ethnicity and age in the United States | 4 | 2226 | 77.2 ± 6.6 | 67.7 | 21 | Willet’s SFFQ (Cambridge, MA) administered by trained interviewers | Cohort, age, sex, education, ethnicity, baseline cognitive performance, baseline MeDi, time, and MeDi × time interaction | Global cognition3 | Sample included those with AD |
| Trichopoulou et al. (28) adapted for the United States | Greater per-unit adherence to the MeDi was associated with 0.3% of an SD less decline per year | |||||||||
| Tangney et al., 2011 (40) | CHAP study: sample of community-dwelling adults aged ≥65 y in the United States | 7.6 | 3790 | 75.4 ± 6.2 | 61.7 | 19 | Modified Harvard FFQ (assessed intake over the past year) either self-administered or by interview | Age, sex, race, education, participation in cognitive activities, energy intake, and MeDi × time interaction | Global cognition3 | Results remained significant when those in the bottom 10% of baseline cognitive scores were excluded |
| Panagiotakos et al. (34) | Results also remained significant when those with heart disease or stroke were excluded | |||||||||
| Tangney et al., 2014 (41) | Rush MAP: sample of residents of >40-y retirement communities and senior public housing units in the United States | 4.1 | 826 | 81.5 ± 7.1 | 74 | 22 | MAP FFQ modified from CHAP study FFQ and self-administeredPanagiotakos et al. (34) | Age, sex, education, energy intake, and cognitive activities | Global cognition3, episodic memory3, executive function2, processing speed2, semantic memory2, working memory2 | MeDi was assessed as a continuous variable |
| When MeDi was examined in tertiles, the highest tertile was significantly associated with rates of change in global cognition and episodic, semantic, and working memory (P = 0.003) | ||||||||||
| There was little change in results when those in the bottom 10% of baseline cognitive scores or possible dementia at baseline were excluded | ||||||||||
| Trichopoulou et al., 2015 (10) | EPIC study: subsample from European cohort study of adults aged ≥65 y based in Greece | 6.6 | 401 | 74 (NA) | 64.1 | 21 | Validated FFQ administered by an interviewer | Age, sex, years of education, BMI, physical activity, smoking status, diabetes, hypertension, cohabiting, and total energy intake | Global cognition3 | MeDi was significantly associated with mildly (−4 to −1 points) and substantially (≤−5 points) lower MMSE score (P = 0.012 and 0.025, respectively) |
| Trichopoulou et al. (28) | Among participants aged ≥75 y only, the association was significant with substantially (P = 0.013) but not mildly (P = 0.059) lower scores | |||||||||
| Wengreen et al., 2013 (42) | CCSMHA: population-based sample of community-dwelling adults aged ≥65 y in the United States | 10.6 | 3580 | 74.1 ± 9.9 | 57.1 | 20 | Self-administered; FFQ based on Harvard FFQ | Age, sex, education, BMI, physical activity, multivitamin/mineral supplement use, alcohol and smoking status, diabetes, heart attack, and stroke | Global cognition2 | Those with dementia were excluded from analysis |
| Adapted MeDi score | Subjects in the highest 4 quintiles of MeDi had significantly higher scores compared to the lowest quintile (P-trend = 0.0022) at baseline; these differences were maintained and there was no significant difference between quintiles in rate of change | |||||||||
AD, Alzheimer disease; AIBL, Australian Imaging, Biomarkers and Lifestyle; apoE ε4, apolipoprotein E genotype; BVRT, Benton Visual Retention Test; CCSMHA, Cache County Study on Memory, Health and Aging; CCVFFQ, Cancer Council of Victoria FFQ; CHAP, Chicago Health and Aging Project; CHNS, China Health and Nutrition Survey; CSIROFFQ, Commonwealth Scientific and Industrial Research Organization FFQ; CVD, cardiovascular disease; EPIC, European Prospective Investigation into Cancer and Nutrition; FCSRT, Free and Cued Selective Reminding Test; HABC, Health, Aging and Body Composition; MAP, Memory and Aging Project; MCI, mild cognitive impairment; MeDi, Mediterranean diet; MIND, Mediterranean–DASH (Dietary Approach to Systolic Hypertension) Diet Intervention for neurodegenerative delay; NA, not available; NHS, Nurses’ Health Study; PATH, Personality & Total Health; RCFT, Rey Complex Figure Test; RCT, randomized controlled trial; ref, reference; SES, socioeconomic status; SFFQ, semi-quantitative food frequency questionnaire; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SUN, Seguimiento Universidad de Navarra; TICS, Telephone Interview for Cognitive Status; TICS-mod, Telephone Interview for Cognitive Status–modified; TRELONG, Treviso Longeva; WHICAP, Washington Heights–Inwood Columbia Aging Project; WHS, Women’s Health Study.
No association with rate of change in cognitive function.
Association with reduced rate of decline in cognitive function.
Two RCTs were eligible for inclusion, with 309 participants in the MeDi intervention groups and 162 in the control groups (Table 2). Participants in the experimental group were typically advised on dietary changes. Supplementary foods or vouchers were given or residential kitchen staff members were advised on appropriate changes. Participants in control groups were either advised on diet or given vouchers. Compliance was assessed through biomarkers and questionnaires. Both studies reported good compliance. One study focused on participants at risk for cardiovascular disease but excluded those with cardiovascular disease and thus was deemed eligible for inclusion.
TABLE 2.
Characteristics of included randomized controlled trials1
| Participants |
||||||||||
|
n |
Age, y: mean ± SD |
Sex, % women |
||||||||
| Study, year (ref) | Intervention | Methods | EG | CG | EG | CG | EG | CG | Outcome of interest | Additional notes |
| Knight et al., 2015 (43) | MeDi intervention versus habitual diet controlAdelaide, Australia | Participants were advised on which group they had been allocated to and were monitored on a fortnightly basis by a dietitian to check that the diet was followed to standards. Food was provided to the EG and vouchers to the CG to promote compliance; compliance was checked through biomarkers and questionnairesFollow-up: 3 and 6 mo | 70 | 67 | >65: 72.1 ± 4.9 | 72.0 ± 5.0 | 47.1 | 59.7 | Attention (Stroop2, TOL2), episodic memory2, immediate recall (BVRT2, DSF2), processing speed (WAIS IV SS2 and Coding2), verbal fluency (ELF2, ILF2),working memory (DSB2, LNS2), global cognition2 | In multivariable-adjusted models, EGs did not perform significantly better than CG for executive functioning (P = 0.33), processing speed (P = 0.15), memory (P = 0.50), visual-spatial ability (P = 0.48), or global cognition (P = 0.19) |
| Valls-Pedret et al., 2015 (44) | 3 conditions: MeDi plus EVOO (EG1), MeDi plus nuts (EG2), and low-fat diet advice (CG)Barcelona, Spain | EGs were given quarterly sessions on how to follow the MeDi; supplemental foods (EVOO and mixed nuts) were provided at no extra cost. CG participants were scheduled for yearly visits and given advice on low-fat diet for 3 y; the CG then received personalized advice and group sessions for remainder of trial and received small nonfood gifts; compliance was checked through biomarkers and questionnairesFollow-up: median 4.1 y | 127 (EG1) and 112 (EG2) | 95 | 55–80: 67.9 ± 5.4 (EG1) and 66.7 ± 5.3 (EG2) | 65.5 ± 5.8 | 52.8 (EG1) and 48.2 (EG2) | 51.6 | Attention3, delayed recall2, episodic memory4, global cognition2, immediate recall2, paired associates2, processing speed4, verbal fluency2, working memory2 | Significant improvement in memory composite for MeDi plus nuts vs. CG (P = 0.04); significant improvement in frontal (P = 0.003) and global cognition (P = 0.005) composites for MeDi plus EVOO vs. CG |
BVRT, Benton Visual Retention Test; CG, control group; DSB, Digit Span Backward; DSF, Digit Span Forward; EG, experimental group; ELF, excluded letter fluency; EVOO, extra-virgin olive oil; ILF, initial letter fluency; LNS, letter number sequencing; MeDi, Mediterranean diet; ref, reference; SS, symbol search; TOL, Tower of London; WAIS, Wechsler Adult Intelligence Scale.
No improvement in function.
Slower rate of decline.
Improved function.
Cohort studies
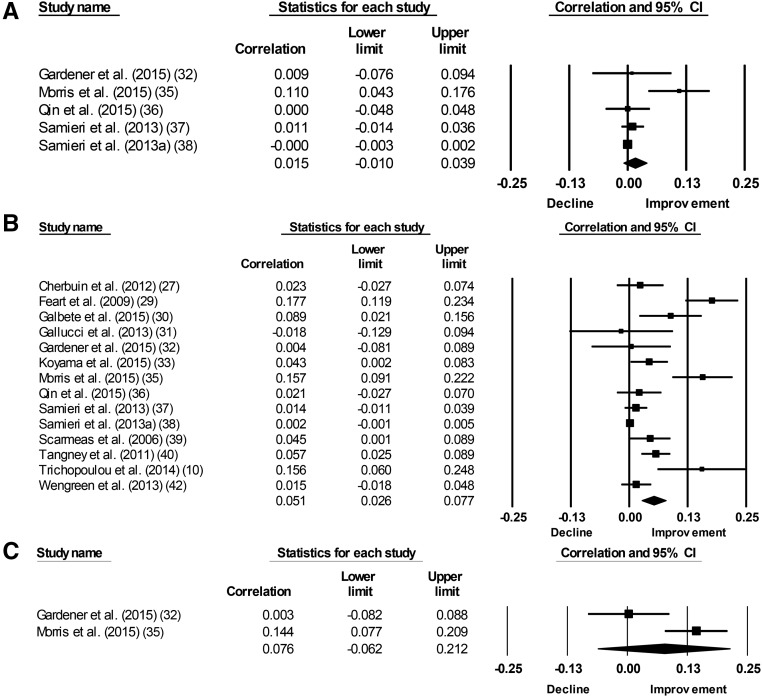
Meta-analysis revealed a significant association between the MeDi and episodic memory (P = 0.03) and global cognition (P < 0.001), but not working memory (P = 0.93) or semantic memory (P = 0.28) (Figure 2, Table 3). There was statistically significant heterogeneity for all 4 outcomes included in the meta-analysis. Inconsistency was moderate for episodic memory and large for global cognition, semantic memory, and working memory. With each group deleted from the model once, results remained the same across all deletions for episodic memory and global cognition (Supplemental Figure 1). Cumulative meta-analysis, ranked by year, showed that episodic memory was not significantly associated with the MeDi for all years examined, but global cognition has been significantly associated with the MeDi since 2011 (Table 4, Supplemental Figure 2). Analysis on small study effects was conducted for global cognition only. Qualitative analysis using a funnel plot demonstrated moderate to no asymmetry (Supplemental Figure 3). Further quantitative analysis with Egger’s test of the intercept found statistically significant and thus potential small study effects. No further analysis was conducted on semantic memory or working memory because there were only 2 outcomes.
FIGURE 2.
Forest plots for observational studies showing plots for episodic memory (A), global cognition (B), and semantic memory (C).
TABLE 3.
Mediterranean diet and cognitive function: main cohort results1
| Variable | Studies, n | Participants, n | r | 95% CI | Z | P | Q | Q (P) | I2, % |
| Attention | 1 | 527 | 0.025 | −0.06, 0.11 | 0.58 | 0.56 | 0 | >0.99 | 0 |
| Episodic memory | 5 | 25,369 | 0.015 | −0.01, 0.039 | 1.16 | 0.24 | 11.13 | −0.03 | 64.05 |
| Global cognition | 13 | 41,492 | 0.051 | 0.026, 0.077 | 3.95 | <0.001 | 91.7 | <0.001 | 85.82 |
| Immediate recall | 1 | 1177 | 0.029 | −0.03, 0.088 | 0.96 | −0.34 | 0 | >0.99 | 0 |
| Processing speed | 1 | 960 | 0.146 | 0.079, 0.212 | 4.24 | <0.001 | 0 | >0.99 | 0 |
| Reasoning | 1 | 960 | 0.107 | 0.039, 0.173 | 3.09 | −0.002 | 0 | >0.99 | 0 |
| Semantic memory | 2 | 1487 | 0.076 | −0.062, 0.212 | 1.079 | −0.28 | 6.51 | −0.01 | 84.63 |
| Verbal fluency | 1 | 1177 | 0.043 | −0.016, 0.102 | 1.42 | −0.16 | 0 | >0.99 | 0 |
| Working memory | 2 | 1487 | 0.007 | −0.15, 0.164 | 0.09 | −0.93 | 8.5 | −0.004 | 88.24 |
I2, inconsistency; Q, heterogeneity; Z, z score.
TABLE 4.
Mediterranean diet and cognitive function: results of further analysis for cohort studies1
| Egger’s test of the intercept |
One study removed |
Cumulative analysis |
|||||
| Variable | β02 | 95% CI | df | P, 1-tailed | Study | Point difference, smallest to largest (%) | Significant since |
| Episodic memory | NA | NA | NA | NA | ND | 0.028 (100) | NS |
| Global cognition | 2.17 | 0.98, 3.37 | 12 | 0.001 | ND | 0.017 (29.8) | 2011 |
NA, not applicable; ND, no difference.
Intercept (results remained statistically significant when each study was deleted from the model).
Meta-regression was used to examine study length, STROBE risk of bias, age (mean and minimum), and sex (percentage of women) for episodic memory (Supplemental Table 5). None of the results were significant. Study length, STROBE risk of bias, age (mean and minimum), sex (percentage of women), and education (percentage of tertiary education) were examined for global cognition (Supplemental Table 6). Only the result for STROBE risk of bias was significant (P = 0.01). For global cognition, there were also sufficient data to examine country per region of the study, whether dementia participants were removed from analysis, and whether the following covariates were controlled for: race/ethnicity, education (mean years and level), vascular factors, BMI, and smoking. No result was significant (Supplemental Table 7). There were insufficient data to examine variables for the remaining outcomes of interest or the method used to calculate MeDi score in moderator analysis. We examined the pooled result for any method with ≥2 global cognition outcomes. The pooled results for the methods of Trichopoulou et al. (28) and Panagiotakos et al. (34) were each significant, whereas the results for those using their own scoring method were not.
In individual studies, significant associations were reported between the MeDi and processing speed (P ≤ 0.001) and reasoning (P = 0.002), but not attention (P = 0.56), verbal fluency (P = 0.16), episodic memory (P = 0.24), immediate recall (P = 0.34), or semantic memory (P = 0.28). Data were not available for the remaining outcomes of interest of recognition and face-name recall.
RCTs
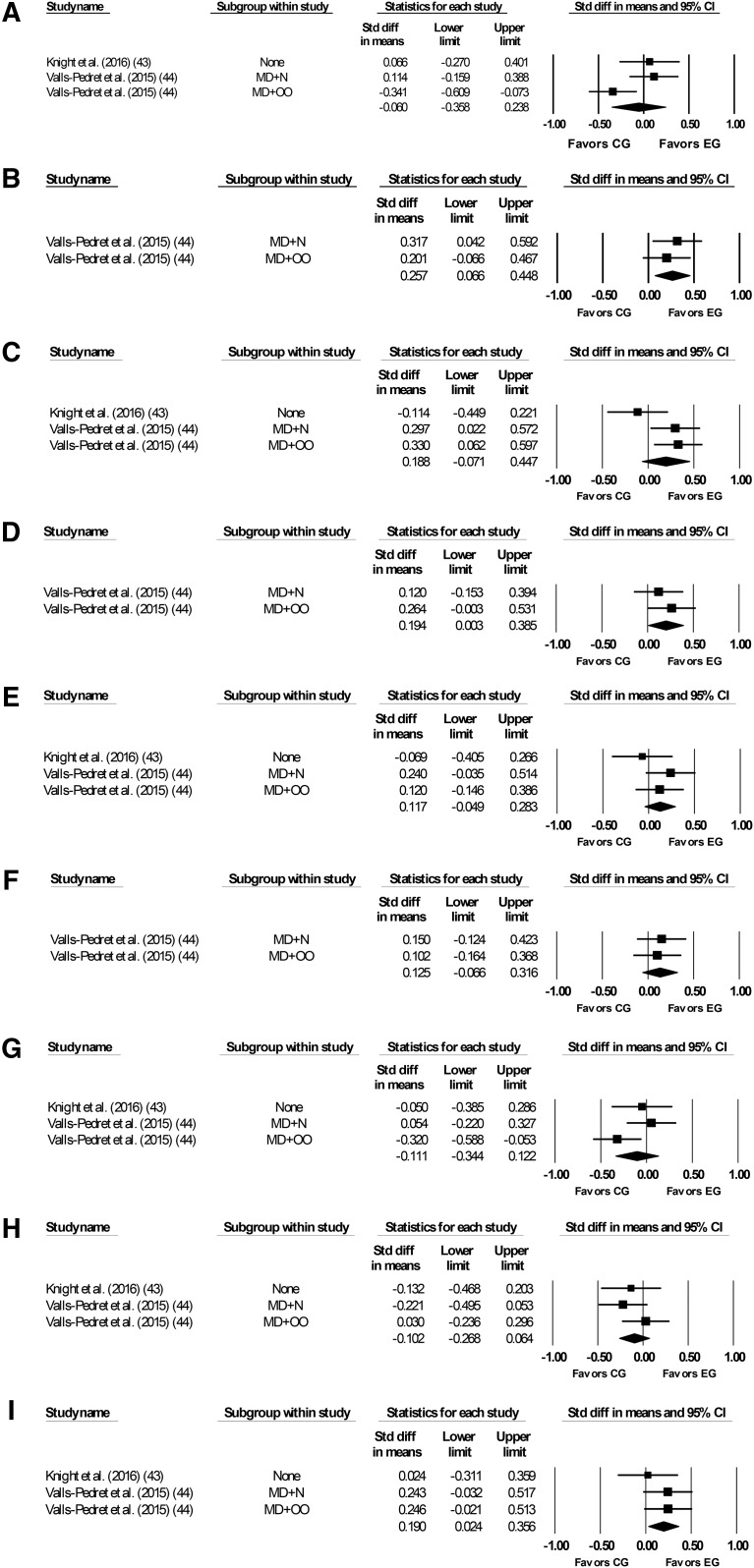
Meta-analysis results revealed that compared with controls, the MeDi group had significantly improved performance on measures of delayed recall (P = 0.01), working memory (P = 0.03), and global cognition (P = 0.047). There were no significant differences between groups on measures of episodic memory (P = 0.15), immediate recall (P = 0.17), paired associates (P = 0.2), attention (P = 0.69), processing speed (P = 0.35), or verbal fluency (P = 0.12) (Figure 3, Table 5). Data were not available for the remaining outcomes of interest, including recognition, face-name recall, reasoning, or semantic memory.
FIGURE 3.
Forest plots for randomized controlled trials showing plots for attention (A), delayed recall (B), episodic memory (C), global cognition (D), immediate recall (E), paired associates (F), processing speed (G), verbal fluency (H), and working memory (I).CG, control group; EG, experimental group; MD, Mediterranean Diet; N, nuts; OO, olive oil.
TABLE 5.
Mediterranean diet and cognitive function: main randomized controlled trial results1
| Variable | Study orsubgroup, n | Participants2, n | 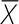 |
95% CI | Z | P | Q | Q (P) | I2, % |
| Attention | 3 | 566 | −0.06 | −0.036, 0.24 | −0.4 | −0.69 | 6.3 | −0.04 | 68.23 |
| Delayed recall | 2 | 429 | 0.26 | 0.07, 0.45 | 2.63 | −0.01 | 0.35 | −0.55 | 0.0 |
| Episodic memory | 3 | 566 | 0.19 | −0.07, 0.45 | 1.42 | −0.15 | 4.75 | −0.09 | 57.85 |
| Global cognition | 2 | 429 | 0.19 | 0.003, 0.39 | 1.99 | −0.047 | 0.54 | −0.46 | 0.0 |
| Immediate recall | 3 | 566 | 0.12 | −0.05, 0.28 | 1.39 | −0.17 | 1.96 | −0.38 | 0.0 |
| Paired associates | 2 | 429 | 0.13 | −0.07, 0.32 | 1.29 | −0.2 | 0.06 | −0.81 | 0.0 |
| Processing speed | 3 | 566 | −0.11 | −0.34, 0.12 | −0.93 | −0.35 | 3.87 | −0.15 | 48.28 |
| Verbal fluency | 3 | 566 | −0.1 | −0.27, 0.06 | −1.2 | −0.23 | 1.71 | −0.43 | 0.0 |
| Working memory | 3 | 566 | 0.19 | 0.02, 0.36 | 2.24 | −0.03 | 1.26 | −0.53 | 0.0 |
There was statistically significant heterogeneity for attention and episodic memory. Inconsistency was moderate for attention and episodic memory, low for processing speed, and very low for the remaining domains assessed. With each group deleted from the model once, results remained the same across all deletions for all domains assessed (Supplemental Figure 4). Cumulative meta-analysis, ranked by year, showed that episodic memory was significant before 2016 from 2015 and working memory has been significant since 2015 (Supplemental Figure 5). Because of the small number of trials available, no analysis was conducted on small study effects. No further analysis on delayed recall, global cognition, and paired associates was conducted because there were only 2 outcomes. There were insufficient data to examine any moderators or covariates.
MeDi components
None of the trials examined the association between individual components of the MeDi with outcomes on cognitive measures. However, 1 trial (44) examined the impact of 2 variations of the diet in 2 clinical groups: one group was given the MeDi with extra-virgin olive oil, and the other was given the MeDi with mixed nuts. Significantly different results in favor of the extra-virgin olive oil group were reported for the executive measure of attention (P = 0.02). Differences between groups were not significant for delayed recall (P = 0.51), episodic memory (P = 0.87), global cognition (P = 0.46), immediate recall (P = 0.54), paired associates (P = 0.81), processing speed (P = 0.06), verbal fluency (P = 0.2), and working memory (P = 0.97).
Among cohort studies, 3 articles examined the association of components of the MeDi with change in cognitive function (27, 37, 38). Samieri et al. (37, 38) reported a higher ratio of monounsaturated to saturated fats as being associated with less decline in episodic memory and global cognition (P ≤ 0.05). Conversely, Cherbuin and Anstey (27) reported a higher ratio of monounsaturated to saturated fats as being significantly associated with greater decline in global cognition (P = 0.008). Other components significantly associated with reduced global cognitive decline included higher intake of fish (P = 0.011) (27), whole grains (P = 0.02) (37), and vegetables (P = 0.04) (38). No other component of the MeDi examined, including intake of alcohol, fruit, legumes, meat , or nuts, was associated with global cognition (27, 37, 38) or episodic memory (37, 38).
Discussion
This review examined the association in cohort studies and RCTs of the MeDi with the cognitive function of older adults without cognitive impairment. Although several meta-analytic reviews on the MeDi and cognition have been published, the current review differs in terms of the targeted population (healthy older adults) and the included outcomes (different domains of cognitive function). To our knowledge, this is the first meta-analysis to provide a detailed examination of the relation between the MeDi and healthy older adults’ recognition, immediate recall, delayed recall, face-name recall, paired associates, semantic memory, working memory, verbal fluency, reasoning, attention, processing speed, and global cognitive function. Among cohort studies, there were sufficient data to pool results for episodic memory, global cognition, semantic memory, and working memory. Greater adherence to the MeDi was associated with improved function on measures of global cognition only. However, results were not consistent across studies and there was significant heterogeneity. Single results for attention, immediate recall, and verbal fluency were not significant, but effect sizes for processing speed and reasoning reached significance. Sensitivity analyses were conducted examining moderators and covariates where possible; a significant association was found between greater effect size and higher STROBE score only. Among RCTs, the MeDi was associated with improved delayed recall, global cognition, and working memory, but not attention, episodic memory, immediate recall, paired associates, processing speed, or verbal fluency. Heterogeneity was nonsignificant for delayed recall, global cognition, and working memory with very low inconsistency. There were insufficient data for moderator or meta-regression analysis.
Cohort studies
Across cohort studies, pooled analysis resulted in a significant association between the MeDi and global cognition only, although analysis of individual studies showed an association between the MeDi and processing speed and reasoning. This finding is somewhat surprising, given the conclusions of previous systematic reviews in this area (13–16). This may, however, be explained by the inclusion of cross-sectional data in all other reviews apart from one (14). For example, one included study did not show an association between the MeDi and change in global cognition and episodic memory; but secondary analysis of the association of the MeDi with the mean score of global cognitive function across 4 time points resulted in a significant cross-sectional association (P < 0.005) (38). We only included cohort studies that reported cognitive change scores across time. Furthermore, there was considerable heterogeneity and inconsistency for all pooled outcomes, which we explored using sensitivity analysis but found no possible explanations apart from a greater effect size for global cognition in those studies with a lower risk of bias. Factors that may contribute to our findings are discussed in more detail below.
RCTs
We found only 2 RCTs examining the effect of the MeDi on the cognitive function of healthy older adults, thus making any conclusions tentative. To our knowledge, this is the first meta-analysis of RCTs using the MeDi as an intervention. Meta-analysis of 9 pooled outcomes from these RCTs showed significant improvement for the intervention groups compared with controls on measures of delayed recall, global cognition, and working memory. A previous systematic review reported that the MeDi was associated with improved outcomes on tests of global cognition and executive function (16). However, because of a lack of RCTs, these results were observed in single trials rather than in pooled analyses. It should be noted that results for delayed recall and global cognition, as well as 2 of the 3 results for working memory, came from 2 intervention groups in the same trial (44). This was the only study that reported significant between-group differences. This discrepancy may be attributable to differences in methodology or samples. This study reported a median follow-up of 4.1 y, whereas the second study was of shorter duration (6 mo) (43). It may take longer-term adherence to the MeDi to observe any changes in cognitive function similar to the impact of exercise (20). There was a large difference in minimum age (55 y compared with 65 y) between studies but mean age was closer (66.8 y compared with 72.1 y), which may have implications for the effectiveness of a dietary intervention, particularly if the mechanisms (e.g., vascular mechanisms) are age dependent. Previous reviews on cardiovascular and cardiometabolic factors and cognitive decline suggest an age-dependent association, particularly in midlife (40–59 y), with a weaker association among those age ≥75 y (45).
The results across both observational and RCT studies consistently indicate that the MeDi benefits global cognition. There were, however, differences between observational and RCT data in relation to the impact of the MeDi on specific cognitive domains. Results from cohort studies showed associations between the MeDi and processing speed and reasoning, whereas RCTs reported that the MeDi improved delayed recall, working memory, and executive function compared with controls. These divergent findings could simply be a result of the distinct study designs and follow-up periods. It should be noted, however, that executive functions include processing speed, working memory, and reasoning (46); although there were some specific differences, both study designs consistently report an association between the MeDi and some aspect of executive function. Relatedly, outcome measures used in included RCTs to measure “executive function” (43) were included as measures of “working memory” in cohort studies (40). Such variations in terms of reporting cognitive tests and domains measured may result in incorrect assumptions of inconsistent results. All of the studies in this review included a measure of global cognition; however, only some measured either memory or executive function, and the specific ability area measured within each subdomain varied largely across studies. Undoubtedly, greater homogeneity across selected outcome measures would help to address this issue. Further RCTs using similar cognitive test batteries are needed to corroborate our findings and to further explore possible reasons for differences in outcomes. We outline several potential areas of interest for future trials below.
MeDi components
Only a small number of cohort studies performed secondary analysis examining the association between components of the MeDi with cognitive function (27, 37, 38). Although all results for ratios of monounsaturated to saturated fats were significant, they were contradictory, possibly because of differences in geographic location. Interestingly, one of the included RCTs examined whether supplementation with extra-virgin olive oil would affect outcomes (44). Results showed that compared with controls, the MeDi plus olive oil group showed significantly greater improvements to memory and executive function. There was no significant difference for another intervention group in the same trial, which had the MeDi supplemented with mixed nuts. This is consistent with other studies that have examined the effects of olive oil and mono- and polyunsaturated fats on age-related cognitive changes in memory, executive function, and global cognition (47–49). Furthermore, in cohort studies, higher intake of fish and vegetables was also significantly associated with reduced cognitive decline. Other studies examining these individual components support this finding (50–53). No other components of the MeDi were reported to have a significant association with cognitive function. Specific MeDi components, rather than the overall pattern, may be beneficial for cognitive function. Thus, examining the association between dietary patterns and cognition, rather than individual components, may contribute to inconsistency in results and mask effects on brain health. However, these conclusions are limited, because no RCTs to date have examined the association of MeDi components with cognition.
Possible mechanisms
The MeDi provides a rich source of antioxidants, vitamins, and unsaturated FAs that may affect possible biological mechanisms of neurocognitive aging (54–56). These mechanisms might include better neurovascular health (57) or a reduction of oxidative stress, metabolic factors, or reduced chronic inflammation (56, 58).
There is epidemiological evidence of reduced levels of inflammatory and oxidative markers and a reduced risk of cardiodiabesity with greater conformity to the MeDi (59, 60). Support for a vascular mechanism comes from neuroimaging studies, including the North Manhattan Study, which reported a beneficial impact of the MeDi on white matter hyperintensities (61), and the Bordeaux Three-City Study of 146 participants, which reported an association between the MeDi and preserved white matter microstructure and structural connectivity, related to improved episodic memory, executive function, and global cognition (62). Further support favoring improved cognition through vascular mechanisms comes from a New York study, which reported that higher adherence of 707 elderly people to the MeDi was associated with reduced cerebrovascular disease burden (63), and from the PREDIMED study, which reported an association between the MeDi and stroke prevention (64).
Promotion of cerebrovascular health through a MeDi may facilitate more efficient clearance of amyloid β from the brain (65, 66). Interestingly, our moderator analysis found no significant differences in effect size for global cognition between those that controlled for vascular factors and those that did not among cohort studies. This is consistent with a neuroimaging study by Scarmeas et al. (58), who found no evidence for a vascular mediation between MeDi with AD outcome, an association that they reported as significant. Further support for nonvascular mechanisms of brain protection by the MeDi comes from ancillary analyses of the PREDIMED cohort, in which the MeDi was associated with a reduction in depression and increased levels of circulating brain-derived neurotrophic factor (44).
Neuroimaging research examining components of the MeDi reports that higher fish intake and lower meat intake were linked with higher total brain, gray matter, and white matter volume (67). In addition, low consumption of meat and meat products was linked to better global cognition and greater total brain volume (68). Conversely, a recent study examining the MeDi and structural brain changes in a Scottish cohort of 73- to 76-y-olds failed to replicate previously reported associations between meat and fish consumption and total brain or gray matter volume (69). Luciano et al. (69) considered the possibility that the discrepant findings might be explained by variations in the quantity and type of meat and fish consumed across studies. Most MeDi studies employ self-report FFQs, which often exclude descriptions of the distribution of meat consumption (65–67). FFQs are also largely subjective and are therefore at risk of differential reporting regarding actual dietary practices. The availability of different types of food in different regions may affect study results (70), and country-specific lifestyles might lead to misclassification regarding adherence to the MeDi (71). An examination of studies conducted in traditional Mediterranean countries would be beneficial, because elderly populations living in Mediterranean countries are more likely to adhere to a homogenous and strict MeDi (72, 73).
Limitations
A substantial problem in using the meta-analytic approach is the heterogeneity in methodology, population, and outcome measurement between studies. We attempted to minimize this by rigorous selection criteria, data preparation (e.g., allocation of tests to appropriate cognitive domains), and planned extensive sensitivity analyses. Typically, a limitation of meta-analysis of cognition in cohort studies is that causal effects cannot be inferred from correlations, but we also conducted a meta-analysis of RCTs to examine possible causal effects on cognition. However, the small number of RCTs retrieved from our search is a limitation. We only included published data, thus running the risk of overestimating intervention effects; however, one of the included trials was published despite no evidence for any intervention effect in any of the cognitive outcomes assessed.
Conclusions and recommendations
The analysis of pooled data from 15 cohort studies and 2 RCTs suggests that adherence to the MeDi might benefit global cognition for healthy older adults. Results also showed evidence of some benefit of the MeDi in domains of delayed recall, working memory, processing speed, and reasoning. Some clear limitations provided guidelines for future studies; future RCTs should consider including older adults from a broad age range, particularly middle-aged (>50 y) and older (>75 y) adults, to examine differences in the impact of the MeDi owing to any possible age-dependent associations. Our sensitivity analysis of cohort studies showed no effects for mean or minimum age on episodic memory or global cognition. However, the lowest mean age among our included cohort studies was 61.9 y, which may be too late to detect any age differences in rate of cognitive decline.
Future observational studies and trials should examine the influence of individual components of the MeDi with cognitive outcomes. Our review indicates that only some components, including olive oil, fish, and vegetables, have beneficial effects. Standardization of study protocol and outcome measurement would be beneficial. Because it may take a long-term intervention to observe changes or maintenance effects in cognition, future RCTs should consider an intervention term of ≥2 y. Studies examining the impact of the MeDi on biomarkers that reflect inflammation would give further insight into any potential mechanism underpinning the effects of the MeDi on cognition. This would guide future trials taking a multitherapeutic approach to enhance modification of these mechanisms (i.e., an intervention using the MeDi in conjunction with exercise) (74).
Acknowledgments
We thank Ian Robertson for guidance and support, Niamh Aspell for proofreading the manuscript and providing expertise on nutrition and diet, and Brian Pennie for contributing to the discussion. The authors’ responsibilities were as follows—DGL, SL, and MEK: contributed to the acquisition of the data; DGL and MEK: designed and conducted the statistical analyses; DGL, SL, and MEK: drafted the manuscript with critical revision for important intellectual content from all authors; BAL, SB, and MEK: supervised the study; and all authors: contributed to the study concept and design and read and approved the final manuscript.
Footnotes
Abbreviations used: AD, Alzheimer disease; CMA, Comprehensive Meta-Analysis; MeDi, Mediterranean diet; RCT, randomized controlled trial; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
References
- 1.Prince M, Wimo A, Guerchet M, Ali G, Wu Y, Prina M. World Alzheimer report 2015: the global impact of dementia an analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International; 2015.
- 2.Salthouse TA. Major issues in cognitive aging. New York: Oxford University Press; 2010. [Google Scholar]
- 3.Royall DR, Chiodo LK, Polk MJ. Correlates of disability among elderly retirees with “subclinical” cognitive impairment. J Gerontol A Biol Sci Med Sci 2000;55:M541–6. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. [DOI] [PubMed] [Google Scholar]
- 5.Kuczmarski MF, Allegro D, Stave E. The association of healthful diets and cognitive function: a review. J Nutr Gerontol Geriatr 2014;33:69–90. [DOI] [PubMed] [Google Scholar]
- 6.Serra-Majem L, Roman B, Estruch R. Scientific evidence of interventions using the Mediterranean diet: a systematic review. Nutr Rev 2006;64 Suppl 1: S27–47. [DOI] [PubMed] [Google Scholar]
- 7.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr 1995; 61(6 Suppl): 1402S–6S. [DOI] [PubMed] [Google Scholar]
- 8.Keys A, Aravanis C, Blackburn HW, Van Buchem FS, Buzina R, Djordjević BD, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, et al. Epidemiological studies related to coronary heart disease: characteristics of men aged 40–59 in seven countries. Acta Med Scand Suppl 1966;460:1–392. [PubMed] [Google Scholar]
- 9.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulou A, Kyrozis A, Rossi M, Katsoulis M, Trichopoulos D, La Vecchia C, Lagiou P. Mediterranean diet and cognitive decline over time in an elderly Mediterranean population. Eur J Nutr 2015;54:1311–21. [DOI] [PubMed] [Google Scholar]
- 11.Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, Roberts RO. Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2014;39:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry 2015;172:323–34. [DOI] [PubMed] [Google Scholar]
- 13.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology 2013;24:479–89. [DOI] [PubMed] [Google Scholar]
- 16.Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr 2016;7:889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight A, Bryan J, Murphy K. The Mediterranean diet and age-related cognitive functioning: a systematic review of study findings and neuropsychological assessment methodology. Nutr Neurosci 2016 May 18 (Epub ahead of print; DOI: 10.1080/1028415X.2016.1183341). [DOI] [PubMed]
- 18.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol 2013;74:580–91. [DOI] [PubMed] [Google Scholar]
- 19.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014;15:28–43. [DOI] [PubMed] [Google Scholar]
- 20.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Res Rev 2014;16:12–31. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collaboration; 2011. [updated 2011 Mar; cited 2016 Apr 20]. Available from: http://www.cochrane-handbook.org.
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 26.Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. J Clin Epidemiol 1995;48:45–57, discussion 9–60. [DOI] [PubMed] [Google Scholar]
- 27.Cherbuin N, Anstey KJ. The Mediterranean diet is not related to cognitive change in a large prospective investigation: the PATH Through Life study. Am J Geriatr Psychiatry 2012;20:635–9. [DOI] [PubMed] [Google Scholar]
- 28.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 29.Féart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA 2009;302:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galbete C, Toledo E, Toledo JB, Bes-Rastrollo M, Buil-Cosiales P, Marti A, Guillén-Grima F, Martínez-González MA. Mediterranean diet and cognitive function: the SUN project. J Nutr Health Aging 2015;19:305–12. [DOI] [PubMed] [Google Scholar]
- 31.Gallucci M, Mazzuco S, Ongaro F, Di Giorgi E, Mecocci P, Cesari M, Albani D, Forloni GL, Durante E, Gajo GB, et al. Body mass index, lifestyles, physical performance and cognitive decline: the “Treviso Longeva (TRELONG)” study. J Nutr Health Aging 2013;17:378–84. [DOI] [PubMed] [Google Scholar]
- 32.Gardener SL, Rainey-Smith SR, Barnes MB, Sohrabi HR, Weinborn M, Lim YY, Harrington K, Taddei K, Gu Y, Rembach A, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry 2015;20:860–6. [DOI] [PubMed] [Google Scholar]
- 33.Koyama A, Houston DK, Simonsick EM, Lee JS, Ayonayon HN, Shahar DR, Rosano C, Satterfield S, Yaffe K. Association between the Mediterranean diet and cognitive decline in a biracial population. J Gerontol A Biol Sci Med Sci 2015;70:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panagiotakos DB, Pitsavos C, Arvaniti F, Stefanadis C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med 2007;44:335–40. [DOI] [PubMed] [Google Scholar]
- 35.Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, Aggarwal NT. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin B, Adair LS, Plassman BL, Batis C, Edwards LJ, Popkin BM, Mendez MA. Dietary patterns and cognitive decline among Chinese older adults. Epidemiology 2015;26:758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samieri C, Grodstein F, Rosner BA, Kang JH, Cook NR, Manson JE, Buring JE, Willett WC, Okereke OI. Mediterranean diet and cognitive function in older age. Epidemiology 2013;24:490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samieri C, Okereke OI, Devore EE, Grodstein F. Long-term adherence to the Mediterranean diet is associated with overall cognitive status, but not cognitive decline, in women. J Nutr 2013;143:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol 2006;59:912–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tangney CC, Li H, Wang Y, Barnes L, Schneider JA, Bennett DA, Morris MC. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014;83:1410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wengreen H, Munger RG, Cutler A, Quach A, Bowles A, Corcoran C, Tschanz JT, Norton MC, Welsh-Bohmer KA. Prospective study of dietary approaches to stop hypertension- and Mediterranean-style dietary patterns and age-related cognitive change: the Cache County Study on Memory, Health and Aging. Am J Clin Nutr 2013;98:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight A, Bryan J, Wilson C, Hodgson J, Murphy K. A randomised controlled intervention trial evaluating the efficacy of a Mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: the MedLey study. BMC Geriatr 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martinez-Gonzalez MA, Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó J, et al. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med 2015;175:1094–103. [DOI] [PubMed] [Google Scholar]
- 45.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol 2015;12:267–77. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Kang Y, Yu K-H, Lee B-C. Disproportionate decline of executive functions in early mild cognitive impairment, late mild cognitive impairment, and mild Alzheimer’s disease. Dement Neurocognitive Disord 2016;15:159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berr C, Portet F, Carriere I, Akbaraly TN, Feart C, Gourlet V, Combe N, Barberger-Gateau P, Ritchie K. Olive oil and cognition: results from the three-city study. Dement Geriatr Cogn Disord 2009;28:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Torres F, Rizzo C, Capurso A, Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging 2006;27:1694–704. [DOI] [PubMed] [Google Scholar]
- 49.Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, Quintana M, Corella D, Pinto X, Martínez-González MÁ, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis 2012;29:773–82. [DOI] [PubMed] [Google Scholar]
- 50.Crichton GE, Elias MF, Davey A, Alkerwi A, Dore GA. Higher cognitive performance is prospectively associated with healthy dietary choices: the Maine Syracuse Longitudinal Study. J Prev Alzheimers Dis 2015;2:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enomoto M, Yoshii H, Mita T, Sanke H, Yokota A, Yamashiro K, Inagaki N, Gosho M, Ohmura C, Kudo K, et al. Relationship between dietary pattern and cognitive function in elderly patients with type 2 diabetes mellitus. J Int Med Res 2015;43:506–17. [DOI] [PubMed] [Google Scholar]
- 52.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev 2015;48:1–9. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr 2016;103:330–40. [DOI] [PubMed] [Google Scholar]
- 54.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Effectiveness of the Mediterranean diet: can it help delay or prevent Alzheimer’s disease? J Alzheimers Dis 2010;20:795–801. [DOI] [PubMed] [Google Scholar]
- 55.Feart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive health: an update of available knowledge. Curr Opin Clin Nutr Metab Care 2015;18:51–62. [DOI] [PubMed] [Google Scholar]
- 56.Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, Solfrizzi V. Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis 2010;22:715–40. [DOI] [PubMed] [Google Scholar]
- 57.Peters R. The prevention of dementia. Int J Geriatr Psychiatry 2009;24:452–8. [DOI] [PubMed] [Google Scholar]
- 58.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol 2006;63:1709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr 2016;146:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Fernández E, Rico-Cabanas L, Rosgaard N, Estruch R, Bach-Faig A. Mediterranean diet and cardiodiabesity: a review. Nutrients 2014;6:3474–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gardener H, Scarmeas N, Gu Y, Boden-Albala B, Elkind MS, Sacco RL, DeCarli C, Wright CB. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch Neurol 2012;69:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelletier A, Barul C, Feart C, Helmer C, Bernard C, Periot O, Dilharreguy B, Dartigues JF, Allard M, Barberger-Gateau P, et al. Mediterranean diet and preserved brain structural connectivity in older subjects. Alzheimers Dement 2015;11:1023–31. [DOI] [PubMed] [Google Scholar]
- 63.Scarmeas N, Luchsinger JA, Stern Y, Gu Y, He J, DeCarli C, Brown T, Brickman AM. Mediterranean diet and magnetic resonance imaging-assessed cerebrovascular disease. Ann Neurol 2011;69:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 65.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci 2015;7:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merrill DA, Siddarth P, Raji CA, Small G. Relation of diet, exercise, and body mass index to a brain imaging biomarker of plaques and tangles in non-demented middle-aged and older adults. Neuropsychopharmacology 2013;38:S210–1. [Google Scholar]
- 67.Gu Y, Brickman AM, Stern Y, Habeck CG, Razlighi QR, Luchsinger JA, Manly JJ, Schupf N, Mayeux R, Scarmeas N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015;85:1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Titova OE, Ax E, Brooks SJ, Sjogren P, Cederholm T, Kilander L, Kullberg J, Larsson EM, Johansson L, Ahlström H, et al. Mediterranean diet habits in older individuals: associations with cognitive functioning and brain volumes. Exp Gerontol 2013;48:1443–8. [DOI] [PubMed] [Google Scholar]
- 69.Luciano M, Corley J, Cox SR, Valdés Hernández MC, Craig LCA, Dickie DA, Karama S, McNeill GM, Bastin ME, Wardlaw JM, et al. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology 2017;88:449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci Rep 2017;7:41317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martínez-Lapiscina EH, Clavero P, Toledo E, San Julián B, Sanchez-Tainta A, Corella D, Lamuela-Raventós RM, Martínez JA, Martínez-Gonzalez MÁ. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging 2013;17:544–52. [DOI] [PubMed] [Google Scholar]
- 72.Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol 2013;28:67–77. [DOI] [PubMed] [Google Scholar]
- 73.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation Into Cancer and Nutrition). Public Health Nutr 2008;11:1054–62. [DOI] [PubMed] [Google Scholar]
- 74.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. A randomised controlled trial investigating the effects of Mediterranean diet and aerobic exercise on cognition in cognitively healthy older people living independently within aged care facilities: the Lifestyle Intervention in Independent Living Aged Care (LIILAC) study protocol [ACTRN12614001133628]. Nutr J 2015;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]