Abstract
Cartilage fatigue may be a factor in the precocious development of degenerative changes in the temporomandibular joint (TMJ). This cross-sectional study estimated potential for cartilage fatigue via TMJ energy densities (ED) and jaw muscle duty factors (DF), which were combined to calculate mechanobehavioral scores (MBS) in women with (+) and without (–) bilateral TMJ disc displacement (DD). All subjects gave informed consent to participate and were examined using Diagnostic Criteria (DC) for Temporomandibular Disorders (TMD) and magnetic resonance (MR) and computed tomography (CT) images. Forty-seven subjects were categorized into +DD (n = 29) and –DD (n = 18) groups. Dynamic stereometry (MR images combined with jaw-tracking data) characterized individual-specific data of TMJ stress-field mechanics to determine ED (ED = W/Q mJ/mm3, where W = work done, Q = volume of cartilage) during 10 symmetrical jaw-closing cycles with a 20-N mandibular right canine load. Subjects were trained to record masseter and temporalis electromyography over 3 days and 3 nights. Root mean square electromyography/bite-force calibrations determined subject-specific masseter and temporalis muscle activities per 20-N bite-force (T20 N, µV), which defined thresholds. Muscle DF (DF = % duration of muscle activity/total recording time) were determined for a range of thresholds, and MBS (ED2 × DF) were calculated. Intergroup differences in ED, DF, and MBS were assessed via analyses of variance with Bonferroni and Tukey honest significant difference post hoc tests. Average ED for contralateral TMJs was significantly larger (P = 0.012) by 1.4-fold in +DD compared to –DD subjects. Average DF were significantly larger (all P < 0.01) for +DD compared to –DD subjects by 1.7-, 2.5-, and 1.9-fold for day, night, and overall, respectively. Daytime MBS were significantly larger (all P < 0.04) by up to 8.5-fold in +DD compared to –DD subjects. Significantly larger ED, DF, and MBS were shown in women with compared to women without bilateral TMJ DD.
Keywords: biomechanical phenomena, cartilage, degenerative joint disease, electromyography, human, temporomandibular joint
Introduction
The etiology of the precocious development of degenerative soft and hard tissue changes in the temporomandibular joint (TMJ) has yet to be clearly elucidated (Chantaracherd et al. 2015). Furthermore, with respect to temporomandibular disorders (TMD), the mechanisms that explain progression versus reversal of diagnoses over time are unknown (Schiffman et al. 2017). As in all synovial joints, failure of the articulating TMJ tissues is thought to involve an interactive process between mechanical fatigue, oxidative stresses, and inflammation (Shi et al. 2010). The biomechanical and biochemical integrity of articular tissue is likely to be dependent on the magnitude and frequency of applied mechanical work imposed on a volume of cartilage, also known as energy density (mJ/mm3). Biphasic modeling of contact mechanics has demonstrated that energy density produced by plowing tractional forces is balanced by an internal strain energy in the collagen-glycosaminoglycan matrix and pressurization of the interstitial fluid (Spilker et al. 2009; Guo et al. 2012; Wu et al. in press). Stress-field translation, which is required for tractional forces (Ftraction = sum of frictional and plowing forces), has been shown to occur on the TMJ disc along the mediolateral axis during jaw function (Gallo et al. 2000) where the disc is more susceptible to mechanical fatigue failure (Beatty et al. 2001, 2003; Detamore and Athanasiou 2003; Tanaka et al. 2003). Stress-field translation velocity (y) averaged 47 mm/s during jaw closing in heathy humans (Gallo et al. 2000), which was similar to healthy and arthritic human knees, where at heal strike average peak y = 29 and 44 mm/s, respectively (Farrokhi et al. 2016), whereas in dogs, average y = 50 mm/s in healthy knees but increased by 2.5-fold in an arthritic model after severing of the anterior cruciate ligament (Anderst and Tashman 2010). Hence, candidate etiological factors that may lead to degenerative changes in the TMJ can be identified and possibly measured.
One such etiological factor is mechanical fatigue of the articulating surfaces, the rate of which is determined by the frequency, magnitude, and concentration of mechanical strains plus innate tissue susceptibilities. Why destruction of articular tissues occurs earlier in the TMJ compared to knees and hips may be due, at least in part, to these variables (Nickel, Spilker, et al. 2009). In addition, evidence that there are significant differences in TMJ loads (Iwasaki et al. 2009) and energy densities (ED) (Gallo et al. 2015) among TMD diagnostic groups suggests that there may be consequent differences in mechanical strains imposed on the articulating tissues during jaw function that could lead to differential tissue fatigue in these groups. Diversity in durations and intensities of jaw function between groups, as evidenced by significant differences in jaw muscle duty factors (DF) (Iwasaki, Gonzalez, et al. 2015), could further differentiate amounts of mechanical work imposed on the TMJ tissues.
The objectives of the current project were to test the hypotheses that 1) TMJ ED, 2) jaw muscle DF, and 3) the combination of these variables in the form of mechanobehavioral scores (MBS = ED2 × DF) are significantly larger in women with bilateral TMJ disc displacement (+DD) compared to healthy women without disc displacement (–DD). The between-group effects of time period (day, night) and TMJ side (ipsilateral, contralateral relative to the jaw load) on the 3 main variables were also investigated. Women have a higher incidence of TMD compared to men (Slade et al. 2016) and hence were the current focus.
Materials and Methods
Subjects
Adult subjects were recruited at the same site from 2011 to 2014 and gave written informed consent to participate, and their rights were protected by 2 institutional review boards. This study was performed in accordance with “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines for human research investigations. Subjects were categorized by calibrated examiners using Diagnostic Criteria for TMD (DC/TMD) (Schiffman et al. 2014), including magnetic resonance (MR) and computed tomography (CT) images (Ahmad et al. 2009) as either +DD or –DD. Women with unilateral TMJ DD and a history of frank trauma to and evidence of degenerative hard tissue changes in the TMJ were excluded. Subjects made 5 visits as summarized in the Table and described below. Investigators were blinded to group categorization of subjects while collecting and analyzing data for ED, DF, and MBS calculations.
Table.
Summary of Methods.
| Activity [Location] | Purpose | Application |
|---|---|---|
| Initial visit [UB] | Collected:
● Informed consent ● Dental impressions ● DC/TMD examination results, completed forms ● Head CT |
Permitted:
● Protection of subjects’ rights ● Fabrication of oral appliances used in dynamic stereometry ● Group categorization (±DD) using DC/TMD, inclusion/exclusion criteria ● 3-dimensional craniomandibular anatomy for numerical modeling |
| Imaging visit 1 [PIC] | Collected bilateral MR images of TMJs (1.5 T machine) | Permitted group categorization (±DD) using DC/TMD |
| Imaging visit 2 [PIC] | Collected bilateral MR images of TMJs and reference system (using surface coils) | Characterized 3D TMJ anatomy for dynamic stereometry |
| Laboratory visit 1 [UB] | Recorded
● Jaw tracking with reference system during static and dynamic jaw tasks ● Masseter and temporalis EMG bilaterally and bite forces during biting tasks Trained subject for ambulatory masseter and temporalis EMG |
Combined:
● Jaw tracking with MR images for dynamic stereometry to determine: ● Q, ΔD, x, y (to calculate ED) ● Eminence shapes (for numerical modeling) ● Subject- and muscle-specific EMG/bite-force data to determine T20 N for laboratory visit 1 Subjects self-recorded EMG unilaterally in their natural environments for 3 days, 3 nights |
| Laboratory visit 2 [UB] | Collected ambulatory EMG equipment and data
Recorded masseter and temporalis EMG bilaterally and bite forces during biting tasks (repeated) |
Inspected and filtered completed ambulatory recordings
Determined T20 N for laboratory visit 2; averaged T20 N results for 2 visits and applied to ambulatory EMG for calculation of DF |
| Data analysis [UB, UMKC, UZ] | Tested for ±DD group differences using:
● TMJ ED (from dynamic stereometry and numerical modeling) ● Jaw muscle DF (from ambulatory EMG recordings and T20 N) ● MBS (ED2 × DF) |
|
CT, computed tomography; ΔD, distance of stress-field translation, mm; ±DD, with/without disc displacement; DC/TMD, diagnostic criteria for temporomandibular disorders; ED, energy density; EMG, electromyography; MBS, mechanobehavioral scores; MR, magnetic resonance; PIC, private imaging center; Q, cartilage volume, mm3; T20N, average threshold of EMG activity for 20 N of bite force; TMJ, temporomandibular joint; UB, University at Buffalo School of Dental Medicine; UMKC, University of Missouri–Kansas City School of Dentistry; UZ, University of Zurich School of Dental Medicine; x, product of aspect ratio and compressive strain3; y, velocity of stress-field translation, mm/s.
TMJ ED
Using previously described approaches (Gallo et al. 2015), ED were calculated for each TMJ in all subjects via the following equation:
where W = mechanical work done (mJ), Q = volume of cartilage under the stress field (mm3), ΔD = change in the mediolateral stress-field position (mm), f = tractional coefficient (Ftraction/Fnormal), and Fnormal = TMJ-specific normal (perpendicular) force. Because human TMJ discs were not available, porcine TMJ discs were used in ex vivo experiments, as previously reported (Nickel et al. 2004, 2006; Nickel, Iwasaki, et al. 2009). The results showed that f was nonlinearly related (R2 = 0.85) to y (mm/s) and the product of aspect ratio and compressive strain3 (x = a/h × (Δh/h)3; a = radius of the stress field, h = instantaneous thickness of the TMJ disc) and provided an empirical formula (Nickel, Spilker, et al. 2009):
where a, b, c, x0, and y0 were constants measured in the experiments. Thus, for each TMJ, the magnitudes of the variables needed to estimate ED were determined using dynamic stereometry for Q, ΔD, y, and x and validated numerical modeling for Fnormal.
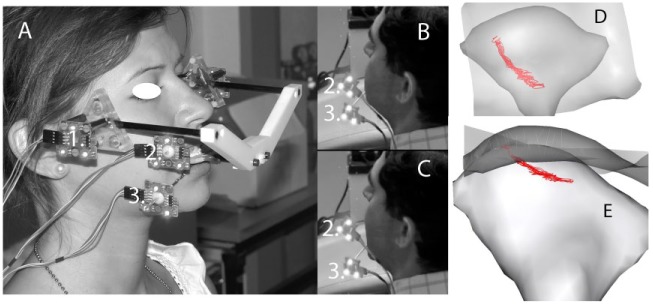
Dynamic stereometry, as previously described (Gallo et al. 2015), involved 3-dimensional image-processing software to reconstruct in vivo anatomical structures captured in MR images of both TMJs and animate these structures using jaw-tracking data, linked through a common reference system. The reference system was connected to a custom oral appliance and worn briefly by each subject during both MR imaging and jaw tracking (Fig. 1A). An opto-electronic system facilitated jaw tracking (Fig. 1A–C) via sets of 3 light-emitting diodes (LED) attached to the maxilla and mandible on one side at a time via custom splints that were luted temporarily to the vestibular tooth surfaces (Fig. 1B, C; Appendix Fig. 1) and to right and left sides of the reference system (Fig. 1A). Relative positions of LED were recorded on one side by a set of 3 fixed cameras at a sampling frequency of 200 Hz and accuracy within 5 µm, then recorded on the other side. These recordings provided the positions of the jaws relative to the reference system statically plus the time-varying jaw positions during 10 symmetrical opening-closing movements performed by the subjects (Fig. 1B, C). Once TMJ anatomy was traced and segmented slice by slice from MR images, then reconstructed in 3 dimensions, the location of the reference system in both MR and jaw-tracking data sets was used to animate the movement of the TMJ components (Fig. 1D, E) via mathematical transformations to an accuracy of within 0.9%. From the reconstructed images of a given TMJ, 30 of the smallest adjacent condyle-fossa/eminence distances (Fig. 1D) measured between segment vertices were identified and averaged to determine h. The centroid of the area that comprised these 30 minimum distances determined the stress field, and the standard deviation of the positions about the centroid determined a. Then, the TMJ-specific animations were used to calculate the magnitudes of Q, ΔD, y, and x averaged over 5-ms time intervals during jaw closing.
Figure 1.
Jaw-tracking data collection for dynamic stereometry where (A) shows a subject wearing the custom oral appliance connected to the reference system with light-emitting diodes (LED) (1). LED are also connected to the maxillary (2) and mandibular (3) teeth via custom splints affixed temporarily to the teeth. A subject’s jaw position at (B) maximum intercuspation and (C) maximum opening is shown during jaw movement as this is tracked and recorded by cameras located outside the field of view to the subject’s left. Dynamic stereometry results in (D) superior and (E) frontal views after reconstruction of temporomandibular joint (TMJ) anatomy and position data are combined and centroid of the stress field identified at each position. Right TMJ condyle and semi-transparent image of fossa and eminence are shown, where the paths of the centroid of the stress field between maximum intercuspal position and maximum jaw opening can be seen in red.
Each subject’s 3-dimensional craniomandibular anatomy from CT images, eminence shapes from dynamic stereometry, and an objective function of minimization of muscle effort were employed in a previously described computer-assisted numerical model (Iwasaki et al. 2009; Iwasaki, Liu, et al. 2015) to predict TMJ forces for a spectrum of 324 loading angles of 20 N on the right mandibular canine. Results were averaged for right and left sides to establish ipsilateral and contralateral Fnormal, respectively.
Instantaneous ED calculations over 5-ms time intervals during symmetrical jaw closing with a 20-N load on the right mandibular canine were thus calculated for the ipsilateral and contralateral TMJ in each subject.
DF
As previously described (Iwasaki, Gonzalez, et al. 2015), ambulatory electromyography recorded by subjects in their natural environments was calibrated via muscle- and subject-specific electromyography thresholds for a 20-N bite force (T20 N) established from 2 laboratory visits to determine jaw muscle DF using the following equation:
where # of windows were the number of 128-ms time windows during a recording where the ambulatory electromyography was at the specified thresholds. These thresholds were <50% T20 N based on previous evidence that higher magnitudes of jaw muscle activities were rare during recordings made in subjects’ natural environments (Iwasaki, Gonzalez, et al. 2015) and in sleep studies (Raphael et al. 2013). After jaw tracking, subjects performed a set of static and dynamic molar bites using low to moderate effort on a bite force transducing device held on one side at a time. During the biting tasks, bilateral masseter and temporalis electromyography were recorded using standard laboratory techniques and surface electrodes (Iwasaki, Gonzalez, et al. 2015) paired on the muscles and a single ground electrode on the right mastoid process. The recorded signals were later analyzed using customized software (MATLAB; MathWorks), and root mean square muscle activities (RMS-electromyography [EMG], mV) were plotted versus bite force (N) for each biting task, muscle, and side. At the same visit, subjects were trained to apply surface electrodes and use custom portable recorders for masseter and temporalis electromyography on the side of their choice (Appendix Fig. 2) as previously presented (Iwasaki, Gonzalez, et al. 2015). Subjects were asked to record for ≥5 h for each of 3 days and 3 nights, use a separate data storage card for each recording, and complete a diary form. At a second laboratory visit, subjects returned data, supplies, and recording equipment and repeated the biting task protocol.
RMS-EMG for 20 N of bite force was established using the linear regression slope for each plot of RMS-EMG versus bite force and averaged for right and left sides and 2 laboratory visits to establish an average threshold activity (T20 N, mV) for each subject’s masseter and temporalis muscles. Each ambulatory electromyography recording was inspected and filtered to eliminate low-level noise and identify and exclude predominantly noisy or blank signals using commercial software (WavePad Sound Editor Master Edition) and then processed using customized software (MATLAB 7.9 R2009b; MathWorks) to identify and count the time windows where the RMS-EMG was 1%–9%, 10%–24%, and 25%–49% T20 N for a given muscle and subject. Thus, subjects’ DF at 3 thresholds, separately and combined, were calculated for each muscle (masseter, temporalis) and time period (day, night).
MBS
MBS for ipsilateral and contralateral TMJs were calculated using an equation with a power function that was consistent with previously reported energy density modeling (Carter et al. 1987):
Thus, MBS were estimated for each subject’s TMJs (ipsilateral, contralateral), threshold (1%–9%, 10%–24%, 25%–49% T20 N), muscle (masseter, temporalis), and time period (day, night).
Factorial analysis of variance was used to investigate if predictors (TMJ side, threshold, muscle, and time period) affected outcomes (ED, DF, and MBS). Simple effect analyses identified the influence of a single predictor. Bonferroni corrections and Tukey honest significant difference post hoc tests were employed, based on research data properties, to keep the type I error rate nominal. Thus, the pairs of dependent and independent variables analyzed with post hoc tests for significant diagnostic group differences were 1) ED and TMJ side (ipsilateral, contralateral); 2) DF and threshold (1%–9%, 10%–24%, 25%–49% T20 N), muscle (masseter, temporalis), and time period (day, night); and 3) MBS and TMJ side (ipsilateral, contralateral), threshold (1%–9%, 10%–24%, 25%–49% T20 N), muscle (masseter, temporalis), and time period (day, night), respectively. Significance was defined by P < 0.05. All analyses were performed with commercial software (SPSS version 23; SPSS, Inc.).
Results
Screening of 98 women resulted in 39 who met exclusion criteria, 3 who quit the study for personal reasons, and 47 who met inclusion criteria and completed all study protocols. These 47 were grouped based on diagnoses into 29 +DD subjects and 18 –DD (healthy control) subjects. Mean ± standard deviation ages of the +DD and –DD groups were 34 ± 14 and 31 ± 10 years, respectively, and not significantly different.
Instantaneous ED averaged over 10 closing cycles for individual TMJs ranged from 0.1 to 81.6 mJ/mm3. Mean ± standard error ED for +DD versus –DD groups was not significantly different in ipsilateral TMJs (P = 0.134; 10.5 ± 0.6 vs. 13.3 ± 1.7 mJ/mm3) but was 1.4-fold and significantly larger for +DD versus –DD groups in contralateral TMJs (P = 0.012; 11.5 ± 1.0 vs. 8.2 ± 0.9 mJ/mm3; Fig. 2).
Figure 2.
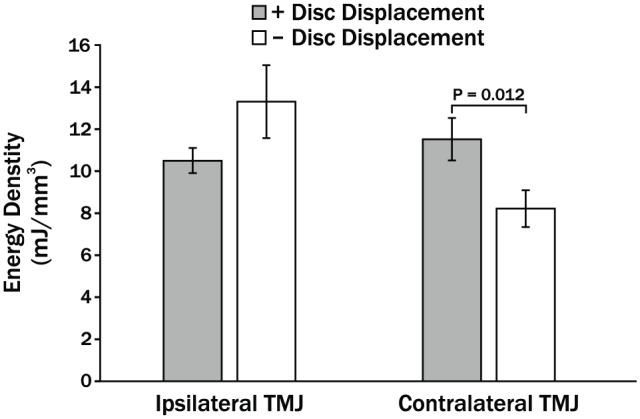
Mean energy densities (ED) in ipsilateral and contralateral temporomandibular joints (TMJs) of women with (+) and without (–) bilateral TMJ disc displacement (DD). Vertical bars indicate standard errors above and below the mean values.
Subjects produced 267 ambulatory electromyography recordings of acceptable quality with mean ± standard deviation durations of 6.8 ± 1.9 and 7.6 ± 1.6 hours for day and night periods, respectively (example in Appendix Fig. 2). Missing or noisy recordings totaled 15 (of 282), representing 5% to 6% of the expected number of recordings per group. Because all subjects had some acceptable day and night recordings and previous tests of self-recorded EMG using the same protocols showed overall reliabilities were high for masseter muscles (intraclass correlation coefficient [ICC] = 0.74) and moderate for temporalis muscles (ICC = 0.42) (Nickel et al. 2015), no subjects were excluded.
Mean ± standard error DF for both muscles, and all 3 thresholds combined were significantly larger (all P < 0.0001) for +DD versus –DD groups overall by 1.9-fold (0.91% ± 0.06% vs. 0.49% ± 0.02%), during the day by 1.7-fold (1.26% ± 0.09% vs. 0.73% ± 0.05%), and at night by 2.5-fold (0.61% ± 0.06% vs. 0.25% ± 0.02%) (Fig. 3A). The largest mean ± standard error DF occurred at the lowest intensity threshold (1%–9% T20 N) for the masseter muscle during the day and were 14.4% ± 0.8% and 4.9% ± 0.3% for the +DD and –DD groups, respectively (Fig. 3B). These DFs represented averages of about 59 and 20 min, respectively, of low-level masseter muscle activity per daytime recording. Mean low-level masseter DF (1%–9% T20 N) were significantly larger (all P < 0.05) in +DD compared to –DD subjects by 2.9-fold and 4.5-fold during the day and night, respectively (Fig. 3B). Mean masseter DFs at 10% to 24% T20 N were ≤2.4% (≤10 min per recording period) and, similarly, were significantly larger (all P < 0.05) during the day and night for +DD compared to –DD subjects (Fig. 3B). Mean temporalis DF at all thresholds were at most 2% and lower than mean masseter DF by up to 7-fold (Fig. 3B, C). Nevertheless, temporalis DF were significantly larger (all P < 0.05) in +DD compared to –DD groups during the day for the 2 lower thresholds (1%–9%, 10%–24% T20 N), while no significant between-group differences were shown during the night (Fig. 3C).
Figure 3.
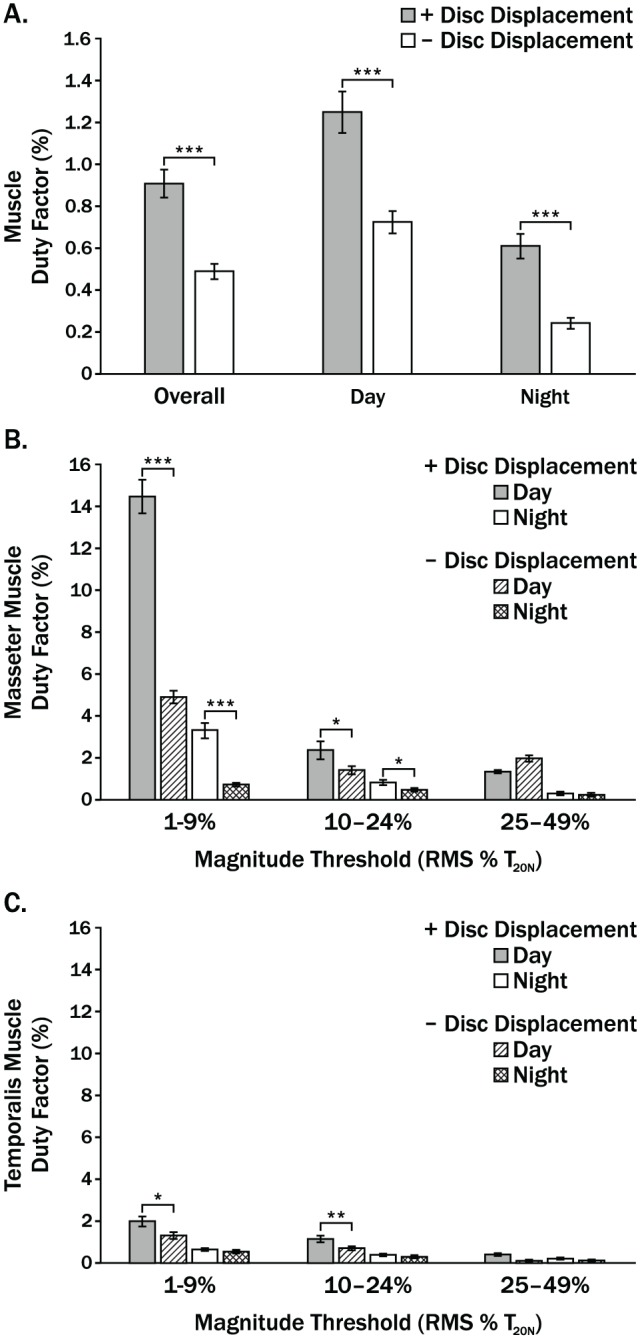
Mean DF for women with (+) and without (–) bilateral temporomandibular joint (TMJ) disc displacement (DD) during overall, day, and night periods where (A) shows results for masseter and temporalis muscles and all thresholds combined; (B, C) show results for masseter and temporalis muscles, respectively, for 3 magnitude thresholds relative to root mean square electromyography for a 20-N bite force (RMS % T20 N). Vertical bars indicate standard errors above and below the mean values. *P < 0.05. **P < 0.001. ***P < 0.0001.
Mean MBS calculated using average DF for both muscles were larger for +DD than –DD subjects for both TMJs, both time periods, and all 3 thresholds as well as for combined TMJs, time periods, and thresholds (Fig. 4A–C). These between-group differences were significantly larger (all P < 0.05) for TMJs, time periods, and thresholds combined; for TMJs and thresholds combined during the day and night; and for TMJs combined at all 3 thresholds during the day and at the lowest threshold at night (Fig. 4A). The largest mean ± standard error MBS for each TMJ occurred during the day at the lowest threshold (1%–9% T20 N); for the +DD group, these were 1,442 ± 572 and 789 ± 377 for the contralateral and ipsilateral TMJs, respectively. In the –DD group, for the same period and threshold, the MBS were significantly smaller (all P < 0.05) by 8.5- and 2.7-fold for the contralateral and ipsilateral TMJs, respectively. Mean MBS for the contralateral TMJ were also significantly different between groups for 10% to 24% T20 N during the day and for combined thresholds and times, as well as combined thresholds during the day and night (all P < 0.05; Fig. 4B). Mean MBS for the ipsilateral TMJ were larger in +DD than –DD subjects but not significantly so (Fig. 4C).
Figure 4.
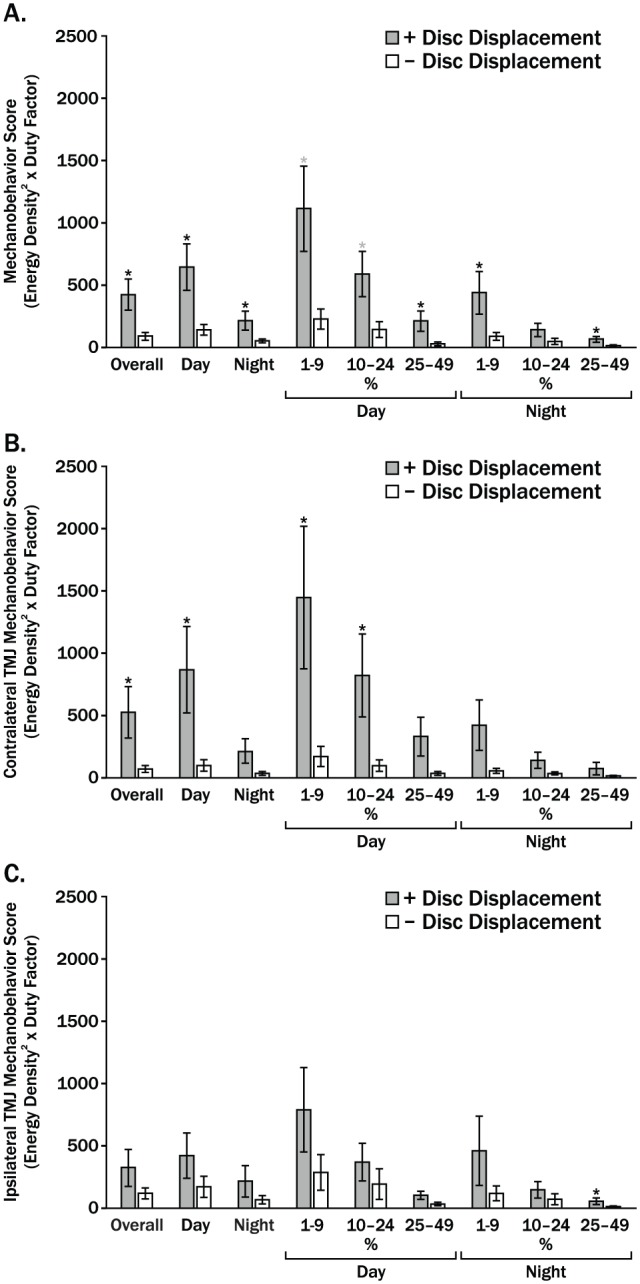
Mean mechanobehavior scores for women with (+) and without (–) bilateral temporomandibular joint (TMJ) disc displacement (DD) are shown for (A) TMJs combined, (B) contralateral TMJs, and (C) ipsilateral TMJs. Overall indicates results for time periods and thresholds combined; day and night results show the 3 thresholds combined as well as individual thresholds of 1% to 9%, 10% to 24% T20 N. Vertical bars indicate standard errors above and below the mean values. *P < 0.05.
Discussion
Mechanical work imposed on the TMJ articular surfaces is a consequence of plowing tractional forces caused by stress-field translation within the joint during function (Nickel, Iwasaki, et al. 2009; Guo et al. 2012). Although the dynamic mechanics of stress-field translation moves fluids through the disc to ensure adequate nutrition to disc fibroblasts (Wright et al. 2013), the combination of concentration of mechanical work (ED) and frequency of loading (DF), as represented by the MBS, potentiates the fatigue of the disc’s collagen fibers. This is especially likely along the mediolateral axis of the disc due to the anisotropic nature of disc fatigue (Beatty et al. 2003). The significantly larger ED, DF, and MBS in bilaterally affected (+DD) compared to bilaterally unaffected (–DD) subjects found in the current study are consistent with the prevailing mechanical fatigue model of TMJ DD (Nickel, Spilker, et al. 2009).
Limitations of the current study include the focus on women only; sample sizes too small to divide further into TMD subcategories with and without pain or psychosocial diagnoses; application of an empirical formula for tractional coefficients that was based on ex vivo studies on porcine TMJ discs; use of a bite-force measurement device that required a gape of 8 mm, which may have tended to underestimate associated EMG calibration activities compared to in vivo behaviors (Morneburg et al. 2014); and subjects who were unmonitored during their self-recordings.
Future work could address some of the current study’s limitations. However, although women are more afflicted with TMD than men, the variables of ED, DF, and MBS are not sex specific. A future study with an expanded sample size that includes both sexes could allow for comparison of healthy subjects with the various TMD subgroups, per DC/TMD physical assessment (Axis I) and psychosocial (Axis II) data, to test further if between-group differences in ED, DF, and MBS exist. Furthermore, if unpreserved human TMJ discs from different stages of development could be tested in the future, the current empirical formula with content validity could be further improved or verified using new reference standards. Although gape size during bite-force measurements for calibration of ambulatory EMG was kept consistent, potential effects of gape size as a matter of scale between subjects of different sizes could be addressed by improved materials and technology that would allow reduced dimensions of the measurement device. Means of confirming subject compliance with self-recording protocols would be beneficial; however, previous tests have shown moderate to high reliabilities of self-recorded EMG using the same protocols (Nickel et al. 2015). Given the past reports of differences in stress-field translation velocities in healthy and arthritic joints, secondary analyses of diagnostic group differences in this variable should also be conducted in future.
With time, individuals with DD may remain stable, improve to develop normal TMJ architecture, or progress to develop degenerative joint disease (Naeije et al. 2013; Schiffman et al. 2017). However, the ability to predict future TMJ conditions for individuals is not presently possible. To build on the current study, whether or not the measured MBS predict directional changes of repair and homeostasis versus degeneration of the integrity of the TMJ surfaces should be tested. Hence, this group of subjects should be followed longitudinally to determine if higher MBS are associated with degenerative joint disease whereas lower MBS are associated with no change or improvement in TMJ architecture with time. If MBS have sufficient predictive value, they could furthermore be used to evaluate the effectiveness of behavioral therapies to ameliorate and prevent degenerative changes in TMJ tissues.
Conclusion
TMJ ED, masseter and temporalis muscle DF, and MBS were significantly larger in women with bilateral TMJ DD compared to healthy women without DD and support a general fatigue model for TMJ DD.
Author Contributions
L.R. Iwasaki, J.C. Nickel, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y.M. Gonzalez, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; Y. Liu, contributed to data analysis and interpretation, critically revised the manuscript; H. Liu, M. Markova, L.M. Gallo, contributed to data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This research was supported by the National Institute of Dental and Craniofacial Research (R01 2DE016417, JN-PI). Stefan Erni, Alessandro Gallo, Vera Colombo, and Eveline Studer contributed to computer programing and data analysis at the University of Zurich.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, John MT, Schiffman EL. 2009. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 107(6):844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderst WJ, Tashman S. 2010. Using relative velocity vectors to reveal axial rotation about the medial and lateral compartment of the knee. J Biomech. 43(5):994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty MW, Bruno MJ, Iwasaki LR, Nickel JC. 2001. Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J Biomed Mater Res. 57(1):25–34. [DOI] [PubMed] [Google Scholar]
- Beatty MW, Nickel JC, Iwasaki LR, Leiker M. 2003. Mechanical response of the porcine temporomandibular joint disc to an impact event and repeated tensile loading. J Orofac Pain. 17(2):160–166. [PubMed] [Google Scholar]
- Carter DR, Fyhrie DP, Whalen R, Orr TE, Schurman DJ, Rapperport DJ. 1987. Control of chondro-osseous skeletal biology by mechanical energy. In: Bergmann G, Kolbel R, Rohlmann A, editors. Biomechanics: basic and applied research. Dordrecht (Netherlands): Martinus Nijhoff. p. 219–224. [Google Scholar]
- Chantaracherd P, John MT, Hodges JS, Schiffman EL. 2015. Temporomandibular joint disorders’ impact on pain, function, and disability. J Dent Res. 94(Suppl 3):79S–86S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detamore MS, Athanasiou KA. 2003. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 125(4):558–565. [DOI] [PubMed] [Google Scholar]
- Farrokhi S, Voycheck CA, Gustafson JA, Fitzgerald GK, Tashman S. 2016. Knee joint contact mechanics during downhill gait and its relationship with varus/valgus motion and muscle strength in patients with knee osteoarthritis. Knee. 23(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LM, Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Nickel JC. 2015. Diagnostic group differences in temporomandibular joint energy densities. Orthod Craniofac Res. 18(Suppl 1):164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LM, Nickel JC, Iwasaki LR, Palla S. 2000. Stress-field translation in the healthy human temporomandibular joint. J Dent Res. 79(10):1740–1746. [DOI] [PubMed] [Google Scholar]
- Guo H, Nickel JC, Iwasaki LR, Spilker RL. 2012. An augmented lagrangian method for sliding contact of soft tissue. J Biomech Eng. 134(8):084503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Crosby MJ, Gonzalez Y, McCall WD, Marx DB, Ohrbach R, Nickel JC. 2009. Temporomandibular joint loads in subjects with and without disc displacement. Orthop Rev (Pavia). 1(2):90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Gonzalez YM, Liu H, Marx DB, Gallo LM, Nickel JC. 2015. A pilot study of ambulatory masticatory muscle activities in temporomandibular joint disorders diagnostic groups. Orthod Craniofac Res. 18(Suppl 1):146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki LR, Liu H, Gonzalez YM, Marx DB, Nickel JC. 2015. Modeling of muscle forces in humans with and without temporomandibular joint disorders. Orthod Craniofac Res. 18(Suppl 1):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morneburg TR, Döhla S, Wichmann M, Pröschel PA. 2014. Afferent sensory mechanisms involved in jaw gape-related muscle activation in unilateral biting. Clin Oral Investig. 18(3):883–890. [DOI] [PubMed] [Google Scholar]
- Naeije M, Te Veldhuis AH, Te, Veldhuis EC, Visscher CM, Lobbezoo F. 2013. Disc displacement within the human temporomandibular joint: a systematic review of a ‘noisy annoyance’. J Oral Rehabil. 40(2):139–158. [DOI] [PubMed] [Google Scholar]
- Nickel J, Spilker R, Iwasaki L, Gonzalez Y, McCall WD, Ohrbach R, Beatty MW, Marx D. 2009. Static and dynamic mechanics of the temporomandibular joint: plowing forces, joint load and tissue stress. Orthod Craniofac Res. 12(3):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Marx DB. 2004. Laboratory stresses and tractional forces on the TMJ disc surface. J Dent Res. 83(8):650–654. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Marx DB. 2009. Tractional forces on porcine temporomandibular joint discs. J Dent Res. 88(8):736–740. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Iwasaki LR, Beatty MW, Moss MA, Marx DB. 2006. Static and dynamic loading effects on temporomandibular joint disc tractional forces. J Dent Res. 85(9):809–813. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Liu Y, Gonzalez Y, Liu H, Gallo LM, Iwasaki LR. 2015. Reliability of masseter and temporalis EMG recorded in habitual environments. J Dent Res. 94(SI A):Abstract #1842. [Google Scholar]
- Raphael KG, Janal MN, Sirois DA, Dubrovsky B, Wigren PE, Klausner JJ, Krieger AC, Lavigne GJ. 2013. Masticatory muscle sleep background electromyographic activity is elevated in myofascial temporomandibular disorder patients. J Oral Rehabil. 40(12):883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, et al. ; International RDC/TMD Consortium Network, International Association for Dental Research; Orofacial Pain Special Interest Group, International Association for the Study of Pain. 2014. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J Oral Facial Pain Headache. 28(1):6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman EL, Ahmad M, Hollender L, Kartha K, Ohrbach R, Truelove EL, Zhang L, Hodges JS, Sommers E, Anderson GC, et al. 2017. Longitudinal stability of common TMJ structural disorders. J Dent Res. 96(3):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Kuo J, Bell PD, Yao H. 2010. Anisotropic solute diffusion tensor in porcine TMJ discs measured by frap with spatial Fourier analysis. Ann Biomed Eng. 38(11):3398–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, et al. 2016. Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res. 95(10):1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker RL, Nickel JC, Iwasaki LR. 2009. A biphasic finite element model of in vitro plowing tests of the temporomandibular joint disc. Ann Biomed Eng. 37(6):1152–1164. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Hanaoka K, van Eijden T, Tanaka M, Watanabe M, Nishi M, Kawai N, Murata H, Hamada T, Tanne K. 2003. Dynamic shear properties of the temporomandibular joint disc. J Dent Res. 82(3):228–231. [DOI] [PubMed] [Google Scholar]
- Wright GJ, Kuo J, Shi C, Bacro TR, Slate EH, Yao H. 2013. Effect of mechanical strain on solute diffusion in human TMJ discs: an electrical conductivity study. Ann Biomed Eng. 41(11):2349–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cisewski SE, Wei F, She X, Gonzales TS, Iwasaki LR, Nickel JC, Yao H. In press. Role of interstitial fluid pressurization in tractional force formation on temporomandibular joint disc: a biphasic finite element analysis. Orthod Craniofac Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.