Abstract
The etiology and treatment of temporomandibular joint (TMJ) osteoarthritis (TMJOA) remain complex and unclear. Based on clinical observations, we hypothesized that low condylar bone quality is significantly correlated with TMJOA and explored this association in a cross-sectional study with human patients. A total of 254 postmenopausal female participants were included in this study. Radiographic findings from cone beam computed tomography (CBCT) and clinical symptoms were used to classify each TMJ data sample as healthy control (n = 124) or TMJOA (n = 130). Condylar bone mineral density (BMD) (computed tomography Hounsfield unit [CT HU]) and bone volume fraction (BV/TV) were measured and modeled as predictors of healthy control versus TMJOA status in multilevel logistic regression analyses. Both CT HU (adjusted odds ratio [AOR] = 0.9989, interquartile odds ratio [IOR] = 0.4206) and BV/TV (AOR= 0.8096, IOR = 0.1769) were negatively associated with TMJOA (P = 0.049, 0.011, respectively). To assess the diagnostic performance of CT HU and BV/TV for identification of TMJOA, receiver operating characteristic (ROC) curves were plotted. The estimated areas under the curve (AUC) were 0.6622 for BV/TV alone, 0.6074 for CT HU alone, and 0.7136 for CT HU and BV/TV together. The model incorporating CT HU and BV/TV together had a significantly higher AUC than the models using BV/TV alone (P = 0.038) or HU alone (P = 0.021). In conclusion, we found that low condylar bone quality was significantly correlated with TMJOA development and that condylar CT HU and BV/TV can be used together as a potential diagnostic tool for TMJOA. Careful clinical evaluation of the condyle coupled with appropriate radiographic interpretation would thus be critical for the early detection of TMJOA.
Keywords: mandibular condyle, bone volume, cone beam computed tomography, micro-computed tomography, multivariate analysis, cross-sectional study
Introduction
Temporomandibular joint (TMJ) osteoarthritis (TMJOA) is one of the most common diseases of the TMJ. The disease affects around 3 million people within the United States (Chen et al. 2009) and has a strong predilection for females and the elderly (de Leeuw et al. 1996). Patients with TMJOA may complain of crepitus, jaw stiffness with pain, or progressively increasing anterior open bite. In the early stages of TMJOA, plain radiography or computed tomography (CT) may show condylar flattening. In the advanced stages, however, radiography may reveal osteophyte formation or condylar sclerosis, suggestive of dysfunctional change.
Once the TMJ develops degenerative osteoarthritic changes, various nonoperative and operative approaches can be taken to relieve the symptoms. These treatments include physical therapy, trigger point injections, medication, lifestyle modifications, the fabrication of mouth guards, and surgery. However, current treatment modalities have limited patient satisfaction and are unable to completely restore degenerative cartilage (Schiffman et al. 2014). Hence, understanding the disease etiology is critical for the early detection and prevention of TMJOA.
The etiology of the majority of TMJOA cases is unclear and multifactorial (Wang et al. 2015). Various etiological factors of TMJOA include trauma, severe malocclusion, jaw asymmetry, and muscle overuse, which can lead to aberrant mechanical loading, hormonal imbalances, and altered joint extracellular matrix development (Tanaka et al. 2008; Krisjane et al. 2012). However, to date, no comprehensive research examines the closely associated factors of TMJOA, especially the association between condylar bone quality and TMJOA in human subjects.
Given that the health and integrity of the TMJ articular cartilage depends on the mechanical properties of its bony bed, it is reasonable to suspect that a change in condylar bone structure is associated with TMJOA development (Radin and Rose 1986). This raises 2 important questions. First, is TMJOA development correlated with condylar bone quality as measured by bone mineral density (BMD) and bone volume (BV)? And second, does condylar bone loss influence the progression of TMJOA?
Recent advancements in imaging techniques and analyses have provided us the ability to quantify with precision the BMD and BV of the condyle. For example, a 3-dimensional (3D) image with minimal distortion can be produced via cone beam computed tomography (CBCT) (dos Anjos Pontual et al. 2012). In turn, data acquired from CBCT have been shown to effectively diagnose multiple changes in bone that might influence the TMJ (Ludlow et al. 2006).
To date, only a few studies have analyzed the bony changes in the TMJ using computed tomography (CT) analyses (de Bont et al. 1993; dos Anjos Pontual et al. 2012; Boeddinghaus and Whyte 2013; Tsiklakis et al. 2014; Pahwa et al. 2015), while no studies have explored the association between condylar bone quantification and TMJOA in human subjects. In the present study, we hypothesized that low condylar bone quality would be significantly correlated with TMJOA development. For the first time, micro-CT software was implemented for the accurate targeted analysis of human condylar CBCT images. Unlike previous studies, statistical quantification was used in place of visual diagnosis (Barghan et al. 2012; dos Anjos Pontual et al. 2012) to achieve a more objective comparison.
Materials and Methods
Data Collection and Patient Grouping
Institutional review board approval was received for this cross-sectional retrospective study. In total, 2,146 high-resolution TMJ CBCT image sets were gathered between January 2011 and March 2016 from a tertiary referral hospital in China. Additional data were obtained from patient charts and phone interviews as summarized in Appendix Table 1.
Regarding the inclusion criteria, only female patients between the ages of 50 and 65 y were included in this study. In total, 884 male data sets were excluded, and 967 female data sets outside the 50- to 65-y age range were also excluded. This was done not only to minimize the effects of age and sex for a more homogeneous study population but also to focus on the principal TMJOA-affected population of elderly postmenopausal women (Alexiou et al. 2009). Of the 295 remaining patients, 41 were excluded for various additional reasons.
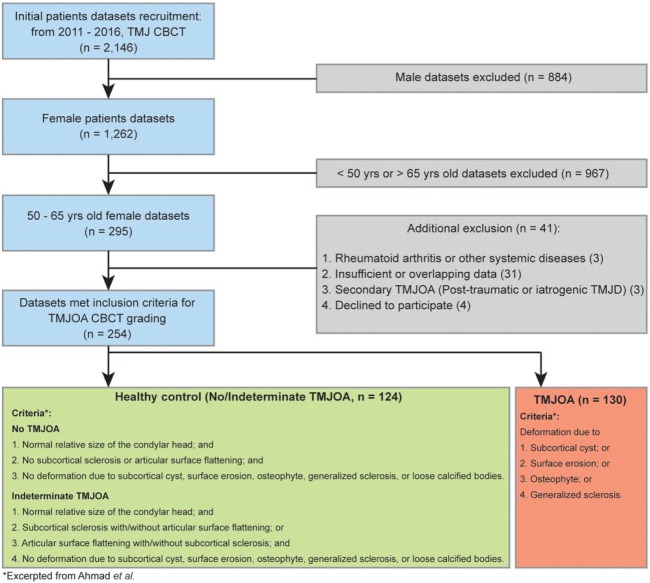
Ultimately, 254 TMJ CBCT image sets were selected for the study. In determining an association between condylar bone loss and TMJOA, we sorted each condyle into 2 groups based on the criteria published by Ahmad et al. (2009): healthy control (normal condyles and indeterminate TMJOA) and TMJOA. Healthy controls included participants who underwent CBCT imaging as healthy volunteers, patients who had other issues unrelated to the TMJ, and patients diagnosed with indeterminate TMJOA or TMJ disorder (temporomandibular disorder [TMD]) only. None of these patients exhibited radiographic abnormalities related to osteoarthritis. While some of the healthy control patients presented with TMD symptoms, they were still classified as such so long as they did not meet the TMJOA image criteria by Ahmad et al. (2009). Patients in the TMJOA group had to demonstrate at least 1 or more TMD symptoms such as clicking, pain, and limited opening of the mouth in addition to radiographic abnormalities. Radiographic bony changes in the TMJ CBCT image sets include erosion, flattening, sclerosis, and the presence of osteophytes as according to the definitions by Akerman et al. (1988), Muir and Goss (1990), Flygare et al. (1992), and Ahmad and Schiffman (2016) (Fig. 1).
Figure 1.
Flowchart for the selection of the study population. CBCT, cone beam computed tomography; TMD, temporomandibular disorder; TMJ, temporomandibular joint; TMJOA, temporomandibular joint osteoarthritis.
Condyle CBCT Analysis
All TMJ images were scanned by the same operator using a CBCT scanner (Morita 3D Accuitomo; J. Morita MFG. CORP) and i-Dixel software (J. Morita MFG. CORP). The following image acquisition protocol was used for each TMJ: 85 kVp, 4 mAs, and 0.25 mm voxel. The images were obtained with the patients in maximum dental intercuspation. The 3D morphometric analysis of the condyles was performed using the micro-CT software, CT-Analyzer (1.16.1.0 SkyScan).
In this study, we focused on the structural changes of the condylar bone as the closely associated factor for TMJOA development. To maintain consistency across our analyses, we customized a region of interest (ROI) for each patient that measured the tip of the condyle to the condyle neck. The condyle neck was defined as the narrowest portion of the condyle process. Based on the micro-CT images, we were able to customize the rectangular-shaped ROI for each patient and further adjusted its position to exclude the temporal bone surrounding the condyle (Appendix Fig. 1, ROI shown as the red shadow box).
All data sets were first subject to Hounsfield unit (HU) calibration within the micro-CT software to reestablish the contrast limits and were then standardized to produce a consistent threshold difference. After reviewing all the data sets between the binarized and raw versions, a global threshold of 85 was applied to all scans to obtain an accurate representation of the bone. Morphometric parameters were then computed from the binarized images using direct 3D techniques. Bone volume/total volume (BV/TV) was generated from a semiautomatic interpolated volume of interest (VOI). The bone volume calculation was based on the binarized contents within the VOI, with the combined ratio constituting our BV/TV calculation. In this study, computed tomography Hounsfield unit (CT HU) was obtained and calibrated by muscle and air as suggested by Liu et al. (2013). After data quantification, 3D-rendered models were generated for better visualization.
Statistics
Frequencies and percentages are used to summarize categorical variables, whereas means and standard deviations (SDs) are used to summarize continuous variables. We employed multilevel logistic regression for all models to assess the association between the dependent variable, healthy versus TMJOA status, and several independent variables. Independent variables include BV/TV, CT HU, and several other possible confounders, including age, body mass index (BMI), and 9 binary factors (Table 1). Multilevel modeling incorporates random effects (intercepts for this study) to capture the correlation among observations. This statistical approach can properly model effects at both the condyle (individual) level and the patient (cluster) level. We first assessed bivariate relationships between TMJOA status (healthy control vs. TMJOA) and each of the independent variables. Then we modeled both BV/TV and CT HU together to estimate each of their effects on TMJOA status, adjusting for the other. Finally, all independent variables were entered into a single model to estimate the effects of CT HU and BV/TV on TMJOA status, adjusting for the confounders.
Table 1.
Demographic Characteristics of All Variables between Healthy Control and TMJOA.a
| Healthy Control (n = 124) | TMJOA (n = 130) | P Valueb | |
|---|---|---|---|
| Age, y | 53.85 ± 8.23 | 56.5 ± 8.49 | 0.101 |
| CT HU | 2,870.93 ± 684.03 | 2,644.478 ± 535.9 | 0.068 |
| Bone volume fraction (BV/TV) | 24.25 ± 6.6 | 20.86 ± 5.95 | 0.006 |
| Poor dental treatment history | 7 (8.24) | 11 (12.5) | 0.774 |
| TMJ dislocation | 3 (3.53) | 5 (5.68) | 0.670 |
| Misaligned teeth | 26 (30.59) | 18 (20.45) | 0.262 |
| Habits related to mastication | 22 (25.88) | 23 (26.14) | 0.633 |
| Hormone replacement therapy | 1 (1.18) | 1 (1.14) | 0.962 |
| Psychological illness | 4 (4.71) | 2 (2.27) | 0.497 |
| Smoking | 10 (11.76) | 9 (10.23) | 0.757 |
| Alcohol | 10 (11.76) | 9 (10.23) | 0.495 |
| Poor socioeconomic status | 4 (4.71) | 3 (4.55) | 0.655 |
| Body mass index | 22.19 ± 3.57 | 21.69 ± 3.6 | 0.509 |
BV/TV, bone volume/total volume; CT HU, computed tomography Hounsfield unit; TMJ, temporomandibular joint; TMJOA, temporomandibular joint osteoarthritis.
Values are presented as number (%) or mean ± standard deviation.
Estimated in a multilevel logistic regression model with each variable as the sole predictor.
To assess the classification performance of CT HU and BV/TV as diagnostic tools for TMJOA, receiver operating characteristic (ROC) curves were plotted and the area under the curve (AUC) was estimated for each of the 4 models: 1) CT HU alone, 2) BV/TV alone, 3) CT HU and BV/TV together, and (4) CT HU, BV/TV, and all confounders together. Specifically, the linear predictor (predicted values from the model, including only fixed effects) from each model was used as the classifier. The χ2 test was used to compare whether the AUC was significantly different between models. The statistical software, Stata 14.0 (StataCorp LP), was used for statistical analyses. Statistical significance was determined at the P < 0.05 level.
Results
Study Population and Bone Quality Difference between Healthy Control and TMJOA
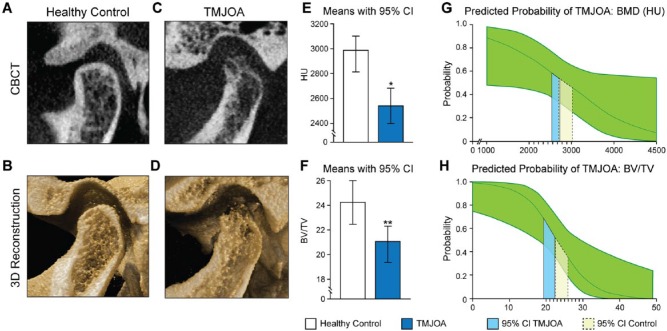
We identified 254 condyles in total, of which 124 were classified as healthy control and 130 as TMJOA. The multilevel logistic regression model was applied to these 2 groups. Bivariate tests between healthy control/TMJOA status and the demographic variables (confounders) showed that there were no significant differences in demographics between the 2 groups (Table 1). However, CBCT analysis showed significant differences between the healthy control and TMJOA groups. Compared to the healthy control, the CT HU and BV/TV values of the TMJOA group were 8.56% lower (P = 0.019) and 16.25% lower (P = 0.003), respectively (Fig. 2). The representative CBCT images of the condyles are shown in Figure 2, revealing thinner and damaged cortical bone and lower trabecular bone density in the TMJOA group.
Figure 2.
Representative images and bone quantification of healthy control and TMJOA. (A–D) The representative CBCT and 3D reconstruction images of healthy control (A and B) and TMJOA (C and D). Compared to the healthy control, the TMJOA images (C and D) show condylar head deformity, lower trabecular bone density, and thinner cortical bone. (E–F) The CT HU (E) and BV/TV (F) of TMJOA condyles were significantly lower than that of healthy condyles. (G–H) Predicted probabilities of TMJOA along the ranges of CT HU (G) and BV/TV (H) observed in the data, averaged over all other covariates and with the subject random effect fixed at zero. The green regions indicate the 95% confidence interval. The solid lines (blue) delineate the interval [mean – SD, mean + SD] for healthy control. The dashed lines (yellow) delineate the interval [mean – SD, mean + SD] for TMJOA. These intervals overlap for CT HU but not for BV/TV. TMJOA, temporomandibular joint osteoarthritis; CBCT, cone beam computed tomography; CT HU, computed tomography Hounsfield unit; BV/TV, bone volume fraction; SD, standard deviation. *P < 0.05. **P < 0.01. This figure is available in color online.
Odds Ratio of Healthy Control versus TMJOA
In the full multilevel logistic regression model, both measures for bone loss, CT HU and BV/TV, were found to be negatively associated with TMJOA after controlling for the confounders. The 11 confounders are age, BMI, poor dental treatment history, TMJ dislocation, misaligned teeth, habits related to mastication, hormone replacement therapy, psychological illness, smoking, alcohol, and poor socioeconomic status. Each unit increase in CT HU was associated with a 0.11% decrease in the odds of TMJOA (P = 0.049). Similarly, each unit increase in BV/TV was associated with a 19.04% decrease in the odds of TMJOA (P = 0.011). Results of statistical analyses are summarized in Table 2.
Table 2.
Odds Ratio for CT HU and BV/TV in TMJOA.
| CT HU |
BV/TV |
|||||
|---|---|---|---|---|---|---|
| OR (IOR) | 95% CI | P Value | OR (IOR) | 95% CI | P Value | |
| Unadjusted | 0.9991 (0.5088) | 0.9982, 1.0001 | 0.068 | 0.8081 (0.1742) | 0.6943, 0.9405 | 0.006 |
| Adjusteda | 0.9989 (0.4206) | 0.9977, 0.9999 | 0.049 | 0.8096 (0.1769) | 0.6884, 0.9520 | 0.011 |
BMI, body mass index; BV/TV, bone volume fraction; CI, confidence interval for OR; CT HU, computed tomography Hounsfield unit; IOR, interquartile odds ratio (odds ratio for increase from 25th percentile to 75th percentile); OR, odds ratio for 1-unit increase; TMJOA, temporomandibular joint osteoarthritis.
Includes all confounding factors (age, smoking, alcohol, BMI, poor socioeconomic status, psychological illness, misaligned teeth, poor dental treatment history, TMJ dislocation, mastication habits).
TMJOA Probabilities Change with CT HU and BV/TV
Because odds ratios (ORs) do not inform us of the actual probability of TMJOA, we estimated model-based predicted probabilities for each observation in the sample by applying the inverse logit transformation on the predicted logit (outcome in log-odds units), p = exp(xb)/(1 + exp(xb)), where p is the predicted probability, and xb is the predicted logit (Agresti 2013; Hosmer et al. 2013). Probabilities of TMJOA were first estimated at the mean values of CT HU and BV/TV in the healthy control (mean CT HU = 2870.926, mean BV/TV = 24.252%) and TMJOA (mean CT HU = 2644.478, mean BV/TV = 20.859%) groups while leaving the independent variables at their observed values. The probabilities were then averaged across observations within the healthy control and TMJOA groups, yielding mean probability estimates at specific values of CT HU and BV/TV but averaged over the other covariates in the model (Kleinman and Norton 2009; Norton et al. 2013). Estimated mean probabilities of TMJOA at the mean values of CT HU and BV/TV in the healthy control were 0.517 and 0.456, respectively, while the probabilities at the mean values of CT HU and BV/TV in the TMJOA group were 0.561 and 0.599, respectively. Graphs of the mean predicted probabilities versus the observed ranges of CT HU and BV/TV exhibit the sigmoidal shape expected from a logistic regression, with inflection points (where the probability = 0.5) at 2,955.5 and 23.21 for CT HU and BV/TV, respectively (Fig. 2G, H).
AUC of 4 Different Models
The estimated AUC values were 0.6074 for CT HU alone, 0.6622 for BV/TV alone, 0.7136 for CT HU and BV/TV together, and 0.7279 for CT HU, BV/TV, and all confounders together. This indicates poor to acceptable discrimination for CT HU alone and BV/TV alone, as well as acceptable to moderate discrimination for BV/TV and CT HU and the full model (Swets 1988). The AUC was significantly greater for CT HU and BV/TV together compared to CT HU alone (χ2 = 5.35, P = 0.021) and BV/TV alone (χ2 = 4.30, P = 0.038). The full model including confounders did not significantly increase the AUC compared to CT HU and BV/TV together (χ2 = 0.83, P = 0.362) (Fig. 3).
Figure 3.
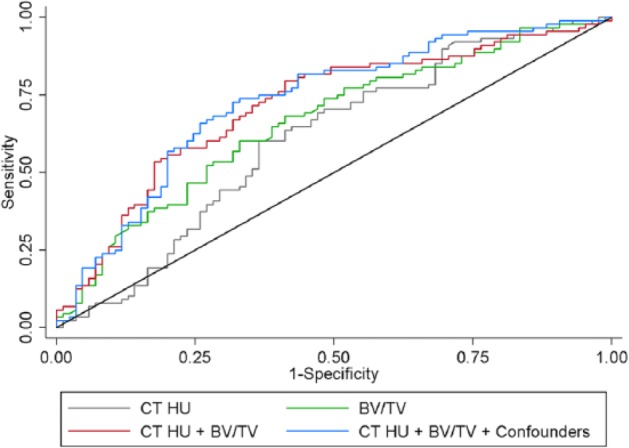
ROC curves for different variables. ROC curves where the classifiers are the fixed-effect predictions (i.e., excluding random effects) from multilevel logistic regression models with the following set of predictors: (1) CT HU alone: gray line, (2) BV/TV alone: green line, (3) CT HU and BV/TV together: red line, and (4) CT HU, BV/TV, and all other confounders: blue line. Based on the sensitivity and specificity of a diagnostic test using ROC curves, the combined model of using CT HU and BV/TV together (AUC = 0.7136) provides significantly better discrimination of case/controls than that of using CT HU (AUC = 0.6074) or BV/TV (AUC = 0.6622) alone. However, adding the additional confounders (AUC = 0.7279) does not significantly improve discrimination over using just CT HU and BV/TV together. AUC, area under the curve; BV/TV, bone volume fraction; CT HU, computed tomography Hounsfield unit; ROC, receiver operating characteristic.
Discussion
In the present study, we used micro-CT software on CBCT data to diagnose and classify TMJOA and to quantify the bone quality after proper calibration. CBCT imaging is useful not only to allow for the early detection of TMJ degenerative processes but also to enable reliable localization and detection of bony changes (Alexiou et al. 2009).
A substantial body of research covers the key factors associated with primary osteoarthritis. These factors include, but are not limited to, sex, repetitive stress, older age, obesity, and genetics (Musumeci et al. 2015). Previous studies, however, mostly analyzed the weight-bearing joint of the hip or knee, which are covered by hyaline cartilage. The TMJ differs from other joints in that the bone of the mandibular condyle is located just beneath the fibrocartilage, making it particularly vulnerable to inflammatory damage (Cevidanes et al. 2014). In addition, due to mechanical loading during growth, the mandibular condylar cartilage undergoes endochondral ossification and vigorously remodels (Kamiya et al. 2013; Owtad et al. 2013). Although the mandibular condyle is one of the most common sites of osteoarthritis (Griffin et al. 1979), there is a substantial deficit in its osteoarthritic disease profile.
Radiographic signs of TMJOA include deformation of the condyle due to subcortical cysts, surface erosion, osteophytes, generalized sclerosis, and loose calcified bodies (Ahmad et al. 2009). Upon searching for TMJOA classification methods, we found that the TMJOA grading system and diagnostic definitions developed by Ahmad et al. (Ahmad et al. 2009; Ahmad and Schiffman 2016) were up-to-date and comprehensive as a current standard in the field. Thus, we grouped and evaluated our patient CBCT images largely based on those standards.
Recently, there has been a renewed focus on the relationship between osteoporosis (low bone quality) and osteoarthritis. Most frequently studied in the hip joint (Lingard et al. 2010), knee joint (Akamatsu et al. 2009), and lumbar spine (Hart et al. 1994), the association between bone quality and osteoarthritis has been examined in a number of cross-sectional studies. Previous studies have suggested that osteoarthritis is directly associated with increased BMD and inversely related to osteoporosis (Hart et al. 1994). However, when individual bones were analyzed, the BMD of the appendicular skeleton in osteoarthritic joints was reported to decrease, especially within the upper extremities (Im and Kim 2014). It can thus be concluded that the relationship between osteoporosis and osteoarthritis is a complex one, dependent on both the site or stage of the disease and the progression of osteoarthritis (Felson and Nevitt 2004). To our knowledge, this is the first study that focuses specifically on the association between bone loss and osteoarthritis in the condylar bone of the TMJ complex.
In this study, we found that the adjusted OR (AOR) of CT HU was 0.9989 but that the AOR of BV/TV was 0.8096. Although the AOR of CT HU is very close to 1, it should be noted that a 1-point change in CT HU is not clinically meaningful due to the huge range of CT HU values (1,206.15–4,653.43). This is especially evident compared to the smaller range of BV/TV values (9.64–47.77). Consequently, a 1-unit change in CT HU is thus miniscule and rather meaningless, as is its associated OR. Instead, we can reexpress the effects of CT HU by estimating the OR for a change in CT HU from the value at the 25th percentile (2,344.13) to the value at the 75th percentile (3,100.75) (Ambrosius 2007; Harrell 2015). This OR is sometimes known as the interquartile OR (IOR) and can be calculated as IOR = ORIQR, where IQR is the interquartile range. The IOR for CT HU was estimated at 0.4206, while the IOR for BV/TV was estimated at 0.1769. Compared to ORs, the IORs provide a fairer and more meaningful comparison between the associations of CT HU and BV/TV with TMJOA. This is because IORs standardize the change in the predictors to represent a change that traverses half (50%) of the sample (Ambrosius 2007).
The smaller IOR for BV/TV suggests that it is overall a stronger predictor of TMJOA than CT HU. Several other results support this finding. First, we can similarly compute changes in predicted probabilities due to increasing CT HU and BV/TV values from the 25th to 75th percentile as we did for the ORs. The interquartile difference in predicted probabilities was estimated at −0.147 for CT HU and −0.334 for BV/TV, indicating steeper drops in probabilities for increasing BV/TV. The steeper curve for BV/TV can be seen in Figure 2G and H. In addition, the regions representing the predicted probabilities for the healthy and TMJOA groups from (mean – SD) to (mean + SD) do not overlap for BV/TV but do overlap for CT HU, once again suggesting that BV/TV is a better discriminator than CT HU. Finally, the AUC from the ROC analysis was higher for BV/TV alone than for HU alone.
There are 3 limitations to this study. First, the assessments of bone density using CBCT may still need further artifact corrections in the aspects of image lag, detector scatter, body scatter, and beam hardening even when using software correction. This is despite the fact that BMD based on CT HU was used as a diagnostic aid for osteoporosis in previous applications (Link et al. 2004; Liu et al. 2013) and that our CT HU analysis was calibrated through the method suggested by Liu et al. (2013). In addition, we have yet to identify a study that focuses on the relationship between the human condyle and CT and CBCT HU values using the same imaging protocol. In our study, we found that CT HU was not as critical and significant for predicting TMJOA as BV/TV. Further imaging studies using human samples are needed to overcome the challenges in the artificial correction of volumetric cone beam projections for quantitative measurements. Second, we were unable to obtain lumbar or femoral neck BMD records, which normally serve as diagnostic criteria for systemic osteoporosis. Therefore, the conclusion that TMJOA is associated with systemic osteoporosis cannot be established. However, according to previous studies (Yamada et al. 1997; Savic Pavicin et al. 2014), mandibular BMD is highly correlated with lumbar BMD, indicating that patients with systemic osteoporosis have a high rate of low condylar BMD and vice versa. Additional prospective studies examining TMJOA in patients diagnosed with osteoporosis are needed to confirm a relationship between systemic osteoporosis and TMJOA. Third, due to the limitations associated with cross-sectional studies, we were unable to definitively suggest a causal relationship between low condylar bone quality and osteoarthritis. Prospective studies will be needed to further elucidate the casual relationship between low condylar bone quality or osteoporosis and TMJOA.
In this study, our results suggest that condylar bone loss is significantly correlated with the development of TMJOA. Therefore, condylar CT HU and BV/TV may be used together as a potential diagnostic tool for detecting TMJOA. Clinicians should inspect not only the joint but also the condylar bone structure when evaluating patients for TMJOA, especially those presenting with TMD symptoms. Although there may be no notable changes in the bone, clinicians should bear in mind that low mandibular condylar bone quality is associated with TMJOA. Consequently, careful clinical evaluation of the condyle coupled with appropriate radiographic interpretation would thus be critical for the early detection of TMJOA.
Author Contributions
J. Shi, S. Lee, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; H.C. Pan, A. Mohammad, A. Lin, W. Guo, E. Chen, A. Ahn, J. Li, contributed to data acquisition, analysis, interpretation, critically revised the manuscript; J.H. Kwak, K. Ting, contributed to conception, design, data acquisition, analysis, interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
The authors thank Dr. Sotirios Tetradis, professor and chair of the Section of Oral and Maxillofacial Radiology at UCLA School of Dentistry, for his technical consultation and guidance in the experimental design; Drs. Hu Wang and Guangning Zhen at the Department of Oral and Maxillofacial Radiology of West China School of Stomatology, Sichuan University for advice on data set collection; and Jiyeon Chung from UCLA College of Letters and Science and Catherine Ding from UCLA School of Dentistry for technical support.
Footnotes
This work was supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) (grant K08DE026805), NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (grants R01 AR066782-01, AR068835-01A1, AR061399-01A1), University of California Los Angeles (UCLA)/NIH CTSI grant UL1TR000124, Center for the Advancement of Science in Space (CASIS) GA-2014-154, AAOF OFDFA award to J.H.K., and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (grant No. 2016M3A9E8941670). This study was also partly funded by National Natural Science Foundation of China to J.L. (grant No. 81500829).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
References
- Agresti A. 2013. Categorical data analysis. Hoboken (NJ): John Wiley. [Google Scholar]
- Ahmad M, Hollender L, Anderson Q, Kartha K, Ohrbach R, Truelove EL, John MT, Schiffman EL. 2009. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): development of image analysis criteria and examiner reliability for image analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 107(6):844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M, Schiffman EL. 2016. Temporomandibular joint disorders and orofacial pain. Dent Clin North Am. 60(1):105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akamatsu Y, Mitsugi N, Taki N, Takeuchi R, Saito T. 2009. Relationship between low bone mineral density and varus deformity in postmenopausal women with knee osteoarthritis. J Rheumatol. 36(3):592–597. [DOI] [PubMed] [Google Scholar]
- Akerman S, Kopp S, Rohlin M. 1988. Macroscopic and microscopic appearance of radiologic findings in temporomandibular joints from elderly individuals: an autopsy study. Int J Oral Maxillofac Surg. 17(1):58–63. [DOI] [PubMed] [Google Scholar]
- Alexiou K, Stamatakis H, Tsiklakis K. 2009. Evaluation of the severity of temporomandibular joint osteoarthritic changes related to age using cone beam computed tomography. Dentomaxillofac Radiol. 38(3):141–147. [DOI] [PubMed] [Google Scholar]
- Ambrosius WT. 2007. Topics in biostatistics. Totowa (NJ): Humana Press. [Google Scholar]
- Barghan S, Tetradis S, Mallya S. 2012. Application of cone beam computed tomography for assessment of the temporomandibular joints. Aust Dent J. 57(Suppl 1):109–118. [DOI] [PubMed] [Google Scholar]
- Boeddinghaus R, Whyte A. 2013. Computed tomography of the temporomandibular joint. J Med Imaging Radiat Oncol. 57(4):448–454. [DOI] [PubMed] [Google Scholar]
- Cevidanes LH, Walker D, Schilling J, Sugai J, Giannobile W, Paniagua B, Benavides E, Zhu H, Marron JS, Jung BT, et al. 2014. 3D osteoarthritic changes in TMJ condylar morphology correlates with specific systemic and local biomarkers of disease. Osteoarthritis Cartilage. 22(10):1657–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gupta T, Barasz JA, Kalajzic Z, Yeh WC, Drissi H, Hand AR, Wadhwa S. 2009. Analysis of microarchitectural changes in a mouse temporomandibular joint osteoarthritis model. Arch Oral Biol. 54(12):1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bont L, Van der Kuijl B, Stegenga B, Vencken L, Boering G. 1993. Computed tomography in differential diagnosis of temporomandibular joint disorders. Int J Oral Maxillofac Surg. 22(4):200–209. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Boering G, van der Kuijl B, Stegenga B. 1996. Hard and soft tissue imaging of the temporomandibular joint 30 years after diagnosis of osteoarthrosis and internal derangement. J Oral Maxillofac Surg. 54(11):1270–1280; discussion 1280–1281. [DOI] [PubMed] [Google Scholar]
- dos Anjos Pontual ML, Freire JS, Barbosa JM, Frazao MA, dos Anjos Pontual A. 2012. Evaluation of bone changes in the temporomandibular joint using cone beam CT. Dentomaxillofac Radiol. 41(1):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson DT, Nevitt MC. 2004. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 30(4):783–797, vii. [DOI] [PubMed] [Google Scholar]
- Flygare L, Rohlin M, Akerman S. 1992. Macroscopic and microscopic findings of areas with radiologic erosions in human temporomandibular joints. Acta Odontol Scand. 50(2):91–100. [DOI] [PubMed] [Google Scholar]
- Griffin CJ, Powers R, Kruszynski R. 1979. The incidence of osteo-arthritis of the temporomandibular joint in various cultures. Aust Dent J. 24(2):94–106. [DOI] [PubMed] [Google Scholar]
- Harrell F. 2015. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Switzerland: Springer International Publishing. [Google Scholar]
- Hart DJ, Mootoosamy I, Doyle DV, Spector TD. 1994. The relationship between osteoarthritis and osteoporosis in the general population: the Chingford study. Ann Rheum Dis. 53(3):158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, Sturdivant RX. 2013. Applied logistic regression. Hoboken, NJ: John Wiley. [Google Scholar]
- Im GI, Kim MK. 2014. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. 32(2):101–109. [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Chen J, Xu M, Utreja A, Choi T, Drissi H, Wadhwa S. 2013. Increased mandibular condylar growth in mice with estrogen receptor beta deficiency. J Bone Miner Res. 28(5):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman LC, Norton EC. 2009. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 44(1):288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisjane Z, Urtane I, Krumina G, Neimane L, Ragovska I. 2012. The prevalence of TMJ osteoarthritis in asymptomatic patients with dentofacial deformities: a cone-beam CT study. Int J Oral Maxillofac Surg. 41(6):690–695. [DOI] [PubMed] [Google Scholar]
- Lingard EA, Mitchell SY, Francis RM, Rawlings D, Peaston R, Birrell FN, McCaskie AW. 2010. The prevalence of osteoporosis in patients with severe hip and knee osteoarthritis awaiting joint arthroplasty. Age Ageing. 39(2):234–239. [DOI] [PubMed] [Google Scholar]
- Link TM, Koppers BB, Licht T, Bauer J, Lu Y, Rummeny EJ. 2004. In vitro and in vivo spiral CT to determine bone mineral density: initial experience in patients at risk for osteoporosis. Radiology. 231(3):805–811. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bäuerle T, Pan L, Dimitrakopoulou-Strauss A, Strauss LG, Heiss C, Schnettler R, Semmler W, Cao L. 2013. Calibration of cone beam CT using relative attenuation ratio for quantitative assessment of bone density: a small animal study. Int J Comput Assist Radiol Surg. 8(5):733–739. [DOI] [PubMed] [Google Scholar]
- Ludlow JB, Davies-Ludlow LE, Brooks SL, Howerton WB. 2006. Dosimetry of 3 CBCT devices for oral and maxillofacial radiology: CB Mercuray, Newtom 3G and I-CAT. Dentomaxillofac Radiol. 35(4):219–226. [DOI] [PubMed] [Google Scholar]
- Muir CB, Goss AN. 1990. The radiologic morphology of asymptomatic temporomandibular joints. Oral Surg Oral Med Oral Pathol. 70(3):349–354. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. 2015. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. 16(3):6093–6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton EC, Miller MM, Kleinman LC. 2013. Computing adjusted risk ratios and risk differences in Stata. Stata J. 13(3):492–509. [Google Scholar]
- Owtad P, Park JH, Shen G, Potres Z, Darendeliler MA. 2013. The biology of TMJ growth modification: a review. J Dent Res. 92(4):315–321. [DOI] [PubMed] [Google Scholar]
- Pahwa S, Bhalla AS, Roychaudhary A, Bhutia O. 2015. Multidetector computed tomography of temporomandibular joint: a road less travelled. World J Clin Cases. 3(5):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin EL, Rose RM. 1986. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. (213):34–40. [PubMed] [Google Scholar]
- Savic Pavicin I, Dumancic J, Jukic T, Badel T, Badanjak A. 2014. Digital orthopantomograms in osteoporosis detection: mandibular density and mandibular radiographic indices as skeletal BMD predictors. Dentomaxillofac Radiol. 43(7):20130366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman EL, Velly AM, Look JO, Hodges JS, Swift JQ, Decker KL, Anderson QN, Templeton RB, Lenton PA, Kang W, et al. 2014. Effects of four treatment strategies for temporomandibular joint closed lock. Int J Oral Maxillofac Surg. 43(2):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets JA. 1988. Measuring the accuracy of diagnostic systems. Science. 240(4857):1285–1293. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. 2008. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 87(4):296–307. [DOI] [PubMed] [Google Scholar]
- Tsiklakis K, Syriopoulos K, Stamatakis HC. 2014. Radiographic examination of the temporomandibular joint using cone beam computed tomography. Dentomaxillofac Radiol. 33(3):196–201. [DOI] [PubMed] [Google Scholar]
- Wang XD, Zhang JN, Gan YH, Zhou YH. 2015. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 94(5):666–673. [DOI] [PubMed] [Google Scholar]
- Yamada M, Ito M, Hayashi K, Sato H, Nakamura T. 1997. Mandibular condyle bone mineral density measurement by quantitative computed tomography: a gender-related difference in correlation to spinal bone mineral density. Bone. 21(5):441–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.