Abstract
Aim
To test the potential benefit of extending cognitive-behavioral therapy (CBT) relative to not extending CBT on long-term abstinence from smoking.
Design
Two-group parallel randomised controlled trial. Patients were randomized to receive non-extended CBT (n=111) or extended CBT (n=112) following a 26-week open-label treatment.
Setting
Community clinic in the USA.
Participants
219 smokers (mean age: 43 years; mean cigarettes/day: 18).
Intervention
All participants received 10 weeks of combined CBT + bupropion sustained release (bupropion SR) + nicotine patch and were continued on CBT and either no medications if abstinent, continued bupropion + nicotine replacement therapy (NRT) if increased craving or depression scores, or varenicline if still smoking at 10 weeks. Half of participants were randomized at 26 weeks to extended CBT (E-CBT) through week 48 and half to non-extended CBT (no additional CBT sessions).
Measurements
The primary outcome was expired CO-confirmed, seven-day point-prevalence (PP) at 52-week and 104-week follow-up. Analyses were based on intention-to-treat.
Findings
PP-abstinence rates at the 52-week follow-up were comparable across non-extended CBT (40%) and E-CBT (39%) groups [OR 0.99; 95% CI (0.55,1.78)]. A similar pattern was observed across non-extended CBT (39%) and E-CBT (33%) groups at the 104-week follow-up [OR 0.79; 95% CI (0.44,1.40)].
Conclusion
Prolonging cognitive-behavioral therapy from 26 to 48 weeks does not appear to improve long-term abstinence from smoking.
Keywords: smoking cessation, clinical trial, cognitive behavioral therapy, extended treatment, adaptive treatment
Introduction
Smoking cessation therapy is typically provided for 8–12 weeks. This paper presents results of an investigation on the efficacy of smoking cessation therapy when extended beyond the conventional 8–12-week treatment regimen. Adult smokers received 26 weeks of open-label treatment with cognitive behavior therapy (CBT) and pharmacotherapy (nicotine patch, bupropion SR, varenicline) followed by 26 weeks of CBT or brief telephone contact.
This investigation is of interest for several reasons. First, most cigarette smokers relapse following treatment (1). A recent systematic review and meta-analysis reported that average abstinence rates (6 months or more) were 17%, 19%, 27% and 31% for NRT, bupropion, varenicline and combination NRT, respectively (2, 3). Therefore, treatments are needed that enable more sustained effects on abstinence.
Second, research indicates drug addiction is a chronic, relapsing brain disorder requiring extended therapy and follow-up (4, 5). However, few studies of extended smoking cessation treatment have been conducted. NRT (6–9), bupropion (10–14), nortriptyline (15) and varenicline (16) have been examined with extended treatment protocols (16). Point-prevalence abstinence rates at 52 weeks for extended treatment ranged from 14–26% for NRT (8, 9), 28–55% for bupropion (12–14), 50% for nortriptyline (15) and 43% for varenicline (16).
We previously reported results from two trials of adult smokers examining extended treatment with open-label consisting of combination bupropion SR + NRT + CBT for 11–12 weeks (12, 13). In the first trial (12), overall point-prevalence abstinence rates in the extended group exceeded 40% at 24-week follow-up and 30% at 52-week follow-up. In the second trial (13), point-prevalence abstinence rates were higher for extended CBT compared to telephone support at 20-week follow-up (extended CBT: 45% vs telephone: 29%). Hall and colleagues examined an extended therapy protocol with bupropion SR and CBT for 12 weeks, then randomized to either 40 weeks of extended CBT combined with bupropion SR, 40 weeks of bupropion SR without CBT, 40 weeks of placebo, or no further treatment (14). The extended CBT plus bupropion SR group had superior point-prevalence abstinence rates than all other treatment groups out to 104-weeks (47%). However, another trial by these investigators randomized smokers to 40 weeks of CBT with or without extended NRT, 40 weeks of NRT alone, 40 weeks of CBT or no further treatment (7). The extended CBT without NRT produced superior abstinence rates out to 104-weeks (55%). Moreover, meta-analyzes reported in a recent Cochrane review suggested that extending the duration of combination CBT and pharmacotherapy can improve long-term (6-month or longer) abstinence rates (17).
Third, there is evidence treatment efficacy could be enhanced by combining more intensive interventions with self-help approaches, telephone counseling and methods for the rapid identification and treatment of smoking lapses (7–9, 12–14, 17–19). Each of these components was incorporated into the multi-factor treatment approach tested in this randomized clinical trial (RCT).
Fourth, we used an adaptive treatment approach to adjust pharmacotherapy based on self-reports of factors known to predict relapse; namely craving and depressive symptoms (20, 21). Adaptive treatment protocols have increased smoking cessation rates beyond conventional approaches for smokers not responding to pre-cessation NRT (20, 21) and when negative mood changes developed after initial treatment (22). As such, the present study used smokers’ self-reports of craving and depressive symptoms to guide the provision of pharmacotherapy.
The primary purpose of this trial was to determine whether patients would benefit from extended duration of CBT. Patients were randomized to receive either non-extended CBT or extended CBT following the adaptive treatment phase. The primary study endpoint was 7-day point-prevalence 104 weeks at which point the only factor that systematically differed between groups was duration of CBT.
Primary hypothesis
Extending CBT from 26 to 48 weeks should produce higher expired-air carbon monoxide (CO)-confirmed 7-day point-prevalence and prolonged abstinence rates at 52- and 104-week follow-ups in treatment-seeking smokers treated with open-label combination bupropion SR plus NRT.
Method
Study Design
A total of 223 cigarette smokers (18–65 years of age; smoking ≥ 10 cigarettes per day or 3.5 packs a week) were randomized between June 1, 2010 to January 22, 2013. The final sample size available for analyses was 219 because 2 participants died (causes unrelated to treatment) and 2 were withdrawn (tachycardia, threatening behavior). With over 100 participants per condition, the trial had 80% power at a two-tailed alpha of 0.05 to detect a difference in abstinence rates of at least 15% over a range of success probabilities (10%, 25% 104-week abstinence). The Stanford University Panel for the Protection of Human Subjects in Medical Research approved the study protocol.
The trial included open-label and extended treatment phases. Follow-ups were conducted at 52 and 104 weeks. The trial was funded by the National Institute on Drug Abuse and registered with clinicaltrials.gov.
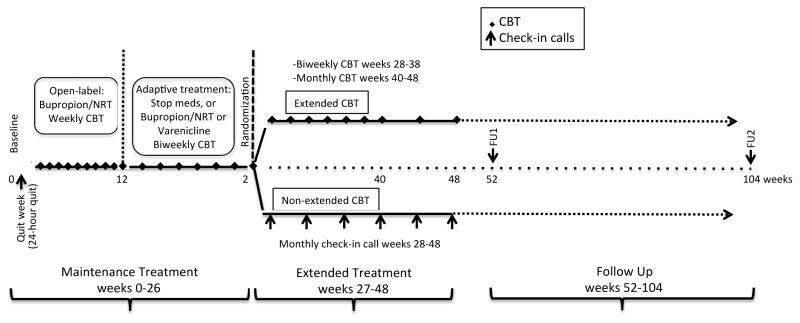
Participants were randomized to extended or non-extended treatment at Wk 1 after a successful 24 hour quit. The randomization was blinded and only revealed to participant and treatment staff after Wk 24 visit. Randomization assignments to extended treatment were generated following the adaptive treatment phase based on intention-to-treat (23). Males and females were randomized separately, using an algorithm that employed a weighted randomization scheme (13). Specifically, if there were equal numbers of males already randomized to the two conditions, the probability that the next male would be randomized to group 1 was 50%. If more males were already randomized to group 1, there was only a 33.33% chance the next participant would be randomized to group 1. If there were fewer males already randomized to group 1, then there was a 66.67% probability that the next participant would be randomized to group 1. The same method was used for females. Study staff and participants were blinded to randomized treatment assignment until the end of the open-label phase. Figure 1 illustrates the study design.
Figure 1. Timing of behavioral and pharmacological therapy and follow-up assessments.
CBT = cognitive behavioral therapy. Expired-air carbon monoxide was checked only for persons reporting abstinence. NRT = nicotine replacement therapy.
Open-label treatment (week 1 – 26)
Individualized, therapist-directed CBT was provided to all participants through week 26. Nicotine patch (21mg) and bupropion SR (300mg) was the standard treatment provided to all participants through week 10 with the exception of certain conditions (i.e., presentation of elevated systolic (≥170) or diastolic (≥110) blood pressure and/or heart rate ≥120, medication interaction or medical contraindication) as determined by the study physician; in which case participants were administered NRT only. At week 10, those who were abstinent (self-report + expired air carbon monoxide <10 parts per million (ppm)) and who reported low cravings and depressive symptoms were withdrawn from NRT and bupropion SR. Those who were abstinent but who reported high cravings or depressive symptoms were continued on NRT and a lower dose bupropion SR through week 26. At week 10, participants who continued to smoke set a new quit date and were switched to treatment with varenicline alone through week 26. Finally, participants who were withdrawn from NRT and bupropion SR but subsequently experienced increases in cravings or depression symptoms during open-label treatment were re-started on NRT and bupropion SR through week 26.
Extended treatment (week 27 – 52)
Half the sample received an additional 24 weeks of CBT (extended CBT; E-CBT) that included both therapist-directed, self-directed components, continued slip detection, and rapid treatment response. Remaining participants (non-extended CBT) received brief telephone calls to check abstinence status on a monthly basis from week 28 to week 52; no therapy was provided.
Study Population
Participants were recruited via advertisements on the radio, television, posted flyers, newspapers, ResearchMatch.org, referrals from local quit lines, advertisements on public transportation, and information tables at local companies. Interested smokers completed a telephone screen, and if eligible, were scheduled for an initial clinic visit where they signed consent forms, completed a structured clinical interview to detect major depression, suicide attempts, and drug abuse; the Modified Fagerström Tolerance Questionnaire (mFTQ) was also administered. The study psychiatrist determined eligibility for those with suicidal ideation. Basic physical exams were paid for by the study and reviewed by the study physician to determine eligibility.
Eligible participants returned to complete questionnaires, provide a refundable $50 deposit, set a quit date for approximately seven days from the baseline visit, and received study medications. The deposit was returned contingent on completion of follow-ups conducted at 26, 52 and 104 weeks. Participants received a $25 gift card upon completion of the 104-week follow-up. Payment was not contingent on successfully quitting smoking. Approval from the study medical director was required to be enrolled in the study. Expired-air CO was measured at all visits using the Bedfont Smokerlyzer (www.bedfont.com).
Screening
Individuals were excluded for pregnancy, current lactation, epilepsy, bipolar disorder, schizophrenia, or reporting current depression or substance abuse, history of heart problems in the previous six months, head trauma leading to unconsciousness in the past year, history of severe head injury resulting in brain surgery or specific neurological problems, current use of bupropion or NRT, or medication contraindicated for use with bupropion or NRT.
Treatments
Health educator training
Counseling was conducted by health educators, who were trained by a PhD-level clinical psychologist and supervised by a project manager, who received tobacco use treatment certification training. Treatment fidelity was evaluated by the clinical psychologist, psychiatrist, and project manager who met with health educators after patient visits (12, 13, 18, 24, 25).
Open-label: CBT
Intensive, manual-based, therapist-directed CBT with a thorough skills mastery experience was provided to participants during the open label phase of treatment. The therapy equipped smokers with the requisite self-regulatory skills to cope with the non-pharmacological factors that maintain cigarette smoking and precipitate relapse (25). Skills mastery is achieved most thoroughly through interventions that employ modeling, guided rehearsal and self-directed application of regulatory skills (29).
At each clinic session, staff met with participants individually for approximately 30 minutes to develop cognitive and behavioral skills to resist urges to smoke. Staff used self-efficacy questionnaires to assess participants’ confidence in their abilities to resist urges to smoke in specific situations and behavioral worksheets to help participants articulate treatment plans to be used in managing their behavior in these situations without smoking. All treatment sessions were led by trained health educators.
During maintenance treatment, intensive training was phased out and participants were shifted to self-directed intervention designed to bolster their confidence in using cognitive behavioral skills with minimal direct therapist aid or contact.
Open-label: pharmacotherapy
Participants received nicotine patch (21mg) and bupropion SR (300mg) through week 10. At week 11, they were switched to one of three groups based on smoking status, craving, and depressive symptoms: (1) No medication. Study medications were discontinued if participant reported not smoking in the past week, expired CO level <10 ppm, and craving and depressive symptoms did not increase from baseline; (2) Bupropion (150 mg) + NRT (21 mg). Participants in this group were continued on bupropion SR + NRT if self reported not smoking in the past week, expired CO level <10 ppm, but reported increased craving or depressive symptoms from baseline; (3)Varenicline. Participants were switched to varenicline and discontinued bupropion and NRT if expired CO level >10 ppm or participant self-reported relapse. A new quit date was set and varenicline was titrated up to 2 mg/day over the course of a week.
Participants in the no medication condition who reported a relapse, increased craving, or depressive symptoms during weeks 12–22 were reinstated on bupropion SR (150 mg) + NRT (21 mg). Those who reported increased craving or depressive symptoms at week 24 were reinstated on NRT (21 mg) through week 26.
Extended CBT (week 27–52)
Participants randomized to receive extended CBT continued to work with staff individually on the development and use of cognitive and behavioral cessation and relapse prevention skills. Treatment sessions were conducted at the San Jose clinic site in weeks 28, 30, 32, 34, 36, 38, 40, 44, 48. Participants made weekly “check-in” telephone calls to a voicemail system to report progress and any smoking or increased cravings or urges in the past 7 days. An affirmative response triggered a return telephone call for telephone-based skills training focused on identifying early relapse warning signs and assisting the participant in gaining cognitive and behavioral skills necessary to avoid relapse or setting new cessation goals and plans for those who had relapsed.
Control: Check-in phone calls
Participants randomized to the non-extended condition received a monthly phone call from the health educator during weeks 28 – 48 to assess smoking status.
Measures
Primary study end-point
The primary study end point was expired-air CO-confirmed 7-day point-prevalence (PP) abstinence evaluated at 52 and 104 weeks. This end-point measure is defined as a report of non-smoking (not even a puff) for seven consecutive days prior to contact plus an expired-air CO level <10 ppm. At all clinic and phone contacts smoking status was determined via the query “Have you smoked a cigarette, even a puff, in the past week?” Expired-air CO was measured at all clinic visits with the Bedfont EC50 Smokerlyzer (Bedfont Scientific Ltd).
Secondary study end point
The secondary end point was prolonged abstinence (PA), defined as self-reported non-smoking following a 2-week grace period. Treatment failure was operationalized as smoking for 7 consecutive days or ≥ 1 day in each of 2 consecutive weeks. Self-reported smoking status was assessed by the question; “Since your first quit date of the study, have you smoked, even a puff, 7 days in a row?”
Individuals who did not provide biochemical verification were classified as smokers with the exception of individuals who moved out of the area during the post-treatment follow-up period.
Measures of Nicotine Dependence
Craving
Craving was measured by taking the average response to the questions: ‘Have you felt cravings for a cigarette?’ and ‘Have you felt strong urges to smoke?’ Participants rated how upsetting their cravings had been in the past 24 hours on a scale from “not upsetting” to “extremely upsetting” (26). Craving was measured through week 26.
Center for Epidemiological Studies Depression scale
The CES-D, a 20-item self-report scale designed to measure depressive symptomology, was administered through week 26 (27).
Modified Fagerström Tolerance Questionnaire (mFTQ)
This 5-item questionnaire was used to quantify level of nicotine dependence. Higher scores indicated greater dependence (28). This measure was taken at baseline.
Self-efficacy
Self-efficacy was measured using a 17-item instrument adapted from Baer, Holt, and Lichtenstein (29). Participants rated their level of confidence in resisting the urge to smoke if they were to quit at that moment. Ratings were made on a scale from 0% – 100%. This measure was taken at baseline.
Physiological measures
Heart rate and blood pressure were measured twice at each clinic visit during the open-label phase with an automated blood pressure device (DINAMAP XL 9300, Johnson & Johnson Medical, Inc). All blood pressure levels were reported to participants and those with elevated levels were advised to see their physicians. Height and weight were also recorded.
Adverse events
Adverse events related to use of study medication was assessed at each visit or by phone during open-label treatment. The study medical director or psychologist reviewed reports of serious adverse events; medication was adjusted accordingly.
Medication compliance
At each assessment during open-label treatment, participants were asked if they were “wearing a patch now”, if they had “taken [their] bupropion [or “Zyban”] in the morning”, and if they had “taken [their] bupropion [or “Zyban”] every day in the past week”. Any deviations in medication compliance from protocol were recorded including date of and reason for deviation.
Analyses
α = 0.05.
Adjusting co-variates
Multicollinearity diagnostics were performed on potentially confounding prognostic characteristics assessed at baseline. Predictors were standardized and entered in a simultaneous multiple linear regression to obtain VIF statistics and eigenvalues. Predictors with large proportions of variation for small eigenvalues tend to be highly correlated and were considered for exclusion or recombination. Predictors identified as independent were used as adjusting co-variates in outcome analyses. Baseline variables identified a priori as having prognostic strength were included whether the imbalances between randomized groups were statistically significant.
Abstinence outcomes
Analyses on abstinence were compared as randomized (intention-to-treat—ITT). Potential effects of E-CBT vs non-extended CBT were tested using the OR with 95% CI. Adjusting co-variate logistic regressions tested the effect of treatment for each follow-up time point.
Adverse event incidences were compared using χ2 tests.
Results
Study Sample
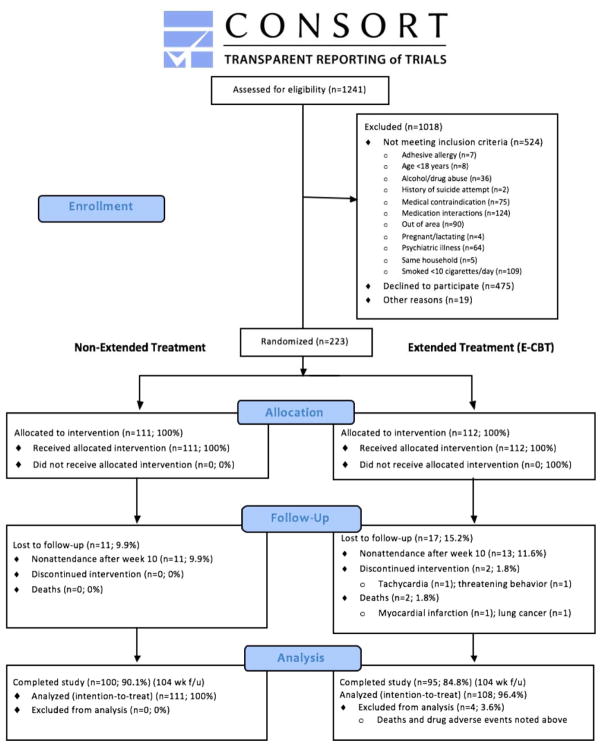
Study staff contacted 1241 individuals interested in participating (see Figure 2 for CONSORT diagram). After learning more about the study 766 remained interested; of these, 223 were randomized. 219 participants were included in the intent-to-treat analysis (E-CBT: n=108; non-extended CBT: n=111) because 2 participants died (causes unrelated to treatment) and 2 were withdrawn (tachycardia, threatening behavior). The ethnic/racial distribution was 78% white (n=171), 3% African-American (n=6), 8% Asian (n=18), 1% American Indian/Alaska native (n=3), 4% more than one race (n=10) and 5% unknown/other (n=8). There was no evidence of a significant difference in follow-up rates between treatment groups, p>0.05.
Figure 2.
Consort diagram
Adjusting co-variates
Table 1 shows baseline measures considered for adjusting in outcome analyses. mFTQ, expired-air CO, and cigarettes per day were highly correlated and were linearly combined to form a single indicator of recent smoking behavior. Adjusting covariates were as follows: age, BMI, CES-D, craving, self-efficacy, sex, recent smoking behavior.
Table 1.
Participant characteristics by group (N=219)
| Non-extended CBT N=111 |
E-CBT N=108 |
|||
|---|---|---|---|---|
|
|
|
|||
| M | SD | M | SD | |
|
| ||||
| Age (years) | 42.1 | 12.1 | 44.0 | 11.6 |
| Cigarettes/day | 16.7 | 5.9 | 18.1 | 7.3 |
| Craving | 3.1 | 1.6 | 3.1 | 1.5 |
| Self-efficacy | 5.6 | 1.7 | 5.5 | 2.0 |
| Expired-air COb | 19.1 | 8.0 | 20.2 | 10.3 |
| mFTQc | 14.7 | 2.6 | 14.5 | 3.5 |
| CES-De | 9.7 | 7.3 | 10.4 | 8.1 |
| BMIf | 27.5 | 6.1 | 27.9 | 5.9 |
Note. All measures were collected at the initial baseline visit except for exhaled CO, CES-D & self-efficacy (week 0/pre-quit) & craving (week 1).
extended cognitive-behavioral therapy
standard deviation
carbon monoxide
modified Fagerström Tolerance Questionnaire
Center for Epidemiological Studies-Depression scale
body mass index
Abstinence during open-label treatment
There were no significant group differences in PP abstinence at 10 weeks [OR 0.98; 95% CI (0.56, 1.72)]. Table 2 shows the 26-week PP abstinence rates for participants allocated to (1) varenicline, (2) bupropion SR + NRT, (3) no medication or (4) discontinuation of medication and then reinstatement of bupropion and NRT; the treatment by drug group interaction was not statistically significant, χ(1)2 = 3.69, p=0.30.
Table 2.
Adaptive treatment allocation and point prevalence abstinence at 26 weeks
| Abstinence rate | % abstinent | p value | |
|---|---|---|---|
|
|
|||
| No medication | 23/38 | 60% | <0.01 |
| Bupropion + NRTa | 53/89 | 60% | <0.01 |
| Vareniclineb | 10/58 | 17% | |
The table shows expired-air carbon monoxide (<10 ppm) at week 26 by medication condition during the adaptive pharmacotherapy phase. Smokers who achieved initial abstinence at 10 weeks received No Medication.
Bupropion + NRT was prescribed if craving or depression had persisted through the open-label and adaptive treatment phases.
Smokers who did not achieve abstinence at 10 weeks were placed on Varenicline, which was the reference group in above analysis.
Chi-square for difference in abstinence between drug groups are reported.
Abstinence outcomes
Table 2 shows no benefit of extending CBT on any primary or secondary outcome measure. PP-abstinence rates at the 52-week follow-up were comparable across non-extended CBT (40%) and E-CBT (39%) groups [OR 0.99; 95% CI (0.55,1.78)]. Similar results were obtained for PP-abstinence rates at the 104-week follow-up across CBT (39%) and E-CBT (33%) groups [OR 0.79; 95% CI (0.44,1.40)]. Unadjusted analyses yielded similar results.
Reclassification
Of self-reported non-smokers, 100%, 81%, 84% and 84% provided biochemical confirmation of abstinence at 10, 26, 52 and 104 weeks, respectively. Those who reported abstinence but failed to provide breath samples for carbon monoxide verification were reclassified as smokers with the following exceptions. The number of participants who had moved out of the area but self-reported abstinence without CO verification were: Week 52: non-extended CBT: n = 4, E-CBT: n = 5; Week 104-non-extended CBT: n = 3, E-CBT: n = 8.
Medication compliance
Weeks 1–10
89% of participants responded ‘yes’ when asked if they had taken their bupropion SR pill at one or more visits and 98% reported using their nicotine patch at one or more visits. The mean number of visits (out of 10) when participants reported taking bupropion was 5.8 (standard deviation (SD): 3.6) and NRT was 6.1 (2.9) in the non-extended group, and 6.1 (3.6) and 6.2 (3) in the E-CBT group, respectively.
Weeks 11–26
Amongst individuals continued on initial treatment, 69% of participants reported taking their bupropion SR pill at one or more visits, 79% reported wearing the patch at one or more treatment session. Regarding participants switched to varenicline, 82% reported taking the medication at one or more treatment sessions. The mean number of visits (out of 9) when participants reported taking bupropion was 1.6 (2.4), NRT was 1.5 (2.3) and varenicline was 3.3 (2.6) in the non-extended group, and 2.4 (2.9), 2.3 (2.8) and 2.9 (2.5) in the E-CBT group, respectively.
CBT session attendance
Weeks 1–26
The non-extended CBT group attended an average of 12.5 sessions while the E-CBT group attended an average of 12.4 sessions (5.3) of the 19 CBT sessions.
Weeks 27–48
The E-CBT group attended an average of 2.8 (3.1) of the 9 sessions.
Adverse Events
One individual was removed from the trial after an episode of tachycardia. The most frequent adverse events in weeks 1–10 were anxiety, dry mouth and insomnia, all which were more common in males. Adverse events were less common in weeks 11–26. There were no statistically significant differences in adverse events between treatment groups (ps > 0.05).
Discussion
The present study tested whether extending CBT from 26 to 48 weeks would enhance long-term abstinence rates. Although we did not find direct support for this hypothesis, the average long-term quit rates observed in the present study were higher than those observed in trials of brief behavioral interventions lacking maintenance treatment and were comparable to studies of maintenance treatment extended up to 26 weeks (30). Based on results from a meta-analysis by the Cochrane Collaboration, estimates of abstinence rates following pharmacological treatment combined with behavioral treatment range from 17% to 31% for different combinations of pharmacotherapies (NRT, bupropion SR, combination NRT, varenicline) at 26 weeks; our treatment produced abstinence rates near 40% and for a longer duration (2).
One explanation for a lack of effect of extended-CBT treatment could be that regular, intensive, in-person CBT beyond about six months produced treatment fatigue and diminished returns of ongoing treatment. The finding that the E-CBT group attended 65% of sessions during weeks 1–26 compared to 31% in weeks 27–48 is consistent with this hypothesis. This account is also consistent with extant trials reporting reduced attendance with extended CBT (14) and declines in efficacy resulting from the combination of multiple behavioral treatments beyond brief interventions (31).
Participants in the extended CBT group may have derived additional benefited from extended pharmacotherapy treatment. Indeed, Hall and colleagues found that smokers who received 12 weeks of combined bupropion SR + NRT followed by 40 weeks of extended CBT and bupropion SR had somewhat higher 104-week abstinence rates than participants in our study. However, other trials have reported when active drug was administered in combination with CBT, effects were lost upon medication termination. Hall and colleagues (7) have also reported extending NRT did not add to the efficacy of CBT. Collectively, these results suggest pharmacotherapies might have a time sensitive threshold of effectiveness. However, the aforementioned studies used standard pharmacotherapy protocols and thus, the potentially positive effects of extending adaptive pharmacotherapy before being switched to non-pharmacological treatments are unknown.
Hall and colleagues found that although patients assigned to extended CBT + active bupropion SR or extended CBT + placebo produced better long-term abstinence rates than standard treatments, they did not differ from one another (14). The authors suggested periodic check-ins might be as effective as an active drug and counseling. Indeed, such an explanation could account for why we did not detect an effect of extending CBT; check-up calls in the control group through week 48 may have been sufficient to maintain abstinence. It is surprising abstinence rates in the present RCT were comparable to those obtained with more aggressive extended treatments.
It is difficult to speak to how this study might inform treatment of lighter smokers because (1) we excluded lighter smokers from the study and (2) there was no relationship between smoking and relapse rates, ps>0.05. Nonetheless, the aggressive treatment plan used in the present study may not be suited for those with lower smoking rates.
Future clinical trials should bear in mind the importance of testing for predictors of treatment failure. For instance, combination treatment studies should better attend to the relationship between medication non-compliance versus CBT nonattendance, how this differs by treatment phase, and for which sub-groups.
In sum, our study suggests CBT treatment beyond 26 weeks does not increase long-term abstinence rates. This could be explained by reduced compliance with extended CBT owing to treatment fatigue. Alternatively, there could be some time threshold wherein additional treatment is not efficacious.
Table 3.
Point-prevalence and prolonged abstinence rates
| Primary Outcome PPb | Non-extended-CBT | E-CBTa | Odds Ratio (95% CI) | ||
|---|---|---|---|---|---|
|
| |||||
| N=111 | N=108 | ||||
| Week 52 | 40% | n=44 | 39% | n=42 | 0.99(0.55,1.78) |
| Week 104 | 39% | n=43 | 33% | n=36 | 0.79(0.44,1.40) |
|
Secondary Outcome PAc
|
|||||
| Week 52 | 35% | n=39 | 31% | n=33 | 0.79(0.43,1.47) |
| Week 104 | 27% | n=30 | 24% | n=26 | 0.87(0.45,1.68) |
Extended treatment group.
7-day Point-prevalence Abstinence (PP) and
Prolonged Abstinence (PA) at 52 and 104 weeks (N=219).
Acknowledgments
The authors of this paper thank Susan W. Bryson for her statistical contribution to the study and dedicate this paper in her memory. We also wish to acknowledge the assistance of Michelle Fujimoto, Connie Watanabe and Sally McCarthy.
This study was funded by grant R01 DA-017441 by the National Institute on Drug Abuse. The first author was supported by a NIDA T32 DA-035165 post-doctoral fellowship. Pfizer provided study medication, varenicline (Chantix™), after the protocol was approved by the Stanford University Institutional Review Board.
Footnotes
The authors declare no conflicts of interest.
Trial Registration: clinicaltrials.gov Identifier: NCT01330043.
Article Information
Author Contributions: JDK conceived the study design. JDK and SPD led the study team. AL and SPD were study physicians. AV and JL conducted statistical analyses. EC and SRB were project managers. DK assisted with data management. All authors contributed to manuscript preparation and final review.
References
- 1.Smith AL, Chapman S. Quitting smoking unassisted: the 50-year research neglect of a major public health phenomenon. JAMA. 2014;311:137–138. doi: 10.1001/jama.2013.282618. [DOI] [PubMed] [Google Scholar]
- 2.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311:193–194. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Tonnesen P, Paoletti P, Gustavsson G, Russell MA, Saracci R, Gulsvik A, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation European Respiratory Society. Eur Respir J. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 7.Hall SM, Humfleet GL, Munoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction (Abingdon, England) 2009;104:1043–1052. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, et al. Long-term nicotine replacement therapy: a randomized clinical trial. JAMA internal medicine. 2015;175:504–511. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Annals of internal medicine. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation a randomized, controlled trial. Annals of internal medicine. 2001;135:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- 11.Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 12.Killen JD, Fortmann SP, Murphy GM, Jr, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. Journal of consulting and clinical psychology. 2006;74:286–294. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 13.Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction (Abingdon, England) 2008;103:1381–1390. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall SM, Humfleet GL, Munoz RF, Reus VI, Prochaska JJ, Robbins J. A Using extended cognitive behavioral treatment and medication to treat dependent smokers. American journal of public health. 2011;101:2349–2356. doi: 10.2105/AJPH.2010.300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry. 2004;161:2100–2107. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 16.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. Jama. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 17.Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killen JD, Fortmann SP, Davis L, Strausberg L, Varady A. Do heavy smokers benefit from higher dose nicotine patch therapy? Exp Clin Psychopharmacol. 1999;7:226–233. doi: 10.1037//1064-1297.7.3.226. [DOI] [PubMed] [Google Scholar]
- 19.Ockene JK, Mermelstein RJ, Bonollo DS, Emmons KM, Perkins KA, Voorhees CC, et al. Relapse and maintenance issues for smoking cessation. Health Psychology. 2000;19:17. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- 20.Collins LM, Murphy SA, Bierman KL. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5:185–196. doi: 10.1023/b:prev.0000037641.26017.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SA. An experimental design for the development of adaptive treatment strategies. Stat Med. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 22.Issa JS, Abe TO, Moura S, Santos PC, Pereira AC. Effectiveness of coadministration of varenicline, bupropion, and serotonin reuptake inhibitors in a smoking cessation program in the real-life setting. Nicotine Tob Res. 2013;15:1146–1150. doi: 10.1093/ntr/nts230. [DOI] [PubMed] [Google Scholar]
- 23.Greenhouse JB, Stangl D, Kupfer DJ, Prien RF. Methodologic issues in maintenance therapy clinical trials. Arch Gen Psychiatry. 1991;48:313–318. doi: 10.1001/archpsyc.1991.01810280029004. [DOI] [PubMed] [Google Scholar]
- 24.Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, et al. Nicotine patch and paroxetine for smoking cessation. Journal of consulting and clinical psychology. 2000;68:883–889. [PubMed] [Google Scholar]
- 25.Killen JD, Fortmann SP, Murphy GM, Jr, Hayward C, Fong D, Lowenthal K, et al. Failure to improve cigarette smoking abstinence with transdermal selegiline + cognitive behavior therapy. Addiction (Abingdon, England) 2010;105:1660–1668. doi: 10.1111/j.1360-0443.2010.03020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Killen JD, Fortmann SP, Newman B, Varady A. Prospective study of factors influencing the development of craving associated with smoking cessation. Psychopharmacology (Berl) 1991;105:191–196. doi: 10.1007/BF02244308. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Kozak J, Fagerstrom KO. High-dose treatment with the nicotine patch. Int J Smok Cessation. 1995;4:26–28. [Google Scholar]
- 29.Baer JS, Holt CS, Lichtenstein E. Self-efficacy and smoking reexamined: construct validity and clinical utility. Journal of consulting and clinical psychology. 1986;54:846–852. doi: 10.1037//0022-006x.54.6.846. [DOI] [PubMed] [Google Scholar]
- 30.Hajek P, Tonnesen P, Arteaga C, Russ C, Tonstad S. Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction (Abingdon, England) 2009;104:1597–1602. doi: 10.1111/j.1360-0443.2009.02646.x. [DOI] [PubMed] [Google Scholar]
- 31.Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction (Abingdon, England) 2016;111:129–141. doi: 10.1111/add.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]