Abstract
Chloroquine (CQ) is highly effective against P. vivax, due to the rapid spread of CQ resistance in P. falciparum parasites; it is no longer the drug of choice against P. falciparum. This study elucidates the scenario of chloroquine efficacy at times that coincided with a new drug policy and especially assessed the chloroquine resistant molecular markers after withdrawal of chloroquine in Kolkata and Purulia, two malaria endemic zones of West Bengal, India. In vitro CQ susceptibility was tested in 781 patients with P. falciparum mono infections between 2008 and 2013, of which 338 patients had received CQ in 2008–2009. Genotyping of the pfcrt and the pfmdr1 gene was carried out in all isolates. Early treatment failure was detected in 114 patients {43 (31·39%) from Kolkata and 71 (35·32%) from Purulia} while recrudescence was identified in 13 (9.49%) and 17 (8.46%) patients from Kolkata and Purulia respectively. In vivo chloroquine resistance was strongly associated with CVMNT-YYSNY (p < 0.01) and SVMNT-YYSNY (p < 0.05) allele in Kolkata. In Purulia chloroquine resistance was associated with CVMNK-YYSNY (P < 0.005), SVMNT-YYSNY (P < 0.01) allele. The proportion of in vitro chloroquine resistance increased in subsequent years to 87.23% and 93·10% in 2013, in Kolkata and Purulia, respectively. Isolates with SVMNT-YFSND, SVMNT-YFSNY, CVIET-YFSND and CVIET-YYSNY haplotypes increased gradually (p < 0.05) from 2010 to 2013, leading to a rise in IC50 (p < 0.05) of chloroquine. An increase in in vitro chloroquine resistance and candidate gene mutations even after five years of chloroquine withdrawal against P. falciparum calls for synchronized research surveillance and proper containment strategies.
Keywords: Plasmodium falciparum, ChloroQuine resistance in India, pfcrt polymorphism, pfmdr1 mutation, In vitro chloroquine resistance
Graphical abstract
Highlights
-
•
Unremitting increase in pfcrt and pfmdr1 polymorphism without CQ drug pressure.
-
•
In vitro CQ resistance was still increase after 5 years of ACT implementation.
-
•
Regional bias in pfcrt and pfmdr1 polymorphism associates CQ resistance.
-
•
Irresponsive self medication (CQ) by civilians made the situation worse day by day.
1. Introduction
Chloroquine (CQ) is cheap, well tolerated and easily administered and has been used as an antimalarial against P. falciparum for more than five decades (Sehgal et al., 1973), but the progressive development of resistance has led to the replacement of CQ by the combination of Artesunate + Sulfadoxine-Pyrimethamine {Artemisinin Combination Therapy (ACT)} in mid 2009 (Government of India, 2009). CQ efficacy was found to be declining in different states of India around 2000. In 1990 only 5·2% of isolates possessed CQ resistance in Madhya Pradesh, which increased to 53% at neighboring state Uttar Pradesh in 2005 (Singh and Shukla, 1990, Wijeyaratne et al., 2005). High rates of CQ treatment failure were also reported in other parts of India such as Orissa and Assam (Satpathy et al., 1997, Dua et al., 2003). The genetic basis of CQ-resistance has now been well studied in P. falciparum. P. falciparum multidrug resistance 1 (pfmdr1) gene located on chromosome 5 encodes a transporter for importing solutes, including different drugs (CQ, quinine, mefloquine) into the food vacuole. Polymorphisms’ leading to the substitution of asparagine with tyrosine at codon 86 of pfmdr1 gene reduces CQ influx into the food vacuole, resulting in CQ resistance (Foote et al., 1990). Some other pfmdr1 polymorphisms, like Y184F, S1034N, N1042D and D1246Y, were implicated in varying degrees to CQ resistance (Duraisingh et al., 2000, Andriantsoanirina et al., 2010). P. falciparum CQ resistance transporter (pfcrt), a key gene for CQ resistance, was identified on chromosome 7. Specific point mutation in 72–76 codon of PFCRT protein promoted CQ resistance by efflux out the CQ from food vacuole (Fidock et al., 2000, Djimdé et al., 2001, Mehlotra et al., 2001, Sharma, 2005).
In 1993, Malawi became the first country in Africa to replace CQ by sulfadoxine-pyrimethamine (SP) (Bloland et al., 1993), and subsequently CQ-resistant mutant pfcrt and pfmdr1 alleles decreased till they became undetectable in 2001, suggesting that CQ might once again be effective. Finally, CQ was reintroduced after 12 years of withdrawal (Laufer et al., 2006) in Malawi. Thus withdrawing the use of CQ in CQ resistant region could result in the re-emergence of CQ-sensitive parasite. In such settings, the molecular markers of CQ-resistance needed to be determined after the introduction of ACT in India. Therefore, the present investigation was conducted to evaluate CQ efficacy prior to ACT treatment and also to assess the CQ resistant molecular markers after withdrawal of CQ in Eastern India.
2. Materials and method
2.1. Study site
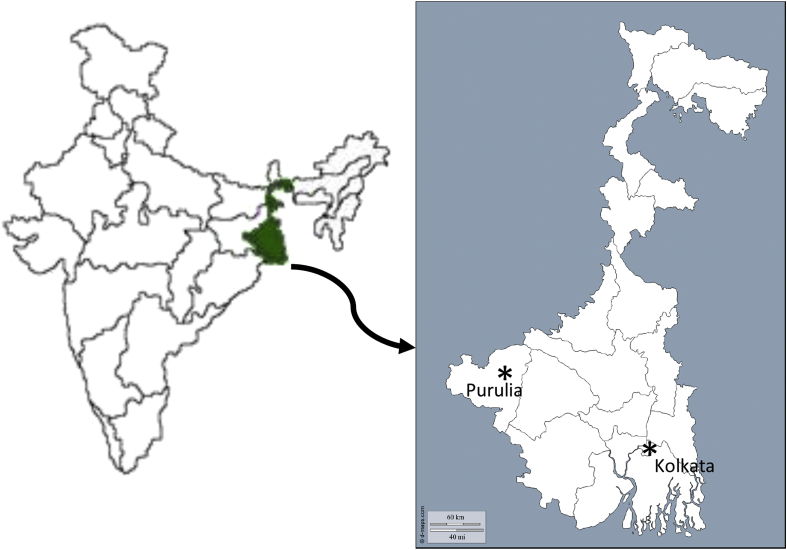
This study deals with the clinical assessment of chloroquine resistance (in vivo as well as in vitro) in India from February 2008 to December 2013, before and after introduction of ACT. Blood samples were collected from Kolkata {Goutam Laboratory (NABL accredited laboratory, ISO 15189:2007-M-0423)}, and Purulia (Purulia district hospital) two highly malaria endemic regions of India. In 2010, there were 134795 cases of malaria in West Bengal. The Kolkata Metropolitan Corporation contributes 96693 (71.73%) malaria cases (both P. falciparum and P. vivax) whereas Purulia contributed more than 75% of P. falciparum infection (Fig. 1) (Annual District wise Epidemiological Report of Malaria of West Bengal, 2006, Annual District wise Epidemiological Report of Malaria of West Bengal, 2010). The experimental design and protocol of this study were duly approved by Vidyasagar University Ethical Committee. Written informed consent was obtained from parents or guardians for child patients.
Fig. 1.
Geographical map of the study sites. Kolkata and Purulia are shown as asterisks.
2.2. Patients’ selection and in vivo CQ treatment
A standard 28-day test of therapeutic efficacy was used (WHO, 2003) to assess treatment of P. falciparum infection in 2008–2009. Clinical isolates collected from 2010 to 2013 were only tested for in vitro CQ susceptibility and polymorphisms in different candidate genes. Patients suffering from fever (body temperature >37.5 °C) with headache, shivering, and vomiting tendency during previous 24 h s were tested for malaria. Two ml of intravenous blood was collected from each of 5210 suspected patients, in an anticoagulant coated (EDTA) vacutainer. Patients with positive rapid diagnostic test results and microscopically confirmed P. falciparum malaria with a parasite density of 1000–200000 asexual parasites/μl blood and no recent history of self-medication with antimalarial drugs received the standard dose of 10 mg/kg CQ on day 1 and day 2, 5 mg/kg CQ on day 3. Study drugs were purchased from standard commercial sources (Resochin; Bayer) and administered under direct observation by trained study nurses. The clinical conditions, hemoglobin and parasite density were monitored on days 0, 1, 2, 3, 7, 14 and 28 (WHO, 2003). Unscheduled follow-up visits were performed at any time between scheduled visits when symptoms of malaria recurred. Patients who vomited the drug (CQ) twice were withdrawn from the study and transferred to Kolkata National Medical College hospital for further care. Patients with signs and symptoms of severe and complicated malaria, pregnant women, lactating mothers, children below the age of 3 years and those with hematocrit <20% were excluded from the study. Therapeutic responses were classified as adequate clinical parasitological response (ACPR) (absence of parasitaemia after day 28 of drug administration; symptoms of malaria subside by day 3, parasitaemia gradually declines and disappears by day 3), early treatment failure (ETF) (Irrespective of axillary temperature, patients having parasitaemia on day 2 higher than from day 0; or parasitaemia on day 3 > 25% of count on day 0 with axillary temperature >37.5 °C) and late treatment failure (LTF) (Recrudescence of parasite within 4–28 days) according to WHO guideline (WHO, 2003). Patients who did not respond to CQ treatment were treated with Artesunate + SP combination.
2.3. In vitro CQ susceptibility
In vitro drug sensitivity assay was performed in all clinical isolates after culture adaptation as a part of the antimalarial susceptibility surveillance during 2008–2013, as described earlier (Trager and Jensen, 1976, Basco and Ringwald, 2000). A synchronized parasite culture was maintained over at least three life cycles prior to drug (CQ) exposure. RPMI1640 was used to prepare stock solutions and dilutions of CQ (Sigma). The IC50 was evaluated using hypoxanthine incorporation assay following our established laboratory protocol (KarMahapatra et al., 2011, Das et al., 2014). Isolates were defined as CQ susceptible when IC50 values were ≤100 nM, and CQ-resistant when IC was >100 nM. The CQ-sensitive strain 3D7 and CQ-resistant strain Dd2 were used as controls.
2.4. DNA extraction, genotyping of candidate gene
Parasite DNA was extracted from 1 ml of infected blood using the phenol-chloroform extraction method as described elsewhere (Basco and Ringwald, 2000). Regions of the pfcrt and pfmdr1 genes surrounding the polymorphism of interest were amplified by polymerase chain reaction using an Eppendorf thermal cycler. Primers were designed as described in our previous laboratory work (Das et al., 2014). Different polymorphisms of pfcrt and pfmdr1 gene were analyzed as described earlier (Lopes et al., 2002, Das et al., 2013, Das et al., 2014). 3D7 and Dd2 strains served as controls. Sequencing was carried out using an ABI Prism Big Dye Terminator cycle sequencing ready reaction kit and run on a model 3730 xl genetic analyzer (Applied Biosystems) (Das et al., 2014). Sequencing was performed at IIT, Kharagpur, and SciGenome Company (Kochi) for cross validation. Sequences were translated using an online translation tool (http://www.expasy.org) and aligned using the multiple sequence alignment tool ClustalW2 (http://www.ebi.ac.uk/clustalw). Mutations were confirmed by reading forward and reverse strands.
2.5. Assessment of isolate clonality
An allelic family-specific nested PCR was used to identify the multiplicity of infection i.e. the highest number of alleles detected in either of the two loci (MAD20 and K1 for pfmsp1 and 3D7 Africa and FC27 for pfmsp-2) (Snounou et al., 1993). It was used to classify the isolates as monoclonal or polyclonal infection and distinguish recrudescence from new infection for all patients failing therapy after day seven (isolates from day 0 and day of recurrence). All amplifications contained a positive control (genomic DNA from strain 3D7) and a negative control (no target DNA).
2.6. Evaluation of antimalarial drug pressure
Cross-sectional surveys were carried out from June 2008 to August 2008 and April 2012 to September 2012 in Kolkata and Purulia. A total of 1440 individuals were interviewed (720 individual each from Kolkata and Purulia). Forty households were randomly selected from three zonal blocks (rural, semi rural and urban division) in each districts, with different socio-economical status. Three randomly selected individuals (age range 19–75) from each household were interviewed about their recent travel and antimalarial drug consumption, as described elsewhere (Gardella et al., 2008).
2.7. Statistical analysis
IC50 values of CQ were expressed as mean ± standard deviation (SD). The relation between treatment efficacies and molecular genotypes was studied by Fisher's exact test and regression analysis. IC50s were expressed as the geometric mean (95% confidence intervals). The Mann-Whitney U test was used to compare between two groups, and the Kruskal-Wallis-test (H-test) was used to compare between more than two groups. Differences were considered statistically significant when p < 0·05. Analyses were performed using the statistical package Origin 6.1, and GraphPad In Stat software 3.0.
3. Results
3.1. Study population
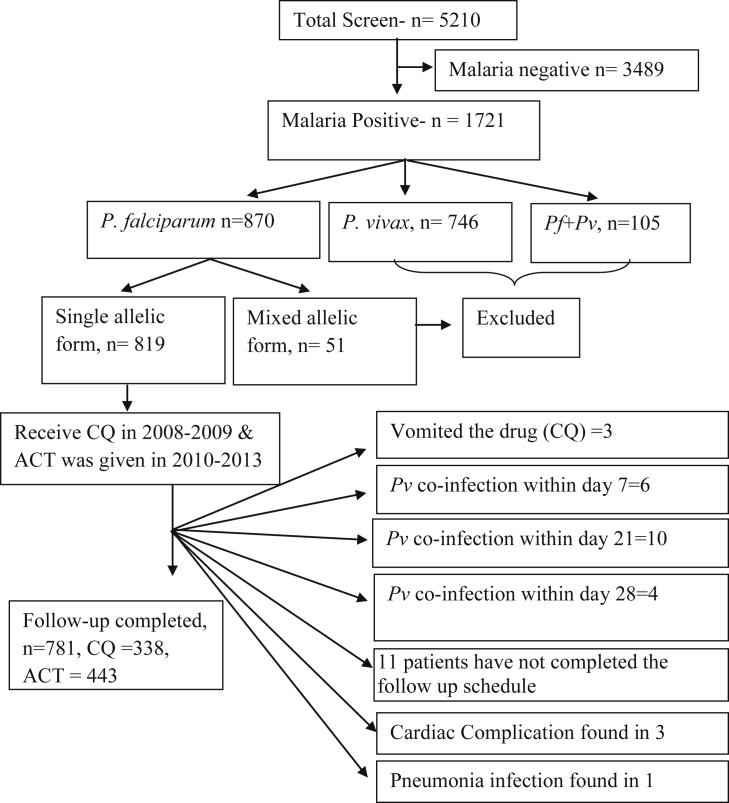
Malaria infection was screened in 5210 suspected cases between 2008 and 2013, of which 1721 patients (33.03%) found malaria positive cases. A total of 870 patients (50.55%) were identified as P. falciparum positive isolates. 746 patients (43.35%) were excluded, as they contained P. vivax infection. Another 105 patients were eliminated due to co-infection with P. vivax. These co-infections were only found in Kolkata. Multiplicity of infection was analyzed for a subset of all 870 P. falciparum positive patients. The proportion of the monoclonal infections was very high. Of the 870 cases, 819 patients (94·14%) contained a single allelic form i.e. either mspI or mspII, and these isolates were enrolled in this study. Finally 781 (95·36%) patients had completed the 28 days follow up treatment (Fig. 2). 63 patients were found to be under the age of five. The characteristics of participants in two study sites during enrolment were quite similar (Table 1) and year wise enrollment information was presented in Supplemental Table 1.
Fig. 2.
Schematic presentation of patients' selection during the study period.
Table 1.
Patient characteristics at enrolment of the study in different study area.
| Patient Characteristics | Kolkata | Purulia |
|---|---|---|
| Age (year) | 28.76 (95%CI, 3–72) | 26.31(95%CI, 3–73) |
| Sex ratio (Women/Men) | 177/168 | 191/245 |
| Axillary Temperature on day 0 (°C) | 39.38 °C (95%CI, 37.71–40.67) | 38.44 °C (95%CI, 37.33–39.89) |
| Parasitaemia (parasite/μl) | 86188 (95%CI, 11500–181000) | 77452 (95%CI, 11000–156000) |
| Mean haemoglobin (g/dl) | 9.6 ± 2.1 | 10.2 ± 1.7 |
3.2. Clinical efficacy of chloroquine
P. falciparum positive patients were received standard dose of CQ in 2008–2009. 338 patients had completed the 28 day follow up treatment (Table 2). Early treatment failure (ETF) was detected in 114 patients {43 patients (31·39%) from Kolkata and 71 (35·32%) patients from Purulia} (33·73%) while 38 patients (11·24%) were identified as late treatment failure (16 patients from Kolkata and 22 patients from Purulia) cases. msp1, msp2 and glurp were analyzed in all 38 apparent LTF cases to differentiate between ‘new infection’ and ‘recrudescence’. Of the 38 cases, 30 patients (13 from Kolkata and 17 from Purulia) were finally identified as true ‘recrudescence’ case. Three LTF cases (2.19%) from Kolkata and five LTF cases (2.49%) from Purulia were classified as a new infection. CQ treatment failure (ETF + recrudescence) increased in subsequent year from 37.09% to 44.0% in 2008–2009, in Kolkata whereas in Purulia treatment failure was observed in 45·33% of patients in 2008, which slightly decreased to 42.96% in 2009 (Table 2).
Table 2.
Distribution of CQ treatment efficacy in Kolkata and Purulia during 2008–2009.
| Year | ACPR |
ETF |
LTF |
|||||
|---|---|---|---|---|---|---|---|---|
| Kolkata | Purulia | Kolkata | Purulia | Kolkata |
Purulia |
|||
| Recrud-escence | New infection | Recrud-escence | New infection | |||||
| 2008 | 38 (61.29%) | 41 (54.67%) | 18 (29.03%) | 28 (37.33%) | 5 (8.06%) | 1 (1.62%) | 6 (8.0%) | 0 (0.00%) |
| 2009 | 40 (53.33%) | 67 (53.17%) | 25 (33.33%) | 43 (34.13%) | 8 (10.67%) | 2 (2.67%) | 11 (8.73%) | 5 (3.97%) |
| Total | 78 (56.93%) | 108 (53.73%) | 43 (31.39%) | 71 (35.32%) | 13 (9.49%) | 3 (2.19%) | 17 (8.46%) | 5 (2.49%) |
3.3. In vitro CQ susceptibility
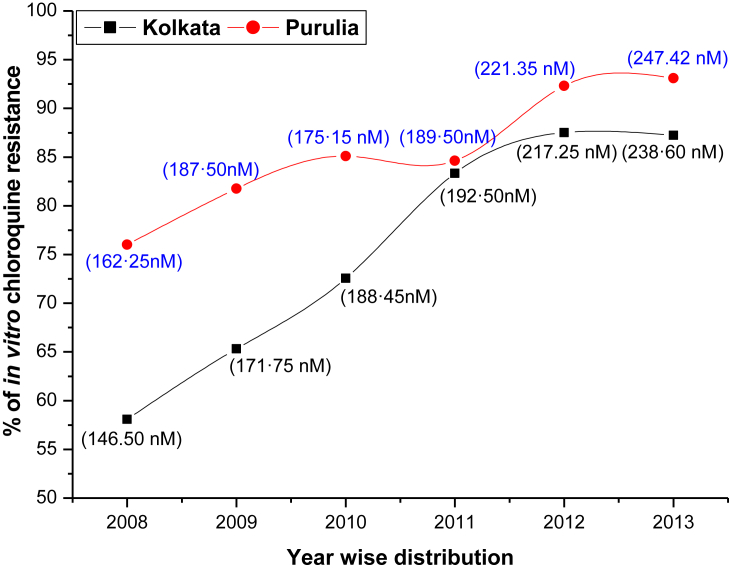
In vitro CQ susceptibility assay was successfully performed in 738 isolates out of 781 cases. Culture adaptations were failed in 12 isolates from Kolkata and 31 isolates from Purulia. The proportion of in vitro CQ resistance (mean IC50 = 146.5 nM, 95%CI, 110–210 nM) in Kolkata was 58.06% in 2008 which was gradually increased in subsequent years to 87.23% in 2013 (mean IC50 = 238·60 nM; 95% CI, 121–321 nM). 76% of isolates in Purulia were highly resistant to CQ (mean IC50 = 162·25 nM; 95%CI, 112–254 nM) in 2008 which was exceedingly increased in consequent years to 93·10% in 2013 (mean IC50 = 247·42 nM; 95%CI, 126–316 nM) (Fig. 3).
Fig. 3.
Proportion of in vitro CQ resistance before and after new national drug policy. Mean IC50 values of CQ in successive years were presented.
3.4. Antimalarial drug pressure assessment
A total of 720 individuals were interviewed (360 individual each from Kolkata and Purulia) in 2008 (mean age = 26·82 years, age range = 21–75 years). An antimalarial treatment was consumed by 17.24% and 21.37% of individual from Kolkata and Purulia respectively in previous 30 days at primary health center or hospital. CQ was the most commonly prescribed antimalarial drug by the clinicians (79%) followed by SP (12%), quinine (QU; 7%) and ACT (2%) in Kolkata. Similarly, CQ (74·5%) was frequently used antimalarial drug after SP (11%) and quinine (QU; 14·5%) in Purulia. A total of 36·45% individuals had taken self medication at home; of them 62% declared that they had used CQ, remaining 28% and 10% of individuals had used QU and SP respectively. In previous 30 days 26.11% of individuals from Purulia travelled to Kolkata whereas only 5.83% of individuals from Kolkata travelled to Purulia. The proportion of individuals who had travelled outside the site in previous 30 days was estimated very high in Purulia (41.11%) than Kolkata (24.72%).
A total of 360 individuals (mean age = 23·41 years, age range = 19–72 years) from each study sites were interviewed again in 2012. ACT was the most commonly prescribed antimalarial drug (86%) by the clinicians followed by chloroquine (9%) and artesunate monotherapy (5%) in Kolkata. Like Kolkata, ACT (72·6%) was also the most preferred antimalarial after artesunate monotherapy (11%), chloroquine (15·4%) and quinine (1%) in Purulia. Self medication (22·5% of total individuals) with CQ, artesunate monotherapy, and ACT had taken by 46%, 31%, and 23% of individuals respectively. The proportion of individuals who travelled outside the site in the previous 30 days was estimated very high in Purulia (46.38%) than Kolkata (29.16%).
3.5. Genotyping of pfcrt and pfmdr1 before new national drug policy
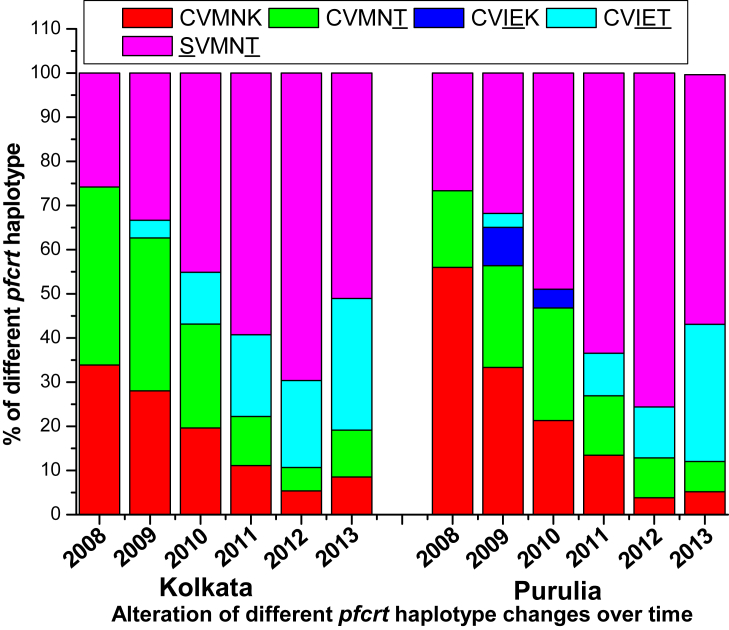
The key polymorphisms leading to the substitution of lysine with threonine at codon 76 of the pfcrt gene was 66.13% in 2008 which increased to 72% in 2009, in Kolkata while K76T polymorphism increased from 44% to 57·94% in 2009, in Purulia (Supplementary Fig. 1). Mutation was absent in 73, 74 and 75 codon of pfcrt gene in 2008. Polymorphisms leading to the substitution of asparagine with tyrosine at codon 86 of pfmdr1gene (N86Y) was recorded 41·94% and 46·67% in 2008 and 2009 respectively from Kolkata while this mutation was identified in 66·97% and 64·28% of isolates from Purulia in 2008 and 2009 respectively. The rates of isolates consisting of mutant D1246Y alleles were very high in 2008 (53·33%) and in 2009 (46·03%) (Supplementary Fig. 2). In Purulia, wild-type pfcrt (CVMNK) haplotype was prevalent (56%), whereas in Kolkata single mutant CVMNT haplotype (40·32%) was more common than wild type CVMNK (33·87%) haplotype in 2008. Interestingly pfcrt CVMNT (34·67%) haplotype was prevalent, followed by SVMNT (33·33%) and CVMNK (28%) haplotype in Kolkata, while in Purulia, SVMNT (31·75%) haplotype was the most common pfcrt allele, followed by wild type CVMNK (29.37%), single mutant CVMNT (23·02%) haplotype, in 2009 (Fig. 4).
Fig. 4.
Frequency of different pfcrt haplotype in Kolkata and Purulia in 2008–2013.
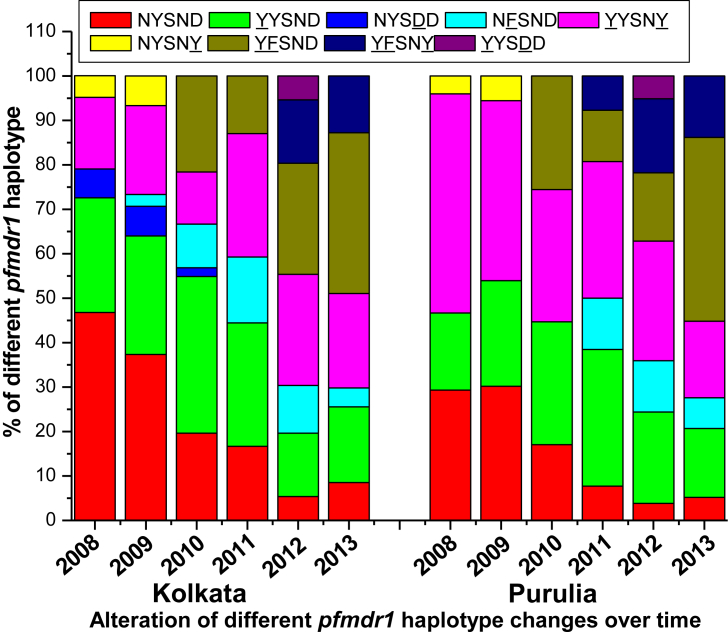
In case of pfmdr1 gene, wild type NYSND haplotype (46·77%) was prevalent followed by single mutant YYSND (25·81%) and double mutant YYSNY (16·13%) haplotype in Kolkata whereas in Purulia YYSNY haplotype (36%) was frequently found after wild type NYSND (29.33%) and YYSND (17·33%) haplotype in 2008. Like 2008, wild type NYSND haplotype (37·33%) was prevalent, followed by YYSND (26·67%) and YYSNY (20%) haplotype in 2009. Just like 2008, pfmdr1 YYSNY haplotype (40.48%) was commonly found after NYSND (30·16%) and YYSND (20·63%) haplotype in 2009 in Purulia (Table 3) (Fig. 5).
Table 3.
Distribution of different pfcrt and pfmdr1 haplotype in relation to in vivo CQ treatment efficacy and in vitro CQ susceptibility in Kolkata and Purulia before new national drug policy.
| Year |
pfcrt haplotype |
Pfmdr1 haplotype |
No of isolates |
CQ treatment efficacy |
In vitro CQ response |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (72–76) | 86,184, 10 34, 1042, 1246 | K | P | ACPR K | ACPR P | ETFK | ETFP | LTFK | LTFP | S K | S P | R K | R P | |
| 2008 | CVMNK | NYSND | 19 | 13 | 19 | 13 | – | – | – | – | 19 | 13 | – | – |
| CVMNK | YYSND | 2 | 6 | 2 | 6 | – | – | – | – | 1 | 2 | 1 | 4 | |
| SVMNT | NYSND | 6 | 3 | 4 | 2 | 1 | 1 | 1 | 1 | – | 5 | 3 | ||
| CVMNT | NYSND | 4 | 6 | 4 | 6 | – | – | – | 2 | 1 | 2 | 5 | ||
| CVMNT | YYSND | 7 | 3 | 2 | 2 | 4 | 1 | 1 | – | 1 | – | 6 | 3 | |
| CVMNT | NYSDD | 4 | – | 3 | – | 1 | – | – | – | 1 | – | 3 | – | |
| CVMNT | YYSNY | 10 | 4 | 2 | 1 | 7 | 3 | 1 | – | 1 | – | 9 | 4 | |
| SVMNT | NYSNY | 3 | 3 | 1 | 3 | 1 | – | 1 | – | – | – | 3 | 3 | |
| SVMNT | YYSND | 7 | 4 | 1 | 3 | 4 | – | 2 | 1 | – | – | 7 | 4 | |
| CVMNK | YYSNY | – | 23 | – | 4 | – | 17 | – | 2 | – | 2 | – | 21 | |
| SVMNT | YYSNY | – | 10 | – | 1 | – | 7 | – | 2 | – | – | – | 10 | |
| 2009 | CVMNK | NYSND | 21 | 5 | 21 | 5 | – | – | – | – | 21 | 5 | – | – |
| SVMNT | NYSND | 7 | 21 | 4 | 17 | 1 | – | 2 | 4 | 2 | 1 | 5 | 20 | |
| CVMNK | YYSNY | – | 37 | – | 5 | – | 26 | – | 6 | – | 4 | – | 33 | |
| CVIEK | YYSNY | – | 11 | – | 1 | – | 8 | – | 2 | – | 1 | – | 10 | |
| CVIET | YYSND | 3 | 4 | – | 2 | 3 | 1 | – | 1 | – | – | 3 | 4 | |
| CVMNT | YYSND | 11 | 12 | 2 | 11 | 6 | 1 | 3 | – | 1 | 4 | 10 | 8 | |
| SVMNT | NYSDD | 5 | – | 5 | – | – | – | – | – | – | – | 5 | – | |
| CVMNT | YYSNY | 15 | 3 | 3 | – | 11 | 3 | 1 | – | – | – | 15 | 3 | |
| SVMNT | NYSNY | 5 | 5 | 4 | 5 | 1 | 1 | 1 | 4 | 4 | ||||
| SVMNT | YYSND | 6 | 14 | 1 | 9 | 3 | 3 | 2 | 2 | 1 | – | 5 | 14 | |
| SVMNT | NFSND | 2 | – | – | – | 1 | – | 1 | – | – | – | 2 | – | |
| CVMNT | NYSND | – | 12 | – | 10 | – | 1 | – | 1 | – | 5 | – | 7 | |
| CVMNT | NYSNY | – | 2 | – | 2 | – | – | – | – | – | 2 | – | – | |
| Total | 137 | 201 | 78 | 108 | 43 | 71 | 16 | 22 | 52 | 41 | 85 | 160 | ||
Bold and underline amino acid are the mutant codon. Here in vitro test responses are classified as sensitive (S) (IC50 value < 100 nM) and Resistant (R) (IC50 value > 100 nM). Here K denotes for Kolkata and P denotes Purulia.
Fig. 5.
Allele frequency of different pfmdr1 haplotype in Kolkata and Purulia in 2008–2013.
3.6. pfcrt and pfmdr1 polymorphisms after new national drug policy
CQ is no longer the drug of choice against P. falciparum after 2009 in India, but the genes (pfcrt, and pfmdr1) which are responsible for CQ resistance continued to increase in number. The proportion of pfcrt K76T polymorphism increased to 94·64% and 96·15% in 2012, in Kolkata and Purulia respectively (Table 4). Like K76T polymorphism, pfcrt C72S mutation also reached to its maximal level of 69·64% and 75·4% in 2012, in Kolkata and Purulia respectively. The rate of isolates consisting of pfmdr1 N86Y and Y184F polymorphisms were markedly increased from 2009 (46·67% and 2·67% respectively) to 2013 (87.23% and 53.19% respectively) in Kolkata. Similarly, polymorphism at N86Y and Y184F allele were found to 87·93% and 62·07% in 2013, in Purulia respectively (Supplementary Fig. 1, Supplementary Fig. 2).
Table 4.
Distribution of different pfcrt and pfmdr1 haplotype in relation to in vitro CQ susceptibility after implementation of ACT.
| Year |
pfcrt haplotype |
Pfmdr1 haplotype |
No of isolates |
In vitro CQ response |
||||
|---|---|---|---|---|---|---|---|---|
| (72–76) | 86,184,1034,1042, 1246 | K | P | S K | S P |
R K | R P |
|
| 2010 | CVMNK | NYSND | 10 | 2 | 10 | 2 | – | – |
| CVIET | YYSND | 6 | - | – | – | 6 | – | |
| CVIEK | YYSNY | – | 2 | – | – | – | 2 | |
| CVMNT | NYSND | – | 6 | – | 3 | – | 3 | |
| CVMNT | YYSND | 6 | 2 | 1 | – | 5 | 2 | |
| SVMNT | NYSDD | 1 | – | – | – | 1 | – | |
| CVMNT | YYSNY | 6 | 4 | 1 | – | 5 | 4 | |
| SVMNT | NFSND | 5 | – | – | – | 5 | – | |
| SVMNT | YYSND | 6 | 11 | 1 | 5 | 11 | ||
| CVMNK | YYSNY | – | 8 | – | 2 | – | 6 | |
| SVMNT | YFSND | 11 | 12 | – | – | 11 | 12 | |
| 2011 | CVMNK | NYSND | 6 | 4 | 6 | 4 | – | – |
| SVMNT | NYSND | 3 | – | 1 | – | 2 | – | |
| CVMNK | YYSNY | – | 3 | – | 1 | – | 2 | |
| SVMNT | YFSND | 7 | 6 | – | – | 7 | 6 | |
| CVIET | YYSND | 4 | – | – | – | – | 4 | |
| CVIET | YYSNY | 6 | 5 | 6 | 5 | |||
| CVMNT | YYSND | 6 | 7 | 2 | 2 | 4 | 5 | |
| SVMNT | YFSNY | – | 4 | – | – | – | 4 | |
| SVMNT | YYSND | 5 | 9 | – | 1 | 5 | 8 | |
| SVMNT | NFSND | 8 | 6 | – | – | 8 | 6 | |
| SVMNT | YYSNY | 9 | 8 | – | – | 9 | 8 | |
| 2012 | CVMNK | NYSND | 3 | 3 | 3 | 3 | – | – |
| CVMNT | YYSND | 3 | 7 | 2 | 3 | 1 | 4 | |
| SVMNT | YYSNY | 7 | 12 | 1 | – | 6 | 12 | |
| SVMNT | YYSND | 5 | 9 | – | – | 5 | 9 | |
| SVMNT | NFSND | 6 | 9 | 1 | – | 5 | 9 | |
| SVMNT | YYSDD | 3 | 4 | – | – | 3 | 4 | |
| CVIET | YYSNY | 7 | 9 | – | – | 7 | 9 | |
| CVIET | YFSND | 4 | – | – | – | 4 | – | |
| SVMNT | YFSND | 10 | 12 | – | – | 10 | 12 | |
| SVMNT | YFSNY | 8 | 13 | – | – | 8 | 13 | |
| 2013 | CVMNK | NYSND | 4 | 3 | 4 | 3 | – | – |
| CVMNT | YYSND | 5 | 4 | 2 | 1 | 3 | 3 | |
| SVMNT | YYSND | 3 | 5 | – | – | 3 | 5 | |
| SVMNT | YFSND | 9 | 13 | – | – | 9 | 13 | |
| SVMNT | YFSNY | 6 | 8 | – | – | 6 | 8 | |
| SVMNT | NFSND | 2 | 4 | – | – | 2 | 4 | |
| CVIET | YYSNY | 6 | 7 | – | – | 6 | 7 | |
| CVIET | YFSND | 8 | 11 | – | – | 8 | 11 | |
| SVMNT | YYSNY | 4 | 3 | – | – | 4 | 3 | |
After 2010 wild type pfcrt CVMNK haplotype rapidly reduced to only 8·51% and 5·17% of isolates in 2013, in Kolkata and Purulia respectively. The frequency of CVMNT haplotype decreased while SVMNT haplotype highly increased and reached its highest pick in 2012. The most vulnerable pfcrt CVIET haplotype was rare in 2008–2009, but this haplotype gradually increased in subsequent years to 29.78% and 31.03% in 2013, in Kolkata and Purulia respectively (Fig. 4). Predominance of pfmdr1 YFSND allele was observed both in Kolkata (36·17%) and in Purulia (41·38%) in 2013. Wild type NYSND and YYSNY haplotypes were gradually decreased in subsequent years while triple mutant YFSNY allele progressively increased (Fig. 5).
3.7. Genotypic correlation of CQ efficacy in vivo and in vitro
CQ resistant pattern was observed much different in Purulia from Kolkata. CQ treatment failure was strongly correlated to pfmdr1 86Y+1246Y mutation (r2 = 0.9990, p < 0.0011) but not with the pfcrt gene in Purulia, while in vivo CQ treatment efficacy was strongly correlated to pfcrt76T + pfmdr186Y polymorphism or pfcrt 76T + pfmdr186Y+1246Y mutation in Kolkata (Fisher test: CQ, p < 0.01 for pfcrt 76T, pfmdr1 86Y codon; p < 0.05 for 1246Y codon) (Table 3). The phenotype of in vitro CQ sensitivity was also highly correlated to codon 76 and 72 of pfcrt gene and codon 86, and 1246 of the pfmdr1 gene (CQ, p < 0.05 for codon 76 and 72 and p < 0.01 for codon 86, and 1246) in 2008–2009.
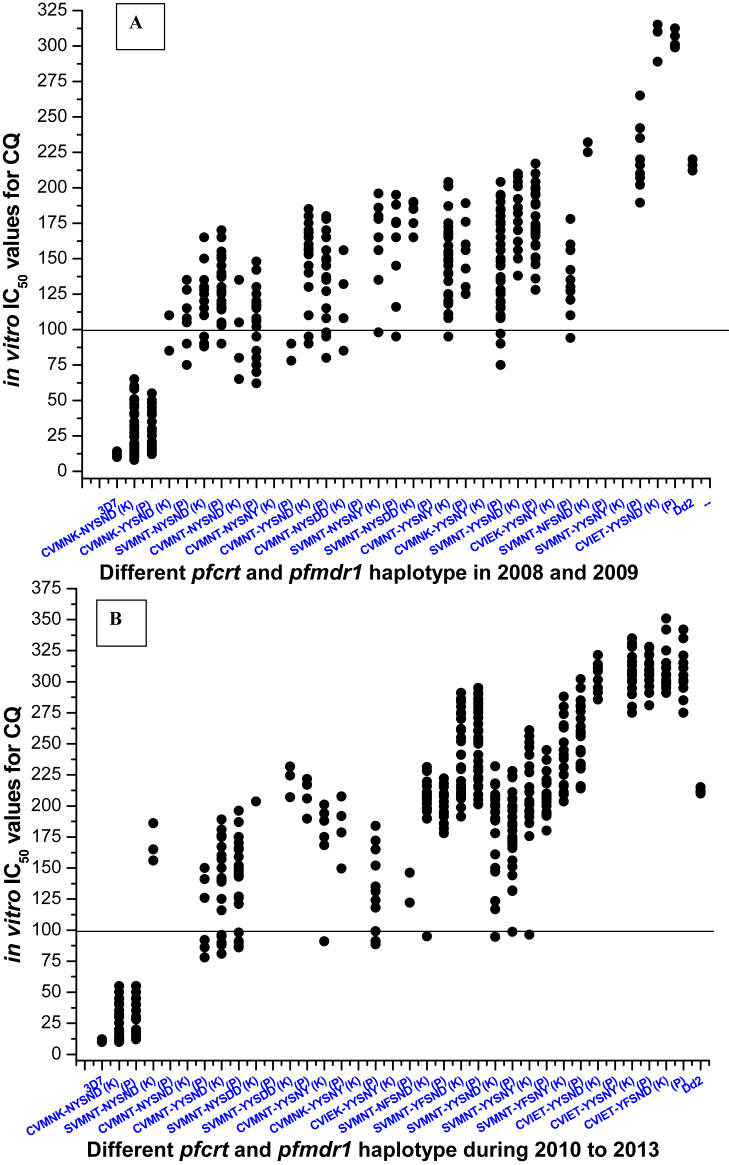
Patients with wild type CVMNK-NYSND haplotype always produced ACPR after CQ treatment. Isolates with CVMNT-NYSND, SVMNT-NYSND and CVMNT-NYSNY haplotype presented low to moderate IC50 values of CQ and were not associated with an ETF (p = 0·74, Kruskal-Wallis test). Triple mutant SVMNT-NYSNY, SVMNT-NYSDD haplotype were found to have moderate to high IC50 of CQ but these haplotypes were not correlated with an ETF (p < 0·01, Mann-Whitney U test). Isolates with CVMNT-YYSNY haplotype were highly correlated with ETF in Kolkata in 2008–2009 (p < 0·005). Three out of five LTF were observed to have this triple mutant haplotype in Kolkata. Isolates containing CVMNK-YYSNY and SVMNT-YYSNY haplotype was found to have association with ETF in 2008 as well as in 2009 (p < 0·001). Isolates with CVMNT-YYSND, and SVMNT-YYSND haplotype represented very high IC50 of CQ (p < 0·001) and found to have less susceptible to CQ in vivo (p < 0·05). Isolates with quadruple mutant CVIET-YYSND haplotype was observed to have highest IC50 of CQ and proved to correlated with CQ treatment failure (p < 0·05) (Fig. 6A). Alteration of molecular genotyping, CQ treatment efficacy and in vitro CQ resistance were found to have strongly correlated (r2 = 0·9993, p < 0·015).
Fig. 6.
AAlteration of in vitro IC50 values for CQ against different pfcrt and pfmdr1 haplotype in Kolkata and Purulia in 2008–2009. The solid line (corresponding to 100 nM of CQ) is hypothetical and shows the level of chloroquine resistance in vitro. ‘K’ represents for Kolkata and ‘P’ represents the haplotypes of Purulia. B: Variations of in vitro CQ, IC50 values in relation to different pfcrt and pfmdr1 haplotype in Kolkata and Purulia during 2010–2013. The solid line is hypothetical and shows the level of chloroquine resistance in vitro. ‘K’ represents for Kolkata and ‘P’ represents the haplotypes of Purulia.
3.8. Haplotype diversity in relation to in vitro CQ susceptibility in 2010–2013
The frequency of wild type CVMNK-NYSND allele was found to have decreased after 2010. Most of the haplotype was observed to have IC50 > 100 nM of CQ proving in vitro CQ resistance. Isolates with SVMNT-YYSND, SVMNT-NFSND, CVMNT-YYSNY and SVMNT-YFSND haplotype increased gradually from 2010 to 2013, leading to a rise in IC50 of CQ (p < 0·05) in Kolkata as well as in Purulia (Fig. 6B). Isolates with quintuple mutant SVMNT-YFSNY and CVIET-YYSNY haplotype were found to have exceedingly high IC50 of CQ (p < 0·005) while quintuple mutant CVIET-YFSND haplotype represented the highest IC50 of CQ in Kolkata as well as in Purulia (Supplementary Fig. 3, Table 4).
3.9. Regional bias and chronological diversity in pfcrt and pfmdr1 polymorphism
In Kolkata, 30·65% and 28% of patients were found to have the wild type CVMNK-NYSND haplotype combination in 2008 and 2009 respectively while only 17·33% and 3·96% of isolates represented this wild haplotype in Purulia. The proportion of this wild type haplotype decreased gradually in subsequent years to 8.51% and 5.17% in 2013, in Kolkata and Purulia respectively. Isolates with double mutant CVMNK-YYSNY haplotype was frequently found in 2008 (30·67%) and in 2009 (29·37%), although it decreased in subsequent years to 17.02% and 5.76% in 2010 and 2011 respectively but it was never observed after 2012 in Purulia. Surprisingly, this haplotype combination was never found in Kolkata. Quadruple mutant CVIEK-YYSNY haplotype (8·73%) was found in Purulia but it was absent in Kolkata. Isolates with double mutant CVMNT-YYSND and triple mutant CVMNT-YYSNY haplotypes were the most common mutant haplotype found in Kolkata whereas quite unlike SVMNT-NYSND and SVMNT-YYSND mutant allele was most frequently found haplotype in 2008–2009, in Purulia. Isolates with quintuple mutant SVMNT-YFSNY haplotype was observed initially in 2011, in Purulia (7.69%), further it spread in subsequent years to Kolkata (14.29% and 12.76% in 2012 and 2013 respectively). Similarly, isolates containing CVIET-YFSND haplotype was observed primarily in 2012, in Kolkata (7.14%), which spread rapidly in 2013, in Kolkata (17.02%) as well as in Purulia (18.97%). Isolates with quadruple mutant CVIET-YYSND haplotype was commonly found haplotype up to 2011, in Kolkata but after 2009 this haplotype had never observed in Purulia. (Table 3, Table 4).
4. Discussion
The findings of this study have provided the evidence of high proportion of CQ treatment failure (in vivo) in both the study sites which supported the change of previous national guidelines for the treatment of uncomplicated P. falciparum malaria (Government of India, 2009). The high rates of CQ treatment failure indicated the enormous CQ pressure over this parasite population, as more than 40% of cases were produced in vivo CQ resistance (Das et al., 2014). The second major finding of this study have provided the evidence of increased in vitro chloroquine resistance as well as rapid rise in candidate gene mutations (pfcrt and pfmdr1) in subsequent years, after withdrawal of chloroquine. These major findings were not corroborated with the previous findings from Malawi (Laufer et al., 2006). The rationale provided for this approach was to evaluate the chloroquine efficacy at times that coincided with the new national drug policy and especially assessed the chloroquine resistant molecular markers even after withdrawal of chloroquine. In our study, we have used multiple approaches, like in vivo CQ treatment efficacy, in vitro drug susceptibility assessment and also molecular genotyping of pfcrt and pfmdr1 genes to confirm this hypothesis.
Our study provides a comprehensive evidence of distinct variation of in vivo CQ resistant pattern in two study sites, however it was quite different from that of previously reported in India. Generally in India, in vivo CQ resistance was solely associated with polymorphisms of pfcrt, CVMNT or SVMNT or CVIET haplotype, by promoting drug efflux from the parasite digestive vacuole (Duraisingh et al., 2000, Vinayak et al., 2003, Vathsala et al., 2004, Mittra et al., 2006), while pfmdr1 gene mutation at N86Y codon (YYSND) modulated the degree of resistance, i.e. increase in IC50 of CQ (Fidock et al., 2000, Duraisingh and Cowman, 2005). Our study confirmed that, CQ resistance was highly associated with CVMNT-YYSND and CVMNT-YYSNY haplotype in Kolkata, which was identical with the previous finding reported from different parts of India (Vinayak et al., 2003, Sharma, 2005, Das et al., 2014). In Purulia, CQ treatment failure (60.71% ETF and, 33.33% LTF, in 2008; 60.47% ETF and 37.5% LTF in 2009) was found to associated with CVMNK-YYSNY allele, which was quite uncommon in India. CQ non responding patients (33.33%) with wild type pfcrt CVMNK allele was previously observed in 2005, in Madhya Pradesh, India (Bharti et al., 2010). These findings suggested that pfmdr1 polymorphism at N86Y and D1246Y codon possessed a pivotal role in CQ resistance in 2008–2009, in Kolkata as well as in Purulia. The association flanked by the pfmdr1 genotypes and CQ resistance habitually generated convincing results in Africa and Southeast Asia (Duraisingh et al., 2000, Sa et al., 2009, Andriantsoanirina et al., 2010), but in India, most of the studies did not support these findings (Vinayak et al., 2003, Mittra et al., 2006). On the basis of our finding, it is postulated that the assessment of pfcrt-pfmdr1 combination mutation was very important to unfold the CQ resistant pattern, in this part of India, as this combination mutation depended on the genetic background of the strain as well as variation of antimalarial drug pressure (Sharma, 2005, Mittra et al., 2006). Polymorphisms of pfmdr1 gene at codon 86, 184, and 1246 were quite uncommon in India, although it was frequently found in Madagascar (Andriantsoanirina et al., 2009, Andriantsoanirina et al., 2010). The extensive and haphazard use of CQ and quinine over the parasite population might employ some specific selective pressure over this parasite population. Therefore, detection of pfcrt genotype alone would not be sufficient to predict the CQ resistant scenario in this part of India.
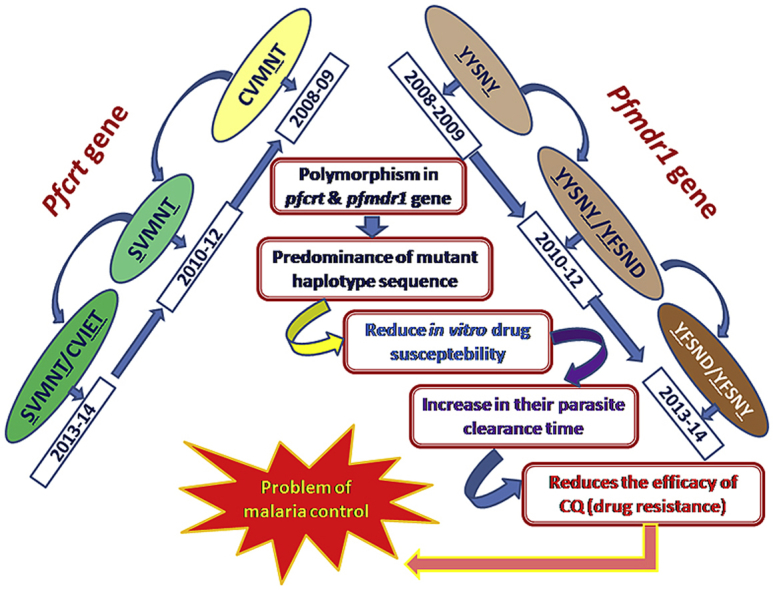
CQ is no longer the drug of choice against P. falciparum after 2009 but in vitro CQ resistance increased in subsequent years to 87.23% and 93.10% in 2013 in Kolkata and Purulia respectively (Fig. 3). Isolates containing wild type pfcrt CVMNK and pfmdr1 NYSND haplotype were drastically reduced in both the place. Isolates with SVMNT-YFSND, SVMNT-YFSNY, CVIET-YFSND and CVIET-YYSNY haplotypes increased gradually (p < 0·05) from 2010 to 2013, leading to a rise in IC50 of CQ (p < 0·05) (Table 4). High malaria transmission and rapid population mix up, might helps to outspread of these vulnerable parasite haplotype in Kolkata (Gardella et al., 2008). Previous study suggested that higher the number of pfcrt mutations, higher will be the levels of CQ resistance (Lim et al., 2003, Durrand et al., 2004). It was reported from different parts of the world, including North-eastern India, that P. falciparum isolates with CVIETS haplotype showed higher levels of in vitro CQ resistance rather than the isolates presenting SVMNTS and CVMNTS haplotype (Fidock et al., 2000, Durrand et al., 2004, Mittra et al., 2006). We had found that isolates with SVMNT-YFSND, SVMNT-YFSNY, CVIET-YFSND and CVIET-YYSNY haplotype seemed to have an advantage over those with the CVMNT-NYSND, SVMNT-NYSND, CVMNT-YYSND and SVMNT-YYSND haplotype, as they could able to survive in the high drug pressure (Supplementary Fig. 3) (Mittra et al., 2006, Das et al., 2014). We implied from our study that, acquisition of CQ resistance is a stepwise process, where CVMNT haplotype of pfcrt gene might occur first, followed by the SVMNT haplotype, whereas CVIET haplotype might take place independently with an increase in drug pressure. In case of pfmdr1 gene, YYSND haplotype might be the first mutation to occur followed by YYSNY and YFSND, whereas YFSNY haplotype seemed to be independent with an increase drug pressure. Additional mutations in pfcrt-pfmdr1 gene would give rise to a higher level of CQ resistance.
Finally the most important question would be raised regarding the deciding factors that promoted or somehow increased the spreading of these vulnerable pfcrt-pfmdr1 combination haplotypes, since AS + SP was recommended as a first line of drug against uncomplicated falciparum malaria. It was reported that P. vivax is more prevalent than P. falciparum while large numbers of isolates were found to have Pv + Pf mixed infection in India (Annual District wise Epidemiological Report of Malaria of West Bengal, 2010). CQ was the principle drug of choice against P. vivax. Genetic cross breeding of mixed Pf + pv infection, leading to a rise in CQ pressure over P. falciparum. On the contrary, in absence of quinoline derivatives only artemisinin derivatives might elicit partial pressure on pfmdr1 gene. It was reported previously that polymorphism in 86Y and 184F codon in re-infecting parasites after artemether + lumifantrine treatment might constitute a first step toward resistance (Sisowath et al., 2005). Finally drug pressure assessment confirmed the irresponsible use of CQ by the private practitioners as well as haphazard and random self medication (CQ) by the civilians made the situation worse day by day.
In conclusion, proper knowledge in treating malaria (both Government and private practitioners) and awareness of common civilians is much crucial to cope up with this drug resistant scenario. Further exploration of whole genome sequencing would provide greater knowledge of parasite genome, which might elucidate the evolutionary history and consequent spreading of a resistant parasite in this part of India. Government surveillance in treatment of malaria, awareness of pharmaceutical shops (stop selling antimalarial without proper prescription of doctor) and synchronized thorough research, surveillance, as well as containment strategies would be very essential to cope up with this drug resistance burden.
Funding source
This work was supported by personal research grant (PRG_SR2008-PRG_SR2013) of Corresponding author from the Vidyasagar University, India as well as personal grant of the first author from Council of Scientific and Industrial Research, India, having sanction no: 09/599 (0055)2K1-EMR-I.
Transparency declaration
All authors declare that we have no conflict of interest.
Acknowledgement
The authors express gratefulness to Vidyasagar University, Midnapore for providing the facilities to execute the study. We are very much thankful to Council of Scientific and Industrial Research (CSIR), India for providing personal grants for first author. We are thank full to The Gautam Laboratories and Imaging, Kolkata, India (NABL accredited laboratory, ISO 15189:2007-M-0423). Under their supervision, in vivo tests were done in collaboration with Vidyasagar University, Midnapore. We are thankful to Mr. Bismay Roy, In charge, Publication Dept, Govt of West Bengal, for his assistance in language editing and proofreading of the manuscript. Finally, we are thank to Purulia District Hospital for their assistance in completing the work.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2017.06.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Andriantsoanirina V., Ratsimbasoa A., Bouchier C., Jahevitra M., Rabearimanana S., Radrianjafy R., Andrianaranjaka V., Randriantsoa T., Rason M.A., Tichit M., Rabarijaona L.P., Mercereau-Puijalon O., Durand R., Ménard D. Plasmodium falciparum drug resistance in Madagascar: facing the spread of unusual pfdhfr and pfmdr1 haplotypes and the decrease of dihydroartemisinin susceptibility. Antimicrob. Agents Chemother. 2009;53:4588–4597. doi: 10.1128/AAC.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriantsoanirina V., Ratsimbasoa A., Bouchier C., Tichit M., Jahevitra M., Rabearimanana S., Raherinjafy R., Mercereau-Puijalon O., Durand R., Ménard D. Chloroquine clinical failures in P. falciparum malaria are associated with mutant Pfmdr1, not Pfcrt in Madagascar. PLoS One. 2010;5:e13281. doi: 10.1371/journal.pone.0013281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annual District wise Epidemiological Report of Malaria of West Bengal, 2010. www.wbhealth.gov.in/Health_Stat/2010_2011/8/VIII.1.3.pdf.
- Annual District wise Epidemiological Report of Malaria of West Bengal, 2006.
- Basco K.L., Ringwald P. Molecular epidemiology of malaria in Yaounde, Cameroon VI. Sequence variations in the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene and in vitro resistance to pyrimethamine and cycloguanil. Am. J. Trop. Med. Hyg. 2000;62(2):271–276. doi: 10.4269/ajtmh.2000.62.271. [DOI] [PubMed] [Google Scholar]
- Bharti P.K., Alam M.T., Boxer R., Shukla M.M., Gautam S.P., Sharma Y.D., Singh N. Therapeutic efficacy of chloroquine and sequence variation in pfcrt gene among patients with falciparum malaria in central India. Trop. Med. Int. Health. 2010;15:33–40. doi: 10.1111/j.1365-3156.2009.02425.x. [DOI] [PubMed] [Google Scholar]
- Bloland P.B., Lackritz E.M., Kazembe P.N., Were J.B., Steketee R., Campbell C.C. Beyond chloroquine: implications of drug resistance for evaluating malaria therapy efficacy and treatment policy in Africa. J. Infect. Dis. 1993;167 doi: 10.1093/infdis/167.4.932. 932–927. [DOI] [PubMed] [Google Scholar]
- Das S., Chakraborty S.P., Hati A.K., Roy S. Association between prevalence of chloroquine resistance and unusual mutation in pfmdr1 and pfcrt gene in India. Am. J. Trop. Med. Hyg. 2013;88(5):828–834. doi: 10.4269/ajtmh.11-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., KarMahapatra S., Tripathy S., Chattopadhyay S., Dash S.K., Mandal D., Hati A.K., Roy S. Double mutation in the pfmdr1 gene is associated with emergence of chloroquine-resistant plasmodium falciparum malaria in eastern India. Antimicrob. Agents Chemother. 2014;58(10):5909–5915. doi: 10.1128/AAC.02762-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djimdé A., Doumbo O.K., Cortese J.F., Kayentao K., Doumbo S., Diourte Y., Diourté Y., Coulibaly D., Dicko A., Su X.Z., Nomura T., Fidock D.A., Wellems T.E., Plowe C.V. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 2001;344(4):257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Dua V.K., Dev V., Phookan S., Gupta N.C., Sharma V.P., Subbarao S.K. Multi-drug resistant Plasmodium falciparum malaria in Assam, India: timing of recurrence and anti-malarial drug concentrations in whole blood. Am. J. Trop. Med. Hyg. 2003;69:555–557. [PubMed] [Google Scholar]
- Duraisingh M.T., Cowman A.F. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94:181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Duraisingh M.T., Jones P., Sambou I., VonSeidlein L., Pinder M., Warhurst D.C. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol. Biochem. Parasitol. 2000;108:13–23. doi: 10.1016/s0166-6851(00)00201-2. [DOI] [PubMed] [Google Scholar]
- Durrand V., Berry A., Sem R., Delabre J.F., Jesic Z.Z., Le, Bras J. Variations in the sequence and expression of the Plasmodium falciparum and chloroquine resistance transporter (Pfcrt) and their relationship to chloroquine resistance in vitro. Mol. Biochem. Parasitol. 2004;136:273–285. doi: 10.1016/j.molbiopara.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Fidock D.A., Nomura T., Talley A.K., Cooper R.A., Dzekunov S.M., Ferdig M.T., Ursos L.M., Sidhu A.B., Naudé B., Deitsch K.W., Su X.Z., Wootton J.C., Roepe P.D., Wellems T.E. Mutations in Plasmodium falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote S.J., Kyle D.E., Martin R.K., Oduola A.M., Forsyth K., Kemp D.J., Cowman A.F. Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990;345:255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- Gardella F., Assi S., Simon F., Bogreau H., Eggelte T., Ba F., Foumane V., Henry M.C., Kientega P.T., Basco L., Trape J.F., Lalou R., Martelloni M., Desbordes M., Baragatti M., Briolant S., Almeras L., Pradines B., Fusai T., Rogier C. Antimalarial drug use in general populations of tropical Africa. Malar. J. 2008;7:124. doi: 10.1186/1475-2875-7-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of India . Government of India; 2009. Guidelines for Diagnosis and Treatment of Malaria in India. NIMR/TRS-06/APR-2009; 2009. [Google Scholar]
- KarMahapatra S., Chakraborty S.P., Das S., Hati A.K., Roy S. Prevalence of severe chloroquine resistance associates the point mutation in pfcrt and pfmdrI gene in eastern India. Asian Pac. J. Trop. Dis. 2011;1(4):263–269. [Google Scholar]
- Laufer M.K., Thesing P.C., Eddington N.D., Masonga R., Dzinjalamala F.K., Takala S.L., Taylor T.E., Plowe C.V. Return of chloroquine antimalarial efficacy in Malawi. N. Engl. J. Med. 2006;355:19959–19960. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- Lim P., Chy S., Ariey F., Incardona S., Chim P., Sem R., Denis M.B., Hewitt S., Hoyer S., Socheat D., Merecreau-Puijalon O., Fandeur T. Pfcrt polymorphism and chloroquine resistance in plasmodium falciparum strains isolated in Cambodia. Antimicrob. Agents Chemother. 2003;47:87–94. doi: 10.1128/AAC.47.1.87-94.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes D., Rungsihirunrat K., Nogueira F., Seugorn A., Gil P.J., do Rosário V.E., Cravo P. Molecular characterisation of drug-resistant Plasmodium falciparum from Thailand. Malar. J. 2002;1:12. doi: 10.1186/1475-2875-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlotra R.K., Fujioka H., Roepe P.D., Maguire J.D., Baird J.K. Evolution of a unique plasmodium falciparum chloroquine resistance phenotype in association with PfCRT polymorphism in papua new guinea and South America. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12689–12694. doi: 10.1073/pnas.221440898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittra P., Vinayak S., Chandawat H., Das M.K., Singh N., Biswas S., Dev V., Kumar A., Ansari M.A. Sharma YD. Progressive increase in point mutations associated with chloroquine resistance in plasmodium falciparum isolates from India. J. Infect. Dis. 2006;193:1304–1312. doi: 10.1086/502979. [DOI] [PubMed] [Google Scholar]
- Sa J.M., Twu O., Hayton K., Reyes S., Fay M.P., Ringwald P., Wellems T.E. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. U. S. A. 2009;106:18883–18889. doi: 10.1073/pnas.0911317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy S.K., Jena R.C., Sharma R.S., Sharma R.C. Status of Plasmodium falciparum resistance to chloroquine in Orissa. J. Commun. Dis. 1997;29:145–151. [PubMed] [Google Scholar]
- Sehgal P.N., Sharma M.D., Sharma S.l., Gopal S. Resistance to chloroquine in falciparum malaria in Assam state, India. J. Commun. Dis. 1973;5:175–180. [Google Scholar]
- Sharma Y.D. Genetic alteration in drug resistance markers of Plasmodium falciparum. Indian J. Med. Res. 2005;121:13–22. [PubMed] [Google Scholar]
- Singh N., Shukla M.M. Response of Plasmodium falciparum to chloroquine in a tribal area of Madhya Pradesh. Indian J. Malariol. 1990;27(3):183–186. [PubMed] [Google Scholar]
- Sisowath C., Stromberg J., Martensson A., Msellem M., Obondo C., Björkman A. In vivo selection of Plasmodium falciparum pfmdr1 86N coding alleles by artemether-lumefantrine. J. Infect. Dis. 2005;191:1014–1017. doi: 10.1086/427997. [DOI] [PubMed] [Google Scholar]
- Snounou G., Zhu X., Siripoon N., Jarra W., Thaithong S., Brown K.N., Viriyakosol S. Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Trans. R. Soc. Trop. Med. Hyg. 1993;93:369–374. doi: 10.1016/s0035-9203(99)90120-7. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vathsala P.G., Pramanik A., Dhanasekaran S., Rangarajan P.N., Padmanaban G. Widespread occurrence of the plasmodium falciparum chloroquine resistance transporter (pfcrt) gene haplotype SVMNT in P. falciparum malaria in India. Am. J. Trop. Med. Hyg. 2004;70:256–259. [PubMed] [Google Scholar]
- Vinayak S., Biswas S., Dev V., Kumar A., Ansari M.A., Sharma Y.D. Prevalence of the K76T mutation in the pfcrt gene of plasmodium falciparum among chloroquine responders in India. Acta Trop. 2003;87:287–293. doi: 10.1016/s0001-706x(03)00021-4. [DOI] [PubMed] [Google Scholar]
- Wijeyaratne P.M., Chand P.B., Valecha N. Therapeutic efficacy of antimalarial drugs along the eastern Indo-Nepal border: a cross-border collaborative study. Trans. R. Soc. Trop. Med. Hyg. 2005;99:423–429. doi: 10.1016/j.trstmh.2004.09.011. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2003. Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. Geneva. (WHO/HTM/RBM/2003.50) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.