Abstract
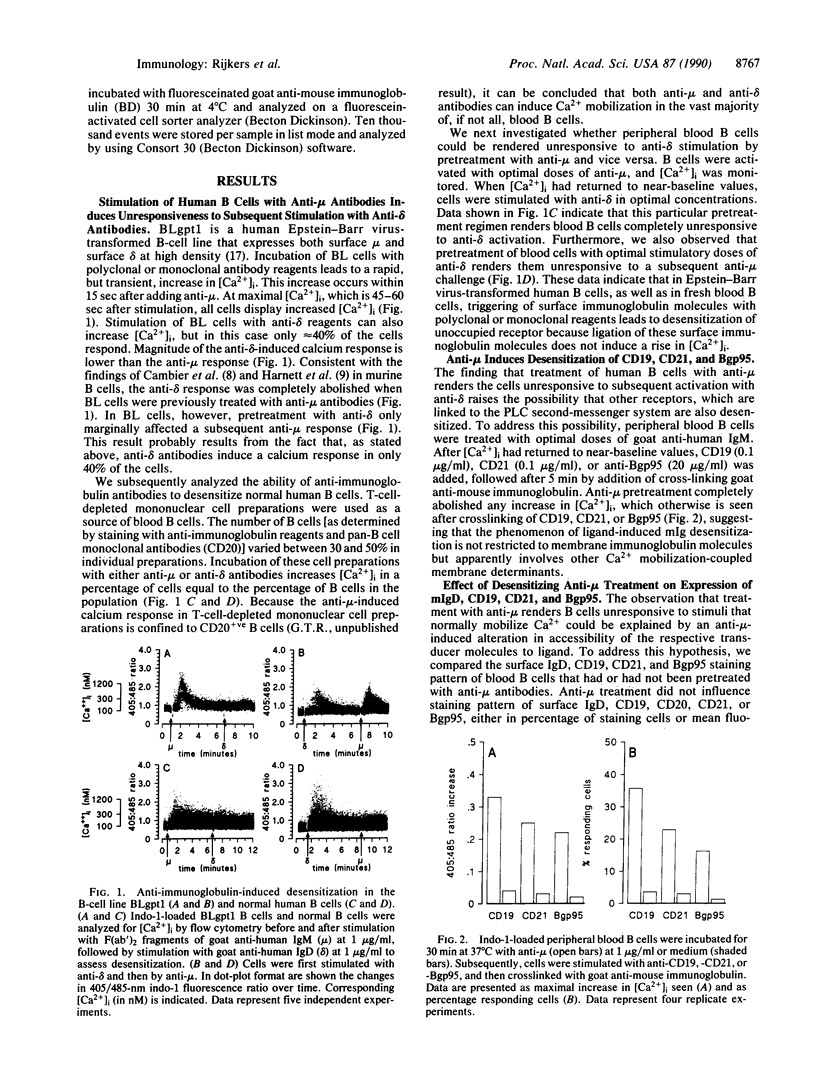
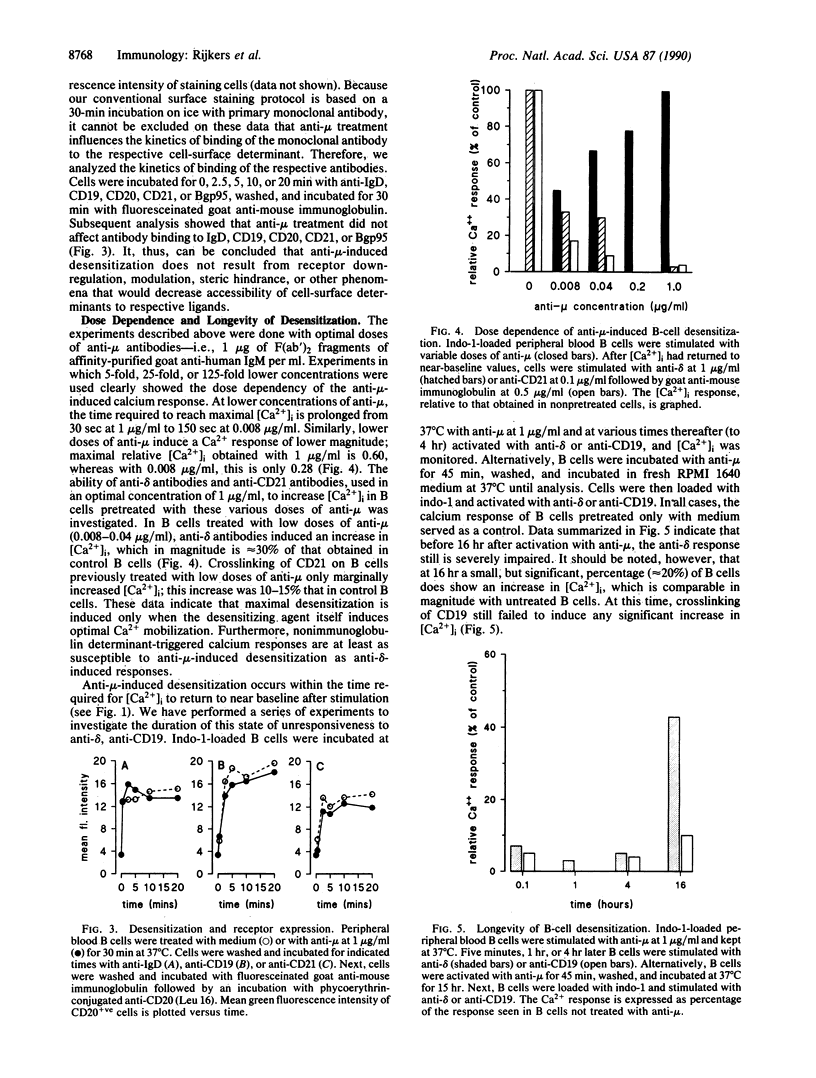
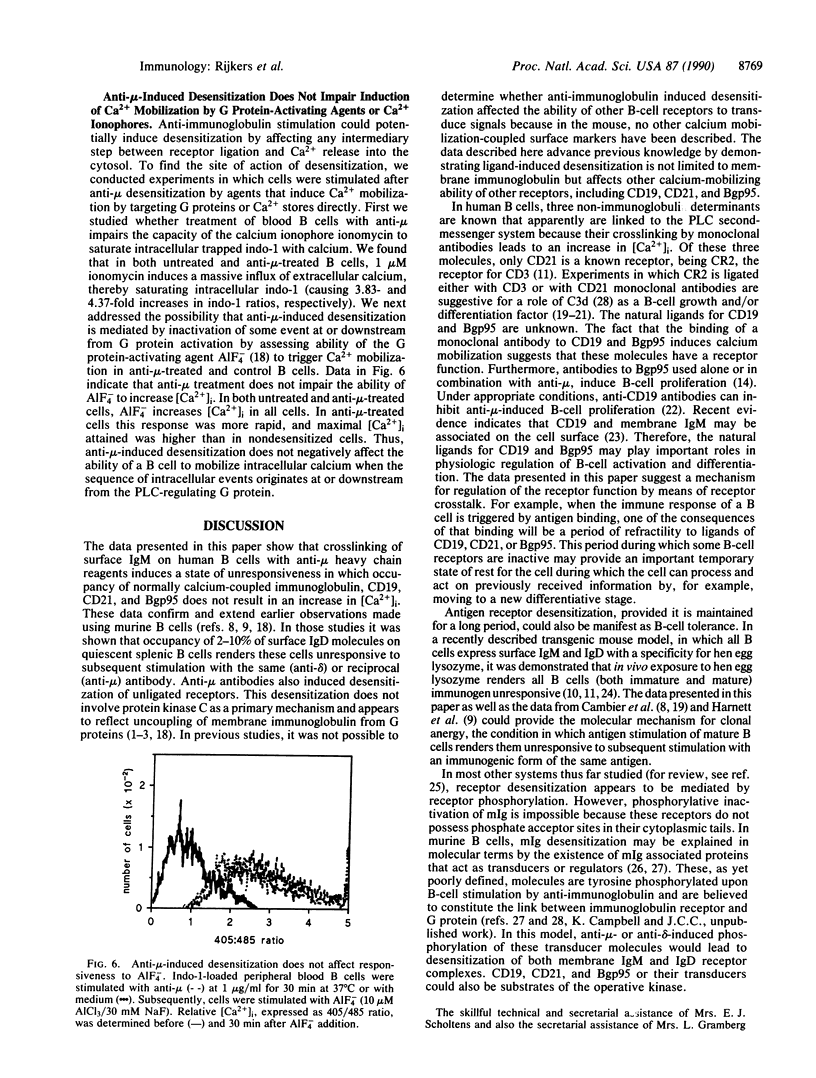
We have examined the ability of membrane immunoglobulin-binding ligands to desensitize several human B-cell surface molecules that normally transduce signals leading to Ca2+ mobilization. Ligation of membrane IgM or IgD leads to heterologous desensitization of the reciprocal receptor in Epstein-Barr virus-transformed B-cell lines and peripheral blood B cells, as evidenced by a failure of cells to mobilize in response to receptor ligation. Under these conditions CD19, CD21, and B-cell gp95 ligation also did not lead to normal Ca2+ mobilization, indicating that these transducers are also desensitized. The desensitization does not reflect receptor modulation from the cell surface or reduced accessibility to ligand and is long lived, lasting greater than 16 hr. Finally, data that indicate that desensitized cells remain responsive to the G protein activating agent AIF4-, as measured by Ca2+ mobilization, suggest that desensitization reflects uncoupling of these receptors from G proteins that are intermediaries in their transduction of signals. We hypothesize that the molecular target of desensitization may be a recently described membrane immunoglobulin-associated and inducibly tyrosine-phosphorylated protein complex that may function as a master transducer in B cells, analogous to CD3 in T cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Bouvier M., Caron M. G., Lefkowitz R. J. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. doi: 10.1146/annurev.cb.04.110188.002201. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Cooper N. R. CR2 ligands modulate human B cell activation. J Immunol. 1988 Oct 15;141(8):2569–2576. [PubMed] [Google Scholar]
- Cambier J. C., Fisher C. L., Pickles H., Morrison D. C. Dual molecular mechanisms mediate ligand-induced membrane Ig desensitization. J Immunol. 1990 Jul 1;145(1):13–19. [PubMed] [Google Scholar]
- Cambier J. C., Justement L. B., Newell M. K., Chen Z. Z., Harris L. K., Sandoval V. M., Klemsz M. J., Ransom J. T. Transmembrane signals and intracellular "second messengers" in the regulation of quiescent B-lymphocyte activation. Immunol Rev. 1987 Feb;95:37–57. doi: 10.1111/j.1600-065x.1987.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Cambier J., Chen Z. Z., Pasternak J., Ransom J., Sandoval V., Pickles H. Ligand-induced desensitization of B-cell membrane immunoglobulin-mediated Ca2+ mobilization and protein kinase C translocation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6493–6497. doi: 10.1073/pnas.85.17.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R. H., Spycher M. O., Ng Y. C., Hoffman R., Fearon D. T. Synergistic interaction between complement receptor type 2 and membrane IgM on B lymphocytes. J Immunol. 1988 Jul 15;141(2):457–463. [PubMed] [Google Scholar]
- Gold M. R., Jakway J. P., DeFranco A. L. Involvement of a guanine-nucleotide-binding component in membrane IgM-stimulated phosphoinositide breakdown. J Immunol. 1987 Dec 1;139(11):3604–3613. [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Jorgensen H., Brink R. A., Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989 Nov 23;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Tursz T., Edwards C. F., Tetaud C., Talbot M., Caillou B., Rickinson A. B., Lipinski M. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL)-like phenotype. J Immunol. 1987 Jul 1;139(1):313–318. [PubMed] [Google Scholar]
- Griffioen A. W., Rijkers G. T., Keij J., Zegers B. J. Measurement of cytoplasmic calcium in lymphocytes using flow cytometry. Kinetic studies and single cell analysis. J Immunol Methods. 1989 Jun 2;120(1):23–27. doi: 10.1016/0022-1759(89)90284-6. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Holman M. J., Klaus G. G. Regulation of surface IgM- and IgD-mediated inositol phosphate formation and Ca2+ mobilization in murine B lymphocytes. Eur J Immunol. 1989 Oct;19(10):1933–1939. doi: 10.1002/eji.1830191026. [DOI] [PubMed] [Google Scholar]
- Harnett M. M., Klaus G. G. G protein coupling of antigen receptor-stimulated polyphosphoinositide hydrolysis in B cells. J Immunol. 1988 May 1;140(9):3135–3139. [PubMed] [Google Scholar]
- Imboden J. B., Pattison G. Regulation of inositol 1,4,5-trisphosphate kinase activity after stimulation of human T cell antigen receptor. J Clin Invest. 1987 May;79(5):1538–1541. doi: 10.1172/JCI112986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keij J. F., Griffioen A. W., The T. H., Rijkers G. T. INCA: software for consort 30 analysis of flow cytometric calcium determinations. Cytometry. 1989 Nov;10(6):814–817. doi: 10.1002/cyto.990100623. [DOI] [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Haldar S. Involvement of a specific guanine nucleotide binding protein in receptor immunoglobulin stimulated inositol phospholipid hydrolysis. Biochim Biophys Acta. 1989 Oct 9;1013(3):273–278. doi: 10.1016/0167-4889(89)90146-8. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., McNaughton M. E., Cooper N. R. Binding of monoclonal antibody to the Epstein Barr virus (EBV)/CR2 receptor induces activation and differentiation of human B lymphocytes. J Immunol. 1985 Nov;135(5):3068–3073. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pesando J. M., Bouchard L. S., McMaster B. E. CD19 is functionally and physically associated with surface immunoglobulin. J Exp Med. 1989 Dec 1;170(6):2159–2164. doi: 10.1084/jem.170.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Boyd A. W., Nossal G. J. Clonal anergy: the universally anergic B lymphocyte. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2013–2017. doi: 10.1073/pnas.79.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Jaszcz W., Ambrus J. L., Fauci A. S., Gajl-Peczalska K., Song C. W., Wick M. R., Myers D. E., Waddick K., Ledbetter J. A. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988 Jan;71(1):13–29. [PubMed] [Google Scholar]
- Valentine M. A., Clark E. A., Shu G. L., Norris N. A., Ledbetter J. A. Antibody to a novel 95-kDa surface glycoprotein on human B cells induces calcium mobilization and B cell activation. J Immunol. 1988 Jun 15;140(12):4071–4078. [PubMed] [Google Scholar]
- Wienands J., Hombach J., Radbruch A., Riesterer C., Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990 Feb;9(2):449–455. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



