Abstract
Background
Aspects of impulsivity have been implicated in the development, or maintenance, of Alcohol Use Disorder (AUD). The brain dopamine system is implicated in both reward processing/memory (typically subcortical) and in brain inhibitory control mechanisms (typically cortical). Using a validated clinical lab paradigm, the dopamine/serotonin “stabilizing” drug, aripiprazole was evaluated in non-treatment-seeking AUD individuals based on their level of impulsivity/ self-control.
Methods
Ninety-nine individuals (77% male; mean age 27; 7.5 drinks per day; 83% heavy drinking days) meeting DSM-IV criteria for alcohol dependence were randomized to aripiprazole (N=47 evaluable) or placebo (N=48 evaluable) based on their Barratt Impulsiveness Scale (BIS-11) score (above or below 68). Aripiprazole, or similar placebo, was titrated to 15mg over 8 days. Drinking was recorded over 6 days under natural conditions. On day 8, after one day of required abstinence, individuals participated in a bar-lab paradigm that included a priming drink (BAC target 0.02–0.03 g/dL) and free-choice consumption of up to 8 drinks (max BAC 0.1 g/dL) in exchange for a “bar-credit” of $2 per drink (max $16). Endpoints were drinks per day under natural conditions and drinks consumed in the bar-lab after the priming drink.
Results
There was no significant main effect of aripiprazole or interaction with BIS-11 score during the natural drinking period. However, there was a main effect of aripiprazole on bar-lab drinking (p=0.04) and aripiprazole reduced the total number of drinks consumed more among individuals with low self-control (p=0.034) and increased latency to consume those drinks (p=0.045) more among those with high impulsivity. Relative to placebo, aripiprazole caused more side effects and increased alcohol-induced sedation, but neither significantly influenced its interaction with impulsivity/self-control scores on drinking.
Conclusions
This paradigm forced a choice between immediate drinking reward and delayed monetary reward. In those with high impulsivity and/or low self-control, aripiprazole shifts the balance away from immediate drinking towards a later reward. Medications targeting cortical dopamine/serotonin balance might show clinical benefit of reduced drinking, among individuals with impulsivity/low self-control.
Keywords: Alcohol, Impulsivity, Aripiprazole, Pharmacotherapy, Alcohol Use Disorder
INTRODUCTION
Alcohol abuse and dependence affect over 18 million Americans (Grant et al., 2004) and constitute one of the highest burdens of illness worldwide (Rehm et al., 2014). Data suggest that the peak prevalence of alcohol use disorders (AUDs) occurs in the early 20s (Grant et al., 2016), and while many individuals no longer meet diagnostic criteria as they grow older, others maintain a trajectory of increasing alcohol consumption and eventually seek treatment in their 30s and 40s. A number of studies have tried to evaluate what characteristics distinguish those who drink heavily at an early age and/or go on to develop AUDs. One characteristic that has received increasing attention is broadly defined as “impulsivity.” This multidimensional construct can be defined and measured in a variety of ways; recent data support a tripartite latent structure comprised of impulsive choice, impulsive action, and impulsive personality traits (MacKillop et al., 2016). One sub-dimension of impulsivity that may hold special relevance for addictive behavior is self-control, which can be defined as the ability to inhibit prepotent responses and regulate behavior (de Ridder et al., 2012). In AUDs, drinking becomes a prepotent response, and impaired self-control may thus be a particular risk factor for the progression of these disorders (Leeman et al., 2014).
It has been postulated that high impulsivity significantly contributes to the development of addiction (Bechara, 2005, Perry and Carroll, 2008, Verdejo-Garcia et al., 2008) and plays a decisive role in the switch to compulsive drug use (Belin et al., 2008). Individuals (particularly adolescents and young adults) who are more impulsive drink more heavily and are more likely to have, or progress to, an AUD (Dougherty et al., 2004, Simons et al., 2004, von Diemen et al., 2008, Rubio et al., 2008). Impulsive individuals have poor decisional balance (Wilcox et al., 2014) and are more likely to accept a smaller immediate reward than a later, more lucrative, reward. Therefore, impulsive individuals might be more likely to choose alcohol when presented with an opportunity to drink even though a “price” would have to be paid in the future (e.g., hangover, missing work, spouse’s anger). It is also possible that impulsive individuals will have more craving for alcohol than non-impulsive individuals, thus being unable to resist alcohol cue-elicited urges to drink (Papachristou et al., 2012), something we have indirectly observed in our preliminary studies (Voronin et al., 2008). Individuals with higher impulsivity also have more difficulty ceasing drug/alcohol use and relapse more quickly if they do (Perry and Carroll, 2008, Bowden-Jones et al., 2005), and co-occurring heavy alcohol use and impulsivity leads to increased mortality over time (Blonigen et al., 2011). Thus, impulsivity is a significant risk factor for the development and progression of AUDs.
Given these clinical observations, it is not surprising that impulsivity has been assessed as an intermediate phenotype for addiction (Dick et al., 2010, Moffitt et al., 2011), and has attracted study of its neurochemical, neuroanatomical, and genetic underpinnings (Cardinal, 2006, Congdon and Canli, 2005, Kreek et al., 2005). In animal studies, new models of impulsive behavior have given support to older assumptions that the brain dopamine, and to some extent the brain serotonin, systems are likely involved (for review see (Jentsch et al., 2014). Trait-impulsive rats self-administer cocaine to a significantly larger degree than non-impulsive rats and, importantly, have lower dopamine D2 and D3 receptor availability in the nucleus accumbens (Dalley et al., 2007). Similarly, rats lacking D2 receptors also administer cocaine in larger amounts (Caine et al., 2002) while chronic stimulant users show a downregulation in dopamine release and D2/D3 receptor availability ((Ashok et al., 2017)). Of note, either reduction or blockade of D3 receptors reduces alcohol intake in rodents ((Bahi and Dreyer, 2014),(Thanos et al., 2005) or alters reward processing in humans (Murphy et al., 2017). Interestingly, the D3 receptor has been suggested to play a more salient role in motivated alcohol/drug responding in the context of higher environmental contingencies (for review see (Le Foll et al., 2005). While these studies implicate the dopamine system in the reward pathway as mediating a role between impulsivity and reward seeking, it is also clear that dopamine plays a major role in cortical function, response inhibition, and possibly some aspects of impulsivity in humans (for review see (Cardinal, 2006, Congdon and Canli, 2005). A number of lesion, stimulation, and pharmacological studies done in rodents indicate that the nucleus accumbens (Nac) and orbitofrontal cortical areas, in particular, play a large role in choice of delayed versus immediate reinforcement (one aspect of impulsive responding), and that dopaminergic transmission plays a crucial role in this effect (for review see (Cardinal, 2006, Winstanley, 2007) It has also been hypothesized, based on available data, that either too much or too little dopamine system function in these crucial brain areas might lead to impulsive behavior and/or responding (Winstanley, 2007). Other brain areas implicated in animals and humans are inferior frontal cortex, dorsolateral cortex, anterior and posterior cingulate and dorsal striatum. It should be noted that many of these regions are also activated by substance cues, including alcohol cues or the drugs themselves. For instance, there is direct relationship between dopamine receptor availability in the ventral striatum and alcohol craving, cue activation of the medial prefrontal cortex (Heinz et al., 2004), and level of intoxication (Yoder et al., 2005). In addition, at least for alcohol, direct effects on frontal areas might increase risk for impulsive choices (Crews and Boettiger, 2009). For instance, an electrophysiological study in humans showed that alcoholics had reduced frontal lobe activity and that reduced frontal lobe activity correlated with impulsivity traits as measured by the Barratt Impulsiveness Scale (Chen et al., 2007). There is also considerable work (primarily in animals) implicating the brain serotonin system in impulsive behaviors (for Review see (Kirby et al., 2011) (Jentsch et al., 2014)) including effects of serotonin depletion on worsening action inhibition, waiting, and delayed risk decision making. Conversely, increasing serotonin does the opposite. Importantly, there is a suggestion of opposing actions of 5HT2A and 5HT2C receptors such that antagonism of 5HT2A receptors reduces various aspects of impulsivity while the opposite occurs with 5HT2c antagonism.
Given the possible link between impulsivity/self-control and brain/serotonin pathways, as well as the role dopamine in particular plays in alcohol reward and reinforcement (Volkow et al., 2007), medications with dopaminergic effects might be useful for treating AUDs. One such medication is aripiprazole, a putative dopamine stabilizer medication. Aripiprazole has been approved by the FDA for the treatment of schizophrenia (Marder et al., 2003, DeLeon et al., 2004), bipolar disorder (Keck et al., 2006), and most recently, as an adjunct to treat depression. Its pharmacological profile differs from other dopamine antagonists. It acts either as a primary brain dopamine system stabilizer (Lawler et al., 1999), or potentially more universally “stabilizes” dopamine-serotonin balance (Burris, 2002) perhaps by its serotonin 5HT2A antagonism effects. However, aripiprazole has been postulated to work primarily as a partial dopamine agonist in that it reduces dopamine actions when dopamine concentrations are high, while producing dopamine agonist-like effects in the absence, or at low concentrations, of dopamine. This might occur directly by reducing dopamine effects at post-synaptic receptors, or, indirectly, by pre-synaptic dopamine receptor blockade (thereby removing the inhibition of dopamine transmission). The net effect of either of these mechanisms is to keep dopamine “balanced.” The clinical utility of this unique pharmacology is that aripiprazole behavioral effects could be obtained while off-target effects such as extrapyramidal effects, or tardive dyskinesia might be minimalized (Marder et al., 2003). In the nucleus accumbens, aripiprazole reduces dopamine release at higher doses but increases prefrontal dopamine release at lower doses, (Li et al., 2004). However, its elevation of prefrontal dopamine appears specific, since serotonin and norepinephrine do not seem to be increased (Zocchi et al., 2005). This unusual pharmacological profile might be particularly useful in the treatment of addiction, either by reducing reward saliency, or by increasing cortical efficiency - potentially leading to more “self-control” and less impulsive drinking behavior. Aripiprazole’s 5HT2A antagonist effects could also reduce impulsive responding in humans as postulated by animal studies (Jentsch et al., 2014).
Aripiprazole was found to reduce alcohol use in animals (Ingman et al., 2006) and, in a clinical laboratory paradigm, to decrease the euphoric effects of alcohol in humans (Kranzler et al., 2008). While high dose side effects limited interpretation of the results, a large US clinical trial indicated that, while aripiprazole did not promote abstinence, it significantly reduced drinks per drinking day and alcohol dependence symptoms, ameliorated many of the effects of alcohol consumption, and normalized a biological marker (%CDT) of heavy alcohol use (Anton et al., 2008). Recent reviews have suggested that, while aripiprazole might be useful for the treatment of AUDs, there are not enough data to draw firm conclusions (Kishi et al., 2013, Litten et al., 2016, Martinotti et al., 2016).
While clinical trials are expensive and take time to conduct, especially when evaluating medication effects on AUD endophenotypes, clinical lab studies can often provide an initial signal of medication efficacy that might inform clinical trial development (Ray et al., 2010). We have developed a clinical lab paradigm to evaluate various medications, including naltrexone (Drobes et al., 2003, Anton et al., 2012), nalmefene (Drobes et al., 2003), and gabapentin (Myrick et al., 2007). We previously evaluated the effects of aripiprazole in this paradigm, and found that it reduced alcohol consumption and alcohol-induced stimulation (Voronin et al., 2008). Importantly, post-hoc analyses indicated that aripiprazole reduced drinking only among individuals who rated themselves low on the self-control subscale of the Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995). To our knowledge, no other data is reported on this intriguing finding, which might suggest a more “targeted” pharmacotherapeutic use for aripiprazole in those with AUDs.
The purpose of the current study was to replicate and extend our previous findings in a prospective study. To accomplish this, non-treatment seeking individuals meeting DSM-IV criteria for Alcohol Dependence, who were either low and high in trait impulsivity/self-control, were randomized to aripiprazole or placebo in a double-blind fashion and evaluated for effects on drinking and related-behaviors using the same subacute natural drinking and alcohol self-administration paradigm as used previously (Voronin et al., 2008). Specifically, we hypothesized that 1) aripiprazole would reduce drinking under natural conditions and/or in a bar lab setting and 2) that those with more trait impulsivity and/or less self-control would show a greater response to aripiprazole compared to placebo. Secondarily, we wanted to explore the effect of aripiprazole vs. placebo on alcohol induced stimulation and sedation, and also whether impulsive individuals would drink more slowly while on aripiprazole compared to placebo.
MATERIALS AND METHODS
Participants
Between April 2011 and December 2015, 137 non-treatment seeking alcoholics between the ages of 21 and 40, recruited from ads in newspapers and social media, were screened for this study and 99 who met DSM-IV criteria for Alcohol Dependence (American Psychiatric Association, 1994) were randomized into the double-blind protocol.
Exclusion criteria were as follows: current DSM-IV criteria for non-alcohol substance dependence (excluding nicotine) by verbal report and urine drug screens, other major DSM-IV Axis I disorders, psychoactive medication or substance use (except marijuana) in the past 30 days or a positive urine drug screen, current suicidal or homicidal ideation, past history of alcohol-related medical illness, liver enzymes ≥ 2.5 times above normal, or significant health problems.
Baseline Assessment Procedures
The Medical University of South Carolina Institutional Review Board approved this study and subjects signed informed consent. After initial phone screening and informed consent, further evaluation was done utilizing a demographic form, the SCID (First MB et al., 1997), the timeline follow-back interview (TLFB) (Sobell et al., 1988) to quantify drinking during the preceding 90 days, the Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995), the Obsessive-Compulsive Drinking Scale (OCDS) (Anton et al., 1996) and the Alcohol Dependence Scale (ADS) (Skinner and Horn, 1984). Finally, a urine drug screen, blood tests for health screening, and a physical exam were conducted. DNA from blood white cells was collected for genetic analysis (data will be reported separately).
Randomization and Medication Dosing
Participants who passed all screening and eligibility criteria were stratified into high and low impulsivity groups on the basis of their BIS-11 scores. In our previous work among non-treatment-seeking individuals with Alcohol Dependence (Voronin et al., 2008, Anton et al., 2012), the median BIS-11 score was 68 (about 10 points higher than age/sex matched social drinkers at our site), consistent with other reports of greater impulsivity among younger alcohol-dependent individuals (von Diemen et al., 2008). Thus, the “high” impulsivity group comprised those participants with a BIS-11 score >= 68, and the “low” group those participants with a score <=67. Individuals within each impulsivity strata were further urn-randomized, based on sex and smoking status, to receive aripiprazole (5 mg on Day 1, 10 mg on Days 2–3, and 15 mg on Days 4–8) or identical placebo capsules in a blinded fashion. All study medications were taken each morning from a blister pack and administered in identical standard gel caps with 25 mg riboflavin added. The final dose level of aripiprazole (15 mg) was chosen based on the putative minimal effective dose in schizophrenia and mania, and low enough to avoid the extensive side effects observed in a multisite AUD study (Anton et al., 2008). It also showed some efficacy in reducing heavy drinking in a 14-day subacute observation study in our hands (Myrick et al., 2010). The titration schedule was chosen to replicate our previous work (Voronin et al., 2008). The last dose of aripiprazole was always taken in front of research personnel at the same time prior to lunch and the bar lab procedure (below) in order to 1) ensure compliance and to standardize food ingestion effects on absorption, and 2) to ensure that the time between ingestion and priming drink was standardized at peak aripiprazole concentration 3–5 hours after dosing (Mallikaarjun et al., 2004).
Experimental Procedures and Outcome Assessments
During medication dosing, participants were given no explicit instructions regarding their drinking behavior for Days 1 – 6 but were required to abstain completely on Day 7 and the morning of Day 8. On Day 7, subjects were assessed for alcohol withdrawal using the Clinical Institute Withdrawal Assessment for Alcohol – Revised (CIWA-Ar) (Sullivan et al., 1989) and a urine sample was collected to ascertain riboflavin levels (based on quantitative spectrofluorometric assay) for medication compliance. The majority of the CIWA-Ar scores were 0 (72% of subjects), and 97% were below 5. Drinking over the previous six days was assessed with the TLFB. On that evening, they underwent an fMRI alcohol cue-reactivity paradigm (similar to (Myrick et al., 2010)), that will be reported separately.
On Day 8 of study medication participants completed a 21-item side-effect checklist, ingested the last dose of study medication under observation at 11:30 am, and 30 minutes later were provided a standard caloric lunch (weight and gender adjusted). At 2:00 pm they consumed a standard dose of spirits (vodka, gin, rum or bourbon) calculated to achieve a breath alcohol concentration (BAC) level of 0.02–0.03 g/dL (Watson, 1989). The drink was 1 part spirits diluted in 3 parts fruit juice (both of the subject’s choosing) and consumed over 5 minutes. Serial BAC measurements were done at 10, 20, and 30 minutes after drink consumption. At each of these time points, the Biphasic Alcohol Effects Scale (BAES) (Martin et al., 1993) was administered to assess stimulation and sedation. At 40 minutes after the initial drink, the curtain in the bar lab was opened to reveal bar-like cues and participants were brought the first tray of 4 “mini-drinks” (each consisting of 1/2 the alcohol consumed in the initial drink and calculated to produce a BAC of 0.015 g/dL) and were told that they could purchase (see below) and consume as many as they desired over the next hour period. In a second hour period, another 4 “mini-drinks” were made available for consumption. A BAC assessment was done at the end of this 2-hour drinking period. As per our previous paradigms and consistent with that of O’Malley (O’Malley et al., 2002), participants were given a “bar credit” of $16, with which they could purchase mini-drinks (as a cost of $2 each) to assess alcohol consumption in the context of an alternative reinforcer. Any money from the bar credit that participants did not use to purchase drinks was given to them the following day. Participants had access to one bag of potato chips (28.3 gram/bag) per tray to simulate a normal bar environment and water was also provided. The number of drinks consumed over the two-hour bar lab period was the main dependent variable for analysis.
After the procedure, participants remained until 10:00 pm and were given dinner and could read, listen to music, or watch videos - but were also provided educational materials regarding alcohol effects to motivate a change in drinking behavior. A breathalyzer reading below 0.02 g/dL was required at departure and they were driven home by a friend or taxi. The following day, a counseling session was given to educate participants about alcohol harm and to increase motivation to reduce drinking or seek treatment. Participants were compensated $360 for their participation, plus any unspent money from their bar credit.
Sample Size, Outcome Variables, and Statistical Approach
The effect size from our previous work (Voronin et al., 2008) for the interaction between aripiprazole and BIS-11 self-control on drinks per day during the 6-day natural observation period indicated that a sample size of 25 subjects per group (total N = 100) would yield 85% power to detect an interaction of the same magnitude (equivalent two-group d = 0.6). While not significant in our previous study, power for detecting an interaction between BIS-11 group and medication for the limited access drinking paradigm was based on our previous effect size (Cohen’s d = 0.78 for aripiprazole over placebo); assuming the aripiprazole effect was only present in the low self-control group, power to detect the interaction was greater than 50% with a sample size of 100.
The main a priori defined dependent variables were 1) drinking under natural conditions (drinks per day during the 6 days prior to the fMRI and bar-lab experiment) and 2) total drinks consumed after the priming drink in the bar-lab paradigm. Using SPSS v. 23, the general linear model (GLM) was used to test the effects of medication, BIS-11 group (high vs. low), and their interaction on these drinking variables. Because our previous work (Voronin et al., 2008) found an interaction between aripiprazole and the BIS-11 self-control subscale, this interaction was also examined; a median split on this subscale score (median = 13) was used to divide participants into high- and low-self-control groups. For the analysis of drinking under natural conditions, baseline drinks per day was covaried; for the analysis of bar-lab drinking, baseline drinks per drinking day was covaried. There were also four exploratory analyses. The first used the GLM to evaluate the interactions between continuously distributed total BIS-11 and BIS-11 self-control scores and medication group on bar lab drinking. The second used the GLM to evaluate the interaction between BIS-11 self-control quartiles and medication group on bar lab drinking. The third used the GLM to examine the effects of medication, BIS-11 group, and their interaction on the subjective effects of the priming drink (BAES stimulation and sedation). Finally, the fourth used Cox proportional hazard regression (conditional risk set model in Stata v. 13) to evaluate the effects of medication, BIS-11 group, and their interaction on latency to drink (time between drinks) in the bar lab. This model tests the difference in survival times between multiple events, conditioned on the existence of these events. Because there was relatively little drinking from the second tray of drinks in the high BIS/aripiprazole group, only the first tray (up to 4 drinks) was used for the latency to drink analysis.
RESULTS
Demographics and Drinking Variables
Of the 99 individuals randomized to study medication, 4 individuals did not progress to the alcohol ingestion paradigm (one was lost to follow-up, one was incarcerated, one sustained a concussion in a car accident as a passenger, and one had a positive BAC on Day 7). That left 95 evaluable participants (47 on aripiprazole and 48 on placebo), whose baseline demographics and drinking characteristics are provided in Table 1. By chance, baseline drinking was greater in the aripiprazole group than the placebo group; however, baseline drinking did not significantly differ across all four impulsivity/medication groups. ADS and OCDS scores suggested mild-moderate AUD severity and did not significantly differ between groups. BIS-11 scores also did not significantly differ, since groups were a priori stratified as a function of this variable.
Table 1.
Demographics and drinking data. The top table provides data based on the full BIS-11 scores and the bottom table is based on the median split of BIS-11 self-control subscale scores.
| Low BIS | High BIS | pa | pb | |||
|---|---|---|---|---|---|---|
| APZ | PLA | APZ | PLA | |||
| N | 23 | 28 | 24 | 20 | - | |
| Gender, M, N (%) | 16 (69.6) | 21 (75.0) | 20 (83.3) | 16 (80.0) | 0.96 | 0.62 |
| Age | 25.2 (4.4) | 26.9 (5.5) | 27.8 (6.4) | 27.2 (5.2) | 0.66 | 0.28 |
| Education, years | 13.6 (2.2) | 14.1 (3.3) | 13.2 (3.4) | 14.8 (2.2) | 0.09 | 0.36 |
| BIS | 55.6 (8.2) | 55.4 (6.9) | 76.4 (9.6) | 79.1 (10.6) | 0.74 | 0.44 |
| BIS self-control | 10.4 (2.4) | 11.3 (2.6) | 16.0 (3.1) | 16.5 (2.9) | 0.86 | 0.76 |
| ADS | 11.3 (4.9) | 9.5 (5.4) | 14.5 (6.2) | 14.6 (6.7) | 0.31 | 0.42 |
| OCDS | 16.6 (8.1) | 13.5 (5.8) | 19.7 (8.5) | 20.3 (9.8) | 0.29 | 0.28 |
| Drinks per day | 8.5 (3.7) | 6.1 (2.0) | 8.0 (3.5) | 7.7 (3.4) | 0.03 | 0.11 |
| Drinks per drinking day | 10.2 (3.2) | 8.0 (2.9) | 10.1 (3.6) | 9.7 (3.8) | 0.04 | 0.19 |
| Heavy drinking days, % | 88.9 (16.4) | 74.0 (23.5) | 88.6 (22.2) | 80.1 (26.9) | 0.009 | 0.50 |
| High Self-Control | Low Self-Control | pa | pb | |||
|---|---|---|---|---|---|---|
| APZ | PLA | APZ | PLA | |||
| N | 26 | 28 | 21 | 20 | - | |
| Gender, M, N (%) | 19 (73.1) | 21 (75.0) | 17 (81.0) | 16 (80.0) | 0.96 | 0.87 |
| Age | 25.8 (4.9) | 27.3 (5.7) | 27.5 (6.4) | 26.7 (4.9) | 0.66 | 0.31 |
| Education, years | 13.7 (2.2) | 14.4 (3.2) | 13.0 (3.5) | 14.3 (2.4) | 0.09 | 0.59 |
| BIS | 58.0 (9.9) | 56.4 (8.1) | 76.3 (10.8) | 77.7 (12.3) | 0.74 | 0.47 |
| BIS self-control | 10.3 (1.9) | 10.9 (2.2) | 17.0 (2.4) | 17.0 (2.3) | 0.86 | 0.59 |
| ADS | 12.2 (5.2) | 9.5 (5.4) | 13.9 (6.3) | 14.6 (6.7) | 0.31 | 0.18 |
| OCDS | 17.9 (7.9) | 13.4 (5.6) | 18.5 (9.1) | 20.5 (9.7) | 0.29 | 0.06 |
| Drinks per day | 8.4 (3.4) | 6.2 (2.2) | 8.0 (3.8) | 7.5 (3.3) | 0.03 | 0.20 |
| Drinks per drinking day | 10.5 (3.3) | 8.2 (3.2) | 10.2 (3.4) | 8.7 (3.4) | 0.04 | 0.17 |
| Heavy drinking days, % | 90.1 (16.3) | 74.1 (25.6) | 87.0 (23.0) | 78.9 (24.1) | 0.009 | 0.35 |
Abbreviations: BIS, Barratt Impulsiveness Scale; APZ, aripiprazole; PLA, placebo; ADS, Alcohol Dependence Scale; OCDS, Obsessive Compulsive Drinking Scale. Figures are means (standard deviations) unless otherwise indicated. Statistics for differences between groups refer to the significance of the Pearson and Wald chi-squared statistics for categorical variables and the t and F statistics for continuous variables.
pa = test for difference between medication groups
pb = test for interaction between medication and BIS groups
Drinking Under Natural Conditions
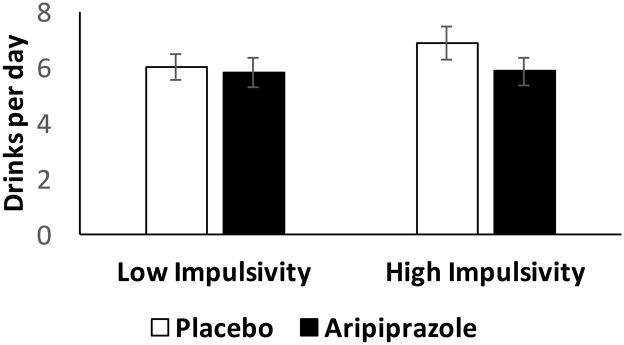
There were no significant main effects of BIS-11 group (F(1, 90) = 0.74, p = 0.39), BIS-11 self-control group (F(1, 90) = 0.11, p = 0.74), or medication (F(1, 90) = 1.30, p = 0.26) on drinks per day under natural conditions, nor were there significant interactions between BIS-11 group and medication (F(1, 90) = 0.60, p = 0.44) or BIS-11 self-control group and medication (F(1, 90) = 0.51, p = 0.48) on this variable (Figure 1).
Figure 1.
Drinks per day (mean +/− SEM) during the 6-day natural observation period in those with low or high impulsivity (BIS-11) scores while taking placebo or aripiprazole. See text for statistical analysis. Figures are adjusted for baseline drinks per day.
Bar Lab Drinking
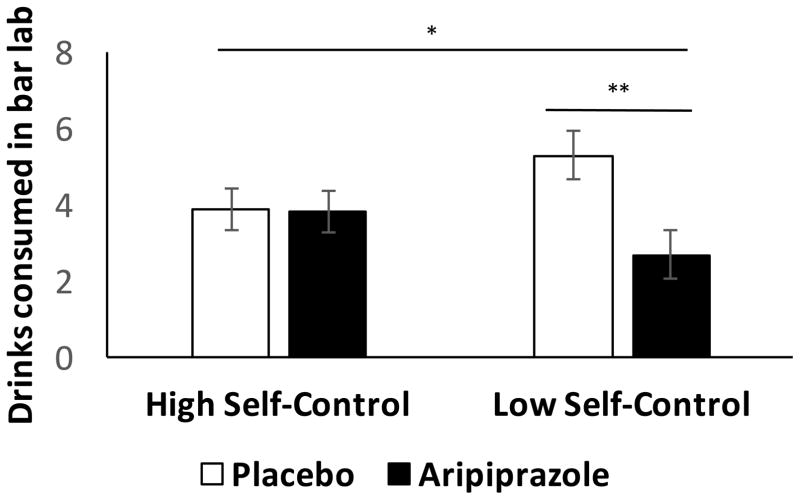
There were no significant main effects of medication or BIS-11 score nor an interaction between these factors on the decision to drink; 75% of low BIS/placebo, 87% of low BIS/aripiprazole, 80% of high BIS/placebo, and 83% of high BIS/aripiprazole subjects opted to drink. While there was no significant main effect of BIS-11 group on the total number of drinks consumed in the bar lab (F(1, 90) = 0.095, p = 0.76), there was, however, a significant main effect of medication (F(1, 90) = 4.32, p = 0.041, partial η2 = 0.046), such that individuals who received aripiprazole (M = 4.6 drinks, 95% CI = [3.7–5.4]) drank less than those who received placebo (M = 3.3 drinks, 95% CI = [2.5–4.2]). There was no significant interaction of BIS-11 group and medication on drinking (F(1, 90) = 1.92, p = 0.165) (Figure 2). However, the interaction between BIS-11 self-control group and medication was significant (F(1, 90) = 4.64, p=0.034, partial η2 = 0.049), such that, among participants with low self-control, those who received aripiprazole (M = 2.7 drinks, 95% CI = [1.5–3.9]) drank less than those who received placebo (M = 5.3 drinks, 95% CI = [4.1–6.5]), while among those with high self-control, drinking was similar in both medication groups (aripiprazole M = 3.8 drinks, 95% CI = [2.7–4.9]; placebo M = 3.9 drinks, 95% CI = [2.8–5.0]) (Figure 3). Essentially, participants with low self-control who received aripiprazole consumed about 50% less alcohol than those who received placebo.
Figure 2.
Free choice drinks (mean +/− SEM) consumed in the bar lab after a priming drink in those with low or high impulsivity (BIS-11) scores while taking placebo or aripiprazole. See text for statistical analysis. Figures are adjusted for baseline drinks per drinking day.
Figure 3.
Free choice drinks (mean +/− SEM) consumed in the bar lab after a priming drink in those with low or high self-control scores (BIS-11 subscale) while taking placebo or aripiprazole. See text for complete statistical analysis. * significant interaction of self-control level and medication (p < 0.05), ** significant difference in medication effect in low self-control group (p = 0.01). Figures are adjusted for baseline drinks per drinking day.
Two further analyses were conducted to evaluate the interaction between continuous BIS-11 total and self-control scores and medication group. Covarying for sex and smoking status, which were significant independent predictors of bar-lab drinking in these models, the interaction between total BIS-11 score and medication (F (1, 89) = 2.80, p = 0.098, partial η2 = 0.031) and the interaction between self-control score and medication (F (1, 89) = 2.62, p = 0.11, partial η2 = 0.029) were not significant; however, both were in the same direction as the analyses that used the median-split variables (i.e., aripiprazole, relative to placebo, reduced drinking more among subjects with greater impulsivity/less self-control). Also, to better understand how specific level of self-control affected the medication effect, self-control scores were grouped by quartile (Table 2). Covarying for baseline drinks per drinking day, the omnibus interaction between self-control quartile and medication was significant (F (3, 86) = 3.08, p = 0.032, partial η2 = 0.097). Post-hoc analysis indicated that the simple effect of medication on bar-lab drinking was significant only among individuals in the highest quartile (lowest self-control) (F(1, 86) = 10.15, p = 0.002, partial η2 = 0.11).
Table 2.
Medication effects on bar-lab drinking by BIS self-control scale quartile (higher scores imply less self-control).
| BIS Self-Control Score (Range) | Medication Group | Bar Lab Drinks Mean (SE) | Post-hoc test for difference | |
|---|---|---|---|---|
| Quartile 1 | 6–11 | Placebo | 4.7 (0.7) | F(1, 86) = 1.27, p = 0.26 |
| Aripiprazole | 3.6 (0.7) | |||
| Quartile 2 | 12–13 | Placebo | 3.0 (0.8) | F(1, 86) = 1.47, p = 0.23 |
| Aripiprazole | 4.5 (1.0) | |||
| Quartile 3 | 14–16 | Placebo | 4.0 (1.0) | F(1, 86) = 0.63, p = 0.43 |
| Aripiprazole | 3.0 (0.9) | |||
| Quartile 4 | 17–23 | Placebo | 6.2 (0.8) | F(1, 86) = 10.15, p = 0.002 |
| Aripiprazole | 2.5 (0.8) |
The omnibus interaction between BIS self-control quartile and medication was significant (F(3, 86) = 3.08, p = 0.032). Figures are estimated marginal means +/− standard errors, covarying for baseline drinks per drinking day.
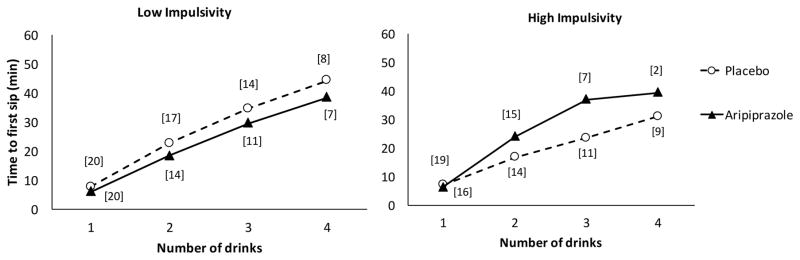
There was no significant main effect of medication on latency to drink in the bar lab, but the main effect of BIS-11 group approached significance (B = −0.34 [SE = 0.20], 95% CI = [−0.74–0.053], z = −1.70, p = 0.089), such that more impulsive participants tended to have shorter latencies between drinks. The interaction between medication and BIS-11 group was significant (B = 0.61 [SE = 0.30], 95% CI = [0.014–1.20], z = 2.01, p = 0.045), such that, among participants with greater impulsivity, those treated with aripiprazole, relative to placebo, had longer latencies between drinks, while among participants with lower impulsivity, there was no difference between aripiprazole and placebo (Figure 4). The interaction between medication and BIS-11 self-control group on latency to drink was in the same direction as the medication by BIS-11 group interaction but not significant (B = 0.47 [SE = 0.30], 95% CI = [−0.012–1.06], z = 1.57, p = 0.12).
Figure 4.
Mean time to first sip (min) of successive drinks from the first tray of free-choice drinks as a function of BIS-11 scores and medication. Brackets indicate the number of participants in each group who drank that number of drinks. The interaction between BIS-11 group and medication on latencies between drinks was significant (p < 0.05), such that aripiprazole, relative to placebo. increased latencies between drinks among high BIS, but not low BIS, subjects.
Participants who received aripiprazole had slightly, but significantly, higher peak BACs after the priming drink than those who received placebo (aripiprazole M = 0.025 (SD = 0.0013) g/dL; placebo M = 0.022 (SD = 0.0013) g/dL; F(1, 93) = 8.78, p = 0.004). However, both the main effect of medication (F(1, 89) = 4.69, p = 0.033, partial η2 = 0.05) and the interaction with BIS-11 self-control group (F(1, 89) = 4.55, p = 0.036, partial η2 = 0.049) on bar-lab drinking remained significant when peak BAC was covaried. The effect of peak BAC on latency to drink approached significance (B = −0.028 [SE = 0.015], z = −1.86, p = 0.063), such that participants with higher BACs had shorter latencies to drink, but the interaction between medication and BIS-11 group remained significant when peak BAC was covaried (B = 0.73 [SE = 0.31], 95% CI = [0.012–1.35], z = 2.33, p = 0.020).
Subjective Effects of Alcohol
There were no significant main effects or interactions of BIS-11 group, BIS-11 self-control group, or medication, on peak BAES stimulation after the priming drink. However, aripiprazole caused more sedation after the priming drink (M = 11.8, 95% CI = [8.9–14.6]) than placebo (M = 6.0, 95% CI = [3.2–8.8] (F(1, 90) = 8.14, p = 0.005, partial η2 = 0.083), but this was not modified by BIS-11 group. Importantly, covarying peak sedation did not influence our main finding of the BIS-11 self-control by medication interaction on bar lab drinking that remained significant (F(1, 89) = 4.45, p = 0.038).
Compliance and Reported Adverse Effects
Based on Day 7 riboflavin levels, approximately 75% of participants were judged adherent with study medication, with no significant difference between medication groups. Table 3 details the adverse effects reported by individuals taking aripiprazole compared to placebo. Trouble sleeping, daytime sleepiness, and low energy were reported significantly more frequently in the aripiprazole group, with some effects reported as severe. Irritability, trouble concentrating, nausea/vomiting, dizziness, and difficulty reaching orgasm were also reported significantly more frequently in the aripiprazole group, but most of these effects were reported as mild to moderate. It should be noted that no participant discontinued medication due to adverse events. Also, pre-study presence or absence of a complaint was not taken into account in this analysis. Given that more adverse events were reported by those taking aripiprazole, we conducted a sensitivity analysis by including the presence or absence of each of the above side effects in an analysis examining the main effect of medication on bar-lab drinking. Next, the effects that reduced the medication effect below statistical significance (daytime sleepiness, low energy, dizziness, and difficulty reaching orgasm) were entered together into a final analysis examining their impact on our major finding of the interaction between BIS-11 self-control group and medication group on bar-lab drinking. That interaction remained significant (F(1, 86) = 4.91, p = 0.029, partial η2 = 0.054) when these adverse events were covaried, suggesting that their presence was unrelated to the superior effect of aripiprazole in reducing bar-lab drinking among individuals with low self-control.
Table 3.
Adverse events reported.
| Aripiprazole (n = 47) | Placebo (n = 48) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | p1 | p2 | |
| Trouble sleeping | 20 | 10 | 4 | 11 | 8 | 1 | .018 | .003 |
| Daytime sleepiness | 12 | 22 | 9 | 15 | 8 | 0 | <.001 | <.001 |
| Nervousness | 16 | 3 | 1 | 10 | 1 | 1 | .301 | .070 |
| Irritability | 16 | 8 | 0 | 7 | 6 | 0 | .043 | .017 |
| Trouble concentrating | 17 | 12 | 2 | 15 | 2 | 1 | .008 | .006 |
| Feeling depressed | 6 | 3 | 0 | 9 | 2 | 0 | .669 | .652 |
| Abdominal pain | 4 | 0 | 0 | 4 | 0 | 0 | .975 | .975 |
| Nausea/vomiting | 10 | 0 | 0 | 1 | 0 | 0 | .003 | .003 |
| Constipation | 3 | 0 | 0 | 2 | 0 | 0 | .629 | .629 |
| Joint pain | 7 | 2 | 2 | 5 | 0 | 0 | .176 | .091 |
| Headache | 9 | 3 | 0 | 10 | 0 | 0 | .205 | .587 |
| Dizziness | 9 | 2 | 0 | 3 | 0 | 0 | .050 | .018 |
| Low energy | 17 | 17 | 5 | 20 | 0 | 0 | <.001 | <.001 |
| Skin rash | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
| Ears ringing | 3 | 1 | 0 | 3 | 0 | 0 | .596 | .673 |
| Vision blurry | 9 | 1 | 0 | 2 | 1 | 0 | .073 | .033 |
| Itching | 3 | 0 | 0 | 4 | 0 | 0 | .716 | .716 |
| Increased libido | 11 | 3 | 0 | 5 | 2 | 0 | .192 | .074 |
| Decreased libido | 7 | 3 | 0 | 8 | 0 | 0 | .205 | .566 |
| Difficulty reaching orgasm | 7 | 0 | 0 | 1 | 0 | 0 | .025 | .025 |
| Inability to reach orgasm | 2 | 0 | 0 | 1 | 0 | 0 | .545 | .545 |
p1 = Between-groups chi-squared for unequal distribution among 4 responses (none, mild, moderate, severe).
p2 = Between-groups chi-squared for presence or absence of symptom.
DISCUSSION
We previously reported (Voronin et al., 2008) in a small exploratory study that aripiprazole reduced drinking both under sub-acute natural conditions and in a bar lab paradigm, and that some of these effects were most evident in those with low self-control, as measured by the BIS-11. This work extends these findings in a larger experiment in which participants were prospectively randomized to aripiprazole or placebo based on their level of impulsivity (BIS-11 score). While aripiprazole did not significantly affect drinking during the natural observation period, after a priming drinking in the more controlled paradigm of the bar lab, individuals on aripiprazole chose to drink less than those on placebo. Importantly, consistent with our prior work, the aripiprazole effect on drinking was most evident among those with low trait self-control, especially in those with the most extreme low self-control scores. Further, aripiprazole also increased time between drinks among individuals with high trait impulsivity. It should be noted that in this paradigm, individuals were forced to choose between immediate (alcohol) and delayed (money) rewards. As such, the paradigm tests the ability to inhibit an expected immediate rewarding choice.
The brain dopamine system has been implicated both in reward perception and in modulating inhibitory responding (Volkow et al., 2007). For instance, alcohol and alcohol cues increase dopamine release and activity in both animal and human ventral striatum (Oberlin et al., 2013, Boileau et al., 2003) and modification of this effect could influence alcohol choice, drinking, and craving (Myrick et al., 2010). On the other hand, in other brain areas (e.g. prefrontal, orbital frontal) the dopamine system might play a separate and unique role in contextual appraisal and inhibition of pre-potent or salient responses (for review see (Cardinal, 2006, Congdon and Canli, 2005). Also, while it has been observed that alcohol acutely increases synaptic dopamine in both ventral striatum (Gonzales et al., 1998) and prefrontal cortex (Schier et al., 2013), chronic alcohol and substance use can lead to a hypo-dopaminergic state (Koob, 2003). Given this apparent dichotomy, an agent like aripiprazole that putatively “stabilizes” or “normalizes” dopamine transmission could positively modulate different brain areas, to affect drinking behavior. However, we cannot completely rule out either a main or partial contribution of aripiprazole’s 5HT2A antagonism. Possibly, it is the combination of aripiprazole effects on the dopamine and serotonin systems that is most important.
In the current study, aripiprazole did not largely impact drinking under natural conditions, in which participants, who were non-treatment-seeking, had no motivation to drink less. This finding differed from our previous work. Since it is likely that drinking under natural conditions is more variable (because of social influences, motivation, medication compliance, etc.), lack of congruence across studies might be expected. However, aripiprazole did impact the choice to drink under a forced choice reward paradigm. Consistent with its major impact being on choice/inhibition rather than reward saliency, it appeared to work mostly among those who rated themselves a priori as being impulsive and/or most likely to have low self-control. Impulsivity and self-control are overlapping but dissociable constructs; in the current study, total BIS scores were strongly (r = 0.85) but not perfectly correlated with self-control scale scores. One proposed distinction between the two is that self-control reflects the ability (or lack thereof) to employ a “top-down” regulatory strategy, while impulsivity reflects the extent to which “bottom-up” impulses are generated (Duckworth and Kern, 2011). This distinction is perhaps consistent with self-control’s moderating influence on aripiprazole’s effect on the choice to consume more drinks (arguably a behavior reflecting a top-down decisional process), and impulsivity’s moderating influence on aripiprazole’s effect on the time between drinks (arguably a behavior reflecting a bottom-up urge). However, further exploration of the manner in which these personality traits, as well as other measurements of both impulsivity and self-control, may impact medication response is warranted.
The clinical implications of these findings need to be further elaborated and tested. For instance, in a large controlled double blind study, aripiprazole did not have a significant effect on abstinent days but did show some reduction in drinks per day and other aspects of the AUD spectrum (Anton et al., 2008). Neither impulsivity nor self-control was measured in that study; these constructs might have been usefully employed to evaluate differential treatment effectiveness. Future clinical trials might use the relatively easy measurement of impulsivity and self-control provided by the BIS-11 or other personality inventories to select individuals to treat with aripiprazole. If the interaction of aripiprazole with low self-control is further replicated, it might be a useful pharmacotherapy paired with cognitive behavioral therapy or other approaches that encourage individuals to avoid or postpone drinking when tempted by cues or to substitute other behaviors for alcohol consumption. Those with lower self-control might particularly benefit from aripiprazole or other dopamine stabilizer/acting agents during such treatment attempts.
Although these data are consistent with our previous findings (Voronin et al., 2008), several factors limit their interpretation. Both studies employed a specific clinical lab paradigm and enrolled non-treatment-seeking individuals who were compensated for their participation. It is not clear whether these findings extend to more severe treatment-seeking individuals. In addition, the dose of aripiprazole used (15 mg), while moderate, might still have been higher and titrated more rapidly than needed in clinical settings. There were clear differences in the number of adverse effects reported by those on aripiprazole compared to placebo but these did not seem to influence adherence or the final results. Perhaps lower doses, titrated more slowly, might be as efficacious and more tolerable in clinical settings. While these data suggest that aripiprazole should not be considered for general AUD treatment, they do suggest those with more trait impulsivity (admittedly a limited number of all AUD individuals) might benefit from it.
In summary, in a large sub-acute natural drinking and bar lab paradigm, aripiprazole was effective in reducing alcohol consumption, but only when participants were forced to choose whether to drink in the context of a competing reward. Aripiprazole reduced the number of drinks consumed in this context the most among participants who rated themselves as having low self-control, and increased the amount of time between drinks the most among those with high impulsivity. As such, aripiprazole and other medications might be further evaluated for this population, perhaps leading to greater personalized pharmacotherapy for AUDs in the future.
Acknowledgments
This work was supported by NIAAA grants P50 AA010761 and K05AA017435.
The authors wish to acknowledge the efforts of Mark Ghent, BA who assisted in the collection of all data and coordinated the study. Ms. Emily Bristol, BA assisted with paper preparation. Pat Latham, PhD and Sarah Book, MD assisted with subject assessment. Yeong-Bin Im, M.S. performed the urine riboflavin measurements.
Footnotes
Clinicaltrials.gov NCT01292057
Disclosure: Dr. Anton has been a consultant for Alkermes, Eli Lilly, Novartis, Lundbeck, Glaxo Smith Kline, Roche, Techmira, Indivior, CT Pharma. He also received grant funding from Eli Lilly. He is a chair and participant in the Alcohol Clinical Trials Initiative (ACTIVE) that has received support from Abbvie, Alkermes, Arbor, Ethypharm, Glaxo Smith Kline, Indivior, Janssen, Eli Lilly, Lundbeck, Otsuka, Pfizer, Schering.
References
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and Statistical Manual of Mental Disorders (4th edition): DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- ANTON RF, KRANZLER H, BREDER C, MARCUS RN, CARSON WH, HAN J. A randomized, multicenter, double-blind, placebo-controlled study of the efficacy and safety of aripiprazole for the treatment of alcohol dependence. J Clin Psychopharmacol. 2008;28:5–12. doi: 10.1097/jcp.0b013e3181602fd4. [DOI] [PubMed] [Google Scholar]
- ANTON RF, MOAK DH, LATHAM PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- ANTON RF, VORONIN KK, RANDALL PK, MYRICK H, TIFFANY A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin Exp Res. 2012;36:2000–7. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHOK AH, MIZUNO Y, VOLKOW ND, HOWES OD. Association of Stimulant Use With Dopaminergic Alterations in Users of Cocaine, Amphetamine, or Methamphetamine: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2017 doi: 10.1001/jamapsychiatry.2017.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAHI A, DREYER JL. Lentiviral vector-mediated dopamine d3 receptor modulation in the rat brain impairs alcohol intake and ethanol-induced conditioned place preference. Alcohol Clin Exp Res. 2014;38:2369–76. doi: 10.1111/acer.12503. [DOI] [PubMed] [Google Scholar]
- BECHARA A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–63. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- BELIN D, MAR AC, DALLEY JW, ROBBINS TW, EVERITT BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–5. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLONIGEN DM, TIMKO C, MOOS BS, MOOS RH. Impulsivity is an independent predictor of 15-year mortality risk among individuals seeking help for alcohol-related problems. Alcohol Clin Exp Res. 2011;35:2082–92. doi: 10.1111/j.1530-0277.2011.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOILEAU I, ASSAAD JM, PIHL RO, BENKELFAT C, LEYTON M, DIKSIC M, TREMBLAY RE, DAGHER A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–31. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- BOWDEN-JONES H, MCPHILLIPS M, ROGERS R, HUTTON S, JOYCE E. Risk-taking on tests sensitive to ventromedial prefrontal cortex dysfunction predicts early relapse in alcohol dependency: a pilot study. J Neuropsychiatry Clin Neurosci. 2005;17:417–20. doi: 10.1176/jnp.17.3.417. [DOI] [PubMed] [Google Scholar]
- BURRIS KD. Aripiprazole, a Novel Antipsychotic, Is a High-Affinity Partial Agonist at Human Dopamine D2 Receptors. Journal of Pharmacology and Experimental Therapeutics. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- CAINE SB, NEGUS SS, MELLO NK, PATEL S, BRISTOW L, KULAGOWSKI J, VALLONE D, SAIARDI A, BORRELLI E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002;22:2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARDINAL RN. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- CHEN AC, PORJESZ B, RANGASWAMY M, KAMARAJAN C, TANG Y, JONES KA, CHORLIAN DB, STIMUS AT, BEGLEITER H. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcohol Clin Exp Res. 2007;31:156–65. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- CONGDON E, CANLI T. The endophenotype of impulsivity: reaching consilience through behavioral, genetic, and neuroimaging approaches. Behav Cogn Neurosci Rev. 2005;4:262–81. doi: 10.1177/1534582305285980. [DOI] [PubMed] [Google Scholar]
- CREWS FT, BOETTIGER CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLEY JW, FRYER TD, BRICHARD L, ROBINSON ES, THEOBALD DE, LAANE K, PENA Y, MURPHY ER, SHAH Y, PROBST K, ABAKUMOVA I, AIGBIRHIO FI, RICHARDS HK, HONG Y, BARON JC, EVERITT BJ, ROBBINS TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE RIDDER DT, LENSVELT-MULDERS G, FINKENAUER C, STOK FM, BAUMEISTER RF. Taking stock of self-control: a meta-analysis of how trait self-control relates to a wide range of behaviors. Pers Soc Psychol Rev. 2012;16:76–99. doi: 10.1177/1088868311418749. [DOI] [PubMed] [Google Scholar]
- DELEON A, PATEL NC, CRISMON ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26:649–66. doi: 10.1016/s0149-2918(04)90066-5. [DOI] [PubMed] [Google Scholar]
- DICK DM, SMITH G, OLAUSSON P, MITCHELL SH, LEEMAN RF, O’MALLEY SS, SHER K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–26. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGHERTY DM, MATHIAS CW, TESTER ML, MARSH DM. Age at first drink relates to behavioral measures of impulsivity: the immediate and delayed memory tasks. Alcoholism: Clinical and Experimental Research. 2004;28:408–414. doi: 10.1097/01.alc.0000117834.53719.a8. [DOI] [PubMed] [Google Scholar]
- DROBES DJ, ANTON RF, THOMAS SE, VORONIN K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology. 2003;28:755–64. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- DUCKWORTH AL, KERN ML. A Meta-Analysis of the Convergent Validity of Self-Control Measures. J Res Pers. 2011;45:259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRST MB, SPITZER RL, GIBBON M, WILLIAMS JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- GONZALES RA, MCNABB J, YIM HJ, RIPLEY T, BUNGAY PM. Quantitative microdialysis of ethanol in rat striatum. Alcohol Clin Exp Res. 1998;22:858–67. [PubMed] [Google Scholar]
- GRANT BF, DAWSON DA, STINSON FS, CHOU SP, DUFOUR MC, PICKERING RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- GRANT BF, SAHA TD, RUAN WJ, GOLDSTEIN RB, CHOU SP, JUNG J, ZHANG H, SMITH SM, PICKERING RP, HUANG B, HASIN DS. Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry. 2016;73:39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINZ A, SIESSMEIER T, WRASE J, HERMANN D, KLEIN S, GRUSSER-SINOPOLI SM, FLOR H, BRAUS DF, BUCHHOLZ HG, GRUNDER G, SCHRECKENBERGER M, SMOLKA M, ROSCH MN, MANN K, BARTEINSTEIN P. Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- INGMAN K, KUPILA J, HYYTIA P, KORPI ER. Effects of aripiprazole on alcohol intake in an animal model of high-alcohol drinking. Alcohol Alcohol. 2006;41:391–8. doi: 10.1093/alcalc/agl037. [DOI] [PubMed] [Google Scholar]
- JENTSCH JD, ASHENHURST JR, CERVANTES MC, GROMAN SM, JAMES AS, PENNINGTON ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KECK PE, JR, CALABRESE JR, MCQUADE RD, CARSON WH, CARLSON BX, ROLLIN LM, MARCUS RN, SANCHEZ R ARIPIPRAZOLE STUDY G. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry. 2006;67:626–37. doi: 10.4088/jcp.v67n0414. [DOI] [PubMed] [Google Scholar]
- KIRBY LG, ZEEB FD, WINSTANLEY CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–32. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISHI T, SEVY S, CHEKURI R, CORRELL CU. Antipsychotics for primary alcohol dependence: a systematic review and meta-analysis of placebo-controlled trials. J Clin Psychiatry. 2013;74:e642–54. doi: 10.4088/JCP.12r08178. [DOI] [PubMed] [Google Scholar]
- KOOB GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- KRANZLER HR, COVAULT J, PIERUCCI-LAGHA A, CHAN G, DOUGLAS K, ARIAS AJ, ONCKEN C. Effects of aripiprazole on subjective and physiological responses to alcohol. Alcohol Clin Exp Res. 2008;32:573–9. doi: 10.1111/j.1530-0277.2007.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREEK MJ, NIELSEN DA, BUTELMAN ER, LAFORGE KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–7. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- LAWLER CP, PRIOLEAU C, LEWIS MM, MAK C, JIANG D, SCHETZ JA, GONZALEZ AM, SIBLEY DR, MAILMAN RB. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology. 1999;20:612–27. doi: 10.1016/S0893-133X(98)00099-2. [DOI] [PubMed] [Google Scholar]
- LE FOLL B, GOLDBERG SR, SOKOLOFF P. The dopamine D3 receptor and drug dependence: effects on reward or beyond? Neuropharmacology. 2005;49:525–41. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- LEEMAN RF, BESELER CL, HELMS CM, PATOCK-PECKHAM JA, WAKELING VA, KAHLER CW. A brief, critical review of research on impaired control over alcohol use and suggestions for future studies. Alcohol Clin Exp Res. 2014;38:301–8. doi: 10.1111/acer.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z, ICHIKAWA J, DAI J, MELTZER HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- LITTEN RZ, WILFORD BB, FALK DE, RYAN ML, FERTIG JB. Potential medications for the treatment of alcohol use disorder: An evaluation of clinical efficacy and safety. Subst Abus. 2016;37:286–98. doi: 10.1080/08897077.2015.1133472. [DOI] [PubMed] [Google Scholar]
- MACKILLOP J, WEAFER J, JCG, OSHRI A, PALMER A, DE WIT H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology (Berl) 2016;233:3361–70. doi: 10.1007/s00213-016-4372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALLIKAARJUN S, SALAZAR DE, BRAMER SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44:179–87. doi: 10.1177/0091270003261901. [DOI] [PubMed] [Google Scholar]
- MARDER SR, MCQUADE RD, STOCK E, KAPLITA S, MARCUS R, SAFFERMAN AZ, SAHA A, ALI M, IWAMOTO T. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61:123–36. doi: 10.1016/s0920-9964(03)00050-1. [DOI] [PubMed] [Google Scholar]
- MARTIN CS, EARLEYWINE M, MUSTY RE, PERRINE MW, SWIFT RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism Clinical & Experimental Research. 1993;17:140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- MARTINOTTI G, ORSOLINI L, FORNARO M, VECCHIOTTI R, DE BERARDIS D, IASEVOLI F, TORRENS M, DI GIANNANTONIO M. Aripiprazole for relapse prevention and craving in alcohol use disorder: current evidence and future perspectives. Expert Opin Investig Drugs. 2016;25:719–28. doi: 10.1080/13543784.2016.1175431. [DOI] [PubMed] [Google Scholar]
- MOFFITT TE, ARSENEAULT L, BELSKY D, DICKSON N, HANCOX RJ, HARRINGTON H, HOUTS R, POULTON R, ROBERTS BW, ROSS S, SEARS MR, THOMSON WM, CASPI A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci U S A. 2011;108:2693–8. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY A, NESTOR LJ, MCGONIGLE J, PATERSON L, BOYAPATI V, ERSCHE KD, FLECHAIS R, KUCHIBATLA S, METASTASIO A, ORBAN C, PASSETTI F, REED L, SMITH D, SUCKLING J, TAYLOR E, ROBBINS TW, LINGFORD-HUGHES A, NUTT DJ, DEAKIN JF, ELLIOTT R. Acute D3 Antagonist GSK598809 Selectively Enhances Neural Response During Monetary Reward Anticipation in Drug and Alcohol Dependence. Neuropsychopharmacology. 2017;42:1049–1057. doi: 10.1038/npp.2016.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRICK H, ANTON R, VORONIN K, WANG W, HENDERSON S. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31:221–7. doi: 10.1111/j.1530-0277.2006.00299.x. [DOI] [PubMed] [Google Scholar]
- MYRICK H, LI X, RANDALL PK, HENDERSON S, VORONIN K, ANTON RF. The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol. 2010;30:365–72. doi: 10.1097/JCP.0b013e3181e75cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’MALLEY SS, KRISHNAN-SARIN S, FARREN C, SINHA R, KREEK MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- OBERLIN BG, DZEMIDZIC M, TRAN SM, SOEURT CM, ALBRECHT DS, YODER KK, KAREKEN DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–24. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPACHRISTOU H, NEDERKOORN C, HAVERMANS R, VAN DER HORST M, JANSEN A. Can’t stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacology (Berl) 2012;219:511–8. doi: 10.1007/s00213-011-2240-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTON JH, STANFORD MS, BARRATT ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- PERRY JL, CARROLL ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- RAY LA, HUTCHISON KE, TARTTER M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des. 2010;16:2149–58. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- REHM J, DAWSON D, FRICK U, GMEL G, ROERECKE M, SHIELD KD, GRANT B. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res. 2014;38:1068–77. doi: 10.1111/acer.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIO G, JIMENEZ M, RODRIGUEZ-JIMENEZ R, MARTINEZ I, AVILA C, FERRE F, JIMENEZ-ARRIERO MA, PONCE G, PALOMO T. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcohol Clin Exp Res. 2008;32:1681–7. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- SCHIER CJ, DILLY GA, GONZALES RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the Long-Evans rat. Alcohol Clin Exp Res. 2013;37:740–7. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONS JS, CAREY KB, GAHER RM. Lability and impulsivity synergistically increase risk for alcohol-related problems. Am J Drug Alcohol Abuse. 2004;30:685–94. doi: 10.1081/ada-200032338. [DOI] [PubMed] [Google Scholar]
- SKINNER HA, HORN JL. Alcohol Dependence Scale: Users Guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- SOBELL LC, SOBELL MB, LEO GI, CANCILLA A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- SULLIVAN JT, SYKORA K, SCHNEIDERMAN J, NARANJO CA, SELLERS EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- THANOS PK, KATANA JM, ASHBY CR, JR, MICHAELIDES M, GARDNER EL, HEIDBREDER CA, VOLKOW ND. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav. 2005;81:190–7. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- VERDEJO-GARCIA A, LAWRENCE AJ, CLARK L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- VOLKOW ND, FOWLER JS, WANG GJ, SWANSON JM, TELANG F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- VON DIEMEN L, BASSANI DG, FUCHS SC, SZOBOT CM, PECHANSKY F. Impulsivity, age of first alcohol use and substance use disorders among male adolescents: a population based case-control study. Addiction. 2008;103:1198–205. doi: 10.1111/j.1360-0443.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- VORONIN K, RANDALL P, MYRICK H, ANTON R. Aripiprazole effects on alcohol consumption and subjective reports in a clinical laboratory paradigm--possible influence of self-control. Alcohol Clin Exp Res. 2008;32:1954–61. doi: 10.1111/j.1530-0277.2008.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON TE. Total body water and alcohol levels: updating the fundamentals. In: BATT CA, editor. Human Metabolism of Alcoholism. Boca Raton: CRC Press; 1989. [Google Scholar]
- WILCOX CE, DEKONENKO CJ, MAYER AR, BOGENSCHUTZ MP, TURNER JA. Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neurosci. 2014;25:1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINSTANLEY CA. The orbitofrontal cortex, impulsivity, and addiction: probing orbitofrontal dysfunction at the neural, neurochemical, and molecular level. Ann N Y Acad Sci. 2007;1121:639–55. doi: 10.1196/annals.1401.024. [DOI] [PubMed] [Google Scholar]
- YODER KK, KAREKEN DA, SEYOUM RA, O’CONNOR SJ, WANG C, ZHENG QH, MOCK B, MORRIS ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–70. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- ZOCCHI A, FABBRI D, HEIDBREDER CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neuroscience Letters. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]