Abstract
Extra-virgin olive oil (EVOO) has several health promoting effects. Evidence have shown that EVOO attenuates the pathology of amyloid-β (Aβ) and improves cognitive function in experimental animal models, suggesting it’s potential to protect and reduce the risk of developing Alzheimer’s disease (AD). Available studies have linked this beneficial effect to oleocanthal, one of the active components in EVOO. The effect of oleocanthal against AD pathology has been linked to its ability to attenuate Aβ and tau aggregation in vitro, and enhance Aβ clearance from the brains of wild type and AD transgenic mice in vivo. However, the ability of oleocanthal to alter the toxic effect of Aβ on brain parenchymal cells is unknown. In the current study, we investigated oleocanthal effect on modulating Aβ oligomers (Aβo) pathological events in neurons and astrocytes. Our findings demonstrated oleocanthal prevented Aβo-induced synaptic proteins, SNAP-25 and PSD-95, down-regulation in neurons, and attenuated Aβo-induced inflammation, glutamine transporter (GLT1) and glucose transporter (GLUT1) down-regulation in astrocytes. Aβo-induced inflammation was characterized by interleukin-6 (IL-6) increase and glial fibrillary acidic protein (GFAP) upregulation that were reduced by oleocanthal. In conclusion, this study provides further evidence to support the protective effect of EVOO-derived phenolic secoiridoid oleocanthal against AD pathology.
Keywords: Oleocanthal, astrocytes, neurons, amyloid-β, Neuroinflammation
Introduction
Alzheimer’s disease (AD) is considered one of the major dementia-related disorders affecting the elderly. The number of AD patients is expected to increase significantly over the coming decades due to increasing life expectancy. Currently, in the United States, AD affects 5 million individuals. However, it is expected to rise to 16 million by 2050 (Alzheimer’s, 2015). Amyloid-β (Aβ) peptide is considered one of the major pathological hallmarks of AD (Glenner and Wong, 1984, Masters et al., 1985). Amyloid precursor protein (APP) is a membrane-bound protein that with the activity of β- and γ-secretases- produces Aβ as a degradation product (Selkoe, 2001). Depending on secretase-γ site of cleavage, Aβ could be cleaved with a different number of amino acids, including Aβ40 and Aβ42 (Selkoe, 2001). However, Aβ42 is considered more pathological as the addition of last two amino acids increases its hydrophobicity and the ability to form higher molecular weight aggregates including Aβ oligomers (Aβo) and fibrils (Selkoe, 2001). Aβo are involved in synaptic dysregulation and interferes with the function of endogenous ligands on susceptible receptors (Sakono and Zako, 2010), while fibrilar Aβ is involved in plaques formation which presents a typical AD manifestation in several brain regions (Murphy and LeVine, 2010).
Astrocytes are glial cells known for their star-like appearance and play a key role in neurological diseases (Batarseh et al., 2016). Astrocytes anatomical location is distinctive being near brain blood vessels, and play a major role in maintaining the BBB integrity (Abbott, 2002). Furthermore, astrocytes are crucial members of the neurovascular unit, working hand in hand with neurons (Bell and Zlokovic, 2009). As part of their neuro-supportive function, astrocytes are responsible for energy regulation; they express glucose transporter-1 (GLUT1) responsible for glucose uptake. Astrocytes convert glucose into lactate used by neurons as a source of energy (Benarroch, 2014). Additionally, astrocytes play an important role in glutamate rapid clearance from neuronal synapses by glutamate transporter-1 (GLT1) (Perego et al., 2000). Besides, the high levels of Aβo observed the brains of AD patients is associated with pathological activation of astrocytes leading to GLT1 down-regulation (Scimemi et al., 2013), and cytokines and free radicals secretion (Li et al., 2011). Astrocytes are capable of clearing Aβ by multiple mechanisms including: Aβ monomers (Aβm) uptake by the function of several receptors and transporters such as LRP1 (Auderset et al., 2016), by direct degradion via endosomal-lysosomal pathways and secretion of degradation enzymes, such as insulin-degrading enzyme (IDE) into the brain parenchyma (Son et al., 2016), and/or by indirect activation of ATP-binding cassette transporter-A1 (ABCA1) function, which is responsible for the lipidation of apolipoprotein E (ApoE) (Wahrle et al., 2004).
Neuronal cells are mainly responsible for sending and receiving electrical signals through synapses (Busche and Konnerth, 2016). The maintenance of tightly regulated synaptic function is essential for proper neuronal signaling (Busche and Konnerth, 2016). Aβ peptides disrupt synapsis by initiating synaptic loss (Narayan et al., 2014, Busche and Konnerth, 2016). Some of the major down-regulated synaptic markers in AD include the post-synaptic marker PSD-95 and the pre-synaptic marker SNAP-25 (Greber et al., 1999, Tu et al., 2014). In addition to Aβm, Aβo inhibit neuronal long-term potentiation and affect synaptic plasticity (Selkoe, 2008), and initiate tau hyperphosphorylation leading to neuronal apoptosis (Kayed and Lasagna-Reeves, 2013, Sengupta et al., 2016).
S(-)-Oleocanthal is one of the phenolic components of extra-virgin olive oil (EVOO) (Beauchamp et al., 2005). It has been identified as an anti-inflammatory agent (Parkinson and Keast, 2014). Our recent findings suggested a positive role for oleocanthal in enhancing Aβ clearance across the blood-brain barrier (BBB) and reducing Aβ load in wild type and transgenic AD animals (Abuznait et al., 2013, Qosa et al., 2015). Furthermore, it reduced astrocytes activation and interluken-1β levels in vivo (Qosa et al., 2015). Other studies demonstrated the ability of oleocanthal to modify Aβ aggregation pattern, creating less toxic aggregates (Pitt et al., 2009), and to reduce formation of hyperphosphorylated tau aggregates by interfering with the aggregation sites on tau protein (Li et al., 2009). However, the preventive effect of oleocanthal on Aβo-induced pathological changes in astrocytes and neuronal cells is still unknown. This study aims to investigate the role of oleocanthal in rectifying Aβo deleterious effects on neurons and astrocytes in vitro.
Methods
Preparation of synthetic amyloid-β oligomers (Aβo)
Solutions of synthetic biotin tagged Aβ42 peptides (AnaSpec, Inc., CA) were prepared by suspending in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; Sigma-Aldrich, MO) at a concentration of 1 mM and incubated for 1 h at room temperature for complete solubilization, then dried with nitrogen gas. Aβ42 peptide HFIP-film was suspended in anhydrous DMSO to a final concentration of 5 mM. DMSO solution of Aβ42 was diluted with phenol red-free F-12 cell culture media (Gibco, NY) to a concentration of 100 μM, vortexed for 1 min and incubated at 4°C for 24 h. At the end of the incubation period, Aβ42 oligomers solution was centrifuged at 14,000 rpm, 4°C for 10 min, aliquoted and stored at −80°C for the experiments.
Cell Culture
Astrocyte cultures were prepared from CCF-STTG1 human astrocytoma cell line (ATCC, VA). Cells were maintained in RPMI-1640 medium containing 10% fetal bovine serum (FBS; Gibco, NY). For the experiments, CCF-STTG1 cells were seeded on 24-well plates or cell culture dishes. Media was changed every other day until cells are ready for experiments. Neuronal cultures were prepared from SH-SY5Y cell line transfected with APP695 (hereafter SH-SY5Y-APP) or their corresponding non-transfected SH-SY5Y cells. Cells were maintained in DMEM media supplemented with 10% FBS containing geneticin (Gibco) added at 400 μg/ml to SH-SY5Y-APP cells. Cultures were maintained in a humidified atmosphere (5%CO2/95% air) at 37°C and media was changed every other day.
Time and concentration-dependent uptake of Aβ in astrocytes
The uptake of Aβm and Aβo was measured in CCF-STTG1 cells using ELISA. Cells were seeded in 24-well plates. For time-dependent experiments, astrocytes were treated with 1500 nM Aβo or 200 nM Aβm for 0, 5, 15, 30, 60, 120, 180, and 360 min. For concentration-dependent studies, cells were treated with 500, 1000, and 2000 nM of Aβo for 5 min; or 25, 50, 100, 200, and 400 nM Aβm for 15 min. At the end of treatment time cells were washed two times with ice cold 2% bovine serum albumin (BSA) in phosphate buffer saline (PBS) to minimize non-specific binding of Aβ and once with PBS (Qosa et al., 2014, Esparza et al., 2016). Media and cell lysates were loaded on Aβo and Aβm specific ELISA as described below.
Astrocytes treatment with Aβo and oleocanthal
CCF-STTG1 cells grown on cell culture dishes were treated with 100 nM Aβo, 5 μM oleocanthal, or 100 nM Aβo and 5μM oleocanthal for 3 or 7 days. To induce Aβo inflammatory condition, the media of astrocytes were daily spiked with treatments (without changing the medium). Total accumulative dose of DMSO (oleocanthal vehicle) was less than 0.1%. Cells were collected and stored in −80°C for further experiments. Astrocytes conditioned media (ACM) from treated astrocytes with 100 nM Aβo, 5 μM oleocanthal, or 100 nM Aβo and 5μM oleocanthal for 3 and 7 days were collected followed by addition of fresh media for 4 h to collect post treatment produced IL-6.
To investigate the effect of Aβo and oleocanthal treatments on the uptake and degradation of Aβm, in another set of experiments, astrocytes were grown on a 24-well plate and treated with the same treatments described above for 3 or 7 days. At the end of treatment time, media containing Aβo, oleocanthal and their combination were removed. Cells were washed 2-times with ice cold 2% BSA in PBS and once with PBS. Fresh media containing Aβm (200 nM) was then added. Fifteen minutes later, cells and media were collected for analysis by ELISA as described previously (Qosa et al., 2015).
Neuronal cells treatment with Aβo and oleocanthal
SH-SY5Y-APP and SH-SY5Y cells were grown on cell culture dishes, treated with 100 nM Aβo, 5 μM oleocanthal, or 100 nM Aβo and 5 μM oleocanthal for 3 and 7 days. To induce Aβo synaptopathological changes, treatments were daily spiked to the media of neurons (without changing the medium). Total accumulative dose of DMSO (vehicle) was less than 0.1%. Cells were collected and stored in −80°C for analysis. In another set of experiments, SH-SY5Y-APP or SH-SY5Y cells were treated with collected ACM; ACM was changed daily for 3 days then neuronal cells were washed, lysed and stored in −80°C for Western blot analysis.
Aβm, Aβo and IL-6 ELISA
Biotin-tagged Aβm and Aβo levels were determined by two-site sandwich ELISA for Aβm and one-site sandwich ELISA for Aβo. Before the ELISA assay, cells were lysed with RIPA buffer (Thermo-Scientific, NY) containing 1% protease inhibitor (v/v; Thermo-Scientific). Neutravidine (Thermo-Scientific) was used for capturing biotin-tagged Aβo at 1:4000 dilution, while 6E10 Aβ antibody (BioLegend, CA; binds on 3–8 amino acids of Aβ) was used to capture biotin-tagged Aβm at 1:350 dilution. Detection of Aβm was achieved with HRP-conjugated 4G8 monoclonal antibody (BioLegend; binds on 17–23 amino acids of Aβ) at 1:1000 dilution. Aβo detection was achieved with HRP-conjugated streptavidin (Rockland Immunochemicals Inc., PA) at 1:2000 dilution. In this assay, Aβo captured by nutravidin can be detected by HRP-conjugated streptavidin only if there is at least one more accessible epitope to be detected by HRP conjugated streptavidin on biotin-tagged Aβo. For detection of IL-6 levels in astrocytes media, at the end of treatment time, media was replaced with fresh media for 4 h to evaluate treatments effect on IL-6 levels using anti-human IL-6 Quantikine ELISA kit (R&D Systems, MN) used according to the manufacturer’s instructions. All samples were run at least in triplicate.
Western blot analysis
Treated cells were lysed with RIPA buffer containing protease inhibitor on ice for 1 h then centrifuged at 21,000 × g for 10 min at 4oC. The supernatant was collected and stored in −80°C until the time of analysis. For Western blot analysis, samples were resolved on 10% tris-glycine gels in tris-glycine-SDS buffer system and electro-transferred onto a 0.45 μm nitrocellulose membrane. Membranes were blocked with 2% BSA and incubated overnight with monoclonal antibodies; analyzed astrocytes proteins include LRP1 (Abcam, MA), GLT1, IDE, neprilysin (NEP), GLUT1, glial fibrillary acidic protein (GFAP), ABCA1, the housekeeping protein GAPDH (all from Santa Cruz, TX); neuronal proteins include LRP1 (Abcam), SNAP-25 and PSD-95 (GeneTex, MI), APP (4G8), and soluble APPα (sAPPα) and soluble APPβ (sAPPβ) (Immuno-Biological Laboratories, MN). For detection, the membranes were washed free of primary antibody and incubated with HRP-labeled secondary IgG anti-mouse antibody for PSD-95, ABCA1, APP (4G8), sAPPα, GAPDH; anti-rabbit antibody for GLT1, LRP1, GLUT1, NEP, sAPPβ, SNAP-25; and anti-goat antibody for IDE and GFAP (Santa Cruz). The bands were visualized using a Pierce chemiluminescence detection kit (Thermo Scientific). Quantitative analysis of the immunoreactive bands was performed using Li-Core luminescent image analyzer (LI-COR Biotechnology), and band intensity was measured by densitometric analysis.
Statistical analysis
The data were expressed as mean ± SD. Results were statistically analyzed for significant difference using Student’s t-test. Values of P<0.05 were considered statistically significant.
Results
Oleocanthal doesn’t alter Aβo-induced Aβm degradation and ABCA1 up-regulation in astrocytes
Initially, the uptake profiles of Aβm and Aβo by CCF-STTG1 cells were compared. The uptake of Aβm and Aβo was linear and did not reach saturation in the examined ranges (Figs. 1 A and B). Time-dependent uptake studies showed Aβo uptake is faster than Aβm with uptake peak of 5 min for Aβo compared to 15 min for Aβm (Figs. 1C and D). However, the uptake of Aβo was lower with 75% remained in the media 6 h after its addition (Fig. 1F) compared to 40% of Aβm (Fig. 1E). These results suggested that compared to Aβo astrocytes uptake and degradation of Aβm is more efficient. In addition, 3 or 7 days treatment of astrocytes with Aβo significantly reduced Aβm (200 nM) by 19 (Fig. 1G, P<0.001) and 32% (Fig. 1I, P<0.001), respectively, in the media that was associated with reduced cellular levels of intact Aβm by 17% (Fig. 1H, P<0.05) and 23% (Fig. 1J, P<0.001), respectively. These results suggest that astrocytes treatment with Aβo for 3 or 7 days enhanced the degradation of Aβm. Oleocanthal treatment alter the effect of Aβo exposure on Aβm uptake and degradation (Fig. 1 G–J).
Figure 1.
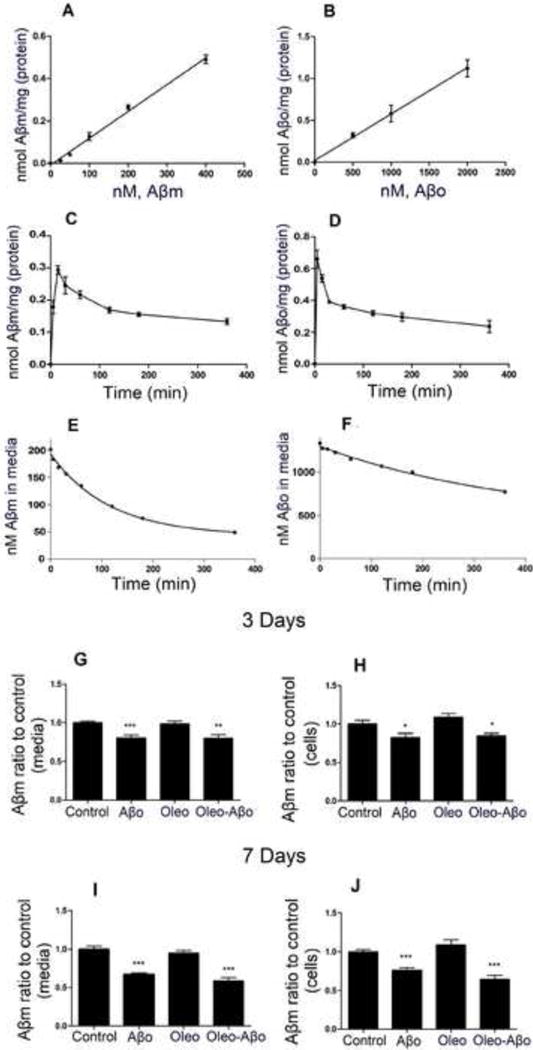
Astrocytes showed linear and rapid uptake of both Aβm and Aβo. (A) Concentration-in cellular uptake of Aβm, (C) time-dependent increase in cellular uptake of Aβm, and (E) time-dependent decrease in Aβm levels in media. (B) Concentration-dependent increase in cellular uptake of Aβo, (D) time-dependent increase in cellular uptake of Aβo, and (F) time-dependent decrease in Aβo levels in media. After 3 and 7 days treatment with Aβo, oleocanthal or combination, Aβm (200 nM) uptake study was initiated for 15 min, (G) effect of treatments on Aβm levels in astrocytes media (3 days treatment), (H) effect of treatments on Aβm levels in cell lysate (3 days treatment), (I) effect of treatments on Aβm levels in astrocytes media (7 days treatment), and (J) effect of treatments on Aβm levels in cell lysate (7 days treatment). Values are normalized to the control. Data is presented as mean ± SD, n= 3 independent experiments.
Next, the expression of astrocytes proteins involved in Aβ clearance including LRP1, ABCA1, NEP and IDE were evaluated. Treatment of CCF-STTG1 cells for 3 and 7 days with Aβo or oleocanthal didn’t alter LRP1, NEP and IDE proteins expression (Fig. 2A); however 3 and 7-days treatments with Aβo induced ABCA1 by 35% (Fig. 2B, P<0.05) and 45% (Fig. 2B, P<0.01), respectively. On the other hand, oleocanthal treatment for 3 or 7 days didn’t change baseline expression of ABCA1 nor modulated the effect of Aβo (Fig. 2B).
Figure 2.
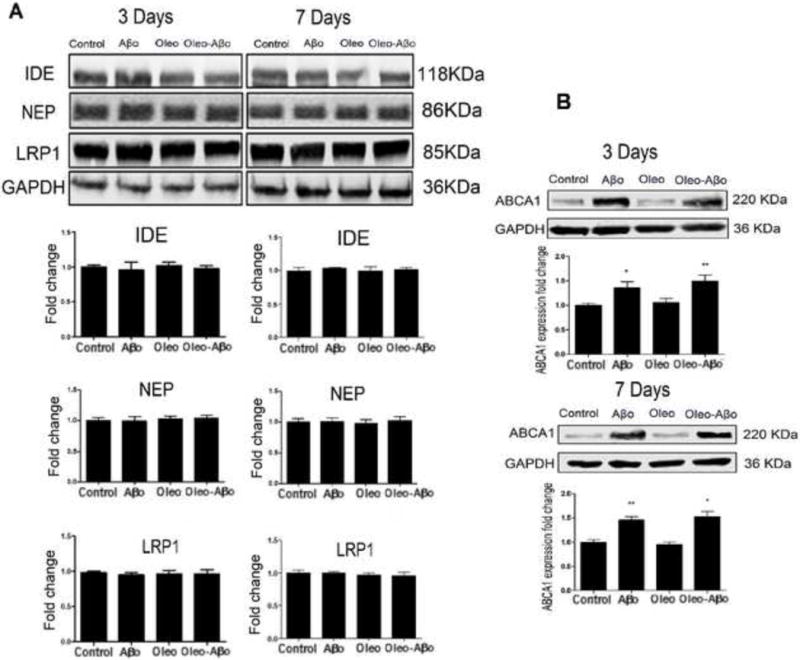
(A) Representative Western blots and densitometry analysis of IDE, NAP and LRP1 in CCF-STTG1 cells after 3 and 7 days treatment with Aβo, oleocanthal, or combination. None of the treatments altered these proteins expression. (B) Representative Western blots and densitometry analysis of ABCA1 in CCF-STTG1 cells after 3 and 7 days treatment with Aβo, oleocanthal, or combination. ABCA1 was significantly up-regulated by Aβo treatment for 3 and 7 days; oleocanthal addition didn’t significantly alter ABCA1 expression. Data is presented as mean ± SD (*P<0.05, **P<0.01, ***P<0.001), n= 3 independent experiments.
Oleocanthal reduces Aβo-induced inflammatory response and attenuates Aβo deleterious effects on the expression of neuro-supportive proteins in astrocytes
We assessed Aβo-induced inflammatory response by measuring the levels of IL-6 and GFAP. As shown in Fig. 3, IL-6 levels were significantly increased in a time-dependent manner in response to Aβo exposure for 3 days by 83% (Fig. 3A, P<0.001) and for 7 days by 147% (Fig. 3B, P<0.001). In the absence of Aβo, oleocanthal treatment (3 and 7 days) reduced IL-6 levels by 26% (Fig. 3A, P<0.01) and 45% (Fig. 3B, P<0.001) respectively, and this effect was maintained in the presence of Aβo where oleocanthal attenuated Aβo inflammatory effect measured by IL-6 levels by 21% (Fig. 3A, P<0.01) and 53% (Fig. 3B, P<0.001), after 3 and 7 days, respectively, when compared to Aβo treatment alone. Three days treatment with Aβo has no effect on GFAP levels (Fig. 3C). However, GFAP expression was significantly increased by 38% (Fig. 3D, P<0.05) following 7 days of Aβo treatment, and oleocanthal was able to restore Aβo-induced GFAP to the control level (Fig. 3D).
Figure 3.
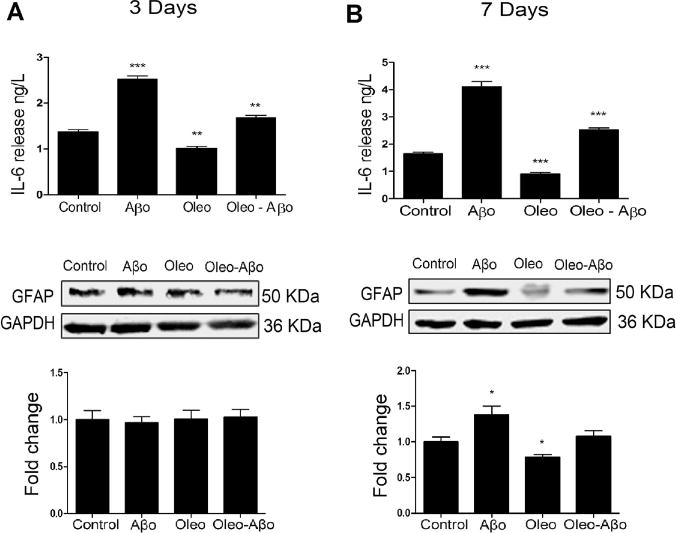
After 3 (A) and 7 days (B) treatment with Aβo, oleocanthal, or combination, ACM was collected for IL-6 measurement using ELISA. Oleocanthal reduced the baseline release of IL-6 and attenuated Aβo-induced secretion of IL-6; values were normalized to the control. The relative expression of astrocytes’ GFAP with the same treatments and duration as above, was determined using Western blot analysis. (C) Representative Western blots and densitometry analysis of GFAP showed significant up-regulation by the 3 days exposure to Aβo; oleocanthal addition attenuated Aβo induced GFAP up-regulation. (D) Representative Western blots and densitometry analysis of GFAP showed a significant up-regulation by the 7 days exposure to Aβo; oleocanthal addition attenuated Aβo induced GFAP up-regulation. Data is presented as mean ± SD (*P<0.05, **P<0.01, ***P<0.001), n= 3 independent experiments.
Furthermore, GLT1 is one of the major astrocytes transporters responsible for the rapid clearance of glutamate from the synaptic area (Perego et al., 2000). Aβo treatment for 3 days didn’t alter GLT1 expression (Data not shown). On the other hand, 7 days treatment with Aβo significantly down-regulated GLT1 in astrocytes by 27% (Fig. 4, P<0.01). Oleocanthal addition rectified GLT1 expression to control level (Fig. 4). Besides glutamate regulation, astrocytes play an essential role in glucose uptake for neuronal energy utilization (Fuller et al., 2010). Our data showed a significant down-regulation of GLUT1 in astrocytes only with 7 days of Aβo exposure by 17% (Fig. 4, P<0.05); oleocanthal addition restored Aβo-induced down-regulation to control level (Fig. 4).
Figure 4.
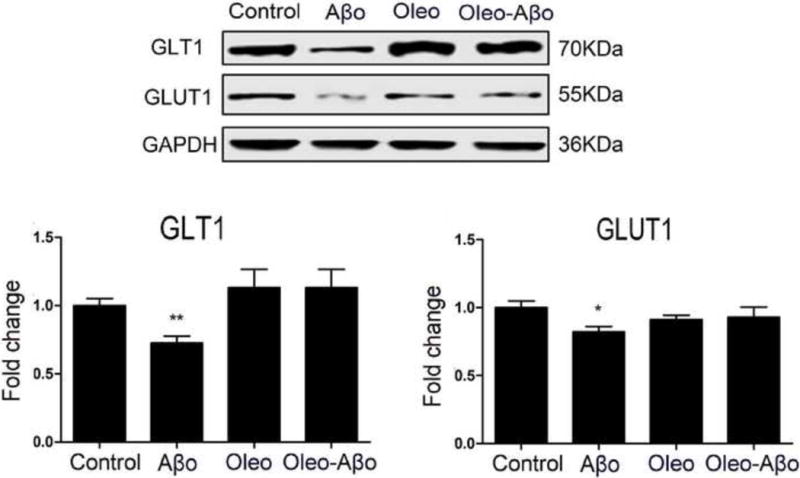
Representative Western blots and densitometry analysis of GLT1 and GLUT1 expressions in astrocytes treated for 7 days with Aβo, oleocanthal, or combination showed that Aβo significantly down-regulated both proteins that were rectified by oleocanthal treatment. Data is presented as mean ± SD (*P<0.05, **P<0.01), n= 3 independent experiments.
Oleocanthal attenuates Aβo-induced synaptic proteins down-regulation but has no effect on Aβ production markers in SH-SY5Y-APP cells
We measured expression of the synaptic proteins GLT1, PSD-95, and SNAP-25. While 3 days treatment with Aβo has no significant effect on synaptic proteins and Aβ production markers (Data not shown), 7 days treatment significantly reduced the expression of GLT1 and PSD-95 by 33% (Fig. 5A, P<0.05) and 19% (Fig. 5A, P<0.05), respectively. Oleocanthal addition induced the baseline expression of GLT1 by 36% (Fig. 5A, P<0.05) and PSD-95 by 49% (Fig. 5A, P<0.05), and maintained their up-regulation in the presence of Aβo exposure. While SNAP-25 expression was not altered by the 3 or 7 days exposure with Aβo (Fig. 5A), oleocanthal 7 days treatment up-regulated SNAP-25 by 29% (Fig. 5A, P<0.05) and maintained this effect in the presence of Aβo (Fig. 5A, P<0.05).
Figure 5.
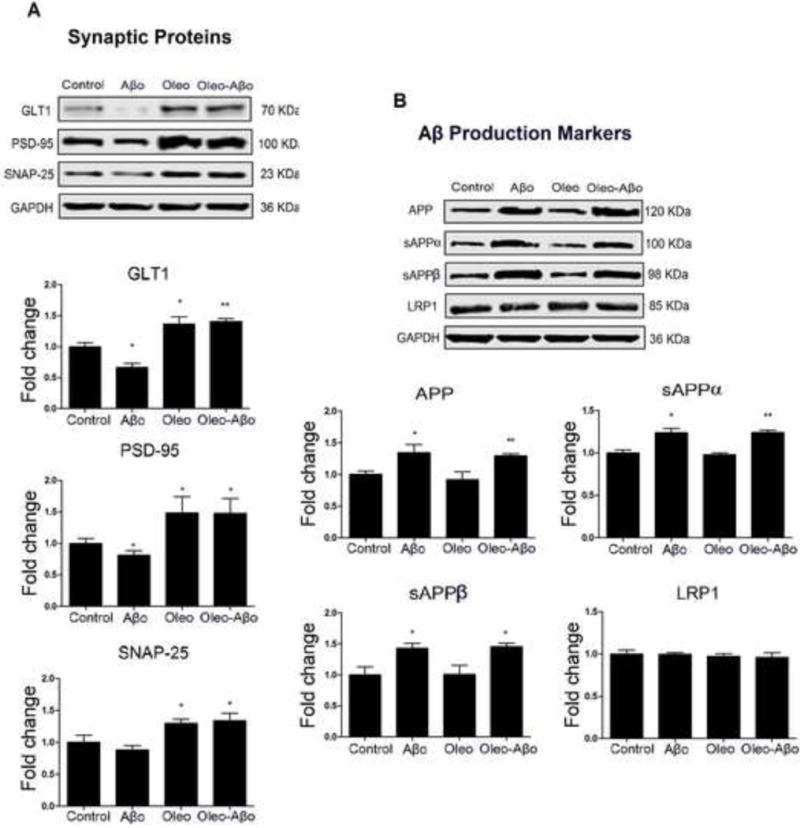
(A) Representative Western blots and densitometry analysis of GLT1, PSD-95, and SNAP-25 following 7 days treatment with Aβo, oleocanthal, or combination. Aβo significantly reduced GLT1 and PSD-95 expressions that were rectified by oleocanthal to levels above their baseline expressions; SNAP-25 was not significantly altered by the 7 days exposure to Aβo. (B) Representative Western blots and densitometry analysis of APP, sAPPα, and sAPPβ; 7 days exposure to Aβo significantly increased APP, sAPPα, and sAPPβ levels that were not modulated by oleocanthal. LRP1 was not significantly altered by the treatments. Data is presented as mean ± SD (*P<0.05, **P<0.01), n = 3 independent experiments.
The expressions of Aβ production markers: APP, sAPPα and sAPPβ, and LRP1 in SH-SY5Y-APP cells were also evaluated to investigate effect of Aβo and oleocanthal on Aβ production. Seven days treatment with Aβo up-regulated the expression of APP by 34% (Fig. 5B, P<0.05), which was associated with increased levels of sAPPα and sAPPβ by 23 and 43%, respectively (Fig. 5B, P<0.05). Oleocanthal treatment has no significant effect on the expression of APP, sAPPα, and sAPPβ, and didn’t rectify their Aβo-induced levels (Fig. 5B). Neuronal LRP1 is associated with Aβ production by binding and internalizing APP (Spuch et al., 2012). LRP1 expression wasn’t significantly changed by any of the treatments (Fig. 5B). Three-day treatments with Aβo, oleocanthal or their combination didn’t significantly affect the expression of these proteins (Data not shown).
SH-SY5Y cells respond differently to Aβo and/or oleocanthal treatments compared to SH-SY5Y-APP cells
The effect of Aβo, oleocanthal and combination on the synaptic proteins GLT1, PSD-95, and SNAP-25 was also studied in SH-SY5Y cells that express endogenous levels of APP. Contrary to SH-SY5Y-APP, none of the treatments altered GLT1, PSD-95, and SNAP-25 in mock cells (Fig. 6). A comparison between SH-SY5Y-APP and SH-SY5Y on the expression of synaptic proteins demonstrated that the base expression of GLT1, PSD-95, and SNAP-25 in SH-SY5Y-APP cells was lower by 31% (Fig. 7, P<0.001), 32% (Fig. 7, P<0.001) and 57% (Fig. 7, P<0.001), respectively, suggesting that chronic Aβ exposure in SH-SY5Y-APP cells down-regulated the expression of synaptic proteins.
Figure 6.
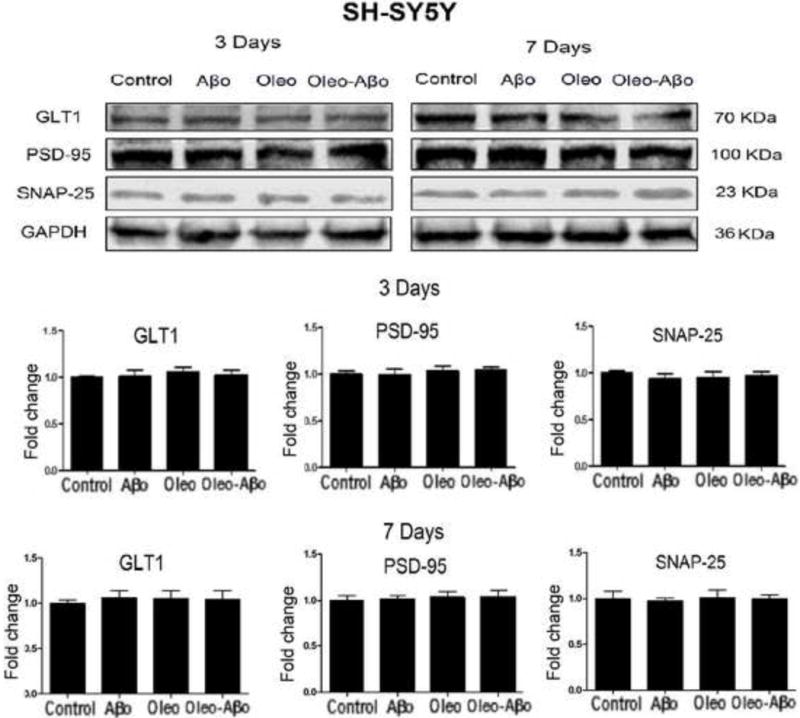
Representative Western blots and densitometry analysis of GLT1, PSD-95, and SNAP-25 in SH-SY5Y after 3 and 7 days treatment with Aβo, oleocanthal, or combination. None of the treatments altered these proteins expression in SH-SY5Y cells. Data is presented as mean ± SD, n= 3 independent experiments.
Figure 7.
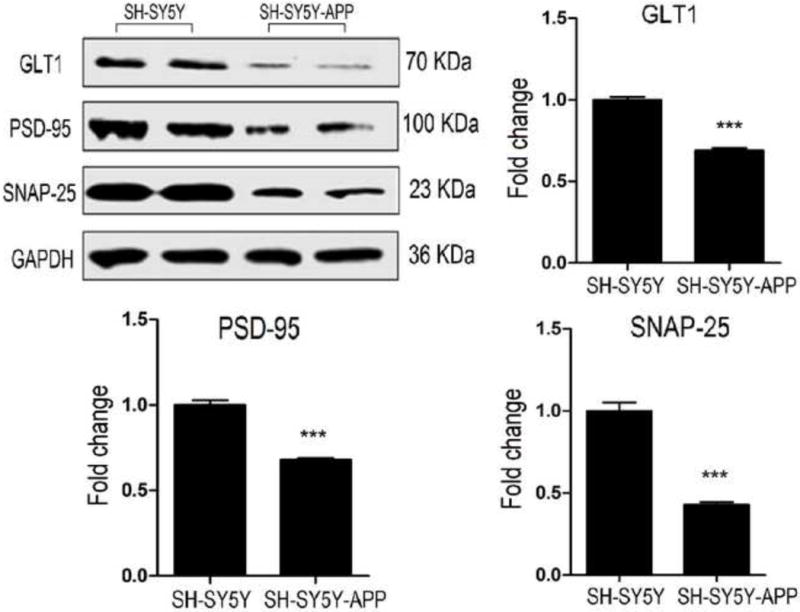
The relative expression of neuronal synaptic proteins GLT1, PSD-95, and SNAP-25 were compared in SH-SY5Y-APP and non-transfected SH-SY5Y cells using Western blot. Densitometry analysis of GLT1, PSD-95, and SNAP-25 revealed significantly lower expressions in SH-SY5Y-APP cells compared to SH-SY5Y cells. Data is presented as mean ± SD (***P<0.001), n= 3 independent experiments.
Oleocanthal doesn’t change astrocytes ACM effect on neuronal synaptic proteins
ACM collected from CCF-STTG1 cells exposed to the treatments for 7 days was used to treat SH-SY5Y-APP and SH-SY5Y cells for 3 days. At the end of 3 days, cells were lysed for Western blotting of the neuronal synaptic proteins GLT1, PSD-95, and SNAP-25. ACM from astrocytes exposed to Aβo enhanced the expression of GLT1, PSD-95, and SNAP-25 in SH-SY5Y by 80% (Fig. 8A, P<0.001), 70% (Fig. 8A, P<0.01) and 71% (Fig. 8A, P<0.001), respectively. On the other hand, in APP transfected cells, ACM from Aβo treated cells only induced SNAP-25 expression by 81% (Fig. 8B, P<0.01). ACM from astrocytes treated with oleocanthal was not able to alter the expression of neuronal synaptic proteins in both neuronal cell lines (Fig. 8), suggesting that the effect of oleocanthal on neuronal synaptic proteins is direct and not mediated by the astrocytes.
Figure 8.
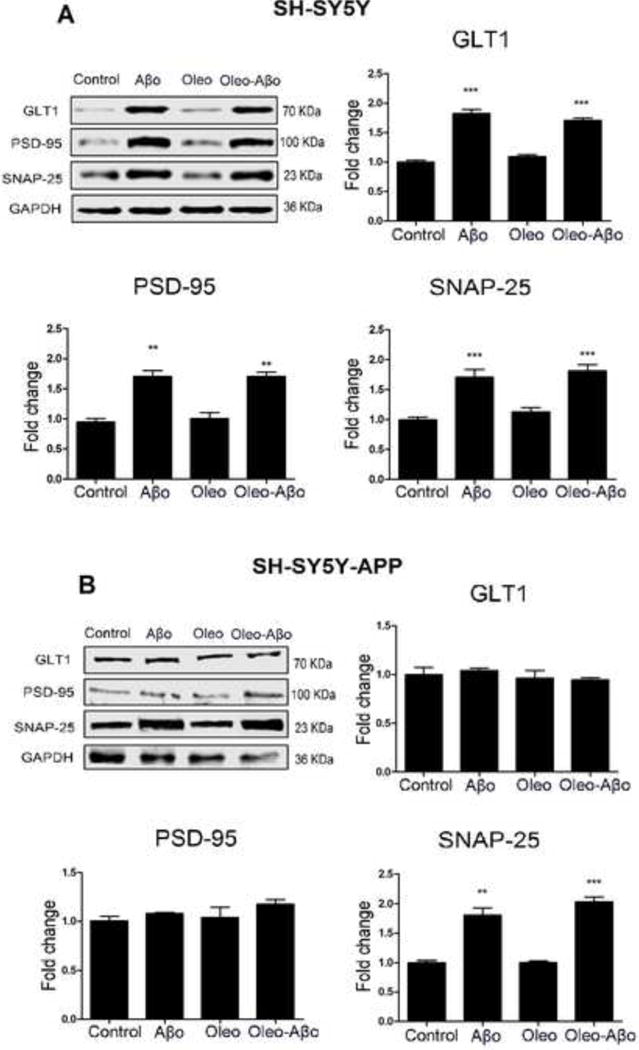
The relative expression of neuronal synaptic proteins GLT1, PSD-95, and SNAP-25 was measured after 3 days of ACM addition to SH-SY5Y and SH-SY5Y-APP cells using Western blot analysis. (A) Representative Western blots and densitometry analysis showed ACM from Aβo and combination treated astrocytes to significantly increase GLT1, PSD-95, and SNAP-25 expressions in SH-SY5Y cells. Oleocanthal treatment has no effect on their expression levels. (B) Representative Western blots and densitometry analysis showed ACM from Aβo, oleocanthal and the combination treated astrocytes have no effect on GLT1 and PSD-95 expressions in SH-SY5Y-APP cells. Only the expression of SNAP-25 was significantly increased by ACM from Aβo and combination treatments. Data is presented as mean ± SD (**P<0.01, ***P<0.001), n= 3 independent experiments.
Discussion
Aβ aggregation plays a significant role in AD pathology. The interaction of Aβ with astrocytes is recognized where astrocytes are capable of Aβ uptake and degradation (Wyss-Coray et al., 2003). To understand astrocytes’ contribution to Aβ pathology (Kayed and Lasagna-Reeves, 2013), it is essential to evaluate astrocytes uptake profiles of different Aβ aggregate forms. While astrocytes showed low uptake capacity to Aβ fibrils (Nielsen et al., 2010), a direct comparison between Aβm and Aβo is lacking. Here, we performed studies to compare Aβm and Aβo time and concentration-dependent uptake profiles by astrocytes. The results suggested astrocytes have efficient uptake capacity for both Aβm and Aβo with linear profiles in the studied concentration ranges. Besides, astrocytes uptake of Aβo was faster with a peak of less than 5 min, compared to 15 min with Aβm. This observation suggests that Aβo may have faster uptake pathways and/or Aβo, such as annular protofibrils, have higher capacity to form hydrophobic interactions with astrocytes cell membrane allowing the rapid attachment (Lasagna-Reeves et al., 2011). On the other hand, compared to Aβm, Aβo showed 35% higher media accumulation after 6 h incubation, suggesting an effecient uptake and/or degradation of Aβm, compared to Aβo by astrcytes. Moreover astrocytes activation by Aβo enhanced Aβm degradation. While Aβo did not alter the expression of Aβm degradation enzymes, IDE and NEP (Fig. 2A), the increased degradation could be related to the effect of Aβo on ABCA1 expression, which was induced. ABCA1 plays important role in the lipidation of ApoE, and its upregulation is expected to increase ApoE lipidation and thus enhancing Aβm degradation via the ApoE clearance pathway (Wahrle et al., 2004). Oleocanthal, however, didn’t alter ABCA1 expression, unlike our previous in vivo finding that showed oleocanthal ability to induce ABCA1 expression in AD transgenic mouse model (Qosa et al., 2015). Such controversy could be related to treatment time were in the in vivo studies mice were treated with oleoacnthal for one month. Oleocanthal’s in vivo effect could also involves a machinery that is absent in the simple in vitro models. Additionally, while oleocanthal showed a significant effect in reducing astrocytes activation, as measured by IL-6 and GFAP levels, it didn’t influence the responsiveness of astrocytes to Aβo on Aβm uptake and degradation (Figs. 1 G–J). This observation supports the neuroprotective effect of astrocytes in reducing excess Aβ (Belanger and Magistretti, 2009), which was not altered by oleocanthal.
Oleocanthal has an anti-inflammatory effect and available studies on chondrocytes and macrophages suggested oleocanthal exerts such effect by reducing IL-6 release (Scotece et al., 2012). Consistently, oleocanthal treatment in the current study reduced the baseline IL-6 level in astrocytes, indicating that oleocanthal extends its anti-inflammatory effects to the brain. Aβo exposure increased astrocytes IL-6 and GFAP levels, and oleocanthal treatment was able to attenuate the Aβo inflammatory effect.
One of the most distinctive features of astrocytes is their ability to multi-task between different neuro-supportive roles in the brain, including synaptic glutamate regulation and glucose homeostasis. Available reportes showed that increased levels of Aβ to down-regulate GLT1 level in astrocytes and interfere with glucose regulation (Perego et al., 2000, Benarroch, 2005, Scimemi et al., 2013, Beglopoulos et al., 2016). Similarly, our findings showed that Aβo down-regulated GLT1 and GLUT1 expressions, that were rectified by oleocanthal treatment.
Multiple studies showed SH-SY5Y cells have dopamine-β-hydroxylase activity, and can convert glutamate to the neurotransmitter GABA (Biedler et al., 1978, Cobos et al., 2007, Brown et al., 2014). Neurons function depends on the synaptic transmission, in which synapse density proteins play a key role; in AD several studies showed synapses dysfunction and loss to occur before neurons loss, thus attention has been paid to synapse-associated proteins, such as PSD-95 and SNAP-25. PSD-95 is a postsynaptic scaffolding protein that regulates synaptic distribution and activity of glutamate receptors (Tu et al., 2014); SNAP-25 is a component of the SNARE complex, which is central to synaptic vesicle exocytosis, and, by directly interacting with different calcium channels subunits, it negatively modulates neuronal voltage-gated calcium channels, thus regulating intracellular calcium dynamics (Antonucci et al., 2016). SH-SY5Y cells express both synaptic proteins, among others, and reduced levels of these synaptic proteins is expected to alter the cells function. Several studies reported that Aβo lead to down-regulation in synaptic markers (Leuba et al., 2008, Feng et al., 2014, Fernandes et al., 2017), and the loss or modification of synaptic proteins directly affects their properties, ultimately impacting synaptic function (Antonucci et al., 2016).
The potential of oleocathal to rectify the toxic effects of Aβo on synaptic markers expression in SH-SY5Y-APP cells was evaluated. While GLT1 is mainly expressed in astrocytes, evidence showed GLT1 is also expressed in neurons and contributes to the neuronal uptake of glutamate (Danbolt et al., 2016, Rimmele and Rosenberg, 2016). Studies tested the effect of Aβo on neuronal GLT1 are lacking. Here, we showed that Aβo down-regulated GLT1 expression in SH-SY5Y-APP cells, an alteration that could affect neurons capacity to clear synaptic glutamate.
Oleocanthal not only prevented Aβo induced GLT1 down-regulation but also induced the baseline expression of GLT1, suggesting an additional protection mechanism against the glutamate toxicity. PSD-95 dysregulation is likely an important intermediate step in the pathological cascade of events caused by Aβ since its expression and function plays a critical role in protein assembly, synaptic development, and neural plasticity. In addition, the expression of SNAP-25 in the brains of AD patients is lower than control subjects with normal cognitive function, suggesting that Aβ may affect SNAP-25 expression (Greber et al., 1999). Consistently, findings from this in vitro study demonstrated that SH-SY5Y-APP cells treatment with Aβo for 7 days down-regulated PSD-95 but not SNAP-25 levels. Our findings also showed that oleocanthal induced the baseline expression of both synaptic markers, and attenuated Aβo toxic effects by maintaining their up-regulation in the presence of Aβo. Collectively suggesting oleocanthal has the potential to rectify Aβo-induced synaptic loss.
Furthermore, our results showed that Aβo treatment increased Aβ production markers in SH-SY5Y-APP cells, which is consistent with available literature studies (Perez et al., 2010). However, our results also suggest that Aβo not only induce the expression of APP, but also modulate the processing of APP where Aβo selectively increased the levels of sAPPβ (a product of secretase-β), compared to sAPPα (a product of secretase-α), which suggest that Aβo induce secretase-β activity and thus further contribute to the pathological load of Aβ. Oleocanthal, however, was not able to rectify the increased production of Aβ markers caused by Aβo, which is consistent with our previous in vivo studies (Qosa et al., 2015). Yet, these findings indicate oleocanthal ability to provide synapto-protective effect in spite of increased levels of Aβ.
With the progression of AD pathology, neurons are subjected to continuous exposure of increasing levels of Aβ compared to healthy brain; due to this pathological brain environment one would predict neuronal cells to respond differently in health and pathology. As expected, the continuous exposure to high levels of Aβ, beyond endogenous levels, SH-SY5Y-APP cells expressed lower levels of GLT1, PSD-95 and SNAP-25 when compared to SH-SY5Y cells that produce much lower concentrations of endogenous Aβ. Interestingly, however, unlike SH-SY5Y-APP cells, SH-SY5Y cells didn’t react to 7 days of exposure to Aβo and/or oleocanthal, which suggests neuronal cells vulnerability to Aβ toxicity and their responsiveness to oleocanthal treatment as the disease progresses.
To investigate neuronal cells response to activated and oleocanthal treated astrocytes, ACM was collected after 7 days of treatment and was added to SH-SY5Y and SH-SY5Y-APP cells for 3 days. Interestingly, the addition of ACM collected from treated astrocytes to neuronal cells demonstrated differential effects between SH-SY5Y-APP and SH-SY5Y cells. ACM from astrocytes exposed to Aβo induced the expression of synaptic proteins in SH-SY5Y; SH-SY5Y-APP cells, however, failed to up-regulate synaptic proteins with the exception of SNAP-25. While further studies are required to explain this observation, available studies reported that astrocytes respond to Aβo by secreting signaling molecules, such as ATP, to counteract Aβo synaptotoxic effect and up-regulate synaptic proteins (Siow et al., 2005, Jung et al., 2012), which could explain SH-SY5Y response to Aβo-ACM; on the other hand the continuous exposure to high levels of produced Aβ in SH-SY5Y-APP cells may desensitized the cells to ACM effect on synaptic proteins. The addition of oleocanthal to astrocytes treatment didn’t change this observation, suggesting that: 1) activated astrocytes, caused by addition of Aβo, released molecules that benefit healthy neuronal cells but not those with Aβ pathology (Fig. 8), and 2) the beneficial effect of oleocanthal on neuronal cells is direct and not mediated by the astrocytes (Fig. 5 and 7).
In conclusion, findings of this study showed that oleocanthal attenuated Aβo induced inflammation, restored astrocytes neuro-supportive function by preventing Aβo down-regulation effects on GLT1 and GLUT1 transporters in astrocytes, and attenuated Aβo induced synaptic proteins down-regulation in SH-SY5Y-APP neurons. The effect of oleocanthal on neuronal cells could be direct and not mediated by astrocytes protective crosstalk.
Highlights.
-
-
Oleocanthal rectified Aβo-induced pathological changes in astrocytes and neurons in-vitro.
-
-
Oleocanthal reduced astrocytes activation and inflammation associated with Aβ oligomers exposure.
-
-
The neuroprotective effect of oleocanthal could be direct and not mediated by astrocytes.
Acknowledgments
This project was supported by grants from the National Institute of General Medical Sciences under grant number P20GM103424 and the National Institute of Neurological Disorders and Stroke under grant number R15NS091934. We thank Dr. Elizabeth A. Eckman, Biomedical Research Institute of New Jersey, NJ, USA for providing the APP transfected SH-SY5Y cell line.
List of Abbreviations
- Aβ
Amyloid-β
- ACM
Astrocytes conditioned media
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- Aβo
Aβ oligomers
- Aβm
Aβ monomer
- ABCA1
ATP-binding cassette transporter-A1
- ApoE
Apolipoprotein E
- BBB
Blood brain barrier
- BSA
Bovine serum albumin
- DMSO
Dimethyl sulfoxide
- EVOO
Extra-virgin olive oil
- GLT1
Glutamate transporter
- GLUT1
Glucose transporter
- GFAP
Glial fibrillary acidic protein
- IL-6
Interleukin-6
- IDE
Insulin-degrading enzyme
- PBS
Phosphate buffer saline
- RIPA
Radioimmunoprecipitation assay
- sAPPα
Soluble APPα
- sAPPβ
soluble APPβ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that no conflict interests exist. dependent increase
References
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. Journal of anatomy. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuznait AH, Qosa H, Busnena BA, El Sayed KA, Kaddoumi A. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: in vitro and in vivo studies. ACS chemical neuroscience. 2013;4:973–982. doi: 10.1021/cn400024q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s A. 2015 Alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions. Frontiers in synaptic neuroscience. 2016;8:7. doi: 10.3389/fnsyn.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auderset L, Cullen CL, Young KM. Low Density Lipoprotein-Receptor Related Protein 1 Is Differentially Expressed by Neuronal and Glial Populations in the Developing and Mature Mouse Central Nervous System. PloS one. 2016;11:e0155878. doi: 10.1371/journal.pone.0155878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh YS, Duong QV, Mousa YM, Al Rihani SB, Elfakhri K, Kaddoumi A. Amyloid-beta and Astrocytes Interplay in Amyloid-beta Related Disorders. International journal of molecular sciences. 2016;17:338. doi: 10.3390/ijms17030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Keast RS, Morel D, Lin J, Pika J, Han Q, Lee CH, Smith AB, Breslin PA. Phytochemistry: ibuprofen-like activity in extra-virgin olive oil. Nature. 2005;437:45–46. doi: 10.1038/437045a. [DOI] [PubMed] [Google Scholar]
- Beglopoulos V, Tulloch J, Roe AD, Daumas S, Ferrington L, Watson R, Fan Z, Hyman BT, Kelly PA, Bard F, Morris RG. Early detection of cryptic memory and glucose uptake deficits in pre-pathological APP mice. Nature communications. 2016;7:11761. doi: 10.1038/ncomms11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Magistretti PJ. The role of astroglia in neuroprotection. Dialogues in clinical neuroscience. 2009;11:281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta neuropathologica. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clinic proceedings. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Brain glucose transporters: implications for neurologic disease. Neurology. 2014;82:1374–1379. doi: 10.1212/WNL.0000000000000328. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer research. 1978;38:3751–3757. [PubMed] [Google Scholar]
- Brown D, Tamas A, Reglodi D, Tizabi Y. PACAP protects against inflammatory-mediated toxicity in dopaminergic SH-SY5Y cells: implication for Parkinson’s disease. Neurotoxicity research. 2014;26:230–239. doi: 10.1007/s12640-014-9468-x. [DOI] [PubMed] [Google Scholar]
- Busche MA, Konnerth A. Impairments of neural circuit function in Alzheimer’s disease. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2016;371 doi: 10.1098/rstb.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos EJ, del Pozo E, Baeyens JM. Irreversible blockade of sigma-1 receptors by haloperidol and its metabolites in guinea pig brain and SH-SY5Y human neuroblastoma cells. Journal of neurochemistry. 2007;102:812–825. doi: 10.1111/j.1471-4159.2007.04533.x. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Furness DN, Zhou Y. Neuronal vs glial glutamate uptake: Resolving the conundrum. Neurochemistry international. 2016;98:29–45. doi: 10.1016/j.neuint.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Datki Z, Juhasz A, Galfi M, Soos K, Papp R, Zadori D, Penke B. Method for measuring neurotoxicity of aggregating polypeptides with the MTT assay on differentiated neuroblastoma cells. Brain research bulletin. 2003;62:223–229. doi: 10.1016/j.brainresbull.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Esparza TJ, Wildburger NC, Jiang H, Gangolli M, Cairns NJ, Bateman RJ, Brody DL. Soluble Amyloid-beta Aggregates from Human Alzheimer’s Disease Brains. Scientific reports. 2016;6:38187. doi: 10.1038/srep38187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Hu P, Lu SJ, Wang R, Du YF. Effects of APP 5-mer peptide analogue P165 on the synaptic proteins and insulin signal transduction proteins. International journal of clinical and experimental medicine. 2014;7:549–557. [PMC free article] [PubMed] [Google Scholar]
- Fernandes LS, Emerick GL, Ferreira RS, Santos NA, Santos AC. High concentration of trichlorfon (1mM) disrupts axonal cytoskeleton and decreases the expression of plasticity-related proteins in SH-SY5Y cells. Toxicology in vitro: an international journal published in association with BIBRA. 2017;39:84–92. doi: 10.1016/j.tiv.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Fuller S, Steele M, Munch G. Activated astroglia during chronic inflammation in Alzheimer’s disease–do they neglect their neurosupportive roles? Mutation research. 2010;690:40–49. doi: 10.1016/j.mrfmmm.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochemical and biophysical research communications. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Greber S, Lubec G, Cairns N, Fountoulakis M. Decreased levels of synaptosomal associated protein 25 in the brain of patients with Down syndrome and Alzheimer’s disease. Electrophoresis. 1999;20:928–934. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<928::AID-ELPS928>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jung ES, An K, Hong HS, Kim JH, Mook-Jung I. Astrocyte-originated ATP protects Abeta(1–42)-induced impairment of synaptic plasticity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:3081–3087. doi: 10.1523/JNEUROSCI.6357-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Lasagna-Reeves CA. Molecular mechanisms of amyloid oligomers toxicity. Journal of Alzheimer’s disease: JAD. 2013;33(Suppl 1):S67–78. doi: 10.3233/JAD-2012-129001. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, Glabe CG, Kayed R. Amyloid-beta annular protofibrils evade fibrillar fate in Alzheimer disease brain. The Journal of biological chemistry. 2011;286:22122–22130. doi: 10.1074/jbc.M111.236257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuba G, Walzer C, Vernay A, Carnal B, Kraftsik R, Piotton F, Marin P, Bouras C, Savioz A. Postsynaptic density protein PSD-95 expression in Alzheimer’s disease and okadaic acid induced neuritic retraction. Neurobiology of disease. 2008;30:408–419. doi: 10.1016/j.nbd.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, Chui D, Yu AC. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Current Alzheimer research. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- Li W, Sperry JB, Crowe A, Trojanowski JQ, Smith AB, 3rd, Lee VM. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. Journal of neurochemistry. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder SD, Veerhuis R, Blankenstein MA, Nielsen HM. The effect of amyloid associated proteins on the expression of genes involved in amyloid-beta clearance by adult human astrocytes. Experimental neurology. 2012;233:373–379. doi: 10.1016/j.expneurol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Murphy MP, LeVine H., 3rd Alzheimer’s disease and the amyloid-beta peptide. Journal of Alzheimer’s disease: JAD. 2010;19:311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan P, Holmstrom KM, Kim DH, Whitcomb DJ, Wilson MR, St George-Hyslop P, Wood NW, Dobson CM, Cho K, Abramov AY, Klenerman D. Rare individual amyloid-beta oligomers act on astrocytes to initiate neuronal damage. Biochemistry. 2014;53:2442–2453. doi: 10.1021/bi401606f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HM, Mulder SD, Belien JA, Musters RJ, Eikelenboom P, Veerhuis R. Astrocytic A beta 1–42 uptake is determined by A beta-aggregation state and the presence of amyloid-associated proteins. Glia. 2010;58:1235–1246. doi: 10.1002/glia.21004. [DOI] [PubMed] [Google Scholar]
- Parkinson L, Keast R. Oleocanthal, a phenolic derived from virgin olive oil: a review of the beneficial effects on inflammatory disease. International journal of molecular sciences. 2014;15:12323–12334. doi: 10.3390/ijms150712323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, Pietrini G. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. Journal of neurochemistry. 2000;75:1076–1084. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- Perez JL, Carrero I, Gonzalo P, Arevalo-Serrano J, Sanz-Anquela JM, Ortega J, Rodriguez M, Gonzalo-Ruiz A. Soluble oligomeric forms of beta-amyloid (Abeta) peptide stimulate Abeta production via astrogliosis in the rat brain. Experimental neurology. 2010;223:410–421. doi: 10.1016/j.expneurol.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Pitt J, Roth W, Lacor P, Smith AB, 3rd, Blankenship M, Velasco P, De Felice F, Breslin P, Klein WL. Alzheimer’s-associated Abeta oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicology and applied pharmacology. 2009;240:189–197. doi: 10.1016/j.taap.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Abuasal BS, Romero IA, Weksler B, Couraud PO, Keller JN, Kaddoumi A. Differences in amyloid-beta clearance across mouse and human blood-brain barrier models: kinetic analysis and mechanistic modeling. Neuropharmacology. 2014;79:668–678. doi: 10.1016/j.neuropharm.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa H, Batarseh YS, Mohyeldin MM, El Sayed KA, Keller JN, Kaddoumi A. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS chemical neuroscience. 2015;6:1849–1859. doi: 10.1021/acschemneuro.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmele TS, Rosenberg PA. GLT-1: The elusive presynaptic glutamate transporter. Neurochemistry international. 2016;98:19–28. doi: 10.1016/j.neuint.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. The FEBS journal. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG. Amyloid-beta1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:5312–5318. doi: 10.1523/JNEUROSCI.5274-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotece M, Gomez R, Conde J, Lopez V, Gomez-Reino JJ, Lago F, Smith AB, 3rd, Gualillo O. Further evidence for the anti-inflammatory activity of oleocanthal: inhibition of MIP-1alpha and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life sciences. 2012;91:1229–1235. doi: 10.1016/j.lfs.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiological reviews. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behavioural brain research. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta U, Nilson AN, Kayed R. The Role of Amyloid-beta Oligomers in Toxicity, Propagation, and Immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son SM, Cha MY, Choi H, Kang S, Choi H, Lee MS, Park SA, Mook-Jung I. Insulin-degrading enzyme secretion from astrocytes is mediated by an autophagy-based unconventional secretory pathway in Alzheimer disease. Autophagy. 2016;12:784–800. doi: 10.1080/15548627.2016.1159375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuch C, Ortolano S, Navarro C. LRP-1 and LRP-2 receptors function in the membrane neuron. Trafficking mechanisms and proteolytic processing in Alzheimer’s disease. Frontiers in physiology. 2012;3:269. doi: 10.3389/fphys.2012.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Abeta-induced synaptic dysfunction in Alzheimer’s disease. Molecular neurodegeneration. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrle SE, Jiang H, Parsadanian M, Legleiter J, Han X, Fryer JD, Kowalewski T, Holtzman DM. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. The Journal of biological chemistry. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nature medicine. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]


