Abstract
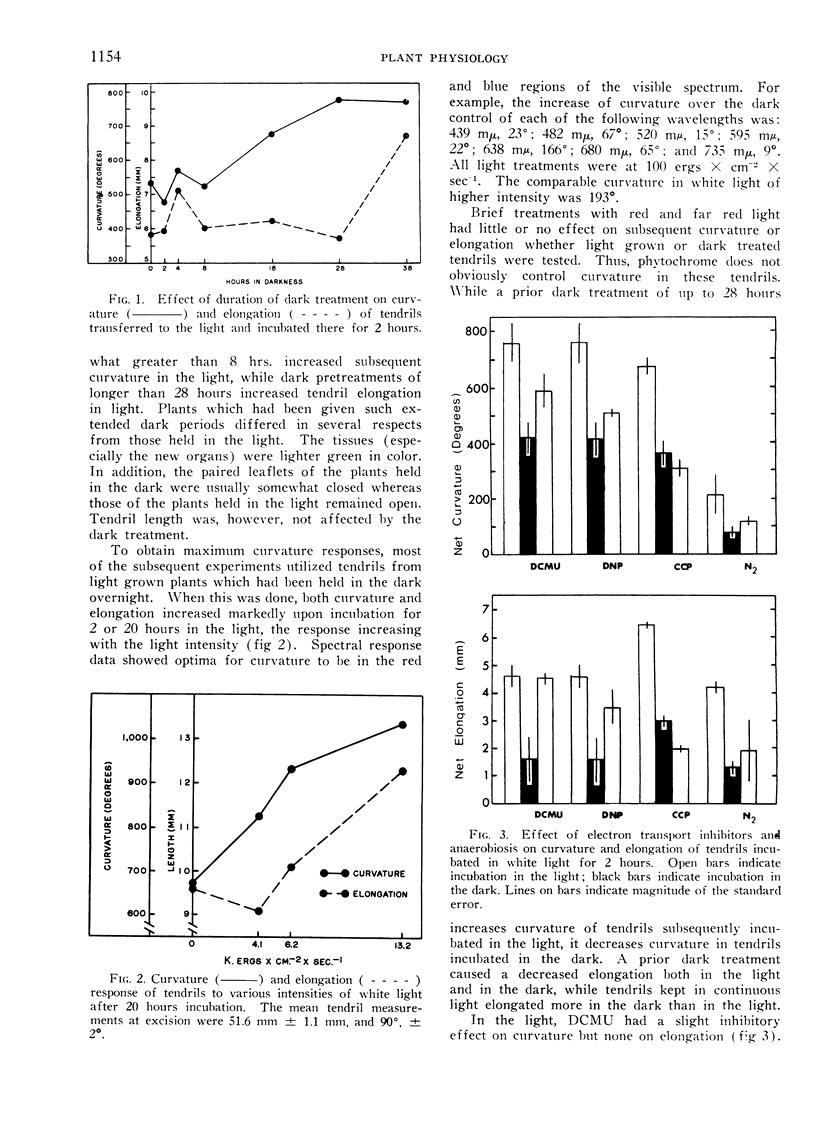
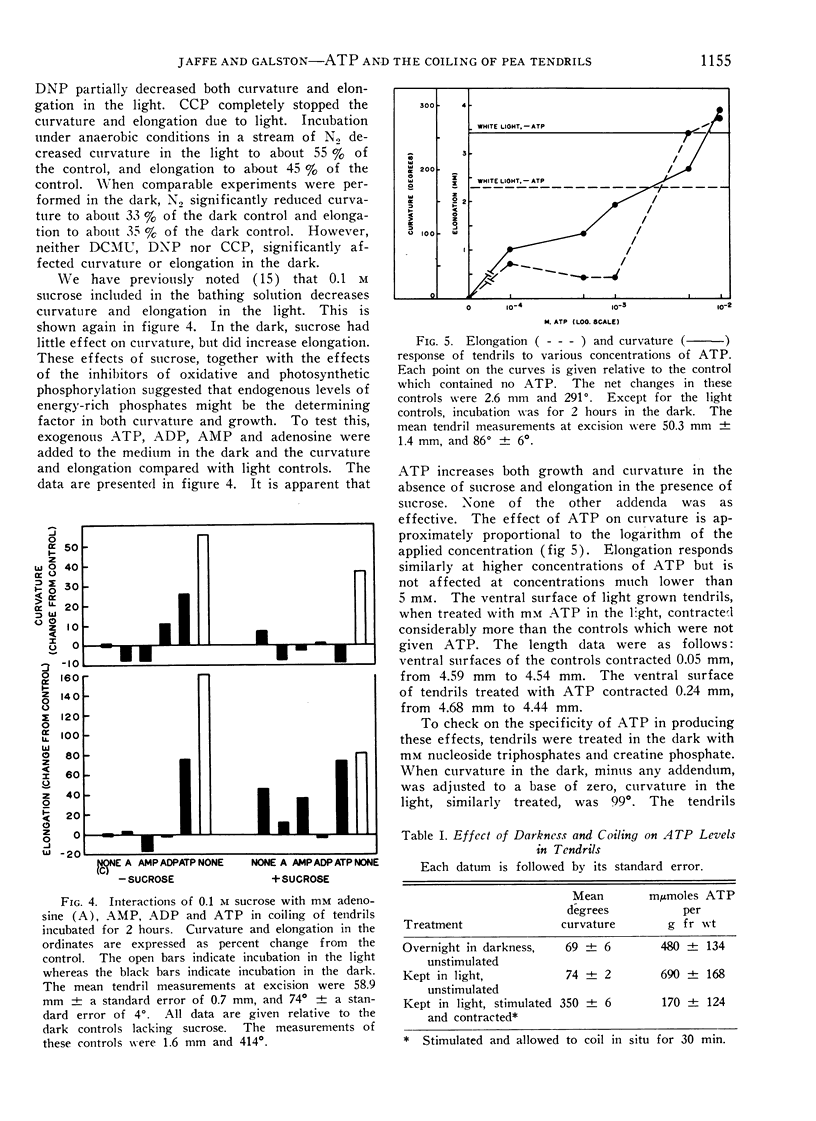
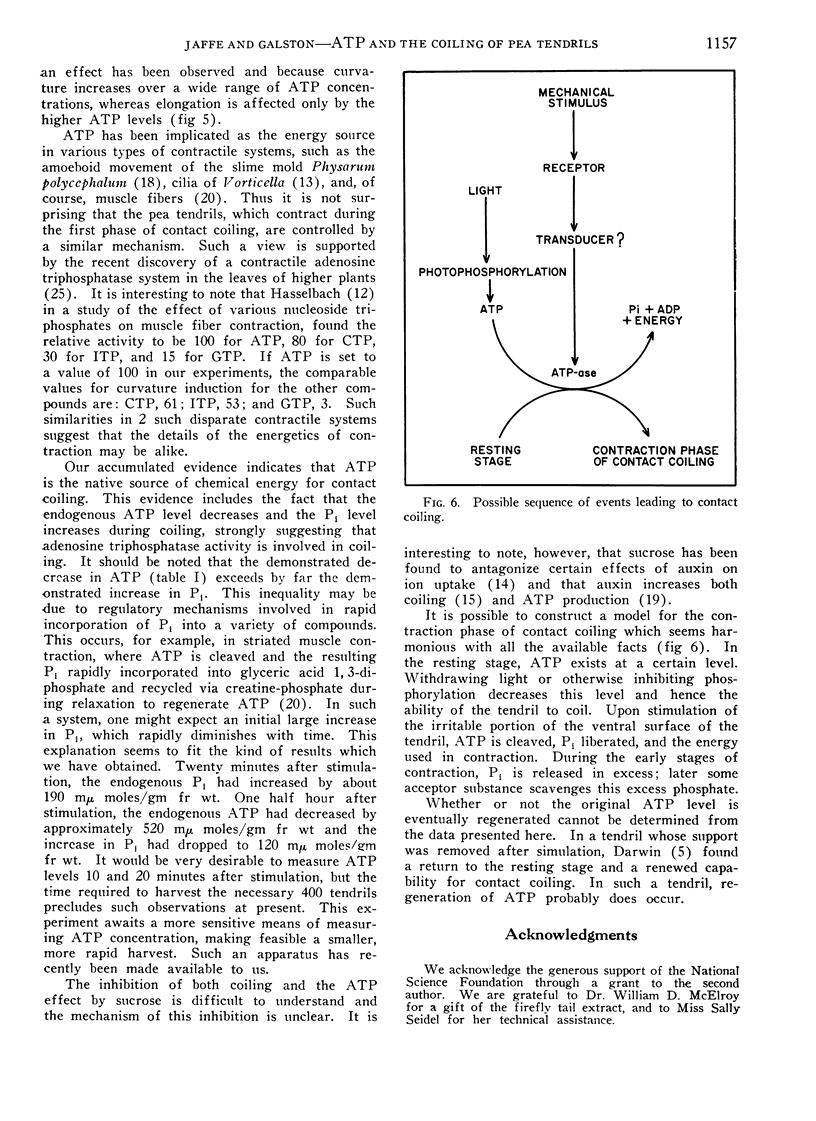
Excised pea (Pisum sativum L.) tendrils incubated in the light coil more than those incubated in the dark. This light effect, which displays spectral responses characteristic of chlorophyll-mediated mechanisms, is increased by at least 8 hours of prior dark incubation of plants from which the tendrils were derived. Considerable evidence indicates a major role of ATP in coiling. For example, inhibitors of ATP production decrease contact coiling. Exogenous ATP increases curvature in the dark, whereas exogenous adenosine, AMP and ADP are practically without effect. The ATP effect can be reversed by the addition of sucrose to the bathing solution. Tendrils of plants placed in the dark overnight have lower ATP levels than those held in the light. One half hour after stimulation, the endogenous ATP level of tendrils on plants kept in the light decreased fourfold. In the same period, the endogenous inorganic phosphate level increased markedly, indicating high adenosine triphosphatase activity.
Curvature is proportional to the logarithm of the molarity of applied ATP between 10−4 and 10−2m, whereas elongation responds only to the higher dosages. It is inferred that endogenous ATP is involved as an energy source in coiling, especially in the initial phase, which involves contraction of the tendril. The existence of a higher plant analog of actomyosin, suggested by others, is supported.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asomaning E. J., Galston A. W. Comparative study of phototropic response & pigment content in oat & barley coleoptiles. Plant Physiol. 1961 Jul;36(4):453–464. doi: 10.1104/pp.36.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of oxygen on photosynthesis, photorespiration and respiration in detached leaves. I. Soybean. Plant Physiol. 1966 Mar;41(3):422–427. doi: 10.1104/pp.41.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSBY R. A., HEYTLER P. G. UNCOUPLING OF OXIDATIVE PHOSPHORYLATION BY CARBONYL CYANIDE PHENYLHYDRAZONES. II. EFFECTS OF CARBONYL CYANIDE M-CHLOROPHENYLHYDRAZONE ON MITOCHONDRIAL RESPIRATION. Biochemistry. 1963 Sep-Oct;2:1142–1147. doi: 10.1021/bi00905a041. [DOI] [PubMed] [Google Scholar]
- HASSELBACH W. Die Wechselwirkung verschiedener Nukleosidtriphosphate mit Aktomyosin in Gelzustand. Biochim Biophys Acta. 1956 May;20(2):355–368. doi: 10.1016/0006-3002(56)90297-9. [DOI] [PubMed] [Google Scholar]
- ILAN I., REINHOLD L. REVERSAL BY SUCROSE OF THE EFFECTS OF INDOLYL-3-ACETIC ACID ON CATION UPTAKE BY PLANT CELLS. Nature. 1964 Feb 15;201:726–726. doi: 10.1038/201726a0. [DOI] [PubMed] [Google Scholar]
- Ozbun J. L., Volk R. J., Jackson W. A. Effects of Light and Darkness on Gaseous Exchange of Bean Leaves. Plant Physiol. 1964 Jul;39(4):523–527. doi: 10.1104/pp.39.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGER H. H., BUCK J. B., FASTIE W. G., MCELROY W. D. THE SPECTRAL DISTRIBUTION OF FIREFLY LIGHT. J Gen Physiol. 1964 Sep;48:95–104. doi: 10.1085/jgp.48.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


