Abstract
Purpose:
It is not clear whether upper limits of the thyrotropin (TSH) reference range should be lowered. This debate can be better informed by investigation of whether variations in thyroid function within the reference range have clinical effects. Thyroid hormone plays a critical role in determining energy expenditure, body mass, and body composition, and therefore clinically relevant variations in these parameters may occur across the normal range of thyroid function.
Methods:
This was a cross-sectional study of 140 otherwise healthy hypothyroid subjects receiving chronic replacement therapy with levothyroxine (L-T4) who had TSH levels across the full span of the laboratory reference range (0.34 to 5.6 mU/L). Subjects underwent detailed tests of energy expenditure (total and resting energy expenditure, thermic effect of food, physical activity energy expenditure), substrate oxidation, diet intake, and body composition.
Results:
Subjects with low-normal (≤2.5 mU/L) and high-normal (>2.5 mU/L) TSH levels did not differ in any of the outcome measures. However, across the entire group, serum free triiodothyronine (fT3) levels were directly correlated with resting energy expenditure, body mass index (BMI), body fat mass, and visceral fat mass, with clinically relevant variations in these outcomes.
Conclusions:
Variations in thyroid function within the laboratory reference range have clinically relevant correlations with resting energy expenditure, BMI, and body composition in L-T4–treated subjects. However, salutary effects of higher fT3 levels on energy expenditure may be counteracted by deleterious effects on body weight and composition. Further studies are needed before these outcomes should be used as a basis for altering L-T4 doses in L-T4–treated subjects.
In hypothyroid subjects receiving L-T4 therapy with normal TSH levels, fT3 levels were directly correlated with BMI, body adiposity, and resting, but not total, energy expenditure.
Serum thyrotropin (TSH) levels provide a sensitive measure of thyroid function, but there is debate over the optimal TSH reference range. The upper TSH reference range may be skewed by subjects with occult mild hypothyroidism, leading to recommendations that it be lowered (1). However, the upper TSH reference range increases with age, suggesting that age-based TSH reference ranges should be used (2). This debate has enormous public health implications. Altering the TSH reference range would affect levothyroxine (L-T4) doses in millions of people with thyroid disease and would label millions more with new thyroid disease.
This debate is better informed by knowledge of the clinical consequences of variations in thyroid function within the reference range. If such consequences exist, this strengthens the argument that the TSH reference range should be more narrowly defined. A number of studies have reported health consequences of variations in thyroid function within the reference range, including cardiovascular risk factors and events, bone density, and fracture risk (3).
Thyroid hormone plays a role in determining energy expenditure, body mass, and body composition, at least in overt hyper- and hypothyroidism (4). Clinically relevant variations in these parameters may also occur across the normal range of thyroid function. In subjects without thyroid disease, some studies have reported correlations between reference-range thyroid function [TSH or free thyroxine (fT4)] and body mass, body composition, weight gain, or energy expenditure. However, the correlations have been in different directions, and other studies have failed to find relationships between thyroid function and these outcomes (5–22).
Many treated patients with hypothyroidism complain of weight gain despite normal TSH levels, leading to requests for higher L-T4 doses or alternate therapies. Data from non–L-T4–treated euthyroid subjects may not pertain to L-T4–treated patients, and there is a paucity of metabolic-related data in the latter group. One small study showed short-term effects of altering L-T4 doses on resting energy expenditure and fat-free mass (23), but it is not clear that varying L-T4 dosages within the TSH reference range actually affected weight or body composition in these patients (24, 25).
To study the components of energy expenditure and body composition in depth in this under-characterized population, we recruited subjects with hypothyroidism receiving replacement L-T4. The study subjects underwent extensive testing of total and resting energy expenditure, thermic effect of food, activity levels, diet intake, and body composition. We hypothesized that L-T4–treated subjects with higher thyroid function within the reference range [lower TSH and/or higher fT4 or free triiodothyronine (fT3) levels] would have increased exergy expenditure and decreased body mass index (BMI), lean body mass, and fat mass compared with subjects with lower thyroid function within the reference range.
Materials and Methods
Experimental Subjects
A total of 140 otherwise healthy hypothyroid subjects receiving L-T4 therapy were recruited as a convenience sample from the authors’ clinics, through reviewer of electronic health records, by flyers, and with mailings. Subjects included 127 women and 13 men aged 21 to 70 years. Their diagnoses were primary hypothyroidism (n = 107), hypothyroidism following I-131 (n = 15) or thionamide therapy (n = 1) for Grave’s disease, postpartum thyroiditis with permanent hypothyroidism (n = 4), or history of thyroidectomy for nodular goiter, hyperthyroidism, or low-risk thyroid cancer (n = 13). All were diagnosed as adults and had past elevated TSH levels, confirming hypothyroidism. They had received L-T4 for 3 months to 50 years (mean, 11.7 years). Two subjects had received L-T4 for 3 months; all others had received L-T4 for at least 6 months. L-T4 doses were stable for at least 3 months (mean duration of current L-T4 dose, 1.9 years).
No subjects had any acute or chronic illness or were on medications that affect thyroid hormone levels, weight, or metabolism. Pregnant or lactating women were excluded. Stable doses of oral contraceptive or estrogen therapy were allowed. Testing was done during the first 14 days after onset of menstrual bleeding or an oral contraceptive cycle.The protocol was approved by the OHSU Institutional Review Board, and subjects gave written informed consent.
Screening visit
Subjects were screened for general health, medicines, and thyroid status by history, physical examination, and laboratory testing.
Testing visit
Within 6 weeks of the screening visit, subjects returned for a testing visit. Subjects were fasting and refrained from taking their L-T4 dose that morning. Serum TSH, fT4, and fT3 levels were obtained after energy expenditure studies were completed.
Anthropometric measurements
Weight was measured with a digital scale (Model 5002; Scale-Tronix, Wheaton, IL) to the nearest 0.01 kg. Height was measured without shoes using a wall-mounted stadiometer (Harpenden Stadiometer; Holtain, Crymych, UK).
Total energy expenditure by doubly labeled water
Subjects consumed water enriched with stable isotopes for hydrogen (2H) (Sigma Aldrich, St. Louis, MO) and oxygen (18O) (Cambridge Isotope Laboratories, Andover, MA). Due to resource limitations, 62 subjects completed total energy expenditure (TEE) measurements (41 in the low-normal and 21 in the high-normal group). Each subject drank a premixed dose of water that provided 1.7 g 2H218O per kilogram of body weight. Spot urine samples were collected predose and at 2 hours, 3 hours, 4 hours, and 7 days. TEE was calculated from the 2H:1H ratio in hydrogen gas and 18O:16O in carbon dioxide gas by standard techniques (Europa 20/20 Isotope Ratio Mass Spectrometer; Dale Scholler, PhD, University of Wisconsin, Madison, WI) (26, 27). The within-subject coefficients of variation (CVs) were 0.2% for 2H18O and 2% for 2H2O. The TEE intrasubject variation was 7.8%.
Resting energy expenditure by indirect calorimetry
Indirect calorimetry was performed at 21.1°C after the participant had fasted for 12 hours and abstained from significant physical activity for 24 hours by standard techniques (VMax Encore 29N Indirect Calorimeter; SensorMedics Viasys Health Care, Yorba Linda, CA). Expired air was sampled and analyzed for the volume of oxygen consumed (VO2) and the volume of carbon dioxide produced (VCO2) each minute for 30 minutes. Resting energy expenditure (REE) was calculated using the modified Weir equation, and macronutrient oxidation was estimated by the equations of Jequier and 24-hour urine nitrogen measurements (28).
Thermic effect of food
The thermic effect of food (TEF) was determined by indirect calorimetry immediately after REE was measured (29). Each participant consumed a standard liquid meal (Ensure, Ross Laboratories) that provided an energy intake of 35% of his or her REE, composed of 14% protein, 31.5% fat, and 54.5% carbohydrate. Postprandial energy expenditure was measured for 15 minutes every half hour for 6 hours using the procedure described for REE. The 6-hour area under the curve was calculated by the trapezoidal method. The result was multiplied by 3.5, a constant representing the typical consumption of three meals and one snack per day, to estimate the total 24-hour TEF.
Body composition by dual-energy X-ray absorptiometry
Body composition was measured by dual-energy X-ray absorptiometry using a QDR Discovery A Densitometer (Hologic, Bedford, MA) following standard procedures. Visceral adipose tissue was estimated using Hologic Horizon DXA System software by a single trained operator (30).
Diet intake
Three 24-hour food recall interviews were conducted by telephone within 1 week of the testing visit by bionutritionists trained in the Nutrition Data System for Research, a software application for the collection of dietary recall information in a standardized fashion (31).
Physical activity
Subjects wore a small, multidirectional accelerometer (Actical; MiniMitter, Bend, OR) at the waist for seven consecutive days within 2 weeks of the testing visit, except during sleep. Data were converted into energy expended and accumulated time during sedentary, light, moderate, and vigorous activities by standard analyses.
Analytic methods
TSH was measured by ICMA (Beckman Coulter) (functional sensitivity, 0.02 mU/L; normal range, 0.34 to 5.60 mU/L; intra-assay CV, 9.5% at 0.03 mU/L and 4.7% at 11.6 mU/L; interassay CV, 11% at 0.04 mU/L, 5% at 0.70 mU/L, and 5.8% at 24.94 mU/L). fT4 was measured by direct equilibrium dialysis (Nichols Institute, San Juan Capistrano, CA) (sensitivity, 0.08 ng/dL; normal range, 0.8 to 2.7 ng/dL; intraassay CV, 5.7% at 0.27 ng/dL and 1% at 4.6 ng/dL; interassay CV, 6.8% at 0.3 ng/dL and 1.6% at 3.8 ng/dL). fT3 was measured by tracer dialysis (Nichols Institute) (sensitivity, 25 pg/dL; normal range, 210 to 440 pg/dL; intra-assay CV, 6%; interassay CV, 4%). Urine nitrogen was measured in a 24-hour urine sample obtained within 1 week of the testing visit by hydrolysis (BioAssay System QuantiChrom kit, Hayward, CA) (functional sensitivity, 0.08 mg/dL; intraassay CV, 2.6%; interassay CV, 5% at 950 mg/dL).
Statistical methods
Subjects were divided into two groups based on serum TSH levels: low-normal TSH (TSH 0.34 to 2.50 mU/L, n = 88, six men) and high-normal TSH (TSH 2.51 to 5.60 mU/L, n = 52, seven men). The TSH cut-off was based on recent debate over restricting the TSH reference range to an upper limit of 2.50 mU/L to achieve a Gaussian distribution in healthy populations (32).
Energy expenditure, body composition, dietary intake, and physical activity outcomes were compared between the two groups. Subscales of each measure were analyzed together using linear repeated measures analyses (R version 3.2.1) using the nlme package lme function (33). This allows for correlation between subscale measures in each subject. Compound symmetric covariance structures were used to adjust for age, sex, estrogen status, time on L-T4, time at current L-T4 dose, and L-T4 dose (μg/kg).
An initial assessment of interaction between group and subscale was obtained for each set of subscales. Likelihood ratio tests were conducted to determine whether models with the interaction were significant at the 0.10 level, in which case a comparison of groups was conducted for each subscale. If the addition of the interaction was not significant, the comparison of groups was conducted for the set of scales as a whole (dropping the interaction from the model). To limit the effect of multiple comparisons, we planned to conduct follow-up comparisons individually only if evidence of a group effect was observed at the 0.05 level. However, because few of these tests were significant, follow-up comparisons for all individual subscales were tested to confirm the lack of significance. Significant P values were adjusted using Bonferroni and false discovery rate procedures.
We also examined relationships between outcomes and TSH, fT4, and fT3 as continuous variables across all subjects using the same repeated measures methodology but substituting, in separate models, the selected hormone for the categorical group variable of low-normal and high-normal TSH.
Results
Clinical parameters and thyroid function tests
Age, sex, estrogen status, duration of L-T4 treatment, and duration at current L-T4 dose did not differ between the low-normal (n = 88) and high-normal (n = 52) TSH groups (Table 1). Mean (± SEM) L-T4 doses were higher in the low-normal compared with the high-normal TSH group (1.51 ± 0.05 vs 1.24 ± 0.06 μg/kg/d; P = 0.001). By design, all subjects had TSH levels within the reference range, with mean TSH levels lower in the low-normal compared with the high-normal TSH group (1.36 ± 0.07 vs 3.73 ± 0.12 mU/L; P < 0.0001).
Table 1.
Demographic, Clinical, and Thyroid Function Variables in the Low-Normal TSH and High-Normal TSH Groups
| Measures | Low-Normal TSH (n = 88) | High-Normal TSH (n = 52) | P Value |
|---|---|---|---|
| Age, y | 48.9 ± 1.2a | 49.5 ± 1.8 | 0.75 |
| Sex | 93% female | 87% female | 0.24 |
| 7% male | 13% male | ||
| Estrogen status | 43% prenone | 47% prenone | 0.89 |
| 11% preon | 9% preon | ||
| 41% postnone | 42% postnone | ||
| 5% poston | 2% poston | ||
| L-T4 time,b y | 12.4 ± 1.1 | 10.7 ± 1.1 | 0.26 |
| L-T4 dose time,c y | 1.64 ± 0.23 | 2.24 ± 0.43 | 0.22 |
| L-T4 dose, μg/kg | 1.51 ± 0.05d | 1.24 ± 0.06 | 0.001 |
| TSH, mU/L | 1.36 ± 0.07 | 3.73 ± 0.12 | <0.0001 |
| fT4, ng/dL | 1.68 ± 0.04 | 1.57 ± 0.05 | 0.09 |
| fT3, pg/dL | 215 ± 5 | 209 ± 6 | 0.49 |
| fT3/fT4 ratio, pg/ng | 136 ± 5 | 137 ± 5 | 0.81 |
| Heart rate, beats/min | 68.0 ± 1.2 | 70.9 ± 1.7 | 0.16 |
| Systolic blood pressure, mm Hg | 116.4 ± 1.6 | 116.7 ± 2.3 | 0.90 |
| Diastolic blood pressure, mm Hg | 68.2 ± 0.9 | 71.6 ± 2.5 | 0.20 |
Abbreviations: Preon, premenopausal on hormone treatment; prenone, premenopausal, no hormone treatment; poston, postmenopausal on hormone treatment; postnone, postmenopausal, no hormone treatment.
Values are means ± SEM.
Duration of L-T4 therapy.
Duration of current L-T4 dose.
dSignificant differences between groups are shown in bold with corresponding P values.
Mean fT4 levels were similar between the two groups, and all were within the reference range. Although mean fT3 levels were also similar between the two groups, 73 subjects had low fT3 levels (range, 118 to 209 pg/dL). Forty-one subjects were in the low-normal (47%) and 32 were in the high-normal TSH group (62%) (P = 0.09 by χ2). The fT3:fT4 ratios were similar in the two groups.
Other clinical characteristics were similar between the two groups, including heart rate and systolic and diastolic blood pressures.
Body composition and energy expenditure
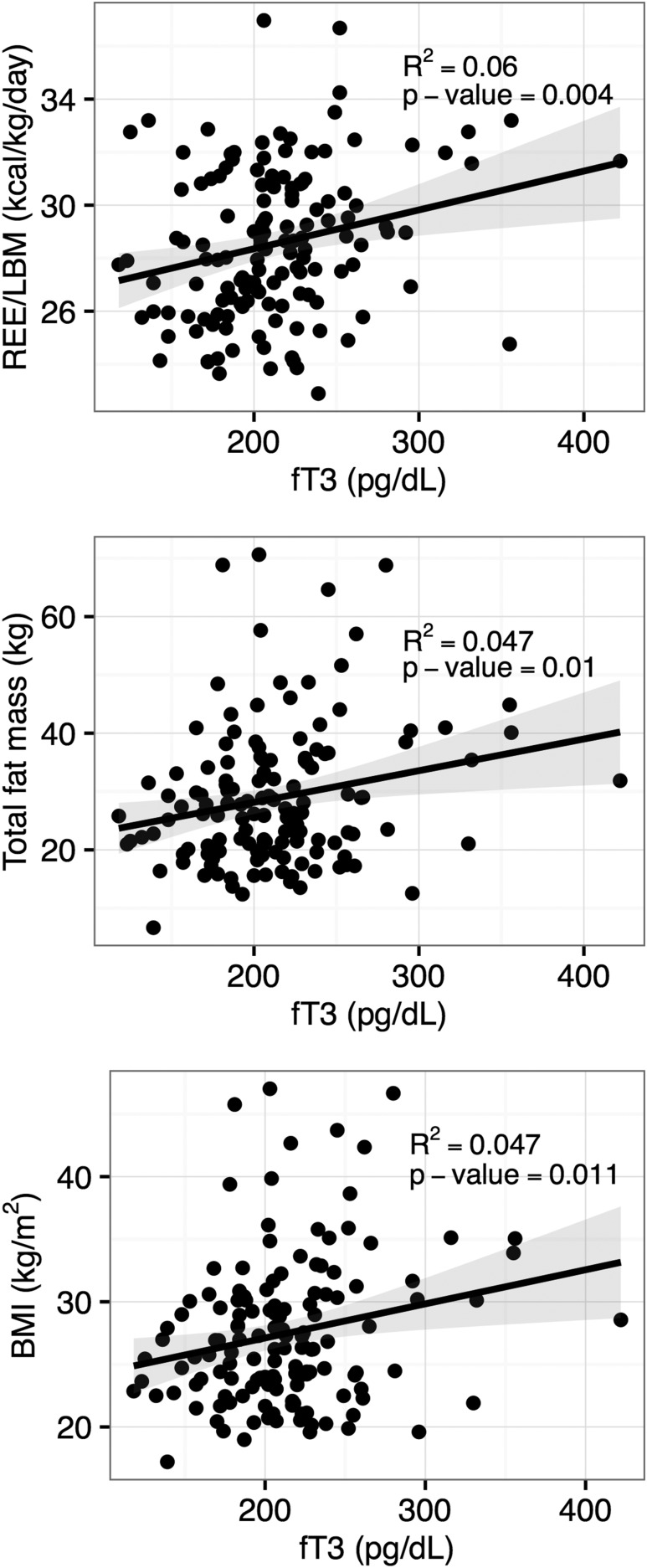
There were no differences in BMI or body composition parameters between the two groups (Table 2). By analysis across both groups with thyroid hormone levels as continuous variables, BMI, fat mass, % fat mass, and visceral fat mass were positively correlated with fT3 levels (P = 0.02, 0.01, 0.01, and 0.03, respectively) (Table 3; Fig. 1). These findings remained significant after adjusting for multiple testing. The magnitudes of the correlations were a 0.23 kg/m2 increase in BMI, a 0.47 kg increase in fat mass, a 0.31% increase in fat mass, and a 10.32 g increase in visceral fat mass for each 10 pg/dL increase in fT3 level. Body composition parameters were not correlated with fT4 or TSH levels.
Table 2.
Body Composition and Energy Expenditure in the Low-Normal TSH and High-Normal TSH Groups
| Measurea | Low-Normal TSH (n = 88) | High-Normal TSH (n = 52) | P Values for Comparing Groupsb |
|---|---|---|---|
| BMI, kg/m2 | 27.3 ± 0.6c | 27.8 ± 0.9 | 0.32 |
| Lean body mass, kg | 43.8 ± 0.8 | 45.1 ± 1.3 | 0.19 |
| Fat mass, kg | 28.0 ± 1.1 | 30.2 ± 1.9 | 0.81 |
| % Fat mass | 37. 0 ± 0.8 | 37.7 ± 1.1 | 0.99 |
| Visceral fat, g in abdominal ROI | 474 ± 29 | 511 ± 43 | 0.44 |
| TEE, kcal/d | 2163 ± 60 | 2273 ± 100 | 0.23 |
| TEE/LBM, kcal/kg/d | 48.1 ± 1.0 | 48.9 ± 1.4 | 0.90 |
| REE, kcal/d | 1309 ± 21 | 1348 ± 33 | 0.76 |
| REE/LBM, kcal/kg/d | 28.5 ± 0.3 | 28.6 ± 0.4 | 0.12 |
| CHO oxidation, g/d | 136 ± 6 | 140 ± 10 | 0.92 |
| Fat oxidation, g/d | 56.5 ± 2.8 | 58.6 ± 4.4 | 0.81 |
| Protein oxidation, g/d | 54.4 ± 2.1 | 58.7 ± 2.5 | 0.52 |
| TEF,d kcal/d | 142 ± 8 | 141 ± 12 | 0.67 |
| TEF peak energy, kcal | 33.3 ± 0.7 | 34.9 ± 1.2 | 0.76 |
| TEF time to peak, h | 1.81 ± 0.11 | 1.62 ± 0.14 | 0.19 |
Abbreviations: CHO, carbohydrate; ROI, region of interest; TEF, thermic effect of food, calculated from 6-hour TEF area under the curve multiplied by 3.5 to estimate total daily TEF.
Body composition values were available for all subjects except for one low normal subject missing visceral fat value. TEE values were available for 41 low normal and 21 high normal subjects. REE values were available for all subjects. Substrate oxidation values were available for all low normal (except for one CHO value) subjects and all but three CHO, two fat, and three protein high-normal values. TEF values were available for 59 low-normal and 29 high-normal subjects. The following measures were analyzed together to allow for correlations between measures: BMI, LBM, and fat mass; TEE and TEE/LBM; REE and REE/LBM; CHO, fat, and protein oxidation; and TEF peak energy and time to peak.
P values were adjusted for age, estrogen status, L-T4 time, L-T4 dose time, and L-T4 dose (μg/kg).
Values are means ± SEM.
TEF models were also adjusted for LBM, %fat, and meal size.
Table 3.
Correlations Between Thyroid Hormone Levels, Body Composition, and Energy Expenditure in L-T4–Treated Subjects
| Measurea | fT4 (n = 139) |
fT3 (n = 139) |
TSH (n = 140) |
|||
|---|---|---|---|---|---|---|
| Coefficientb | P valuec | Coefficient | P value | Coefficient | P value | |
| BMI, kg/m2 | −0.70 | 0.64 | 0.23 | 0.02d | −0.17 | 0.64 |
| Lean body mass, kg | −2.75 | 0.10 | 0.13 | 0.25 | −0.04 | 0.92 |
| Fat mass, kg | −1.20 | 0.68 | 0.47 | 0.01d | 0.06 | 0.93 |
| % Fat mass | 0.55 | 0.76 | 0.31 | 0.01e | −0.15 | 0.73 |
| Visceral fat, g in abdominal ROI | −42.49 | 0.55 | 10.32 | 0.03d | −10.50 | 0.54 |
| TEE, kcal/d | −71.92 | 0.63 | −1.98 | 0.87 | −20.35 | 0.61 |
| TEE/LBM, kcal/kg/d | −0.30 | 0.92 | −0.17 | 0.47 | 0.37 | 0.62 |
| REE, kcal/d | −66.35 | 0.21 | 10.00 | 0.003e | 4.58 | 0.72 |
| REE/LBM, kcal/kg/d | 0.23 | 0.75 | 0.15 | 0.001e | 0.16 | 0.36 |
| CHO oxidation, g/d | −18.97 | 0.28 | 3.06 | 0.01e | 0.53 | 0.90 |
| Fat oxidation, g/d | 1.19 | 0.88 | −0.35 | 0.50 | 0.06 | 0.97 |
| Protein oxidation, g/d | −1.28 | 0.80 | −0.10 | 0.78 | 1.37 | 0.28 |
| TEF, kcal/d | 0.52 | 0.47 | 0.08 | 0.23 | −0.06 | 0.76 |
| TEF peak energy, kcal | 0.36 | 0.24 | 0.02 | 0.51 | −0.12 | 0.12 |
| TEF time to peak, h | −15.35 | 0.45 | 2.08 | 0.24 | −5.72 | 0.27 |
Abbreviation: CHO, carbohydrate.
TEE values were available for 62 subjects. REE values were available for all subjects. Substrate oxidation values were available for all but four subjects. TEF values were available for 88 subjects. TEF was also adjusted for LBM, %fat, and meal size. Body composition values were available for all subjects except for one missing visceral fat value.
Correlations were analyzed by repeated measures methodology using separate models for each hormone. Positive coefficients indicate that the measure increased with increasing hormone levels; negative coefficients indicate that the measure decreased with increasing hormone levels. The magnitude of the coefficient indicates the estimated change in the measure with a one-unit increase in fT4 or TSH or a 10 unit increase in fT3.
P values were adjusted for age, estrogen status, L-T4 time, L-T4 dose time, and L-T4 dose (μg/kg). Significant coefficients are shown in bold with corresponding P values unadjusted for multiple comparisons.
P value is still significant at the 0.05 level when applying multiple testing adjustment using the false discovery rate.
P value is still significant at the 0.05 level both when applying multiple testing adjustments using a Bonferroni correction and when using the false discovery rate.
Figure 1.
Correlations between serum fT3 levels and REE/LBM (top), total fat mass (middle), and BMI (lower) in L-T4–treated subjects.
Total and resting energy expenditure values were similar in the two groups (Table 2). By analysis across both groups with thyroid hormone levels as continuous variables, REE and REE per kilogram lean body mass were directly correlated with fT3 levels (P = 0.003 and 0.001, respectively) but not with fT4 or TSH (Table 3; Fig. 1). These findings remained significant after adjusting for multiple testing. The magnitude of the correlation was a 10 kcal/d or 0.15 kcal/kg/d increase in REE for each 10 pg/dL increase in fT3 level. There were no differences in substrate oxidation rates or TEF parameters between the two groups (Table 2). Across both groups, carbohydrate oxidation rates were positively correlated with fT3 levels (P = 0.01), with a 3.06 g/d increase for each 10 pg/dL increase in fT3 level (Table 3), which remained significant after adjustment for multiple testing. There were no correlations between fT3 levels and TEF parameters or between fT4 or TSH levels and substrate oxidation rates or TEF parameters.
To ensure that we did not overlook significant effects between the low-normal and high-normal TSH groups due to inclusion of subjects with midnormal TSH levels, we also analyzed TEE, TEE per kilogram lean body mass (LBM), REE, REE per kilogram LBM, BMI, LBM, total fat mass, and % fat mass based on tertiles of TSH. TSH tertile cut-offs were 1.31 and 2.60 mU/L. There were 47 subjects in the low, 46 in the middle, and 47 in the high tertile. There were no differences in any of the TEE or REE measures between the lowest and highest tertiles (P values 0.15 to 0.70; data not shown).
Diet intake and physical activity
Total daily energy intake, as well as carbohydrate and fat as percentages of daily intake, were similar in the two groups (Table 4). The low normal TSH group had a slightly lower % protein intake (15.4 ± 0.4 vs. 17.2 ± 0.7; P = 0.01), which was still significant after adjusting for multiple testing (Table 4). By analysis across both groups with thyroid hormone levels as continuous variables, % fat intake was negatively and % protein intake was positively correlated with TSH levels (P = 0.03 and 0.04), but this was no longer significant after adjustment for multiple testing (Table 5).
Table 4.
Dietary Intake and Physical Activity in the Low-Normal TSH and High-Normal TSH Groups
| Measurea | Low-Normal TSH (n = 88) | High-Normal TSH (n = 52) | P Valuesb for Comparing Groups |
|---|---|---|---|
| Daily energy intake, kcal/kg/d | 27.7 ± 1.0c | 26.8 ± 1.2 | 0.88 |
| % CHO intake | 47.5 ± 0.9 | 45.3 ± 1.1 | 0.06 |
| % Fat intake | 34.3 ± 0.7 | 34.3 ± 0.9 | 0.78 |
| % Protein intake | 15.4 ± 0.4 | 17.2 ± 0.7 | 0.01d |
| Total daily PAEE, kcal/d | 625 ± 34 | 566 ± 28 | 0.11 |
| Daily PAEE, per kg LBM | 13.4 ± 0.7 | 12.0 ± 0.5 | 0.29 |
| Daily PAEE—light, kcal/d | 175 ± 7 | 173 ± 7 | 0.20 |
| Daily PAEE—moderate/vigorous, kcal/d | 450 ± 30 | 394 ± 24 | 0.13 |
| Daily time spent in sedentary activities, min | 539 ± 15 | 555 ± 15 | 0.82 |
| Daily time spent in light activities, min | 215 ± 7 | 206 ± 8 | 0.42 |
| Daily time spent in moderate/vigorous activities, min | 155 ± 11 | 128 ± 7 | 0.18 |
| % Daily time spent in sedentary activities | 59.8 ± 1.4 | 62.5 ± 0.14 | 0.38 |
| % Daily time spent in light activities | 23.8 ± 0.7 | 23.2 ± 0.8 | 0.75 |
| % Daily time spent in moderate/vigorous activities | 16.5 ± 1.0 | 14.3 ± 0.8 | 0.25 |
Abbreviation: CHO, carbohydrate.
Diet measures were available in all subjects. Physical activity measures were available in all but two subjects in the low-normal TSH group.
P values were adjusted for age, estrogen status, L-T4 time, L-T4 dose time, and L-T4 dose (μg/kg). The following measures were analyzed together to allow for correlations between measures: % CHO, fat and protein intake; daily PAEE-light and PAEE-moderate/vigorous; daily time spent in sedentary, light, and moderate/vigorous activities; and % daily time spent in sedentary, light, and moderate/vigorous activities. Significant differences between the two groups are shown in bold with corresponding P values unadjusted for multiple comparisons.
Values are means ± SEM.
P value is still significant at the 0.05 level both when applying multiple testing adjustments using a Bonferroni correction and when using the false discovery rate.
Table 5.
Correlations Between Thyroid Hormone Levels and Dietary Intake and Physical Activity Measures in L-T4–Treated Subjects
| Measurea | fT4 (n = 139) |
fT3 (n = 139) |
TSH (n = 140) |
|||
|---|---|---|---|---|---|---|
| Coefficientb | P Valuec | Coefficient | P Value | Coefficient | P Value | |
| Daily energy intake, kcal/kg/d | 2.51 | 0.30 | −0.02 | 0.90 | 0.27 | 0.64 |
| % CHO intake | −0.95 | 0.61 | −0.01 | 0.94 | 0.48 | 0.30 |
| % Fat intake | 3.29 | 0.14 | 0.21 | 0.14 | −1.13 | 0.03d |
| % Protein intake | −0.88 | 0.44 | −0.07 | 0.34 | 0.57 | 0.04d |
| Total daily PAEE, kcal/d | 57.02 | 0.47 | 9.49 | 0.06 | −36.91 | 0.049d |
| Daily PAEE per kg LBM | 2.08 | 0.19 | 0.17 | 0.10 | −0.67 | 0.08 |
| Daily PAEE—light, kcal/d | −9.69 | 0.56 | 4.00 | 0.0002e | −4.55 | 0.26 |
| Daily PAEE—moderate/vigorous, kcal/d | 68.36 | 0.32 | 5.57 | 0.22 | −32.18 | 0.05 |
| Daily time spent in sedentary activities, min | −31.83 | 0.35 | −2.28 | 0.31 | 9.17 | 0.27 |
| Daily time spent in light activities, min | −7.49 | 0.66 | 3.08 | 0.01f | −3.83 | 0.36 |
| Daily time spent in moderate/vigorous activities, min | 7.44 | 0.76 | 1.12 | 0.48 | −9.29 | 0.11 |
| % Daily time spent in sedentary activities | −1.86 | 0.58 | −0.35 | 0.10 | 1.24 | 0.13 |
| % Daily time spent in light activities | 0.12 | 0.95 | 0.29 | 0.01f | −0.33 | 0.43 |
| % Daily time spent in moderate/vigorous activities | 1.50 | 0.49 | 0.10 | 0.47 | −0.93 | 0.07 |
Abbreviation: CHO, carbohydrate.
Diet measures were available in all subjects. Physical activity measures were available in all but two subjects. Correlations were analyzed by repeated measures methodology using separate models for each hormone.
Positive coefficients indicate that the measure increased with increasing hormone levels; negative coefficients indicate that the measure decreased with increasing hormone levels. The magnitude of the coefficient indicates the estimated change in the measure with a one-unit increase in fT4 or TSH or a 10-unit increase in fT3.
P values were adjusted for age, estrogen status, L-T4 time, L-T4 dose time, and L-T4 dose (μg/kg). Significant coefficients are shown in bold with corresponding P values unadjusted for multiple comparisons.
P value is not significant at the 0.05 level both when applying multiple testing adjustments using a Bonferroni correction and when using the false discovery rate.
P value is still significant at the 0.05 level both when applying multiple testing adjustments using a Bonferroni correction and when using the false discovery rate.
P value is still significant at the 0.05 level when applying multiple testing adjustment using the false discovery rate.
There were no differences in physical activity measures between the two groups (Table 4). By analysis across both groups with thyroid hormone levels as continuous variables, total daily physical activity energy expenditure (PAEE) was negatively correlated with TSH levels (P = 0.049), although this was no longer significant after adjustment for multiple testing (Table 5). Daily light PAEE and time spent in light activities were positively correlated with fT3 levels (P = 0.0002 and 0.01, respectively), which remained significant after adjustment for multiple testing. The magnitude of the correlations were 4 kcal/d and 3 minutes of time for each 10 pg/dL increase in fT3.
Discussion
In this large cohort of L-T4–treated subjects who had TSH levels across the full span of the normal laboratory range, we found no differences in BMI, body composition, energy expenditure, or diet intake between subjects with low-normal and high-normal TSH levels. However, across the entire group, we did find significant positive correlations between serum fT3 levels and BMI, fat mass, and resting energy expenditure. We also found minor correlations between fT3 levels and other aspects of energy metabolism, including macronutrient intake, oxidation rates, and physical activity.
In healthy euthyroid (non–L-T4–treated) subjects, variable correlations have been reported between TSH, fT4, or fT3 levels within the reference range and BMI, body composition, or energy expenditure (5–22). However, data from non–L-T4–treated euthyroid individuals are not comparable to data from L-T4–treated hypothyroid subjects, especially given the frequent occurrence of low fT3 levels in the latter group (34, 35). A recent large study reported that L-T4–treated subjects had higher BMI than euthyroid-matched control subjects, but there is a paucity of published data on weight and metabolic function in L-T4–treated subjects (35).
In agreement with two previous small intervention studies (23, 24), our data show a robust direct correlation between fT3 levels (but not fT4 or TSH) and REE (even with adjustment for lean body mass), with a 10 kcal/d increase in REE per 10 pg/dL increase in fT3 levels. This suggests that variations in thyroid function significantly contribute to variations in REE in L-T4–treated subjects.
Hypothyroid patients on L-T4 therapy often complain of altered body composition or difficulty losing weight despite TSH levels within the reference range, and they often request increased L-T4 doses or alternate thyroid preparations despite little support for this approach in the literature (24, 25). We did not find differences in BMI or body composition between the low-normal and high-normal TSH groups, but the similar fT3 levels in the two groups might have obscured any effects. In fact, when we analyzed BMI and body composition measures as continuous variables, we found positive correlations between fT3 levels and BMI, fat mass, % fat mass, and visceral fat mass.
Given the known effects of T3 to stimulate lipolysis and decrease body fat in animal models and increase REE in humans (4), these findings seemingly raise a paradox: How can fT3 levels be simultaneously associated with increases in REE, BMI, and body adiposity? One explanation is that animal models may not accurately represent long-term effects of T3 on human body fat compartments, given other compensatory mechanisms and the relative lack of brown adipose tissue in humans. Another explanation is that compensations in non-REE metabolism (such as TEF and nonactivity thermogenesis) may maintain total daily energy expenditure (TEE), thereby preventing weight loss. Indeed, even though we found evidence of increased total and light activity (PAEE) in association with higher fT3 levels, neither TEF nor TEE was related to fT3 levels.
An intriguing alternative explanation is that increasing body weight or adiposity generates higher fT3 levels in LT-4–treated patients, rather than the other way around. This relationship has been suggested by observational studies in euthyroid subjects (17). If so, fT3 generation from exogenous L-T4 in relevant tissues would need to vary in relation to increasing weight. In mice, insulin stimulates D2-mediated T3 production in skeletal muscle (36), and bile acids enhance fT3 production in adipose tissue and muscle (37). The bile acid pool is expanded in obese subjects and decreases with caloric restriction induced weight loss (38). These observations provide two possible pathways by which elevated fT3 levels are a consequence of, rather than a direct contributor to, body weight.
In either case, our results represent the largest observational study to date to investigate associations between thyroid hormone levels and BMI or body composition in L-T4–treated subjects and are contrary to the widespread clinical assumption that higher T3 levels are associated with lower BMI and decreased body fat. These observations point to the need for more detailed studies of energy expenditure, body composition, and sources of fT3 generation in L-T4–treated subjects.
We also found some minor correlations between thyroid hormone levels and diet macronutrient composition and carbohydrate oxidation rates in these L-T4–treated subjects. Given the number of comparisons we conducted and the small magnitude of our findings, we feel that the most clinically relevant point is that variations in thyroid function within the reference range do not have significant effects on caloric intake.
Fifty-two percent of our subjects had low serum fT3 levels despite normal TSH levels, an observation made in other L-T4–treated subjects (34, 35). This raises the question as to whether L-T3 supplementation might be indicated in L-T4–treated subjects to optimize metabolic function, at least in the subset with low fT3 levels. However, there have been a number of randomized, controlled studies of replacing some of a hypothyroid subject’s L-T4 with L-T3, but none of these studies found clinically significant differences in weight (39). One recent cross-over study did report that hypothyroid subjects lost an average of 1.5 kg after 6 weeks when L-T3 was completely substituted for their L-T4 doses (40). In aggregate, our data and data from published studies suggest that adjusting thyroid function within the reference range or adding L-T3 are not successful strategies to achieve significant weight loss.
There are a number of strengths to our study. Our study included a large and well-characterized group of L-T4–treated subjects and sensitive measures of metabolic function that have not been previously studied in this depth in euthyroid or L-T4–treated subjects. Our study also has limitations, including the cross-sectional design, which does not allow for determination of causality or trends over time. Although our sample size is the largest reported for these outcomes in L-T4–treated subjects, it was still constrained by the availability of volunteers and resources, particularly in the measurement of TEE. Although there were a relatively large number of comparisons for the number of subjects, we corrected for multiple testing. Our subjects were heterogeneous in terms of diagnosis, severity, and duration of hypothyroidism; L-T4 dose requirements; duration of L-T4 treatment; and time at current L-T4 doses, due to the practicalities of recruiting for an intensive clinical study. All but two of our subjects had received L-T4 for at least 6 months, but a few had minor dose adjustments 3 to 6 months prior to study, which may not have been long enough for body composition effects to stabilize. However, 3 months on a stable L-T4 dose is sufficient to observe changes in energy expenditure (23). Subjects less satisfied with their weight or general health may have preferentially volunteered, introducing a selection bias. Most of our subjects were women and tended to be younger and slimmer than the US population, and our results may not be generalizable to men or older or heavier subjects. We attempted to collect blood samples at a consistent time of day, but this was not always possible due to scheduling limitations. In healthy subjects with typical sleep-wake cycles, serum TSH levels decrease slightly between 0700 and 0900 and then remain stable until the evening (41). Thus, there may have been slight variations in TSH levels in our study due to sampling time.
In summary, we found no differences in measures of energy expenditure or body composition in L-T4–treated subjects based on whether their TSH levels were above or below 2.50 mU/L, a level suggested as a target for L-T4 therapy (32). Even though we found clinically significant positive correlations between fT3 levels and resting energy expenditure, we also found similar relationships between fT3 and BMI and body fat, which have not been previously reported in a large cohort of these subjects. These findings, along with published data, do not support the commonly held belief that increasing L-T4 doses or augmenting T3 levels in L-T4–treated subjects mitigates weight gain or improved body composition. Instead, these treatments raise concerns regarding adverse effects (34) and argue for carefully conducted interventional studies using sensitive methodologies to measure energy expenditure and body composition in thyroid hormone–treated patients.
Acknowledgments
We thank the staff of the OHSU Clinical and Translational Research Center for excellent patient care and research support and the Biostatistics & Design Program for data analysis expertise.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK075496 (to M.H.S.) and UL1 RR024120 (OHSU CTSA).
Clinical trial registry: ClinicalTrials.gov no. NCT00565864 (registered 28 November 2007).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- fT3
- free triiodothyronine
- fT4
- free thyroxine
- LBM
- lean body mass
- L-T4
- levothyroxine
- PAEE
- physical activity energy expenditure
- REE
- resting energy expenditure
- TEE
- total energy expenditure
- TEF
- thermic effect of food
- TSH
- thyrotropin.
References
- 1.Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92(11):4236–4240. [DOI] [PubMed] [Google Scholar]
- 2.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. [DOI] [PubMed] [Google Scholar]
- 3.Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98(9):3562–3571. [DOI] [PubMed] [Google Scholar]
- 4.McAninch EA, Bianco AC. Thyroid hormone signaling in energy homeostasis and energy metabolism. Ann N Y Acad Sci. 2014;1311:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toubro S, Sørensen TI, Rønn B, Christensen NJ, Astrup A. Twenty-four-hour energy expenditure: the role of body composition, thyroid status, sympathetic activity, and family membership. J Clin Endocrinol Metab. 1996;81(7):2670–2674. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, Jørgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. [DOI] [PubMed] [Google Scholar]
- 7.Nyrnes A, Jorde R, Sundsfjord J. Serum TSH is positively associated with BMI. Int J Obes. 2006;30(1):100–105. [DOI] [PubMed] [Google Scholar]
- 8.Manji N, Boelaert K, Sheppard MC, Holder RL, Gough SC, Franklyn JA. Lack of association between serum TSH or free T4 and body mass index in euthyroid subjects. Clin Endocrinol (Oxf). 2006;64(2):125–128. [DOI] [PubMed] [Google Scholar]
- 9.Ortega E, Pannacciulli N, Bogardus C, Krakoff J. Plasma concentrations of free triiodothyronine predict weight change in euthyroid persons. Am J Clin Nutr. 2007;85(2):440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox CS, Pencina MJ, D’Agostino RB, Murabito JM, Seely EW, Pearce EN, Vasan RS. Relations of thyroid function to body weight: cross-sectional and longitudinal observations in a community-based sample. Arch Intern Med. 2008;168(6):587–592. [DOI] [PubMed] [Google Scholar]
- 11.Makepeace AE, Bremner AP, O’Leary P, Leedman PJ, Feddema P, Michelangeli V, Walsh JP. Significant inverse relationship between serum free T4 concentration and body mass index in euthyroid subjects: differences between smokers and nonsmokers. Clin Endocrinol (Oxf). 2008;69(4):648–652. [DOI] [PubMed] [Google Scholar]
- 12.Rondeau G, Rutamucero N, Messier V, Burlacu L, Prud’homme D, Mircescu H, Rabasa-Lhoret R. Reference range thyroid-stimulating hormone is associated with physical activity energy expenditure in overweight and obese postmenopausal women: a Montreal-Ottawa New Emerging Team Study. Metabolism. 2010;59(11):1597–1602. [DOI] [PubMed] [Google Scholar]
- 13.Svare A, Nilsen TI, Bjøro T, Asvold BO, Langhammer A. Serum TSH related to measures of body mass: longitudinal data from the HUNT Study, Norway. Clin Endocrinol (Oxf). 2011;74(6):769–775. [DOI] [PubMed] [Google Scholar]
- 14.Waring AC, Rodondi N, Harrison S, Kanaya AM, Simonsick EM, Miljkovic I, Satterfield S, Newman AB, Bauer DC; Health, Ageing, and Body Composition (Health ABC) Study . Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf). 2012;76(6):911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de González A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7(4):e34979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garin MC, Arnold AM, Lee JS, Tracy RP, Cappola AR. Subclinical hypothyroidism, weight change, and body composition in the elderly: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2014;99(4):1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agnihothri RV, Courville AB, Linderman JD, Smith S, Brychta R, Remaley A, Chen KY, Simchowitz L, Celi FS. Moderate weight loss is sufficient to affect thyroid hormone homeostasis and inhibit its peripheral conversion. Thyroid. 2014;24(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren R, Jiang X, Zhang X, Guan Q, Yu C, Li Y, Gao L, Zhang H, Zhao J. Association between thyroid hormones and body fat in euthyroid subjects. Clin Endocrinol (Oxf). 2014;80(4):585–590. [DOI] [PubMed] [Google Scholar]
- 19.Spadafranca A, Cappelletti C, Leone A, Vignati L, Battezzati A, Bedogni G, Bertoli S. Relationship between thyroid hormones, resting energy expenditure and cardiometabolic risk factors in euthyroid subjects. Clin Nutr. 2015;34(4):674–678. [DOI] [PubMed] [Google Scholar]
- 20.Nagel A, Spinneker A, Neuhäuser-Berthold M. Association of thyroid-stimulating hormone with resting energy expenditure in euthyroid elderly subjects: a cross-sectional study. Ann Nutr Metab. 2016;68(1):12–18. [DOI] [PubMed] [Google Scholar]
- 21.Tiller D, Ittermann T, Greiser KH, Meisinger C, Agger C, Hofman A, Thuesen B, Linneberg A, Peeters R, Franco O, Heier M, Kluttig A, Werdan K, Stricker B, Schipf S, Markus M, Dörr M, Völzke H, Haerting J. Association of serum thyrotropin with anthropometric markers of obesity in the general population. Thyroid. 2016;26(9):1205–1214. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Pedley A, Marqusee E, Sutherland P, Hoffmann U, Massaro JM, Fox CS. Thyroid function and cardiovascular disease risk factors in euthyroid adults: a cross-sectional and longitudinal study. Clin Endocrinol (Oxf). 2016;85(6):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.al-Adsani H, Hoffer LJ, Silva JE. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82(4):1118–1125. [DOI] [PubMed] [Google Scholar]
- 24.Boeving A, Paz-Filho G, Radominski RB, Graf H, Amaral de Carvalho G. Low-normal or high-normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study. Thyroid. 2011;21(4):355–360. [DOI] [PubMed] [Google Scholar]
- 25.Dubois S, Abraham P, Rohmer V, Rodien P, Audran M, Dumas JF, Ritz P. Thyroxine therapy in euthyroid patients does not affect body composition or muscular function. Thyroid. 2008;18(1):13–19. [DOI] [PubMed] [Google Scholar]
- 26.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118(11):1278–1289. [DOI] [PubMed] [Google Scholar]
- 27.Compher C, Frankenfield D, Keim N, Roth-Yousey L; Evidence Analysis Working Group . Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. J Am Diet Assoc. 2006;106(6):881–903. [DOI] [PubMed] [Google Scholar]
- 28.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55(2):628–634. [DOI] [PubMed] [Google Scholar]
- 29.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr. 1996;63(2):164–169. [DOI] [PubMed] [Google Scholar]
- 30.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring). 2012;20(5):1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 32.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90(9):5483–5488. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: linear and nonlinear mixed effects models. R package version 3.1-120. http://CRAN.R-project.org/package=nlme. Accessed 25 June 2016.
- 34.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lartey LJ, Werneck-de-Castro JP, O-Sullivan I, Unterman TG, Bianco AC, O-Sullivan I, Unterman TG, Bianco AC. Coupling between nutrient availability and thyroid hormone activation. J Biol Chem. 2015;290(51):30551–30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. [DOI] [PubMed] [Google Scholar]
- 38.Bennion LJ, Grundy SM. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975;56(4):996–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jonklaas J. Risks and safety of combination therapy for hypothyroidism. Expert Rev Clin Pharmacol. 2016;9(8):1057–1067. [DOI] [PubMed] [Google Scholar]
- 40.Celi FS, Zemskova M, Linderman JD, Smith S, Drinkard B, Sachdev V, Skarulis MC, Kozlosky M, Csako G, Costello R, Pucino F. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96(11):3466–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013;34(5):619–657. [DOI] [PubMed] [Google Scholar]