Abstract
Ghrelin plays a key role in appetite, energy homeostasis, and glucose regulation. Recent evidence suggests ghrelin suppresses inflammation in obesity; however, whether this is modulated by the acylated and/or des-acylated peptide is unclear. We used mice deficient in acylated ghrelin [ghrelin octanoyl-acyltransferase (GOAT) knockout (KO) mice], wild-type (WT) littermates, and C57BL/6 mice to examine the endogenous and exogenous effects of acyl and des-acyl ghrelin on inflammatory profiles under nonobese and obese conditions. We demonstrate that in the spleen, both ghrelin and GOAT are localized primarily in the red pulp. Importantly, in the thymus, ghrelin was predominantly localized to the medulla, whereas GOAT was found in the cortex, implying differing roles in T cell development. Acute exogenous treatment with acyl/des-acyl ghrelin suppressed macrophage numbers in spleen and thymus in obese mice, whereas only acyl ghrelin increased CD3+ T cells in the thymus in mice fed both chow and a high-fat-diet (HFD). Consistent with this result, macrophages were increased in the spleen of KO mice on a HFD. Whereas there was no difference in CD3+ T cells in the plasma, spleen, or thymus of WT vs KO mice, KO chow and HFD-fed mice displayed decreased leukocytes. Our results suggest that the acylation status affects the anti-inflammatory properties of ghrelin under chow and HFD conditions.
Ghrelin suppresses inflammation. We examined if acylated and/or des-acylated peptides regulate inflammation and show acylation status affects ghrelin's anti-inflammatory actions on chow and HFDs.
Ghrelin is a peptide hormone produced mainly in the stomach and is primarily involved in food intake, energy homeostasis, and glucose regulation. It is the endogenous ligand for the growth hormone (GH) secretagogue receptor 1a (GHS-R1a), regulating GH release (1, 2). To perform most of its physiological functions, proghrelin is acylated on its third serine residue in the endoplasmic reticulum and packaged to mature acyl ghrelin in the Golgi apparatus. Ghrelin octanoyl-acyltransferase (GOAT) is the enzyme responsible for acylating ghrelin (3, 4), which is necessary to bind GHS-R1a and promote GH secretion, body weight, appetite, motivation, and blood glucose (5–8). After release into the blood stream, acyl ghrelin is readily processed to des-acyl ghrelin by a number of esterases including protein thioesterase (9) or by butyrylcholinesterase (10, 11). Des-acyl ghrelin does not bind to GHS-R1a, although a number of studies suggest it has physiological roles in insulin secretion, osteoblast growth, neuronal cell proliferation, the stress axis (12), and lipid accumulation (13–16), independent from the GHS-R1a.
Des-acyl ghrelin is also thought to antagonize the role of acyl ghrelin in glucose homeostasis as well as sharing functions in cardiovascular and antiproliferative pathways (17, 18). In the central nervous system, ghrelin functions to convey a signal of negative energy balance from the body to the brain to initiate a number of behavioral and physiological changes that promote weight regain, blood glucose production, neuroprotection, and anxiolytic effects (19–24). However, the effect of ghrelin in the body and brain go far beyond metabolic processing, and it is becoming clearer that acyl ghrelin plays an important role in modulating an appropriate inflammatory response. Real-time (RT) polymerase chain reaction (PCR) analysis has revealed ghrelin messenger RNA (mRNA) expression in human lymph nodes, spleen, and thymus (25, 26), and GHS-R and ghrelin are expressed in human T lymphocytes and monocytes (25). In a murine model of colitis, ghrelin attenuated the inflammatory and autoimmune response, restoring immune tolerance and helping treat Crohn’s disease, rheumatoid arthritis, and multiple sclerosis (27). Specifically, ghrelin administration suppresses mRNA levels of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6.
Any anti-inflammatory effect of des-acyl ghrelin remains unknown even though it is found in the plasma in at least three times higher concentrations than acyl ghrelin. In addition, it has recently been documented that patients with nonalcoholic steatohepatitis exhibited twofold higher concentrations of des-acyl ghrelin than patients with alcoholic steatohepatitis (28). In this study, we have examined whether ghrelin or des-acyl ghrelin exhibit differential effects on the immune system and inflammatory markers. To do this, we used exogenous administration of acyl or des-acyl ghrelin peptides to wild-type (WT) mice and examined the phenotype of GOAT knockout (KO) mice, which have no acyl ghrelin but high levels of des-acyl ghrelin (23).
The neuroendocrine ghrelin system is suppressed in an obese state in both humans and mouse models (24, 29, 30). This effect is predominantly due to reduced ghrelin secretion into the blood and reduced GHS-R sensitivity to circulating ghrelin, at least in the brain, resulting in ghrelin resistance (24, 29, 30). Indeed, obesity is often associated with both acute and chronic inflammation and because plasma ghrelin is reduced during obesity, a lack of ghrelin signaling or a change in the ratio of acyl to des-acyl in such conditions may exacerbate the inflammatory response (31, 32). We therefore also examined how obesity affected the function of acyl and des-acyl ghrelin in the immune system and on inflammation.
Materials and Methods
Animals
Naïve C57BL/6 (C57BL/6) mice (6 to 7 weeks old, male) were housed in shared cages (five mice per cage) in the Animal Research Laboratory at Monash University and maintained at a 12-hour light/dark cycle (6 am to 6 pm) at 22°C with free access to food and water. Mice were randomly allocated to receive either a chronic high-fat diet (HFD; 43% of calories from fat, Specialty Feeds, SF04-001) or a chow diet (9% of calories from fat, Barastoc/Specialty Feeds, 8720610/5001) for over 16 weeks. GOAT −/− (KO) mice and littermates (WT) on a C57BL/6 background (6 to 7 weeks old, male) were housed (three mice per cage) at a 12-hour light/dark cycle (6 am to 6 pm) at 22°C with free access to food and water. They were also randomly allocated to either a HFD or chow diet for 7 weeks. Body weights were recorded on a weekly basis. All mice were euthanized by isoflurane, and the blood, spleen, and thymus of both the C57BL/6 and WT/KO mice cohort were perfused and removed for fluorescent-activated cell sorting (FACS) analysis or histology. This was repeated in a different cohort of C57BL/6 mice (aged 6 to 7 weeks) fed a chow or HFD; however, tissue was gathered by fresh tissue collection for PCR analysis.
The KO mice were generated using Velocigene technology. The GOAT gene sequence (ATG-stop) was replaced with a LacZ reporter gene using the target vector, bacterial artificial chromosome (23). These mice originated from C57BL/6/129-targeted embryonic stem cells, and mice were backcrossed onto a C57BL/6 mice background. All experiments were performed with the approval of the Animal Ethics Committee.
Immunohistochemistry (fluorescence)
The spleen and thymus of KO and WT mice were fixed and embedded in paraffin. Sections were then incubated with the primary antibody rabbit antighrelin (1:1000 dilution, Phoenix, H03131). The secondary antibody goat anti-rabbit conjugated to Alexa 488 (1:400, Invitrogen, A11003) was then added and mounted using the fluorescent mounting medium 4′,6-diamidino-2-phenylindole (Dako, S3023). This protocol was repeated for GOAT expression with the exception of using the primary antibody chicken anti-β-galactosidase antibody (1:1000 dilution, Sigma Life Sciences, GW20071F). β-Galactosidase is the product of the LacZ reporter gene used to replace the GOAT sequence in KO mice. The corresponding secondary antibodies used were goat anti-mouse conjugated to Alexa 488 (1:400 dilution, Life Technologies, A11001) and goat anti-chicken conjugated to Alexa 488 (1:400 dilution, Invitrogen, A11039). Positive controls used were stomach tissue (the main site of ghrelin production) and negative controls used were no primary antibody controls or WT mouse tissue (no LacZ expression).
Real-time quantitative PCR
RNA from the spleen and thymus of C57BL/6 mice on HFD or chow diet was isolated and purified using TRIzol reagent and DNase. Complementary DNA (cDNA) was generated from 1 μg RNA using the iScript cDNA Synthesis Kit for RT quantitative PCR (170-8891, Bio-Rad Laboratories, Hercules, CA). All samples were then incubated in a C1000 thermal cycler (Bio-Rad Laboratories) with the following conditions: 5 minutes at 25°C, 30 minutes at 42°C for reverse transcription, and 5 minutes at 85°C for reverse transcription inactivation.
Real-time quantitative PCR was performed using SybrGreen Fast Gene Expression Assay with primers for ghrelin (forward ACCCAGAGGACAGAGGACAA; reverse TCGAAGGGAGCATTGAACCTG), MBOAT4 (GOAT; forward GACAGTGGGCCTCACATTCA; reverse GAGAAAGAGGTACCTGGCCC). GHS-R (forward GCTGCTCACCGTGATGGTAT; reverse ACCACAGCAAGCATCTTCACT), IL-1β (forward AGGAGAACCAAGCAACGACA; reverse CTTGGGATCCACACTCTCCAG), IL-6 (forward GTGGCTAAGGACCAAGACCA; reverse TAACGCACTAGGTTTGCCGA), and TNF-α (forward CGTCGTAGCAAACCACCAAG; reverse TTGAGATCCATGCCGTTGGC), and β-actin (forward CCAGATCATGTTTGAGACCTTC; reverse CATGAGGTAGTCTGTCAGGTCC) was used as a housekeeping gene for normalization.
cDNA was amplified by a Real Plex4 Mastercycler (Eppendorf, Hamburg, Germany) following PCR thermal cycling conditions: a temperature hold step at 50°C for 2 minutes, another temperature hold step at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute allowing quantification.
Blood chemistry
Blood samples were obtained by terminal cardiac puncture under isoflurane anesthesia. Plasma IL-1β, TNF-α, and IL-6 were determined by multiplexed bead assay (Bio-Plex Pro mouse cytokine assay, Bio-Rad) using the MAGPIX instrument (Luminex).
Dual-energy-X-ray absorptiometry
Body composition was measured using a dual-energy x-ray absorptiometry scanner on C57BL/6 mice at 12 weeks on the diet (4.2 months old) and WT and KO mice at 6 weeks on the diet (3.2 months old). Scans were then analyzed using PIXImus2 software.
Oral glucose tolerance test and insulin tolerance test
Glucose tolerance and insulin sensitivity were assessed in C57BL/6 mice at 16 weeks on the diet and in WT and KO mice at 6 weeks on the diet. Mice were fasted for 4 hours and then dosed orally with 2 g/kg glucose solution for the oral glucose tolerance test (GTT). Blood glucose was recorded from the tail blood at 0, 30, 60, and 120 minutes after the glucose bolus. For insulin tolerance test (ITT), mice were fasted for 4 hours and injected intraperitoneal with 0.75 UI/kg of insulin. Blood glucose was recorded from the tail blood at 0, 15, 30, 60, and 120 minutes after the insulin bolus.
Acyl, des-acyl, and saline administrations in C57BL/6 mice
The effect of exogenous acyl and des-acyl ghrelin administration was assessed in nonfasted C57BL/6 mice by randomly selecting 15 mice on HFD and 15 mice on chow to receive an acute intraperitoneal injection of either acyl, des-acyl ghrelin, or saline at 1 mg/kg. One hour after administration, mice were culled and the blood, spleen, and thymus were processed for FACS analysis.
FACS
Blood (treated with 800 U/mL of heparin), spleen, and thymus of both C57BL/6 and WT and KO mice were collected; red blood cells were lysed; and samples were treated with trypan blue, where the number of live cells were recorded by a Countess Automated Cell Counter (Invitrogen, C10281). A concentration of 106 cells/100 μl was used per well. Samples were firstly incubated with aqua live/dead cell viability stain (L34957, Life Technologies, 1:1000). The antibodies—APC anti-mouse CD3 (100312, Biolegend, 1:200), BV605 anti-mouse CD4 (100548, Biolegend, 1:200), PE/Cy7 anti-mouse F4/80 (123113, Biolegend, 1:1000), A700 anti-mouse CD45 (103128, Biolegend, 1:200), Perc/P Cy5.5 anti-mouse CD8a (100734, Biolegend, 1:200), and BV421 anti-mouse CD11b (101235, Biolegend, 1:500)—were then added. After incubating overnight, samples were processed on the flow cytometer machine (LSR-IIa cell analyzer, BD Bioscience) at the FlowCore facility (Monash University). Data were analyzed using FlowJo software (FlowJo vX 10.0.7 for Windows 7) for individual amounts of leukocytes, macrophages, CD3+, CD4+, CD8+, CD4+CD8+, and CD4–CD8– T cells in the blood, spleen, and thymus of WT and KO and C57BL/6 mice.
Statistical analysis
Data are presented as mean ± standard error of the mean, where error bars also indicate standard error of the mean. Statistical analysis was carried out using unpaired Student’s t test or two-way analysis of variance (repeated measures for GTTs and ITTs) followed by Tukey's post hoc test (P < 0.05, P < 0.01, P < 0.001, and P < 0.0001).
Results
Ghrelin and GOAT are localized in the spleen and thymus
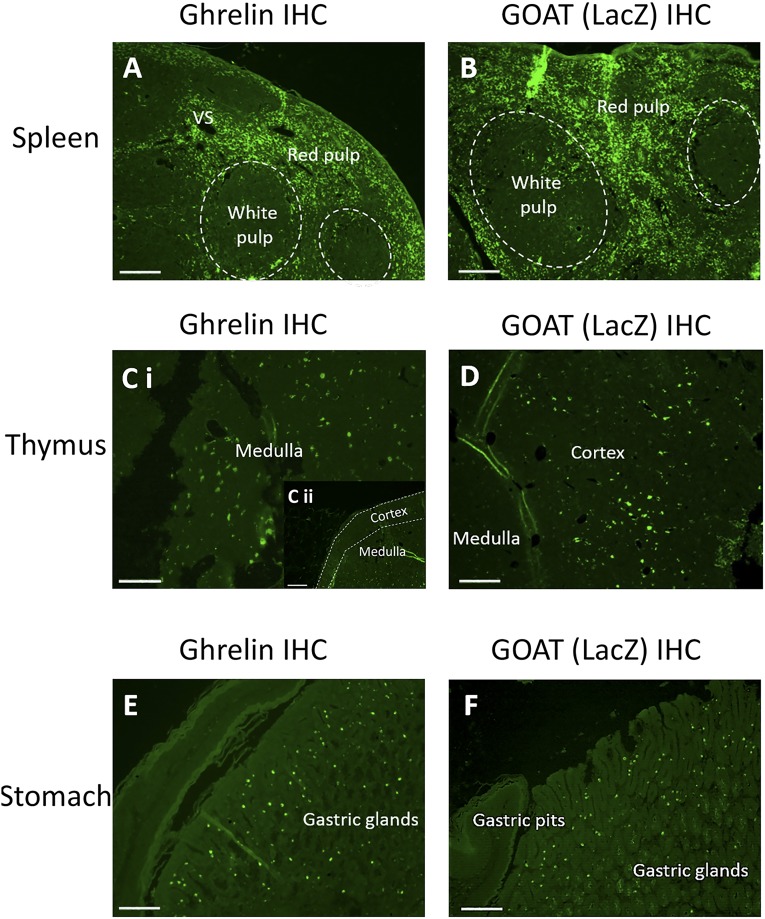
We first examined localization of ghrelin in the primary and secondary lymphoid organs, thymus, and spleen in WT and KO mice, using standard immunohistochemistry techniques. Ghrelin was found predominantly in the red pulp of the spleen and medulla of the thymus [Fig. 1(A), 1(Ci), and 1C(ii)]. The white pulp was defined by dark, spheroidal shapes in the spleen and the red pulp was defined by the extracellular area of connective tissue surrounding the white pulp. To identify the extent of GOAT expression in the same tissues we used the LacZ reporter gene driven from the endogenous promoter in KO mice. β-Galactosidase staining in tissues taken from KO mice showed GOAT was localized primarily in the red pulp of the spleen and to the cortical region of the thymus [Fig. 1(B) and 1(D)]. Staining specificity was confirmed by multiple methods [3,3′-diaminobenzidine, fluorescence, Supplemental Fig. 1(A) and 1(D (115.9MB, tiff) )], various negative controls by omitting primary antibody [Supplemental Fig. 1(B), 1(C), and 1(E–G) (115.9MB, tiff) ] and positive control tissues such as immunolabeled ghrelin and GOAT in the stomach [Fig. 1(E) and 1(F)].
Figure 1.
Representative photomicrograph of ghrelin and GOAT expression. Fluorescence Immunohistochemical staining of ghrelin expression (left side) and GOAT (right side) in the (A, B) spleen, (Ci, Cii, D) thymus, and (E, F) stomach. (F) Stomach tissue was used as the positive control (site of ghrelin synthesis). For GOAT expression, the LacZ reporter gene was detected by an anti β-galactosidase antibody. All photos taken at 10× magnification with scale bars set at 100μm.
HFD increases body weight and fat distribution and induces insulin resistance and glucose intolerance
To examine how acyl and des-acyl ghrelin affect immune function during obesity, both C57BL/6 and WT and KO mice were placed on a HFD. We measured changes in body weight, fat and lean mass, bone mineral density, and insulin and glucose tolerance to confirm metabolic dysfunction in HFD mice. HFD significantly increased body weight; total, fat and lean mass; percentage fat; and bone mineral density/content in C57BL/6 mice [Supplemental Fig. 2(A–D) (81MB, tiff) ]. HFD also significantly worsened blood glucose levels and induced insulin resistance [Supplemental Fig. 2(E–F) (81MB, tiff) ]. In WT and KO mice, HFD again significantly increased body weight; total, fat, and lean mass; percentage fat; and bone mineral density/content, with no genotype effect [Supplemental Fig. 3(A–D) (81.5MB, tiff) ]. Glucose tolerance was significantly worsened in HFD WT mice only [Supplemental Fig. 3(E)] (81.5MB, tiff) , consistent with studies showing that inhibiting GOAT activity improves glucose tolerance in mice (33). Insulin resistance, as assessed by ITTs was induced in both HFD WT and HFD KO mice [Supplemental Fig. 3(F)] (81.5MB, tiff) .
HFD affects ghrelin, GOAT, and GHS-R expression in the thymus
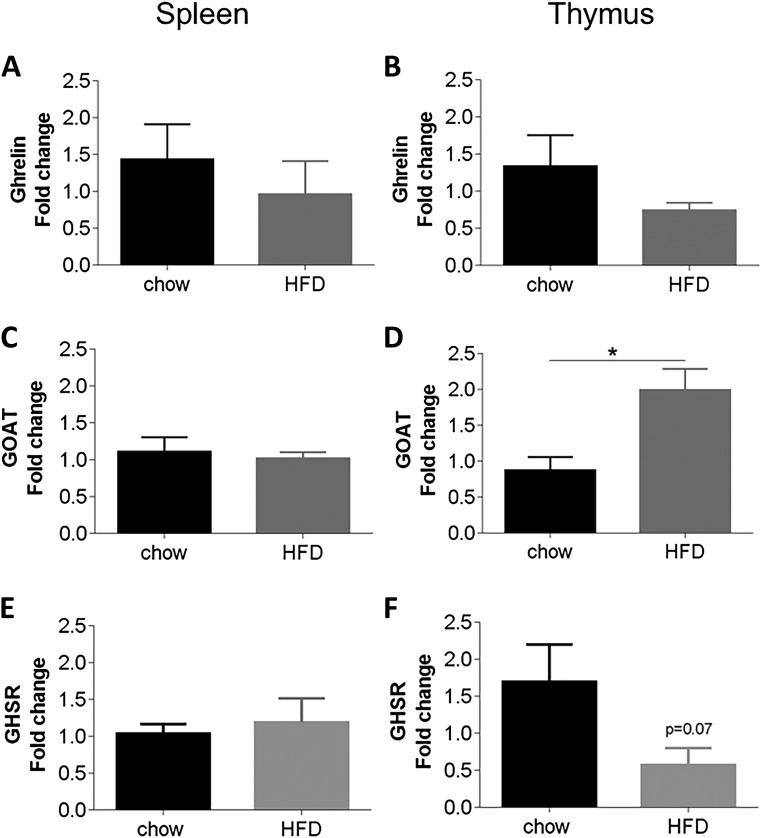
HFD caused a trend toward decreased ghrelin and GHS-R in the thymus [P = 0.2 and P = 0.07, respectively; Fig. 2(B) and 2(F)], which is consistent with a downregulation of ghrelin system during obese states (24). However, thymic GOAT expression significantly increased under HFD conditions [Fig. 2(D)], suggesting that obesity has differential effects on GOAT, ghrelin, and GHS-R gene expression. There were no significant differences of ghrelin, GOAT, or GHS-R in the spleen [Fig. 2(A), 2(C), and 2(E)].
Figure 2.
mRNA expression of ghrelin, GOAT and GHS-R in the spleen (left) and thymus (right) in C57BL/6 mice fed a chow or HFD. Relative mRNA expression of ghrelin, GOAT, and the ghrelin receptor, GHS-R, in the spleen (left) and thymus (right) of C57BL/6 mice fed a chow/HFD (N = 12). Results were normalized to β-actin RNA, and expressed as the fold change vs the mean of the chow diet group using the delta-delta Ct method. Results expressed as mean ± standard error of the mean. Statistical significance was obtained using a Student’s unpaired t test.
Effects of exogenous acyl/des-acyl ghrelin on immune cell profiles of C57BL/6 mice fed a chow/HFD
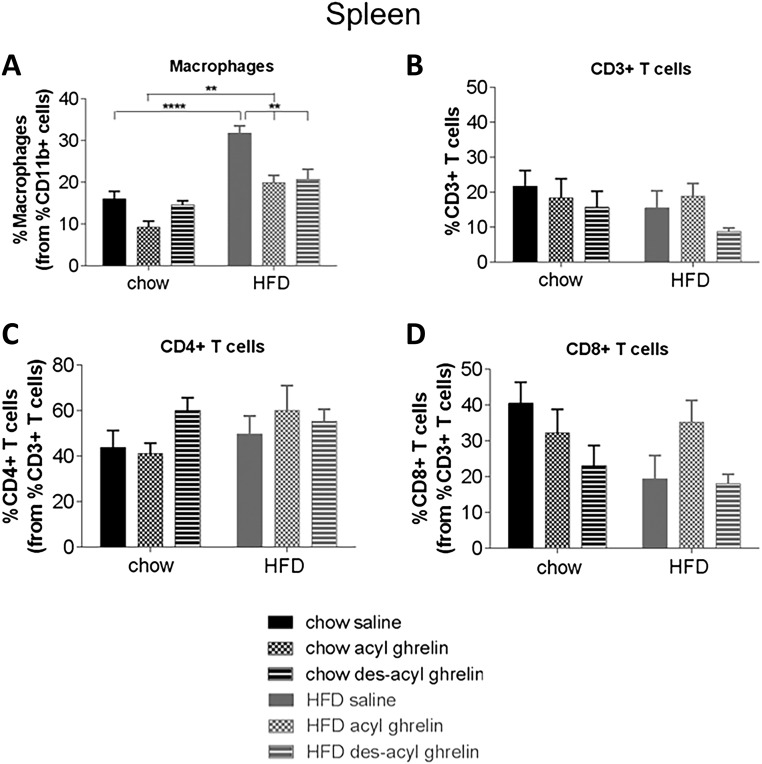
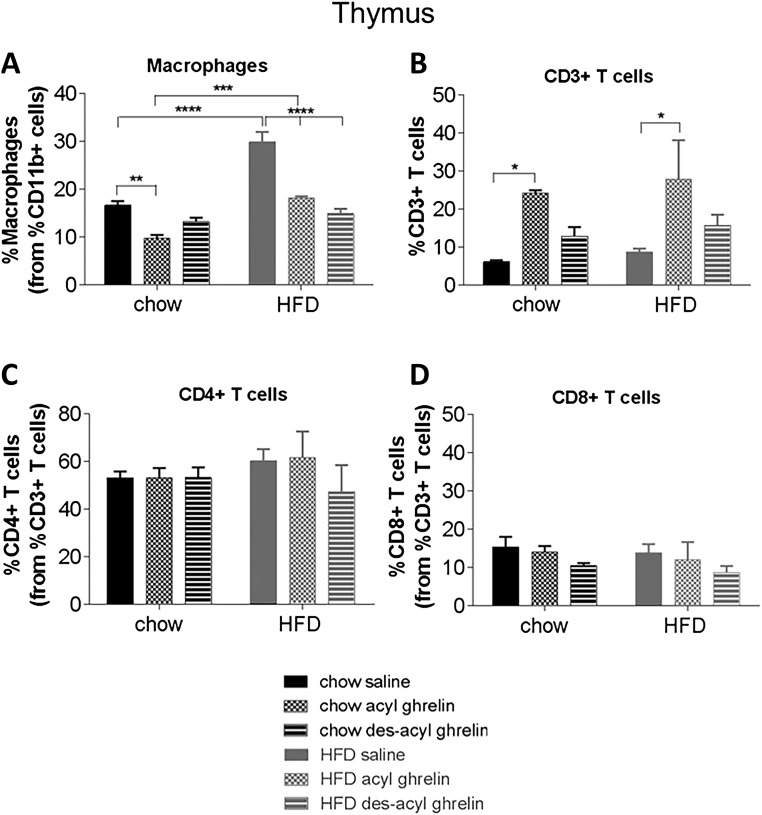
To determine the effect of acyl ghrelin or des-acyl ghrelin on immune function, we treated chow and HFD mice with acute acyl or des-acyl ghrelin and examined various immune cell populations by FACS from the blood, spleen, and thymus. There were no significant differences obtained among all immune cell profiles in the blood (Supplemental Fig. 4 (77MB, tiff) ). Diet and treatment significantly affected the percentage of macrophages in both spleen [Fig. 3(A)] and thymus [Fig. 4(A)]. In the spleen, HFD significantly increased the percentage of macrophages; however, both acyl and des-acyl ghrelin administration significantly decreased percentage of macrophages in comparison with the saline control (P < 0.01 and P < 0.0001, respectively). No significant changes were seen in CD3+, CD4+, or CD8+ T cell populations in the spleen [Fig 3(B–D)]. A similar pattern was observed in the thymus, in which HFD significantly increased percentage of macrophages [Fig. 4(A)]. In both chow and HFD conditions, acyl ghrelin reduced the percentage macrophages; however, only des-acyl ghrelin reduced macrophages in HFD conditions [Fig. 4(A); P < 0.01]. Conversely, acyl ghrelin but not des-acyl ghrelin increased the percentage of CD3+ T cells in the thymus under both chow and HFD conditions [Fig. 4(B); P < 0.05]. No significant changes were seen in CD4+ and CD8+ T cells in the thymus [Fig. 4(C) and 4(D)].
Figure 3.
Immune cell profiles of spleen from C57BL/6 treated with saline, acyl, or des-acyl ghrelin and percentage of immune cells and markers from the parent population of leukocytes, (A) macrophages, (B) CD3+ T cells, (C) CD4+ T cells, and (D) CD8+ T cells in the spleen of C57BL/6 mice. Mice received either a chow (n = 15) or HFD (n = 15) and an intraperitoneal injection of either 1 mg/kg saline, acyl, or des-acyl ghrelin. Results expressed as mean ± standard error of the mean with P values obtained using a two-way analysis of variance followed by Tukey's post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Figure 4.
Immune cell profiles of thymus from C57BL/6 mice treated with saline, acyl, or des-acyl ghrelin and percentage of immune cells and markers from the parent population of leukocytes, (A) macrophages, (B) CD3+ T cells, (C) CD4+ T cells, and (D) CD8+ T cells in the thymus of C57BL/6 mice. Mice received either a chow (n = 15) or HFD (n = 15) and an intraperitoneal injection of either 1mg/kg saline, acyl, or des-acyl ghrelin. Results expressed as mean ± standard error of the mean with P values obtained using a two-way analysis of variance followed by Tukey's post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
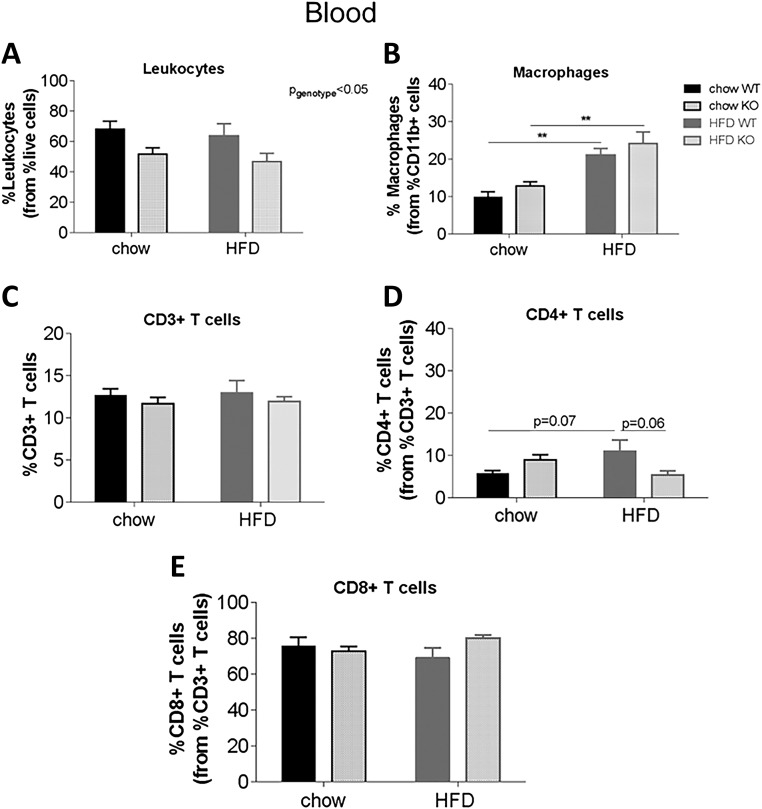
Effects of diet on the blood, spleen, and thymus of WT and KO mice
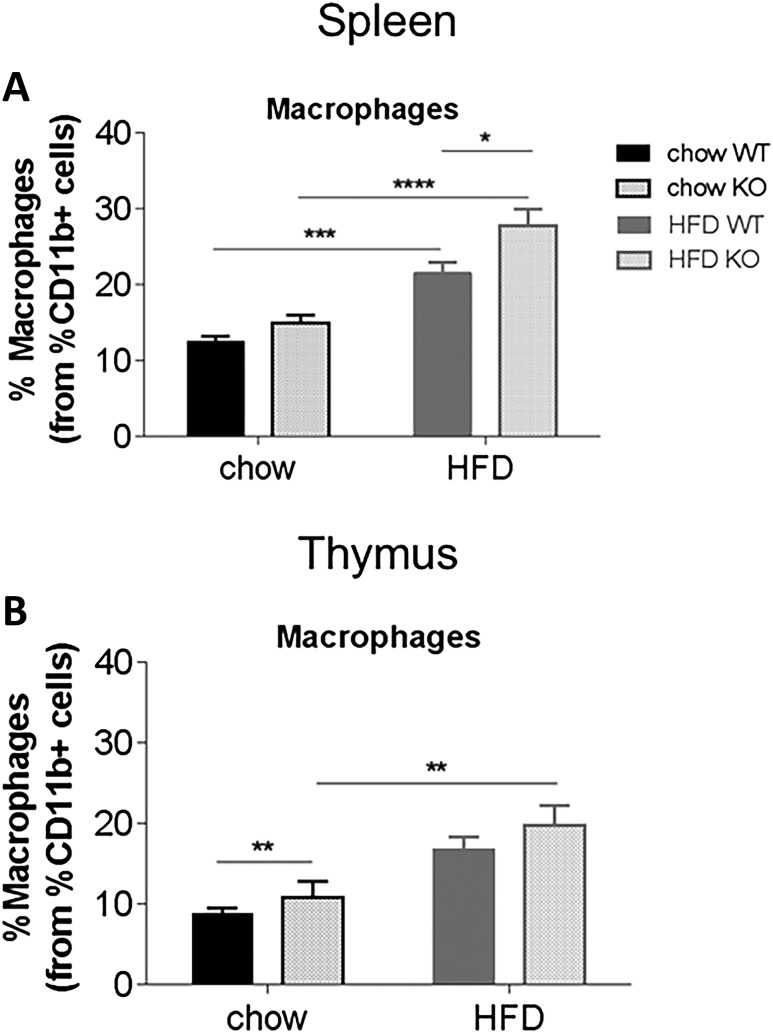
We have observed specific effects of acute acyl or des-acyl ghrelin injection on immune cell profiles, suggesting that acylation status may play an important role on ghrelin’s ability to dampen immune function. To test this idea in a prolonged chronic setting, we used GOAT KO mice, which have no acyl ghrelin and chronically high des-acyl ghrelin. In the blood, there was an overall effect of genotype to decrease the percentage of leukocytes in KO mice [Fig. 5(A); P < 0.05]. HFD significantly increased the percentage of macrophages in WT and KO mice [Fig. 5(B); P < 0.01], although no differential effect of genotype was observed. No significant differences were observed in CD3+ or CD8+ T cells, although HFD increased the percentage of CD4+ T cells in HFD WT to chow WT mice (P = 0.07) and decreased CD4+ T cells in HFD KO to HFD WT mice [P = 0.06; Fig. 5(C–E)]. In the spleen, HFD increased macrophages (main effect of diet), and this was significantly higher in KO compared with WT mice on HFD [P < 0.05; Fig. 6(A)], whereas in the thymus, both diet and genotype increased the percentage of macrophages [P < 0.01; Fig. 6(B)]. In both the spleen and thymus, there were no differences in CD3+, CD4+ or CD8+ T cells (Supplemental Fig. 5 (93.6MB, tiff) ).
Figure 5.
Effect of diet on immune cell profiles in the blood of WT and KO mice. Percentage of immune cells/markers from the parent population of leukocytes, (A) leukocytes, (B) macrophages, (C) CD3+ T cells, (D) CD4+ T cells, and (E) CD8+ T cells in the blood of WT and KO mice. Mice were fed a chow diet (n = 15) or HFD (n = 16) for 7 weeks. Results are expressed as mean ± standard error of the mean with P values obtained using a two-way analysis of variance followed by Tukey's post hoc test. **P < 0.01.
Figure 6.
Effect of diet on percentage of macrophages in the spleen and thymus of WT and KO mice. Percentage of macrophages from the parent population of leukocytes in the (A) spleen and (B) thymus of WT and KO mice. Results are expressed as mean ± standard error of the mean with P values obtained using a two-way analysis of variance followed by Tukey’s post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
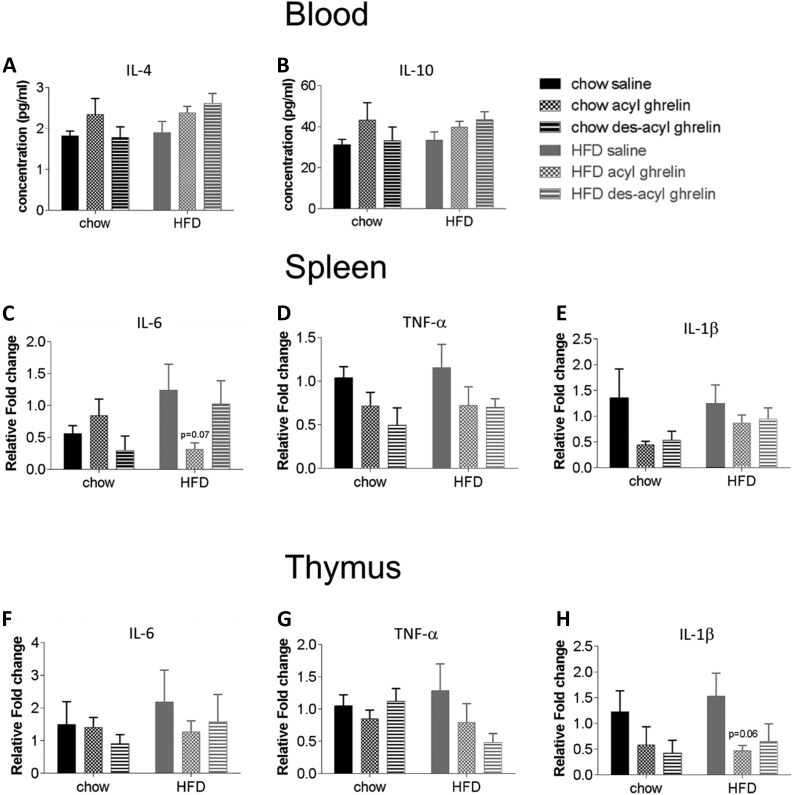
Effects of exogenous acyl/des-acyl ghrelin on proinflammatory and anti-inflammatory cytokine levels
Because acyl ghrelin decreased the percentage of macrophages, we next analyzed the effect of IL-4 and IL-10 in the blood as well as IL-6, TNF-α, and IL-1β in the spleen and thymus of C57BL/6 mice fed a chow diet or HFD. Although there were no significant differences, there was a trend toward increased levels of IL-4 and IL-10 with acyl ghrelin under both chow and HFD conditions and des-acyl ghrelin under HFD conditions in the blood [Fig. 7(A) and 7(B)]. Conversely, acyl ghrelin but not des-acyl ghrelin trended to decrease mRNA expression of IL-6, TNF-α, and IL-1þ in HFD conditions in the spleen and thymus, respectively [Fig. 7(C–H)].
Figure 7.
Expression of anti-inflammatory cytokines in the blood, spleen and thymus of C57BL/6 mice treated with saline, acyl, or des-acyl ghrelin fed a chow or HFD. The serum concentration (pg/mL) of (A) IL-4 and (B) IL-10 anti-inflammatory cytokines in the blood of C57BL/6 mice fed a chow or HFD treated with saline, acyl, or des-acyl ghrelin 1 hour before euthanasia. Relative mRNA expression of (C, F) IL-6, (D, G) TNF-α, and (E, H) IL-1β in the spleen and thymus of C57BL/6 mice fed a chow/HFD (N = 12). Results obtained through RT-PCR of targets were normalized to β-actin RNA, then expressed as the fold change vs the mean of the chow diet group using the delta-delta Ct method. Results are expressed mean ± standard error of the mean. Statistical significance was obtained using a two-way analysis of variance followed by Tukey’s post hoc test.
Discussion
The gut peptide ghrelin has received most attention as a regulator of metabolic events through centrally expressed GHS receptors; however, there is strong evidence of an association with inflammation and a critical role in the regulation of immune cell function (25, 34). Ghrelin is expressed and secreted by resting and activated T cells (25), and its expression declines in the thymus with age (35). The expression of GHS-R on human T lymphocytes and monocytes is well established, as is the action of ghrelin to inhibit splenic T cells proliferation (36) and proinflammatory cytokine expression in various cell types (25, 34, 37). Consistent with previous studies, we show cellular expression profile of ghrelin in immunologically relevant organs but also extend this to the expression of the ghrelin-specific acyltransferase, GOAT, and provide further evidence of a role for acyl and des-acyl ghrelin acylation in immune function (25, 38, 39).
Although previous studies suggest a specific function for ghrelin on immune effector cells (25, 34, 37), we provide data that suggest a broader role of both circulating forms of ghrelin (acyl and des-acyl) and additionally show a high level of staining for both ghrelin and GOAT (via β-galactosidase) in the spleen and thymus. Within the spleen, both ghrelin and GOAT were localized to the red pulp, suggesting a role in removal of foreign material and possibly hematopoietic and macrophage function. A single study has assigned some hematopoiesis activity to ghrelin, reporting an increase in both hematocrit and red blood cell count (40). This was attributed to either a direct effect of ghrelin on bone marrow or indirect effects through adrenocorticotrophic hormone and GH secretion; however, direct splenic ghrelin expression may also be involved, although this requires additional investigation. Both RT-PCR and immunofluorescent detection of ghrelin and GOAT in the spleen support findings by Lim and colleagues (41), who discovered the spleen to be one of the organs with a high expression of GOAT.
Surprisingly, in the thymus, we found a divergence between ghrelin and GOAT staining: ghrelin was found predominantly in the medulla, whereas GOAT was only present in the cortex. Moreover, in HFD conditions, expression of ghrelin, GOAT, and the ghrelin receptor, GHS-R, differ in the thymus but not the spleen. The presence of GOAT in the cortex of the thymus suggests that GOAT may play a role in modulating peptides involved in the regulation of thymocyte development and immune cell function. Finally, within the thymus, acyl ghrelin increased CD3+ T cells, whereas the selective absence of acyl ghrelin (GOAT KO mice) decreased percentage of leukocytes. These results would support a role for acyl ghrelin to regulate leukocytes to ultimately recognize pathogens and prevent infection, in line with previous studies (25, 34, 35, 42).
It has been shown previously that dietary stressors such as a HFD impact the immune system (39, 43). We therefore sought to determine if the effects of both acute and chronic acyl and des-acyl ghrelin in immune cell function were modulated by nutritional status. In the spleen and thymus, there was an effect of treatment, diet, and genotype, whereby acyl ghrelin and des-acyl ghrelin administration decreased the percentage of macrophages in HFD-fed mice in comparison with control saline administration (effect of treatment). Also, HFD mice treated with saline or acyl ghrelin displayed an increased percentage of macrophages in comparison with chow-fed mice treated with saline or acyl ghrelin (effect of diet). Finally, mice on the HFD increased macrophage numbers in comparison with WT and KO mice on the chow diet (effect of diet). KO mice fed a HFD displayed a higher percentage of macrophages in the spleen compared with WT mice on HFD (effect of genotype). This indicates that although diet contributed to the inflammation induced in obese states, acute treatment of acyl and des-acyl ghrelin helped dampen this process by downregulating macrophage levels. Obesity is commonly associated with inflammatory disease, whereby metabolic dysfunction, such as insulin resistance, has been identified in sepsis and inflammatory diseases including rheumatoid arthritis (44, 45). One main feature of inflammation is increased infiltration of macrophages and proinflammatory cytokines. Therefore, it is not surprising that HFD-fed C57BL/6 and WT and KO mice were accompanied with an increased percentage of macrophages. Recently, the ghrelin receptor, GHS-R, has been shown to play an important role in macrophage polarization and adipose tissue inflammation during aging (32). Furthermore, the trend toward decreased mRNA expression of IL-6, TNF-α, and IL-1β under HFD conditions in the spleen and thymus with acute acyl/des-acyl ghrelin treatment further supports the anti-inflammatory effects of ghrelin. However, future studies would benefit to confirm this effect.
The role of acyl ghrelin in immune function was further confirmed in GOAT KO (lacking acyl ghrelin) mice on HFD, as they exhibited a decrease in the percentage of leukocytes in the blood compared with WT mice on HFD. KO mice also displayed a trend toward decreased percentage of CD3+ T cells in the blood compared with WT mice on chow and HFD, implying a role for acyl ghrelin in T cell activation. This trend was also seen in the thymus, where the percentage of CD3+ T cells decreased between genotypes and diet. This is consistent with previous reports showing acyl ghrelin inhibits proliferation of splenic T cells when costimulated by an anti-CD3 monoclonal antibody (35, 36). Therefore, because KO mice have four times higher des-acyl ghrelin levels, this may imply that acyl ghrelin plays a role in enhancing T cell activation; des-acyl ghrelin may act in opposite to decrease T cell activation. Further supporting this, acyl ghrelin increased the percentage of CD3+ T cells in the thymus of C57BL/6 WT mice. The differences between macrophages in KO vs WT mice may be explained by the well-documented anti-inflammatory role of acyl ghrelin, because KO mice lack acyl ghrelin (25, 42, 44). Indeed, Dixit and colleagues (25) found that knockdown of ghrelin in activated T cells increased the secretion of proinflammatory cytokines TNF-α, IFN-y, IL-2, and IL-17 (35).
However, acute des-acyl ghrelin treatment to HFD C57/BL6 mice reduced macrophage number in both the thymus and spleen and therefore we might predict that the high levels of des-acyl ghrelin in KO mice would equally suppress spleen macrophage number on a HFD. As noted above, KO mice on a HFD had significantly higher macrophage number than WT mice on HFD, the exact opposite of that predicted by des-acyl ghrelin administration experiments. It remains possible that chronic high levels of des-acyl ghrelin, as seen in KO mice, induces a proinflammatory response compared with acute des-acyl ghrelin, which produces an anti-inflammatory response. Alternatively, GOAT in the spleen may be required to acylate additional substrates that regulate macrophage production.
Although acute ghrelin treatment and chronic GOAT deletion resulted in mostly consistent effects on immune cell levels, GOAT deletion independently of exogenous acyl/des-acyl administration affected plasma leukocytes, suggesting that GOAT may acylate substrates other than ghrelin, an idea that has also been postulated in the stress axis (12).
Ghrelin was initially reported as an immune enhancing factor; studies by Koo et al. (46) demonstrated that ghrelin treatment reduced tumor development by increasing cytotoxic lymphocytes. Recently, ghrelin has been found to be immunosuppressive both in vitro and in vivo. Dixit et al. (25) demonstrated the anti-inflammatory function of ghrelin against T cells and macrophages in vitro. Ghrelin decreased proinflammatory cytokine levels associated with several disease states, such as inflammatory bowel disease (27), arthritis (47, 48), sepsis, and endotoxemia in vivo (47, 49, 50).
Also, Theil et al. (51) used an animal model of multiple sclerosis to show that exogenous administration of ghrelin suppressed experimental autoimmune encephalomyelitis. Proinflammatory cytokines were also reduced in the microglia of the spinal cord. Microglia are resident macrophage cells and are the first line of active immune defense in the central nervous system, derived from hematopoietic cells (52). Lee and Yune (53) also showed that ghrelin treatment inhibited oligodendrocyte cell death by decreasing pro-NGF and ROS production as well as p38MAPK and JNK activation in activated microglia. Obesity involves the expansion of adipose tissue leading to inflammation via an increase in proinflammatory cytokines from macrophages. Proinflammatory cytokines can cause insulin resistance in adipose tissue, skeletal muscle, and liver by inhibiting insulin signal transduction, thus leading to associated diseases such as diabetes and metabolic syndrome. Therefore, the effect of ghrelin on inflammation is important in reducing the pathogenesis of obesity and diabetes (54).
The exact mechanism by which the ghrelin/GOAT system affects the immune system remains to be defined. Many studies suggest ghrelin permits its anti-inflammatory effects through the vagus nerve, suppressing sympathetic nerve activity (50, 55, 56). Some propose that ghrelin acts on the immune system in an autocrine/paracrine way and indirectly via GH, as GH has shown to improve the development of B cells and antibodies (39, 57, 58). Other studies demonstrated acyl ghrelin acts indirectly by blocking nuclear factor κB and/or STAT3 activation (49, 59, 60), or directly on cytokine mRNA transcription and translation as well as thymic size and cellularity, because it did not affect insulin-like growth factor-1 levels (36).
Regardless of the exact mechanisms, we show cellular expression profile of ghrelin in immunologically relevant organs and extend this to the expression of the ghrelin-specific acyltransferase, GOAT, thus providing further evidence of a role for both acyl and des-acyl ghrelin in immune function. Our results suggest that the acylation status affects the anti-inflammatory properties of ghrelin under chow and HFD conditions. Along with its role in appetite, glucose homeostasis, and neuroprotection, ghrelin provides an attractive therapeutic target in obesity and correlated immune disorders. Further studies are required to address the exact mechanisms involved.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog No, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Ghrelin | Antighrelin | Phoenix, H03131 | Rabbit; polyclonal | 1000 | AB_2314558 | |
| β-galactosidase | Anti-β-galactosidase | Sigma Life Sciences, GW20071F | Chicken; polyclonal | 1000 | AB_2636937 | |
| APC (CD3) | APC anti-CD3 | BioLegend, 100312 | Hamster; monoclonal | 200 | AB_312677 | |
| BV605 (CD4) | BV605 anti-CD4 | BioLegend, 100548 | Rat; monoclonal | 200 | AB_2563054 | |
| PE/Cy7 (F4/80) | PE/Cy7 anti-F4/80 | Biolegend, 123113 | Rat; monoclonal | 1000 | AB_893490 | |
| A700 (CD45) | A700 anti-CD45 | Biolegend, 103128 | Rat; monoclonal | 200 | AB_493715 | |
| Perc/P Cy5.5 (CD8) | Perc/P Cy5.5 anti-CD8a | Biolegend, 100734 | Rat; monoclonal | 200 | AB_2075238 | |
| BV421 (CD11b) | BV421 anti-CD11b | Biolegend, 101235 | Rat; monoclonal | 500 | AB_10897942 |
Abbreviation: RRID, research resource identifier.
Acknowledgments
We would like to acknowledge the histology department, FlowCore, and the Cowley Laboratory at Monash University.
Acknowledgments
Z.B.A. acknowledges a National Health and Medical Research Council Career Development fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- cDNA
- complementary DNA
- FACS
- fluorescent-activated cell sorting
- GH
- growth hormone
- GHS-R
- growth hormone secretagogue receptor
- GHS-R1a
- growth hormone secretagogue receptor 1a
- GOAT
- ghrelin octanoyl-acyltransferase
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- IL
- interleukin
- ITT
- insulin tolerance test
- KO
- knockout
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- TNF
- tumor necrosis factor
- WT
- wild-type.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 2.Smith RG, Pong SS, Hickey G, Jacks T, Cheng K, Leonard R, Cohen CJ, Arena JP, Chang CH, Drisko J, Wyvratt M, Fisher M, Nargund R, Patchett A. Modulation of pulsatile GH release through a novel receptor in hypothalamus and pituitary gland. Recent Prog Horm Res. 1996;51:261–285. [PubMed] [Google Scholar]
- 3.Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. [DOI] [PubMed] [Google Scholar]
- 5.Wang QP, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund E, Elmquist JK, Zigman JM. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2013;3(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott MM, Perello M, Chuang JC, Sakata I, Gautron L, Lee CE, Lauzon D, Elmquist JK, Zigman JM. Hindbrain ghrelin receptor signaling is sufficient to maintain fasting glucose. PLoS One. 2012;7(8):e44089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chacko SK, Haymond MW, Sun Y, Marini JC, Sauer PJ, Ma X, Sunehag AL. Effect of ghrelin on glucose regulation in mice. Am J Physiol Endocrinol Metab. 2012;302(9):E1055–E1062. [DOI] [PubMed] [Google Scholar]
- 8.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115(12):3564–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satou M, Nishi Y, Yoh J, Hattori Y, Sugimoto H. Identification and characterization of acyl-protein thioesterase 1/lysophospholipase I as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology. 2010;151(10):4765–4775. [DOI] [PubMed] [Google Scholar]
- 10.Chen VP, Gao Y, Geng L, Parks RJ, Pang YP, Brimijoin S. Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci USA. 2015;112(7):2251–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vriese C, Gregoire F, Lema-Kisoka R, Waelbroeck M, Robberecht P, Delporte C. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology. 2004;145(11):4997–5005. [DOI] [PubMed] [Google Scholar]
- 12.Stark R, Santos VV, Geenen B, Cabral A, Dinan T, Bayliss JA, Lockie SH, Reichenbach A, Lemus MB, Perello M, Spencer SJ, Kozicz T, Andrews ZB. Des-acyl ghrelin and ghrelin o-acyltransferase regulate hypothalamic-pituitary-adrenal axis activation and anxiety in response to acute stress. Endocrinology. 2016;157(10):3946–3957. [DOI] [PubMed] [Google Scholar]
- 13.Delhanty PJ, Huisman M, Baldeon-Rojas LY, van den Berge I, Grefhorst A, Abribat T, Leenen PJ, Themmen AP, van der Lely AJ. Des-acyl ghrelin analogs prevent high-fat-diet-induced dysregulation of glucose homeostasis. FASEB J. 2013;27(4):1690–1700. [DOI] [PubMed] [Google Scholar]
- 14.Delhanty PJ, Neggers SJ, van der Lely AJ. Des-acyl ghrelin: a metabolically active peptide. Endocr Dev. 2013;25:112–121. [DOI] [PubMed] [Google Scholar]
- 15.Delhanty PJ, Sun Y, Visser JA, van Kerkwijk A, Huisman M, van Ijcken WF, Swagemakers S, Smith RG, Themmen AP, van der Lely AJ. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS One. 2010;5(7):e11749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delhanty PJ, van der Eerden BC, van Leeuwen JP. Ghrelin and bone. Biofactors. 2014;40(1):41–48. [DOI] [PubMed] [Google Scholar]
- 17.Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89(6):3062–3065. [DOI] [PubMed] [Google Scholar]
- 18.Ku JM, Andrews ZB, Barsby T, Reichenbach A, Lemus MB, Drummond GR, Sleeman MW, Spencer SJ, Sobey CG, Miller AA. Ghrelin-related peptides exert protective effects in the cerebral circulation of male mice through a nonclassical ghrelin receptor(s). Endocrinology. 2015;156(1):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, Andrews ZB. Ghrelin-AMPK signaling mediates the neuroprotective effects of calorie restriction in Parkinson’s disease. J Neurosci. 2016;36(10):3049–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology. 2011;93(1):48–57. [DOI] [PubMed] [Google Scholar]
- 21.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11(7):752–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFarlane MR, Brown MS, Goldstein JL, Zhao TJ. Induced ablation of ghrelin cells in adult mice does not decrease food intake, body weight, or response to high-fat diet. Cell Metab. 2014;20(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Goldstein JL, Brown MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zigman JM, Bouret SG, Andrews ZB. Correction to “Obesity impairs the action of the neuroendocrine ghrelin system”: [Trends in Endocrinology and Metabolism, 27 (2016) 54–63]. Trends Endocrinol Metab. 2016;27(5):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixit VD, Schaffer EM, Pyle RS, Collins GD, Sakthivel SK, Palaniappan R, Lillard JW Jr, Taub DD. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Rey E, Chorny A, Delgado M. Therapeutic action of ghrelin in a mouse model of colitis. Gastroenterology. 2006;130(6):1707–1720. [DOI] [PubMed] [Google Scholar]
- 28.Estep M, Abawi M, Jarrar M, Wang L, Stepanova M, Elariny H, Moazez A, Goodman Z, Chandhoke V, Baranova A, Younossi ZM. Association of obestatin, ghrelin, and inflammatory cytokines in obese patients with non-alcoholic fatty liver disease. Obes Surg. 2011;21(11):1750–1757. [DOI] [PubMed] [Google Scholar]
- 29.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151(10):4745–4755. [DOI] [PubMed] [Google Scholar]
- 30.Briggs DI, Lemus MB, Kua E, Andrews ZB. Diet-induced obesity attenuates fasting-induced hyperphagia. J Neuroendocrinol. 2011;23(7):620–626. [DOI] [PubMed] [Google Scholar]
- 31.Dardzińska JA, Małgorzewicz S, Kaska Ł, Proczko M, Stefaniak T, Stankiewicz M, Śledziński Z. Fasting and postprandial acyl and desacyl ghrelin levels in obese and non-obese subjects. Endokrynol Pol. 2014;65(5):377–381. [DOI] [PubMed] [Google Scholar]
- 32.Lin L, Lee JH, Buras ED, Yu K, Wang R, Smith CW, Wu H, Sheikh-Hamad D, Sun Y. Ghrelin receptor regulates adipose tissue inflammation in aging. Aging (Albany NY). 2016;8(1):178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnett BP, Hwang Y, Taylor MS, Kirchner H, Pfluger PT, Bernard V, Lin YY, Bowers EM, Mukherjee C, Song WJ, Longo PA, Leahy DJ, Hussain MA, Tschöp MH, Boeke JD, Cole PA. Glucose and weight control in mice with a designed ghrelin O-acyltransferase inhibitor. Science. 2010;330(6011):1689–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG, Taub DD. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117(10):2778–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixit VD, Yang H, Cooper-Jenkins A, Giri BB, Patel K, Taub DD. Reduction of T cell-derived ghrelin enhances proinflammatory cytokine expression: implications for age-associated increases in inflammation. Blood. 2009;113(21):5202–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia Q, Pang W, Pan H, Zheng Y, Kang JS, Zhu SG. Effects of ghrelin on the proliferation and secretion of splenic T lymphocytes in mice. Regul Pept. 2004;122(3):173–178. [DOI] [PubMed] [Google Scholar]
- 37.Dixit VD, Weeraratna AT, Yang H, Bertak D, Cooper-Jenkins A, Riggins GJ, Eberhart CG, Taub DD. Ghrelin and the growth hormone secretagogue receptor constitute a novel autocrine pathway in astrocytoma motility. J Biol Chem. 2006;281(24):16681–16690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orlova EG, Shirshev SV, Loginova OA. Leptin and ghrelin regulate dendritic cell maturation and dendritic cell induction of regulatory T-cells. Dokl Biol Sci. 2015;462:171–174. [DOI] [PubMed] [Google Scholar]
- 39.Taub DD. Neuroendocrine interactions in the immune system. Cell Immunol. 2008;252(1-2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taati M, Kheradmand A, Tarahi M. Hematopoietic effect of ghrelin in Wistar rats. J Guilan Univ Med Sci. 2008;17:7–13. [Google Scholar]
- 41.Lim CT, Kola B, Grossman A, Korbonits M. The expression of ghrelin O-acyltransferase (GOAT) in human tissues. Endocr J. 2011;58(8):707–710. [DOI] [PubMed] [Google Scholar]
- 42.Dixit VD, Taub DD. Ghrelin and immunity: a young player in an old field. Exp Gerontol. 2005;40(11):900–910. [DOI] [PubMed] [Google Scholar]
- 43.Morrow WJ, Ohashi Y, Hall J, Pribnow J, Hirose S, Shirai T, Levy JA. Dietary fat and immune function. I. Antibody responses, lymphocyte and accessory cell function in (NZB × NZW) F1 mice. J Immunol. 1985;135(6):3857–3863. [PubMed] [Google Scholar]
- 44.Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340(1):44–58. [DOI] [PubMed] [Google Scholar]
- 45.Nishimoto S, Fukuda D, Higashikuni Y, Tanaka K, Hirata Y, Murata C, Kim-Kaneyama JR, Sato F, Bando M, Yagi S, Soeki T, Hayashi T, Imoto I, Sakaue H, Shimabukuro M, Sata M. Obesity-induced DNA released from adipocytes stimulates chronic adipose tissue inflammation and insulin resistance. Sci Adv. 2016;2(3):e1501332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koo GC, Huang C, Camacho R, Trainor C, Blake JT, Sirotina-Meisher A, Schleim KD, Wu TJ, Cheng K, Nargund R, McKissick G. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166(6):4195–4201. [DOI] [PubMed] [Google Scholar]
- 47.Chorny A, Anderson P, Gonzalez-Rey E, Delgado M. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180(12):8369–8377. [DOI] [PubMed] [Google Scholar]
- 48.Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A. Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab. 2005;288(3):E486–E492. [DOI] [PubMed] [Google Scholar]
- 49.Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation. 2004;109(18):2221–2226. [DOI] [PubMed] [Google Scholar]
- 50.Wu R, Dong W, Cui X, Zhou M, Simms HH, Ravikumar TS, Wang P. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245(3):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theil MM, Miyake S, Mizuno M, Tomi C, Croxford JL, Hosoda H, Theil J, von Horsten S, Yokote H, Chiba A, Lin YW, Oki S, Akamizu T, Kangawa K, Yamamura T. Suppression of Experimental Autoimmune Encephalomyelitis by Ghrelin. J Immunol. 2009;183(4):2859–2866. [DOI] [PubMed] [Google Scholar]
- 52.Noto D, Takahashi K, Miyake S, Yamada M. In vitro differentiation of lineage-negative bone marrow cells into microglia-like cells. Eur J Neurosci. 2010;31(7):1155–1163. [DOI] [PubMed] [Google Scholar]
- 53.Lee JY, Yune TY. Ghrelin inhibits oligodendrocyte cell death by attenuating microglial activation. Endocrinol Metab (Seoul). 2014;29(3):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F, Ghè C, Isgaard J, Papotti M, Ghigo E, Muccioli G. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3′,5′-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-kinase/Akt signaling. Endocrinology. 2007;148(2):512–529. [DOI] [PubMed] [Google Scholar]
- 56.Cheyuo C, Wu R, Zhou M, Jacob A, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock. 2011;35(3):258–265. [DOI] [PubMed] [Google Scholar]
- 57.Li B, Zeng M, He W, Huang X, Luo L, Zhang H, Deng DY. Ghrelin protects alveolar macrophages against lipopolysaccharide-induced apoptosis through growth hormone secretagogue receptor 1a-dependent c-Jun N-terminal kinase and Wnt/β-catenin signaling and suppresses lung inflammation. Endocrinology. 2015;156(1):203–217. [DOI] [PubMed] [Google Scholar]
- 58.Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19:187–197. [DOI] [PubMed] [Google Scholar]
- 59.Yuan MJ, Huang CX, Tang YH, Wang X, Huang H, Chen YJ, Wang T. A novel peptide ghrelin inhibits neural remodeling after myocardial infarction in rats. Eur J Pharmacol. 2009;618(1–3):52–57. [DOI] [PubMed] [Google Scholar]
- 60.Zhou X, Xue C. Ghrelin inhibits the development of acute pancreatitis and nuclear factor kappaB activation in pancreas and liver. Pancreas. 2009;38(7):752–757. [DOI] [PubMed] [Google Scholar]