Abstract
The mineralocorticoid receptor (MR) is a member of the steroid-thyroid hormone receptor superfamily of ligand-dependent transcription factors with diverse functions including the biological actions of aldosterone. Identification of the various transcriptional coregulators of MR is essential for understanding the complexity of MR signaling pathways under physiological and pathological conditions. We used a yeast two-hybrid system to find proteins that interact with a full-length MR and found, among other proteins, that MR interacted specifically with receptor for activated C kinase 1 (RACK1), a scaffolding protein. Overexpression of RACK1 using a tetracycline-inducible lentivirus in mouse cortical collecting duct M1 cells stably expressing the rat MR and a Gaussia luciferase gene reporter under a hormone-response element promoter resulted in enhanced agonist-dependent MR transactivation. Knockdown of RACK1 protein expression by short hairpin RNAs led to a significant reduction in MR activation of the reporter gene and the endogenous genes Ctla2α and Psca. We also demonstrated that RACK1 regulation of MR action is mediated through phosphorylation by the PKC-β signaling pathway. MR and RACK1 were coimmunoprecipitated using an MR antibody in male Sprague-Dawley brain tissue and M1-rMR cells, and colocalization in M1-rMR cells and male rat brains was confirmed by immunofluorescence and immunohistochemistry. The scaffolding protein RACK1 is associated with MR under basal and agonist-stimulated conditions and facilitates agonist-stimulated MR actions through PKC-β. These findings indicate that RACK1 is a newly described coactivator of MR.
The MR, a ligand-activated transcription factor, interacts with RACK1 to enhance MR-mediated transactivation of specific genes acting in part with PKC.
Aldosterone and its receptor, the mineralocorticoid receptor (MR), play an important role in salt and water homeostasis and blood pressure regulation. Excessive activation of these MRs causes hypertension and cardiovascular hypertrophy and fibrosis (1, 2). MR is activated by the endogenous ligands aldosterone, cortisol, and corticosterone to mediate effects via genomic and nongenomic pathways. The former alludes to MR action as a ligand-activated transcription factor of the steroid-thyroid hormone receptor superfamily (3). Proteins resulting from MR-mediated transcription and translation of cell-specific genes mediate many of aldosterone’s actions (1).
Several large clinical studies since the Randomized Aldactone Evaluation Study (RALES) and Eplerenone (Post-Acute Myocardial Infarction) Heart Failure Efficacy and Survival Study (EPHESUS) have clearly demonstrated that MR antagonists reduce pathological cardiac, vascular, and renal remodeling in patients (4–6). However, many of the molecular mechanisms of cell- and context-specific activation of gene expression by the aldosterone-bound MR remain unknown. This information is essential for the development of selective therapeutic agents for the treatment of inappropriate activation of MR while avoiding deleterious effects—for example, hyperkalemia produced by antagonizing renal MR effects (4).
The MR consists of ligand-binding, DNA-binding, and N-terminal domains. The DNA-binding domain enables the MR to bind DNA at hormone-responsive elements; the N-terminal domain binds coactivators and corepressors that modulate MR action (7). Under basal conditions, MR is associated with hsp70, hsp90, and FKBP-52 and translocates to the nucleus to activate responsive genes upon ligand binding (1, 8).
Receptor for activated C kinase-1 (RACK1) is a small, ubiquitously expressed adaptor/scaffolding protein containing WD motifs that are highly conserved from Chlamydomonas spp to humans (9). RACK1 exerts its biological activities by binding to numerous partner molecules including Src, JNK, PDE4D, FAK, and PKC, among others (9–11). RACK1 interacts with conventional PKC isoforms, with a preference for PKCβII (12, 13).
In this study, we identified a protein-protein interaction between RACK1 and MR using a yeast two-hybrid system and describe a function of RACK1 as a coactivator of MR that promotes its interaction with PKCβ, resulting in increased MR transcriptional activity.
Materials and Methods
Animal care and use
Male Sprague-Dawley rats were housed in an Association for Assessment and Accreditation of Laboratory Animals–accredited facility at the G.V. (Sonny) Montgomery VA Medical Center, and tissues were obtained under an approved Virginia Institutional Animal Care and Use Committee protocol (Animal Component of Research Protocol).
Yeast two-hybrid system using a rat brain complementary DNA library
A yeast two-hybrid system was used as previously reported (14, 15) to screen a rat brain library with the whole MR sequence as bait; RACK1 was one of the proteins that interacted with the MR.
Cells and tissue samples
M1-murine cortical collecting duct cells kindly provided by Dr. Anikó Náray-Fejes-Tóth (16) were used for in vitro studies. M1 cells were stably transduced with a lentivirus carrying the full-length rat MR [(pFUGW-rMR) the empty vector kindly provided by Dr. David Baltimore] (17) and a lentivirus (pBM14) (18) with the Gaussia luciferase reporter with an artificial promoter containing three repeats of the rat tyrosine aminotransferase (TAT) hormone response element [pBM14-TAT3-Gaussia luciferase (Gluc)]. The resulting M1-rMR TAT3-Gluc cells were maintained in Dulbecco’s modified Eagle media (Sigma-Aldrich, St. Louis, MO) containing 5% Cosmic calf serum (Thermo Fisher Scientific, Waltham, MA). Male Sprague-Dawley rat brains, hearts, and kidneys were used for immunohistochemistry and immunofluorescence.
Luciferase activity
The M1-rMR TAT3-Gluc cells were plated in 96-well plates and, when confluent, were serum-starved overnight. The cells were then incubated with vehicle and different concentrations of aldosterone for 24 hours. After the incubation period, the supernatants were collected for luminescence detection using the substrate coelenterazine (Native; Nanolight, Pinetop, AZ) and read with a BMG FLUOstar Omega reader (19).
Silencing and overexpression of RACK1
The effect of RACK1 on MR transactivation was studied in M1-rMR TAT3-Gluc cells in which the level of RACK1 expression was manipulated; M1-rMR TAT3-Gluc cells were transduced with a lentivirus encoding short hairpin RNA (shRNA) pLKO-RACK1 (MISSION shRNA, NM_008143.3, 0411060MN; Sigma-Aldrich, St. Louis, MO) or a control plasmid to silence the expression of RACK1 protein. Briefly, cells were cultured in six-well plates, and the virus was added and spinoculated at 3000g × 2 hours in the presence of 8 µg/mL polybrene. After 48 hours, transduced cells were selected with puromycin for a week.
To overexpress RACK1, M1 cells were transduced with a tetracycline-inducible RACK1 construct in a lentiviral vector (pCW57.1/RACK1, Addgene plasmid no. 41393; a gift to Addgene by Dr. David Root). Cells were selected with blasticidin (10 µg/mL) 72 hours after transduction. Selected cells were serum-starved for 18 hours with or without doxycycline (300 ng/mL), and then the media was changed to one with vehicle or aldosterone. MR transactivation was assessed by measuring Gaussia luciferase in the media 24 hours after aldosterone treatment.
Endogenous gene expression
M1-rMR TAT3-Gluc cells were transfected with empty (pLKO) and shRNA pLKO-RACK1 vector. Cells were treated with vehicle or aldosterone for 24 hours. Total RNA was isolated from M1-rMR cells transfected with or without RACK1 treated with aldosterone by TRI Reagents (RT-RNA Isolation Kit; Molecular Research Center, Cincinnati, OH) following the manufacturer’s instructions. RNA was quantified using the NanoDrop 2000C (Thermo Fisher Scientific). For gene expression, complementary DNA (cDNA) was synthesized with 1 µg of RNA extracted using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction assay was performed in a Bio-Rad CFX96 Real-Time PCR Detection System. The PCR reaction was performed in a 25-µL final reaction volume containing 200 nmol of each primer and EVAGreen SuperMix (Bio-Rad). All the reactions were performed in 96-well plates in triplicate. A negative control containing all reagents but no cDNA template was included in all runs. Primers were designed from sequences derived from the GenBank database using Primer 3 (Whitehead Institute, MA) and Oligo software (Operon, CA) and were purchased from Eurofins MWG (Louisville, KY). The primers used were Ctla2α_F, CAGACTATGAGCAGGGCAAG, Ctla2α_R, GCAAATCAGGAGCCATTTCTC, and mPsca_F, ACTCTGACCTGTGCAATGTC mPsca_R, TTCACAATCGGGCTATGGTAG. Validation of specificity of quantitative polymerase chain reaction assay was performed by melt-curve analysis and by agarose gel analysis. GAPDH was used as reference gene. For each target gene, a calibration curve was generated with threshold cycle values from serial dilutions of cDNA (from 10 to 106 copies per reaction) to determine reaction efficiencies, linearity, detection, and quantification limits. Data analyses were performed with the Bio-Rad CFX Manager. The comparative cycle threshold method, which compares the difference between groups in cycle threshold values, was used to obtain the relative fold change of gene expression.
Treatment with PKC activator and inhibitor
M1-rMR TAT3-Gluc cells were serum-starved for 18 hours. Cells were then treated with PKC activator phorbol-12-myristate-13-acetate (PMA, 10 nM), a PKC-specific inhibitor staurosporine (100 nM), a PKCβ inhibitor LY333531 (10 nM), and dequalinium chloride (DQA; Cayman Chemicals, Ann Arbor, MI), an inhibitor of the RACK1 and PKC interaction (5 µM) (20) for 24 hours. MR transactivation was measured by luciferase activity. The role of PKCβ was also explored using a shRNA lentivirus (pGIPZ-PRKCB, 5579; GE Dharmacon, Lafayette, CO) to knockdown the expression. M1-rMR TAT3-Gluc cells were transduced with the pGIPZ-PKCβ-shRNA, and 3 days later, cells were serum-starved overnight and then incubated with vehicle or aldosterone (1 nM) for 6 hours, and the luciferase was measured in the supernatant. Control cells were transduced with the pGIPZ-empty lentivirus.
Immunoprecipitation and immunoblotting
M1-rMR TAT3-Gluc cells (1 × 107) were treated with vehicle, aldosterone (1 nM), or aldosterone (1 nM) plus spironolactone (1 µM) for 6 hours. Cells were lysed in RIPA buffer [20 mM tris(hydroxymethyl)aminomethane-HCl, pH 6.6, containing 1% NP-40, 50 mM NaCl, 1 mM EDTA, and 2.5% glycerol (volume-to-volume ratio)] and protease and phosphatase inhibitor solution (Thermo Fisher Scientific, Waltham, MA) for 30 minutes at 4°C. Protein concentration was measured using a bicinchoninic acid kit (BCA; Thermo Fisher Scientific). For immunoprecipitation, 500 µg of whole-cell lysate was incubated overnight with a rat MR rabbit polyclonal antibody (N-terminal) on a rocker, and then MagnaBind immunoglobulin G (IgG) beads were added to the antibody lysate mixture for 3 hours (Thermo Fisher Scientific). Immunoprecipitated proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% gel). Proteins were transferred to polyvinylidine difluoride membranes and then incubated with 1:10,000 anti-RACK1 monoclonal antibody (mAb) (610177; BD Bioscience, San Jose, CA) and anti-mouse immunoglobulin M (IgM) µ-chain–specific HRP-coupled secondary antibody (115-035-020; Jackson Immunoresearch, West Grove, PA) for 1 hour. The membrane was exbiposed on a BioRad ChemiDoc™ imaging system. All antibodies used in this study are shown in Table 1.
Table 1.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised In; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| MR | N-terminal 500 AA | MR | Rabbit, in-house antibody | Rabbit; polyclonal | ||
| MR | 1-18 MR | MR1-18-6G1 | Monoclonal IgG1, in-house antibody (21) | Mouse | 100 | AB_2637093 |
| RACK1 | RACK1 | BD Bioscience, 610177 (IgM) | Mouse; monoclonal | 10,000 | AB_397576 | |
| RACK1 | RACK1 | Epitomics, MABT475, clone EPR7388 | Rabbit; monoclonal | 1000 | AB_11155116 | |
| PKCβ | PKCβ | Santa Cruz, SC-13149, lot: K0515 | Mouse; monoclonal | 500 | AB_628144 | |
| β-tubulin | β-tubulin | DSHB, E-7 | Mouse; monoclonal | 100 | AB_2315513 |
Abbreviations: AA, amino acid; RRID, research resource identifier.
Immunohistochemistry
Paraformaldehyde-fixed, paraffin-embedded rat brain was cut in 5-µm sections and dried, and the paraffin was melted at 56°C for 4 hours. After deparaffination, the slides were subjected to antigen retrieval using citrate buffer in a steamer for 30 minutes. Endogenous peroxidases were inactivated with 0.1% phenyl hydrazine for 15 minutes. The slides were blocked using tris(hydroxymethyl)aminomethane (0.05 M), with 5% goat serum, pH 7.4, for 1 hour. The anti-MR 1-18 6G1 mouse mAb (21) (1/100 dilution) and anti-RACK1 rabbit mAb (MABT475, clone EPR7388; Abcam, Cambridge, MA) were incubated in blocking buffer overnight at 4°C. The slides were washed 5 times in phosphate-buffered saline with 0.2% Tween 20, incubated with secondary antibody, and counterstained with Mayer hematoxylin solution (Vector Laboratories, Burlingame, CA).
Immunofluorescence
Double immunofluorescence was done using mouse monoclonal anti–rMR1-18 6G1 (21) and rabbit monoclonal anti-RACK1 (MABT475, clone EPR7388; Epitomics, Abcam) primary antibodies incubated overnight at 4°C. After washing, mixtures of highly absorbed secondary antibodies were used: goat anti-mouse IgG Alexa Fluor 594 and goat anti-rabbit IgG Alexa Fluor 488 (Jackson Immunoresearch, West Grove, PA) at a dilution 1/1000 for 1 hour. The slides were then washed and mounted with VECTASHIELD HardSet Mounting Medium with 4’,6-diamindino-2-phenylindole (Vector Laboratories, Burlingame, CA). Photomicrographs were made using an Eclipse Nikon Microscope and pseudo-colored.
Statistical analysis
Results were expressed as mean ± standard error of the mean of at least three separate experiments in which each sample was assayed in triplicate or quadruplicate. Differences between groups were analyzed by t test and, for multiple group comparison, by one-way analysis of variance followed by Bonferroni comparisons. The differences were considered to be significant at P < 0.05. Statistical analyses were performed using GraphPad/Prism version 5 for Windows software (GraphPad Software, La Jolla, CA).
Results
Identification of RACK1-MR by yeast two-hybrid screening
To identify potential MR-interacting partners, we performed yeast two hybrid screening with a full-length rat MR as bait for a hypothalamic library as described (15). RACK1 was one of the prominent proteins identified.
Interaction of MR and RACK1 in rat tissues and M1-CCD cells
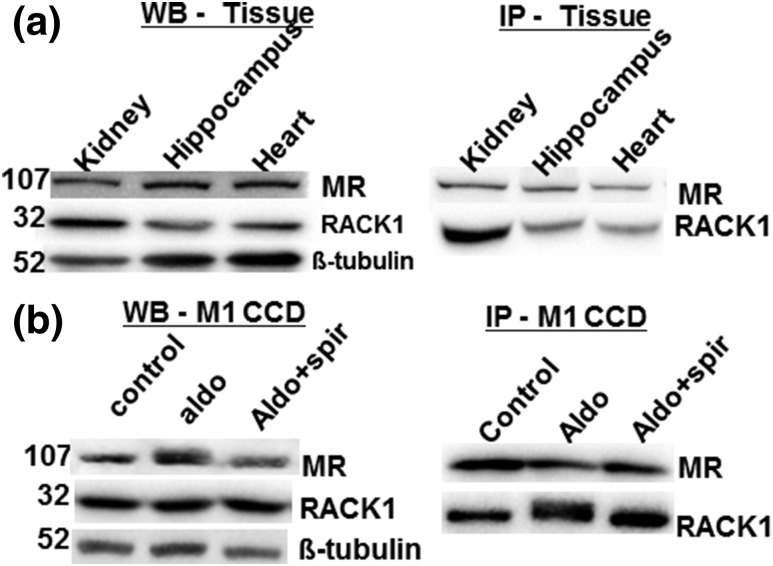
To analyze the physical interaction between MR and RACK1, we performed coimmunoprecipitation assays from endogenous proteins from rat tissues [Fig. 1(a)] and the M1-rMR TAT3-Gluc cells [Fig. 1(b)]. Rat kidney, hippocampus, and heart and M1-rMR TAT3-Gluc cell proteins were precipitated with a rabbit polyclonal MR-N-term antibody, and the presence of RACK1 was then examined by immunoblotting with a mouse monoclonal IgM anti-RACK1 antibody. The endogenous expression of RACK1 was not changed by 24 hours of aldosterone or spironolactone treatment as determined by western blot analysis and immunoprecipitation of RACK1. RACK1 is expressed in association with MR in the rat kidney, hippocampus, and heart [Fig. 1(b)], where MR abundantly expressed.
Figure 1.
RACK1 interacts with MR in rat tissue and mouse collecting duct cells. (a) Coimmunoprecipitation of RACK1 and MR in the rat kidney, hippocampus, and heart samples. Tissue lysates (30 µg) were separated by western blot (WB), and MR (1D5), RACK1, and β-tubulin as internal control (left panel) were immunodetected. For immunoprecipitation (IP), whole-cell extracts (800 µg) were incubated with anti-MR antibody and immunoprecipitates were subsequently analyzed by western blot with anti-RACK1 and anti-MR antibodies (right panel). (b) The M1-rMR TAT3-Gluc cells treated with aldosterone (10 nM) and aldosterone (10 nM) with spironolactone (1 µM) for >3 hours and then total protein (30 µg) were separated, and MR and RACK1 proteins were detected by western blot (left panel). Whole-cell lysates were immunoprecipitated with an anti-MR antibody and detected with an anti-RACK1 antibody (right panel). Aldo, aldosterone; Aldo+spiro, aldosterone with spironolactone.
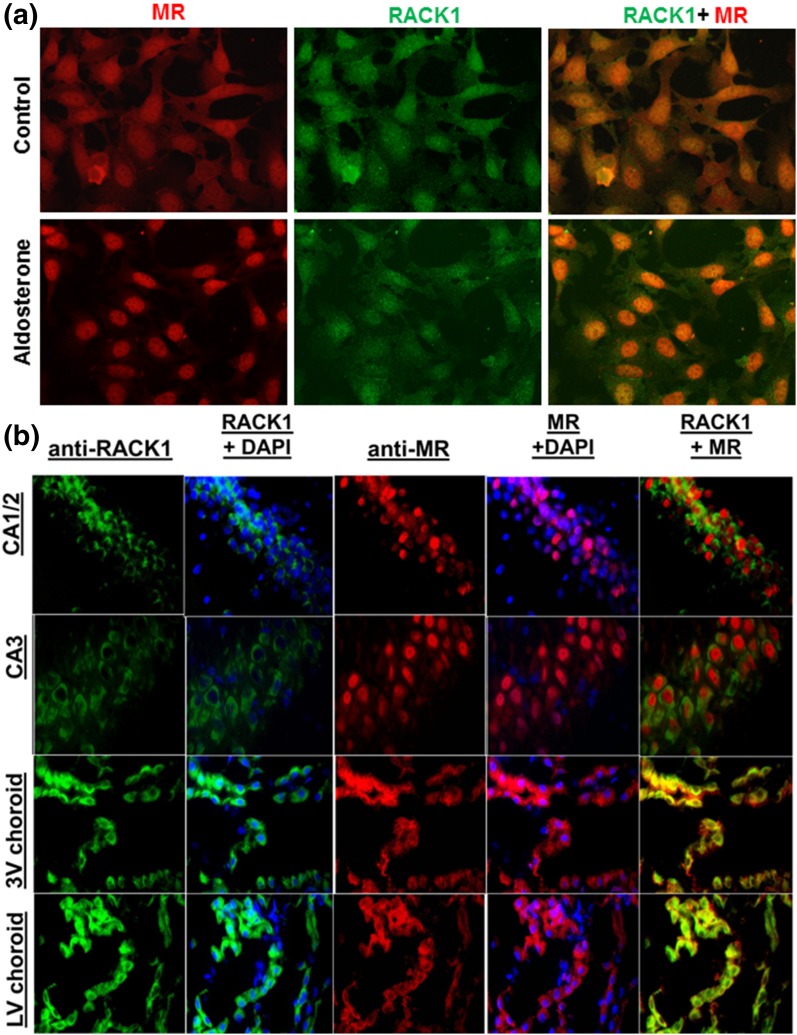
Subcellular localization of MR and RACK1 by immunofluorescence
We next examined whether MR (red) and RACK1 (green) interact within the cellular environment by using serum-starved M1-rMR TAT3-Gluc cells treated with vehicle or aldosterone for 24 hours [Fig. 2(a)]. As expected, aldosterone treatment caused most of the MR to move to the nucleus. A significant amount of RACK1 immunoreactivity also was found to have migrated from the cytoplasm to the nucleus of aldosterone-treated cells [Fig. 2(a)]. Immunofluorescence was used to examine whether the interaction of MR and RACK1 had potential physiological significance. MR and RACK1 are colocalized within the hippocampus and the choroid plexus of the third and lateral ventricles. Using specific antibodies, we detected MR (red) and RACK1 (green) immunoreactivity in the pyramidal cells of the hippocampus and in choroid plexus epithelial cells [Fig. 2(b)]. Strong RACK1 immunoreactivity is found in neurons of the dentate gyrus and CA1 and CA3 regions of the hippocampus, with less in the CA2 region, whereas MR immunoreactivity is strong in the CA1 and CA2 regions of the hippocampus, but modest in dentate gyrus or CA3.
Figure 2.
(a) Subcellular localization of MR and RACK1 in cultured M1-rMR TAT3-Gluc cells. Serum-starved cells were treated with vehicle or aldosterone for 3 hours. Fixed cells were incubated with mouse anti-MR and rabbit anti-RACK1, followed by goat anti-mouse Alexa Fluor 594 (red) and goat anti-rabbit Alexa Fluor 488 (green). RACK1 colocalized with MR in the presence of aldosterone in the nuclei of the transfected M1-CCD cells. (b) Colocalization of RACK1 (green) and MR (red) in rat brain. Paraffin-embedded tissue was incubated with mouse anti-MR antibody, followed by Alexa Fluor 594–conjugated anti-mouse IgG (red) and rabbit anti-RACK1 antibody, followed by Alexa Fluor 488–conjugated anti-rabbit IgG (green). Nuclear staining was performed by using 4′,6-diamidino-2-phenylindole. Scale bar = 50 µM.
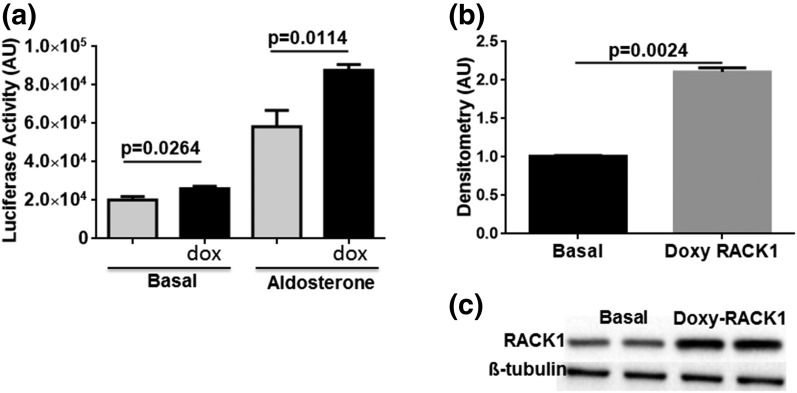
RACK1 enhances MR transactivation in dose-dependent manner
The functional effect of RACK1 on MR-induced transcriptional activity was measured in the M1-rMR TAT3-Gluc cells transduced with tetracycline-inducible RACK1 cDNA. Treatment with doxycycline for 48 hours resulted in a significant increase in RACK1 protein expression and enhanced the transcriptional activity under basal aldosterone-stimulated conditions (Fig. 3).
Figure 3.
RACK1 overexpression increases MR transactivation. (a) M1-rMR TAT3-Gluc cells were transduced with the tetracycline-inducible RACK1 (pCW57.1-RACK1) selected with puromycin. Serum-starved cells were treated with vehicle or aldosterone (10 nM) for 3 hours. After treatment, cell media were assayed for Gaussia luciferase activity. Assay was performed in three separate experiments, each with a minimum of triplicate samples. Statistically significant differences as compared with control conditions are indicated. (b, c) RACK1 expression after doxycycline (300 ng/mL) induction by western blot is shown. AU, arbitrary units; dox, Doxy, doxycycline.
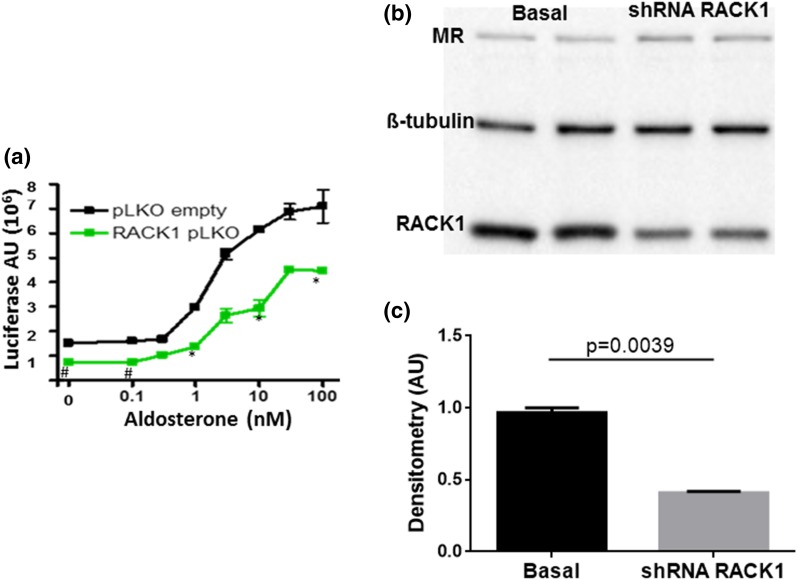
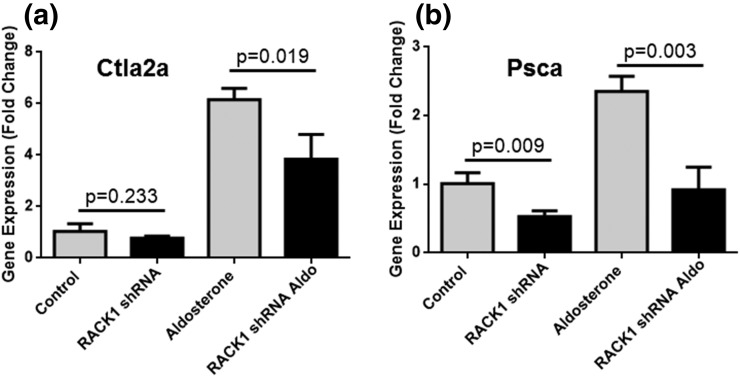
As there is significant endogenous expression of RACK1 in these cells, we transduced them with a lentivirus bearing a shRNA for RACK1, which significantly reduced the basal and aldosterone-stimulated expression of the reporter Gaussia luciferase (Fig. 4). Transduction of RACK1 shRNA effectively reduced the endogenous levels of RACK1 protein but had no effect on the β-tubulin or MR protein level as shown in western blot (Fig. 4). These findings suggest that endogenous RACK1 functions as a transcriptional coactivator for MR-mediated transactivation. Unpublished studies by Drs. Aniko N Fejes-Toth and Geza Fejes-Toth using microarrays and RNA sequencingidentified cytotoxic T lymphocyte-associated protein 2 α (Ctla2α) (22) and prostate stem cell antigen (Psca) (23) as two genes that increased significantly when M1rMR cells are stimulated with low doses of aldosterone. Expression of the MR target genes Ctla2α and Psca increased significantly with 1 nM aldosterone and was significantly suppressed by the RACK1 knockdown (Fig. 5).
Figure 4.
RACK1 knockdown decreases MR transactivation. (a) shRNA RACK1 expression significantly reduces MR transactivation. M1-rMR TAT3-Gluc cells were stably transduced with shRNA RACK1 and control lentivirus. Cells serum-starved overnight were stimulated with aldosterone (10 nM) for 24 hours. The supernatants were collected, and reporter activity was measured. Results represent at least three independent experiments. (b, c) Western blot analysis of RACK1 expression in cells with RACK1-specific shRNA. AU, arbitrary units.
Figure 5.
RACK1 knockdown decreases MR transactivation of endogenous genes. shRNA RACK1 expression significantly decreased the expression of (a) Ctla2α and (b) Psca in M1-rMR TAT3-Gluc cells.
Role of PKCβ in MR transactivation
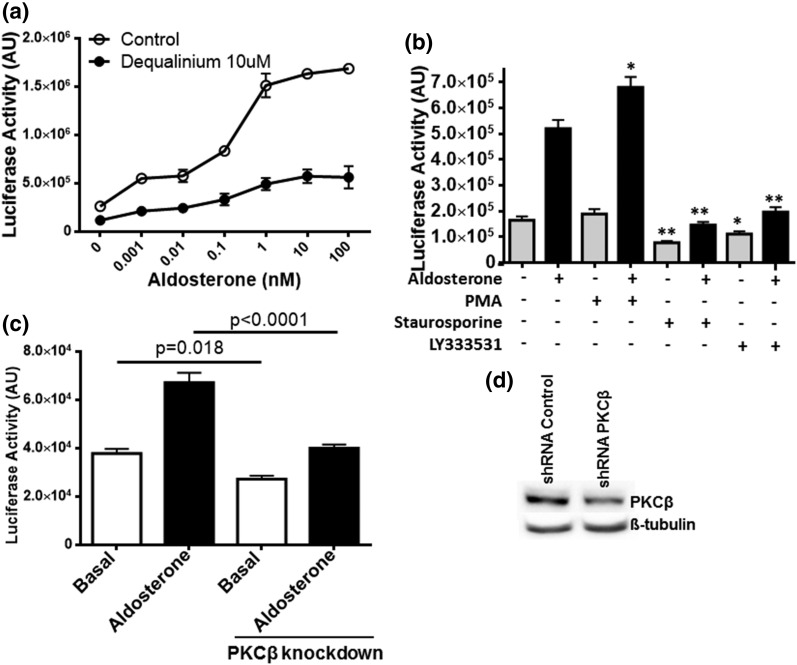
RACK1 is major adaptor protein for PKC, especially the PKCβ isoform (13). To determine the effect of disrupting the interaction of PKC with RACK1 on MR transactivation, serum-starved M1-rMR TAT3-Gluc was incubated with DQA, an inhibitor of the interaction between RACK1 and PKCβ (20), for 24 hours. The basal and agonist-stimulated MR transactivation was significantly reduced by DQA [Fig. 6(a)], indicating a role for PKC-RACK1 interaction in MR genomic action. To confirm the specific PKC isoform involved, M1 cells were treated with the PKC activator PMA, a more general PKC inhibitor staurosporine, and the PKCβ-specific inhibitor LY333531 for 24 hours. MR transactivation was significantly enhanced by the PKC activator, whereas both PKC inhibitors [Fig. 6(b)] and PKCβ knockdown [Fig. 6(c)] reduced MR activation significantly, suggesting a role for PKCβ in MR genomic action.
Figure 6.
RACK1-mediated PKC effect on MR genomic action. (a) M1-rMR TAT3-Gluc cells were seeded on 96-well plates. The cells were serum-starved overnight, media with DQA and with or without different concentrations of aldosterone were added, and the cells were incubated an additional 24 hours. (b) Serum-starved cells were treated with PMA (100 nM), staurosporine (10 nM), or LY333531 (10 nM) for 24 hours (*P < 0.05, **P < 0.001). (c) shRNA knockdown of PKCβ expression significantly reduced the MR transactivation. Serum-starved cells were stimulated with aldosterone 10 nM for 24 hours. The supernatants were collected and measured for reporter activity. Results are representative of at least three independent experiments. (d) PKCβ expression under PKCβ-specific shRNA by western blot.
Discussion
Transcriptional regulation by MR is mediated by recruitment of binding partners such as chaperone proteins, coactivators, and corepressors (24, 25). The Gem (nuclear organelle)-associated protein 4 (GEMIN4) and nuclear transcription factor Y subunit gamma (NF-YC) act as MR corepressors in an agonist-dependent manner (26, 27), whereas Ubiquitin-Conjugating Enzyme E2I (Ubc9), SRC-1, and PGC1α significantly increase MR activation in COS-7 and HEK293 cells (25, 28). Our findings now add RACK1 to the list of MR coactivators.
RACK1 is a seven-sided propeller protein with seven WD40 repeat domains that serves as a scaffold protein for many receptors and signaling proteins (9). It obtained its name because of its interaction with PKC, primarily PKCβ. Activation of PKC by PMA increased, whereas inhibition of PKC by the relative nonspecific PKC inhibitor staurosporine or the PKCβ inhibitor LY333531 significantly inhibited baseline and aldosterone-stimulated transactivation of the reporter gene. Similar effects were found by PKCβ knockdown using an shRNA. Inhibiting the binding of RACK1 to PKC with DQ inhibited MR-induced gene transcription. These results confirm that RACK1 influences MR-induced gene transcription through its interactions with both MR and PKC. This is concordant with reports that high glucose stimulates aldosterone-induced hypertrophy of neonatal rat cardiomyocytes through the MR through a PKC-mediated process (29) and provides evidence for an MR/RACK1/PKC signal transduction system involved in the MR-induced transcriptions. The interaction of the MR with RACK1 would bring together PKC to phosphorylate either the MR or another target involved in MR action, but the specific target(s) remain to be identified.
RACK1 interacts with multiple nuclear receptors, including the androgen receptor (AR) (30, 31) and glucocorticoid receptor (GR) (14, 32), as well as other transcription factors, including p73 and NFAT (9, 14, 30). In contrast to our findings with the MR, PKC activation represses GR and AR transcription. PKC activation promotes the translocation of the AR to the nucleus in the absence of ligand only in the presence of RACK1; however, the complex is unable to associate with gene response elements and activate transcription (10). Tyr-246 in the sixth WD-40 domain of RACK1 can be phosphorylated by Src and mediates the Src-RACK1 interaction (11). PKC activation enhances tyrosine phosphorylation of RACK1 (10), and this posttranslation modification might enable RACK1 to interact with other signaling proteins. The G-proteins β1 and β2 also share similar WD-repeats with RACK1, interact with the NH2-terminal region of the GR, and similarly comigrate into the nucleus and suppress the transcriptional activity of the GR in a dose-dependent manner (14, 33).
The MR is activated by the physiological ligands, aldosterone, and the glucocorticoids cortisol and corticosterone. Prereceptor specificity for aldosterone is mediated by the coexpression of the enzyme 11β-hydroxysteroid dehydrogenase 2 (11-HSD2), which converts cortisol and corticosterone into their inactive 11-dehydro metabolites (1). MR and GR are coexpressed in many cells. In nonaldosterone, target cells such as neurons of the hippocampus cortisol and corticosterone activate both receptors. As the affinity of MR for these ligands is greater than that of GR, MR is occupied at lower concentrations of circulating glucocorticoids. Expression of RACK1 in such cells might enhance MR-mediated activation of gene expression while suppressing GR activation, a very important cell-type–specific control of glucocorticoid effects that merits further study. For example, Sato and Funder (29) showed that the ability of glucose to potentiate the effect of aldosterone in cardiomyocytes depends on its ability to activate PKC. Thus, aldosterone-meditated PKC activation could be through RACK1-MR interaction.
In summary, these results showed that the scaffolding protein RACK1 is a newly identified coactivator of agonist-bound MR and that PKCβ is involved. Further investigation is required to explore the role of RACK1 in the MR function under physiological and pathological conditions.
Acknowledgments
Current affiliation: L.N. Beloate is currently affiliated with the Department of Neurobiology, Medical University of South Carolina, Charleston, South Carolina 29425.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL27255 and National Institute of General Medical Sciences of the National Institutes of Health award 1U54GM115428). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- androgen receptor
- cDNA
- complementary DNA
- Ctla2α
- cytotoxic T lymphocyte–associated protein 2 α
- DQA
- dequalinium chloride
- Gluc
- Gaussia luciferase
- GR
- glucocorticoid receptor
- IgG
- immunoglobulin G
- IgM
- immunoglobulin M
- mAb
- monoclonal antibody
- MR
- mineralocorticoid receptor
- PMA
- phorbol-12-myristate-13-acetate
- Psca
- prostate stem cell antigen
- shRNA
- short hairpin RNA, TAT, tyrosine aminotransferase.
References
- 1.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4(3):965–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaisser F, Farman N, Creteil P. Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol Rev. 2016;68(1):49–75. [DOI] [PubMed] [Google Scholar]
- 3.Bauersachs J, Jaisser F, Toto R. Mineralocorticoid receptor activation and mineralocorticoid receptor antagonist treatment in cardiac and renal diseases. Hypertension. 2015;65(2):257–263. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Sanchez EP. Third-generation mineralocorticoid receptor antagonists: why do we need a fourth? J Cardiovasc Pharmacol. 2016;67(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt B, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, Maggioni AP, Ponikowski P, Voors AA, Zannad F, Nowack C, Kim SY, Pieper A, Kimmeskamp-Kirschbaum N, Filippatos G. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail. 2015;17(2):224–232. [DOI] [PubMed] [Google Scholar]
- 6.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators . Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–1321. [DOI] [PubMed] [Google Scholar]
- 7.Meinel S, Gekle M, Grossmann C. Mineralocorticoid receptor signaling: crosstalk with membrane receptors and other modulators. Steroids. 2014;91:3–10. [DOI] [PubMed] [Google Scholar]
- 8.Bruner KL, Derfoul A, Robertson NM, Guerriero G, Fernandes-Alnemri T, Alnemri ES, Litwack G. The unliganded mineralocorticoid receptor is associated with heat shock proteins 70 and 90 and the immunophilin FKBP-52. Recept Signal Transduct. 1997;7(2):85–98. [PubMed] [Google Scholar]
- 9.Adams DR, Ron D, Kiely PA. RACK1, A multifaceted scaffolding protein: structure and function. Cell Commun Signal. 2011;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66(22):11047–11054. [DOI] [PubMed] [Google Scholar]
- 11.Gallo S, Manfrini N. Working hard at the nexus between cell signaling and the ribosomal machinery: an insight into the roles of RACK1 in translational regulation. Translation (Austin). 2015;3(2):e1120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ron D, Luo J, Mochly-Rosen D. C2 region-derived peptides inhibit translocation and function of β protein kinase C in vivo. J Biol Chem. 1995;270(41):24180–24187. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev. 2008;88(4):1341–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kino T, Tiulpakov A, Ichijo T, Chheng L, Kozasa T, Chrousos GP. G protein β interacts with the glucocorticoid receptor and suppresses its transcriptional activity in the nucleus. J Cell Biol. 2005;169(6):885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bratton MR, Gomez-Sanchez EP, Gomez-Sanchez CE, Subauste JS. The myosin binding protein is a novel mineralocorticoid receptor binding partner. Mol Cell Endocrinol. 2004;217(1-2):221–227. [DOI] [PubMed] [Google Scholar]
- 16.Stoos BA, Náray-Fejes-Tóth A, Carretero OA, Ito S, Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991;39(6):1168–1175. [DOI] [PubMed] [Google Scholar]
- 17.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. [DOI] [PubMed] [Google Scholar]
- 18.Mitta B, Weber CC, Rimann M, Fussenegger M. Design and in vivo characterization of self-inactivating human and non-human lentiviral expression vectors engineered for streptogramin-adjustable transgene expression. Nucleic Acids Res. 2004;32(12):e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4(4):582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotenberg SA, Sun XG. Photoinduced inactivation of protein kinase C by dequalinium identifies the RACK-1-binding domain as a recognition site. J Biol Chem. 1998;273(4):2390–2395. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147(3):1343–1348. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Ahn J, Suh Y, Hwang S, Davis ME, Lee K. Identification of CTLA2A, DEFB29, WFDC15B, SERPINA1F and MUP19 as novel tissue-specific secretory factors in mouse. PLoS One. 2015;10(5):e0124962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, Loda M, Witte ON. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95(4):1735–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, Young MJ. The mineralocorticoid receptor and its coregulators. J Mol Endocrinol. 2009;43(2):53–64. [DOI] [PubMed] [Google Scholar]
- 25.Fuller PJ. Novel interactions of the mineralocorticoid receptor. Mol Cell Endocrinol. 2015;408:33–37. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Fuller PJ, Morgan J, Shibata H, Clyne CD, Young MJ. GEMIN4 functions as a coregulator of the mineralocorticoid receptor. J Mol Endocrinol. 2015;54(2):149–160. [DOI] [PubMed] [Google Scholar]
- 27.Murai-Takeda A, Shibata H, Kurihara I, Kobayashi S, Yokota K, Suda N, Mitsuishi Y, Jo R, Kitagawa H, Kato S, Saruta T, Itoh H. NF-YC functions as a corepressor of agonist-bound mineralocorticoid receptor. J Biol Chem. 2010;285(11):8084–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokota K, Shibata H, Kurihara I, Kobayashi S, Suda N, Murai-Takeda A, Saito I, Kitagawa H, Kato S, Saruta T, Itoh H. Coactivation of the N-terminal transactivation of mineralocorticoid receptor by Ubc9. J Biol Chem. 2007;282(3):1998–2010. [DOI] [PubMed] [Google Scholar]
- 29.Sato A, Funder JW. High glucose stimulates aldosterone-induced hypertrophy via type I mineralocorticoid receptors in neonatal rat cardiomyocytes. Endocrinology. 1996;137(10):4145–4153. [DOI] [PubMed] [Google Scholar]
- 30.Rigas AC, Ozanne DM, Neal DE, Robson CN. The scaffolding protein RACK1 interacts with androgen receptor and promotes cross-talk through a protein kinase C signaling pathway. J Biol Chem. 2003;278(46):46087–46093. [DOI] [PubMed] [Google Scholar]
- 31.Corsini E, Galbiati V, Papale A, Kummer E, Pinto A, Serafini MM, Guaita A, Spezzano R, Caruso D, Marinovich M, Racchi M. Role of androgens in DHEA-induced RACK1 expression and cytokine modulation in monocytes. Immun Ageing. 2016;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto A, Malacrida B, Oieni J, Serafini MM, Davin A, Galbiati V, Corsini E, Racchi M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br J Pharmacol. 2015;172(11):2918–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Vecchio I, Zuccotti A, Pisano F, Canneva F, Lenzken SC, Rousset F, Corsini E, Govoni S, Racchi M. Functional mapping of the promoter region of the GNB2L1 human gene coding for RACK1 scaffold protein. Gene. 2009;430(1-2):17–29. [DOI] [PubMed] [Google Scholar]