Abstract
Although antiretroviral therapy can suppress HIV-1 infection to undetectable levels of plasma viremia, integrated latent HIV-1 genomes that encode replication competent virus persist in resting CD4+ T cells. This latent HIV-1 reservoir represents a major barrier to a cure. Currently, there are substantial ongoing efforts to identify therapeutic approaches that will eliminate or reduce the size of this latent HIV-1 reservoir. In this regard, a sensitive assay which can accurately and rapidly quantify inducible replication competent latent HIV-1 from resting CD4+ T cells is essential for HIV-1 eradication studies. Here we describe a reporter cell-based assay to quantify inducible replication competent latent HIV-1. This assay has several advantages over existing technology in that it: (i) is sensitive; (ii) requires only a small blood volume; (iii) is faster, less labor intensive, and less expensive, and (iv) can be readily adapted to a high-throughput format. Using this assay we show that the size of the inducible latent HIV-1 reservoir in aviremic participants on therapy is approximately 70-fold larger than previous estimates.
The latent HIV-1 reservoir in resting CD4+ (rCD4+) T cells is an obstacle to eradicating HIV-1 infection. This reservoir is small, consisting of approximately 1–10 infectious units per million cells1–3. Therefore it is critical to develop assays that can reproducibly quantify the reservoir size, and changes therein, in participants enrolled in curative intervention strategies. To date, assays to measure cell-associated HIV-1 DNA and RNA have been developed4–8. However, their clinical utility is unclear, because the majority of integrated HIV-1 DNA is replication defective9–11, and measurement of viral mRNA expression might not reflect the amount of replication competent virus. For this reason, the quantitative viral outgrowth assay (Q-VOA)3,12, which quantifies inducible, replication competent HIV-1 from rCD4+ T cells, is considered the gold standard. However, the Q-VOA may provide only a minimal estimate of the size of the latent HIV-1 reservoir because it only detects a fraction of the total integrated pool of replication competent HIV-1, although this may, in part, be due to stochastic reactivation of the latent reservoir following maximum T cell activation 9,10,13. Nevertheless, underestimating the size of the latent reservoir in rCD4+ T cells could result in the misconception that an infected individual is cured when in fact they are not. Additionally, the Q-VOA requires a large volume of blood (120–180 mL)13, is labor intensive, time consuming, and expensive. As such, the development of a rapid, high-throughput, sensitive and validated assay is important for clinical studies evaluating cure strategies, and for researchers to identify new latency reversing agents and to characterize the latent reservoir ex vivo. In this report, we describe the development of a sensitive assay (termed TZA) that utilizes the TZM-bl cell line, which stably expresses CD4, CCR5, and CXCR4, and carries an integrated copy of the β-galactosidase (β-gal) gene under control of an HIV-1 LTR promoter, to quantify inducible replication competent HIV-1. Using this assay we show that the size of the inducible latent HIV-1 reservoir in aviremic subjects on ART is approximately 70-fold larger than previous estimates.
RESULTS
Sensitivity of TZM-bl cells to infection by replication competent HIV-1
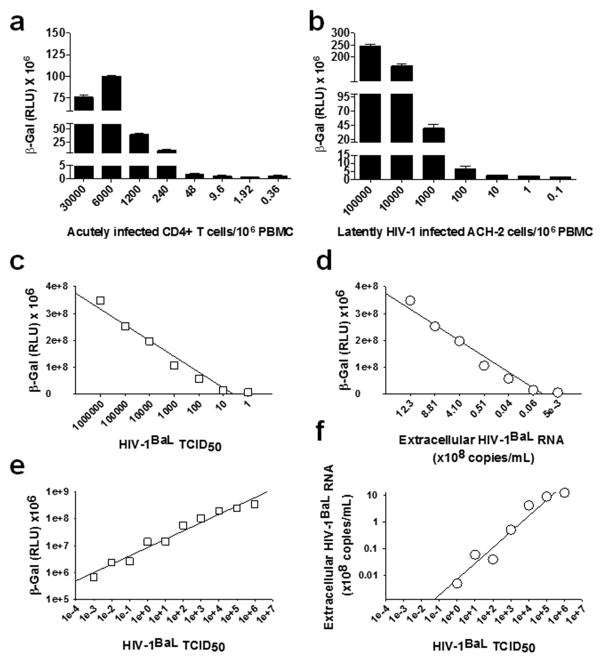
To establish the sensitivity of TZM-bl cells to HIV-1 infection, we first serially diluted CD4+ T lymphocytes that were acutely infected with the laboratory strain HIV-1BaL (30% infection frequency as determined by flow cytometry of intracellular p24) with uninfected CD8+ T cell-depleted peripheral blood mononuclear cells (PBMC), and added 1×105 cells from each serial dilution to 5×104 TZM-bl cells in a 96-well plate. β-gal activity was measured by chemiluminescence 48 h later. We found that the TZM-bl cells reproducibly detected 0.3 infected cells/106 PBMC (Fig. 1a). We also treated the latently HIV-1 infected human T cell line ACH-2 14 with 100nM phorbol 12-myristate 13-acetate (PMA), serially diluted them with uninfected CD8+ T cell-depleted PBMC, and added them to TZM-bl cells. We could detect at least 0.1 infectious HIV-1 producing ACH-2 cells/106 CD8+ T cell-depleted PBMC (Fig. 1b). Next, we determined the relationships between HIV-1BaL 50% tissue culture infectivity doses (TCID50), β-gal activity (measured 48 h post infection) and virus production (measured 10 days post infection by quantitation of extracellular virion-associated HIV-1 RNA) in TZM-bl cells. We found strong correlations between the β-gal activity and TCID50 (r=0.76; P=0.04; TCID50 range: 106–1) and between the β-gal activity and extracellular virus production (r=0.71; P=0.05) (Fig. 1c, 1d). However, when we assessed the dynamic range of the β-gal and virus production signals as a function of TCID50 (range 106–10−3), we observed that the linearity of response extended as far as 0.001 TCID50 (HIV-1BaL) for the β-gal activity (Fig. 1e), but only as far as 1.0 TCID50 for virus production (Fig. 1f). These data show that the β-gal signal correlates with production of replication competent HIV-1in the TZM-bl cells, but highlights a 1000-fold increase in sensitivity for the β-gal signal. To directly compare the ability of TZM-bl cells and activated CD4+ T cells to detect infection events, we calculated the TCID50 for HIV-1BaL in both cell types. For the activated CD4+ T cells, viral p24 production was used as the readout of HIV-1 infection, whereas β-gal activity was used for the TZM-bl cells. The TCID50 in TZM-bl cells (2×106) was found to be 10-fold higher than that in activated CD4+ T cells (1.5×105).
Fig. 1. Sensitivity of TZM-bl cells to replication competent HIV-1.
a) Acutely infected HIV-1BaL-positive CD4+ T lymphocytes (30% infection frequency as determined by flow cytometry of intracellular p24) were serially diluted with uninfected CD8+ T cell- PBMC. 1×105 cells from each serial dilution were added to 5×104 TZM-bl cells in a 96-well plate. β-gal activity was measured by chemiluminesence 48 h later. The relative light units (RLU) for the control (460,000 +/− 56,000) were subtracted from each assay sample. b) Latently HIV-1 infected ACH-2 cells were treated with 100nM PMA, serially diluted with uninfected CD8+ T cell-depleted PBMC, and added to TZM-bl cells, as described above. c) Correlation between the TCID50 for HIV-1BaL and β-gal activity in the TZM-bl cells. TZM-bl cells were infected with HIV-1BaL and β-gal activity was measured 48 h later. d) Correlation between the β-gal activity for HIV-1BaL 48 h post infection and virus production (assessed by extracellular virion associated HIV-1 RNA 10 days post-infection) e) Detection of β-gal activity in the TZM-bl cells as a function of HIV-1BaL TCID50. We observed a linear relationship between β-gal activity and TCID50 down to a TCID50 of 0.001. f) Detection of extracellular virion-associated RNA as a function of HIV-1BaL TCID50. We observed a linear relationship between extracellular virion-associated RNA and TCID50 down to a TCID50 of 1. P values (cited in the text) for c, d, e and f were obtained using the Pearson test.
Sensitivity of TZM-bl cells to infection by replication defective HIV-1
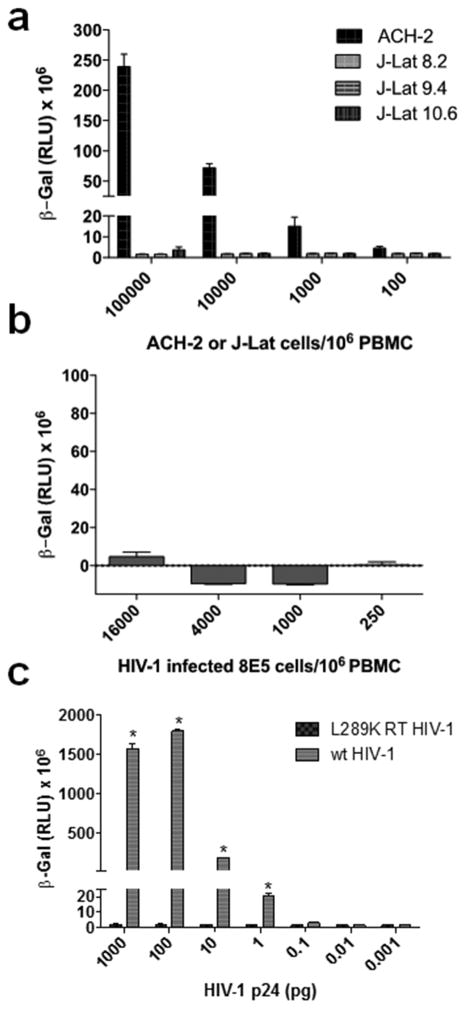
To ascertain whether replication defective HIV-1 particles were detected with the TZM-bl assay, we measured β-gal activity following their incubation with 3 different clones of the latently HIV-1-infected J-Lat Jurkat T cell line15. Each of the J-Lat clones contains a full-length integrated HIV-1 genome that expresses the green fluorescent protein upon activation, and generates defective virions due to a frameshift in the HIV-1 env gene. Additionally, each of the J-Lat clones produces different amount of extracellular virus particles after stimulation with 100 nM PMA (Supplementary Table 1). Our data show that none of the J-Lat clones produced any signal in the TZM-bl cells (Fig. 2a). We also evaluated the chronically infected T cell line 8E5 which contains a single integrated copy of proviral HIV-1LAV DNA and produces defective virus particles that lack reverse transcriptase16. Our data (Fig. 2b) show that even with 4,000 8E5 cells (which produce 8606 pg/mL viral p24 protein), there was no positive β–gal signal. In contrast, as indicated in Fig. 1a and 1b, we can detect a positive signal using less than 1 cell infected with replication competent HIV-1 per106 cells. Finally, we evaluated the ability of a full-length replication defective clone of the HIV-1LAI laboratory strain that harbors the inactivating L289K mutation in the reverse transcriptase gene 17 to infect TZM-bl cells. In comparison to wild type HIV-1, the mutant virus does not induce any β–gal activity in TZM-bl cells even when 1000 pg of p24 equivalent virus was added to the cells (Fig. 2c). Collectively, these data demonstrate that the TZM-bl cells are insensitive to replication defective virus particles with mutations in env or reverse transcriptase.
Fig. 2. TZM-bl cells are insensitive to infection by replication defective HIV-1.
a) J-Lat clones 10.3, 9.2 and 8.4 were stimulated with PHA, serially diluted with uninfected CD8+ T cell-depleted PBMC and added to TZM-bl cells. β-gal activity was measured 48 h later. b) 8E5 cells were serially diluted with uninfected CD8+ T cell-depleted PBMC and added to TZM-bl cells. β-gal activity was measured 48 h later. c) Different p24 amounts of wild type (wt) HIV-1LAI, and a mutant virus containing the L289K mutation in reverse transcriptase that renders the enzyme defective, were added to TZM-bL cells and β-gal activity was measured 48 h later. Statistical comparison of wild type versus mutant HIV-1 β-gal activity was performed using a Student’s T test (*, P < 0.05).
Quantification of inducible replication competent HIV-1 from rCD4+ T cells purified from HIV-1-infected subjects
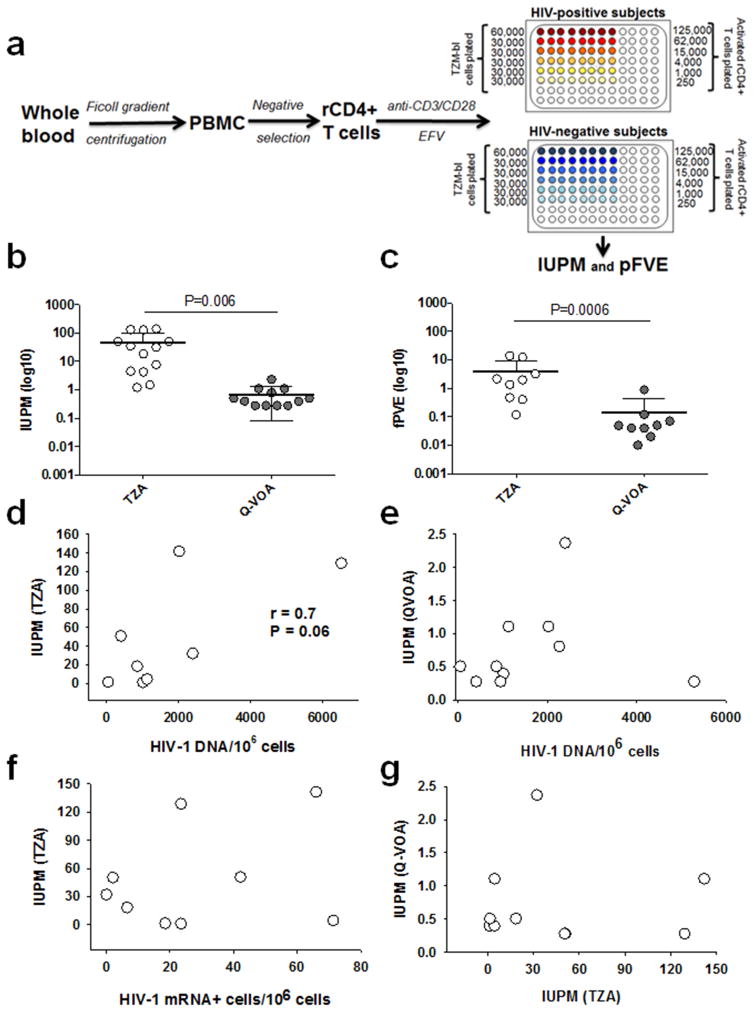
We developed a strategy to quantify inducible replication competent HIV-1 from rCD4+ T cells purified from infected, aviremic individuals on suppressive cART that involved: (i) induction of latent virus using anti-CD3/CD28 monoclonal antibody (mAb)-coated microbeads; and (ii) quantification of the induced replication-competent HIV-1 in TZM-bl cells (Fig. 3a). Blood was obtained from 15 participants who were enrolled in the Pittsburgh clinical site of the Multicenter AIDS Cohort Study (Table 1). rCD4+ T cells were isolated by negative selection from PBMC to >98% purity (Supplementary Fig. 1), and were comprised of naïve (36%), central memory (41%), effector memoryCD45RO+ (19%), and effector memory CD45RA+ (4%) cells. Purified rCD4+ T cells were exposed to anti-CD3/CD28 mAb-coated microbeads for 5 days to induce latent HIV-1 expression (i.e., bulk stimulation). Efavirenz (300nM), a non-nucleoside reverse transcriptase inhibitor, was included in the culture medium to prevent spread of de novo HIV-1 infection. Treatment of rCD4+ T cells with the anti-CD3/CD28 mAb resulted in ~1.6 fold increase in CD4+ T cell proliferation, but approximately 0.8-fold decrease in the frequency of HIV-1 DNA/106 cells (Supplementary Table 2). The mechanisms responsible for the observed decrease in the frequency of HIV-1 DNA/106 cells post T cell activation are unclear, but may be due to the cytopathic effect of the virus produced from the infected activated CD4+ T cells. Next, we added serially diluted rCD4+ T cells to 3–6×104 TZM-bl cells in a 96-well plate and following 48 h co-culture we quantified β-gal expression. As a control, anti-CD3/CD28 mAb treated rCD4+ T cells from healthy uninfected donors were similarly co-incubated with TZM-bl cells and expression of β-gal was measured. Infectious units per million (IUPM) cells ranged from 1.2 to 141.7 (mean 46.9, N=13) for the TZM-bl based assay (TZA) (Fig. 3b, Table 1). The IUPM values calculated for rCD4+ T cells from the same participants using the Q-VOA ranged from 0.28 to 2.37 (mean 0.70, N=12) (Fig. 3b, Table 1), which is almost 70-fold lower (p = 0.006). In this study, the IUPM values calculated from the Q-VOA (0.28–2.37) were similar to values previously reported (0.03–3)3. In a subset of 9 participants, we also determined the fraction of proviruses (fPVE) that can be reactivated to produce infectious virus in both assays, as described previously12. fPVE provides an estimate of the size of the replication competent viral reservoir. fPVE calculated from TZA ( mean 4.03%, range 0.12 – 13.93 %) was 28-fold higher (P=0.0006) than that from Q-VOA (mean 0.14 %, range 0.02–0.9%) (Fig. 3c). We found a weak correlation that was not significant between total HIV-1 DNA/106 rCD4+ T cells and the IUPM calculated from the TZA (r=0.7; P=0.06) (Fig. 3d). In our dataset, there was no correlation between HIV-1 DNA/106 rCD4+ T cells, HIV-1 gag-pol RNA+T cells/106 rCD4+ T cells, and the IUPM calculated from the Q-VOA, or between the TZA and Q-VOA assays (Fig 3e, 3f, 3g).
Fig 3. TZM-bl based assay (TZA) to quantify inducible replication competent HIV-1 from rCD4+ T cells.
a) Schematic overview of the TZA, b) Statistical comparison of the IUPM values determined by TZA or Q-VOA using a parametric unpaired T test (P=0061). c) Statistical comparison of fractional HIV-1 provirus expression (fPVE) determined by TZA or Q-VOA using a parametric ratio paired T test (P=0.0006). d) Correlation between TZA and total HIV-1 DNA in rCD4+ T cells. e) Correlation between Q-VOA and total HIV-1 DNA in rCD4+ T cells. f) Correlation between TZA and intracellular HIV-1 gag-pol mRNA+ cells/106 rCD4+ T cells. g) Correlation between TZA and Q-VOA. P values for all correlations were obtained using the Pearson test.
Table 1.
Characteristics of study participants, including the IUPM values determined by either the TZA or Q-VOA.
| Participant ID | Age | Current CD4+ T cell count (cells/mm3) | Current ART regimen | Years on ART | Current viral load (copies/mL) | IUPM (TZA) | IUPM (Q-VOA) |
|---|---|---|---|---|---|---|---|
| 001 | 63 | 1,264.0 | TDF+FTC+ATV/r | 15 | <15 | 32.2 | 2.4 |
| 002 | 71 | 509.2 | TDF+FTC+NVP | 12 | <15 | 1.2 | 0.4 |
| 003 | 40 | 850.1 | TDF+FTC+RAL | 5 | <15 | 50.8 | 0.3 |
| 004 | 51 | 859.2 | TDF+FTC+ATV/r | 18 | <15 | 18.4 | 0.5 |
| 005 | 52 | 636.4 | RAL+DRV/r | 14 | <15 | 50.4 | 0.3 |
| 006 | 57 | 859.7 | AZT+3TC+NFV | 14 | <15 | 4.3 | 0.4 |
| 007 | 57 | 389.6 | TDF+FTC+EFV | 17 | <15 | 128.9 | 0.3 |
| 008 | 56 | 510.5 | d4T+ddI+NFV | 17 | <15 | 4.6 | 1.1 |
| 009 | 34 | 797.1 | TDF+FTC+EVG/c | 1 | <15 | 1.5 | 0.5 |
| 010 | 57 | 700.6 | d4T+3TC+EFV | 14 | <15 | 141.7 | 1.1 |
| 011 | 66 | 615.1 | AZT+3TC+EFV | 19 | <15 | NA | 0.8 |
| 012 | 52 | 374.4 | TDF+FTC+ATV/r | 13 | <15 | 7.8 | NA |
| 013 | 59 | 1,586.7 | TDF+RAL+ATV/r | 12 | <15 | NA | 0.3 |
| 014 | 53 | 469.3 | ABC+3TC+RAL | 12 | <15 | 132.6 | NA |
| 015 | 54 | 612.7 | TDF+FTC+EFV | 13 | <15 | 35.3 | NA |
| Mean ± S.D. | 54.8 ± 9.2 | 735.6 ± 329.0 | 13.1 ± 4.7 | <15 | 46.9 ± 52.8 | 0.7 ± 0.6 |
Further Characterization of the TZA
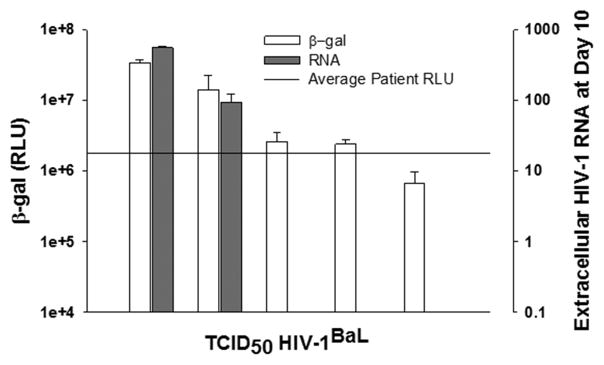
In control experiments, we exposed TZM-bl cells to efavirenz prior to the addition of activated rCD4+ cells, which would block active HIV-1 replication, but allow us to ascertain whether cytokines released from the activated rCD4+ T cells increased TZM-bl cell β-gal activity. In rCD4+ T cells from two HIV-1-infected participants we observed that the IUPM values decreased from 81 and 26 respectively in the absence of inhibitor, to undetectable in the presence of inhibitor. This finding suggests that the higher IUPM values determined using the TZA (compared to the Q-VOA) is not due to cytokines released after T cell activation with the anti-CD3/CD28 mAbs. We also compared our bulk rCD4+ T cell stimulation approach (Fig. 3a) to one where we first serially diluted the rCD4+ T cells prior to adding anti-CD3/CD28 mAb. The latter approach is used in the Q-VOA. We found no significant difference in the IUPM values when using these 2 different approaches with rCD4+ T cells isolated from 3 different HIV-infected participants (Supplementary Fig. 2). Finally, we evaluated whether a positive IUPM in the TZA reflected productive HIV-1 replication. As discussed above (Fig. 1e, 1f), the β-gal activity for detection of HIV-1BaL infection in TZM-bl cells was sensitive down to a TCID50 of 10−2, whereas virus production was only sensitive down to a TCID50 of 1. The average β-gal RLU value for the rCD4+ T cells from the 12 HIV-1-infected donors included in this study was 1.8×106. This value closely approaches the cut-off for detection of virus production (Fig. 4). In this regard, if we evaluate rCD4+ T cells from HIV-infected participants that have a high β-gal RLU value, we can readily detect virus production at day 3 and day 10 post-infection (Supplementary Table 3). However, if the value falls below this RLU, we cannot reproducibly detect virus production.
Fig. 4. Relationship between β-gal RLU signal or extracellular virion-associated HIV-1 RNA and the TCID50 for HIV-1BaL.
The experimental set up was same as described for Fig. 1c, 1d, 1e and 1f. The average patient RLU (n = 12) in our study was 1.8 ×106. This value closely approaches the cut-off for detection of virus production as assessed by extracellular HIV-1 RNA.
DISCUSSION
There is a critical need to develop sensitive assays that can accurately quantify inducible replication-competent latent HIV-1. In this study, we developed a reporter cell-based assay (termed TZA) to quantify inducible replication competent latent HIV-1 in blood. In this regard, control experiments revealed that the TZA is insensitive to virus particles defective in env or reverse transcriptase, and that the β-gal activity is not altered by cytokine release from activated CD4+ T cells. Using the TZA, we show that the size of the inducible latent HIV-1 reservoir in aviremic participants on therapy is approximately 70-fold larger than previous estimates. The larger IUPM (46.9) and fPVE (4.03%) values determined for the TZA are consistent with the fPVE of 1.5% obtained by Cillo et al 19 by quantitation of extracellular virion-association HIV-1 RNA, and the number of multiply spliced HIV-1 RNA cells/million CD4+ T cells (mean, 24) by Procopio et al8 using the tat/rev Induced Limiting Dilution Assay. Furthermore, our IUPM values are in line with the observation that the Q-VOA underestimates the amount of replication competent virus by 60-fold compared to the level of intact proviruses estimated by sequence analysis 9. There are, however, several differences between the TZA and Q-VOA that may contribute toward some of the differences in IUPM values noted in this study. Specifically, the Q-VOA and TZA utilize different measures of replication competent HIV-1: the Q-VOA employs multiple rounds of infection and direct quantitation of extracellular virion-associated HIV-1 RNA whereas the TZA is based on a single round of infection and quantitation of tat induced activation of β-gal activity. There may be some virus particles that can efficiently undergo a single-cycle of replication, but are not capable of undergoing multiple-rounds of infection. In this regard, full-length genome sequencing of virus particles produced in the TZM-bl cells may provide insight into the frequency of detecting partially-attenuated replication competent viruses in the TZA.
The TZA has several advantages in that it (i) is sensitive; (ii) requires only a small blood volume (2×106 rCD4+ cells); (iii) is significantly faster (7 versus 14 days for Q-VOA), less labor intensive, and less expensive ($350/assay versus more than $1,000 for Q-VOA)18. These features may allow the TZA to be used for in vitro screening to identify latency reversing agents, as well as in clinical HIV-1 eradication studies, although the latter will necessitate further validation of the assay. Because of its low cell requirement, the TZA may also be useful for quantification of replication-competent HIV-1 in the pediatric population, as well as in tissue.
ON LINE METHODS
Isolation of rCD4+T cells from HIV-1-infected participants on ART
PBMC were isolated from 200 mL heparinized blood from participants in the Multicenter AIDS Cohort Study (MACS) by ficoll-hypaque density gradient centrifugation. All study participants provided written consent, which was approved by the University of Pittsburgh Institutional Review Board. The study population is predominantly composed of white non-Hispanic Caucasian males with an average age of 62 years (range, 51– 66 years) (Table 1). The plasma viral loads for of all participants were less than 20 copies/mL, as determined by the Roche assay. rCD4+ T cells were isolated from total PBMC by depletion of activated cells expressing CD69, CD25 and HLA-DR+ cells using a one-step rCD4+ T isolation kit (Stem Cell Technologies, Vancouver, BC, Canada), and evaluated for their purity by flow cytometry using a LSRII cytometer and Kaluza flow cytometry analysis software (Beckman Coulter). The purity of the rCD4+ T cells (CD3+, CD4+, HLA-,DC-, CD69-, CD25-) was determined to be on average 98% (n=16).
Quantification of total HIV-1 DNA
Total HIV-1 DNA was quantified by qPCR amplification of the integrase region of the pol gene 20,21. HIV-1 DNA was normalized to the total number of cells assayed by qPCR amplification of the CCR5 gene22. A DNA standard for the quantification of total HIV-1 DNA was used as described previously20. A human genomic DNA control was serially diluted and used as a standard curve for CCR5 DNA quantification (Thermo Fisher). DNA sample and standards were run in triplicate for qPCR amplification on a Roche LightCycler 480 using a previously described protocol22.
Quantitation of intracellular HIV-1 gag-pol mRNA
Intracellular HIV-1 gag-pol mRNA was quantified by simultaneous ultrasensitive subpopulation staining/hybridization in situ (SUSHI), as described previously23.
Activation of rCD4+ T cells by anti-CD3/CD28 antibodies
Two to four ×106 rCD4+ T cells were cultured with anti-human CD3/CD28 mAb-coated microbeads (Dynabeads, Life Technologies) at a 1:1 bead to T cell ratio (as described by the company product insert protocol) for 4 days at 370 C in RPMI medium 1640 containing 10% fetal bovine serum and 300nM efavirenz. Following treatment with anti-CD3/CD28 mAb, cells were counted and washed thoroughly to remove the antibody. Cells were either aliquoted for HIV-1 DNA quantification or used in the TZA or Q-VOA.
TZA assay
Anti-CD3/CD28 antibody-treated rCD4+ T cells from HIV-1-infected participant were serially diluted 4-fold (125,000 -250 cell per well) and seeded (8 replicates per dilution) in a 96-well plate containing 30,000–60,000 TZM-bl cells per well in RPMI medium 1640 containing 10% FBS (Fig. 1). After co-culture for 48 h, the wells were washed and Beta-Glo® reagent (Promega, Madison, WI) was added to the TZM-bl cell monolayers, incubated for 1 h, and the chemiluminescence activity was read using an illuminometer. Control rCD4+ T cells from an uninfected donor were treated in parallel and subjected to the TZA as described above. A sample well (HIV-1-infected participant’s cells) was considered positive if the chemoluminescence signal was above the mean + 2 standard deviations of the signal obtained from control sample (healthy donor cells) well.
Q-VOA
The Q-VOA was carried out as described previously 12. Viral outgrowth was assessed at day 14 by quantification of the virion-associated HIV-1 RNA using a quantitative-PCR for HIV-1 gag RNA, with a sensitivity/cutoff of 10 copies/mL24. A well with HIV-1 gag RNA ≥ 10 copies/ml was considered positive.
IUPM calculations
The maximum likelihood estimate was applied to determine the infectious unit per million (IUPM) cells for both TZA and Q-VOA by using online software, available at http://silicianolab.johnshopkins.edu, developed by Laird et al. 25
fPVE calculations
The fPVE was calculated by dividing the total HIV-1 DNA copies/million cells with the IUPM values, as described by Cillo et al.19
Statistical analyses
A student’s paired or unpaired t test was used to calculate the significance while comparing sets of data whenever necessary. All statistical analysis was performed using PRISM software. A P value of less or equal to 0.05 was considered significant in each of the statistical analysis performed.
Supplementary Material
Acknowledgments
We thank Lori Caruso and Angel Anthony for technical assistance, Dr. John Mellors for plasma viral load measurements and for discussion during development of the assay, Drs. Patrick Tarwater and Chengli Shen for statistical consultation, Mr. William Buchanon for recruitment of the Multicenter AIDS Cohort Study (MACS) participants for the study, and participants of Pittsburgh portion of the MACS for donating blood for this study. This work was supported by NIH grants U01-AI35041 (CRR), R21AI119117 (NSC) and NIH Fogarty training grant fellowship D43TW010039 (AS).
Footnotes
AUTHOR CONTRIBUTIONS
P.G, N.S.-C, A.S, R.N.M, B.K.P, C.R.R, N.J.V and Y.C designed the study, analyzed data and wrote the manuscript. D.R, M.D, A.S, A.C, J.M.Z and N.G performed the experiments. C.R.R provided the MACS specimens.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests in this study.
DATA AVAILABILITY
All primary data used in generating the figures and tables in this report are available by contacting pgupta1@pitt.edu
References
- 1.Bailey JR, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. Journal of virology. 2006;80:6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature medicine. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 3.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 4.Lewin SR, et al. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. Journal of virology. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasternak AO, et al. Cellular levels of HIV unspliced RNA from patients on combination antiretroviral therapy with undetectable plasma viremia predict the therapy outcome. PloS one. 2009;4:e8490. doi: 10.1371/journal.pone.0008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strain MC, et al. Highly precise measurement of HIV DNA by droplet digital PCR. PloS one. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandergeeten C, et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. Journal of virology. 2014;88:12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Procopio FA, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine. 2015;2:872–881. doi: 10.1016/j.ebiom.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruner KM, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nature medicine. 2016;22:1043–1049. doi: 10.1038/nm.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson S, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS pathogens. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods in molecular biology. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 13.Bruner KM, Hosmane NN, Siliciano RF. Towards an HIV-1 cure: measuring the latent reservoir. Trends in microbiology. 2015;23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clouse KA, et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. Journal of immunology. 1989;142:431–438. [PubMed] [Google Scholar]
- 15.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. The EMBO journal. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folks TM, et al. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. The Journal of experimental medicine. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel R, et al. Structure/function studies of HIV-1(1) reverse transcriptase: dimerization-defective mutant L289K. Biochemistry. 1993;32:13012–13018. doi: 10.1021/bi00211a009. [DOI] [PubMed] [Google Scholar]
- 18.Ananworanich J, Mellors JW. How Much HIV is Alive? The Challenge of Measuring Replication Competent HIV for HIV Cure Research. EBioMedicine. 2015;2:786–787. doi: 10.1016/j.ebiom.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cillo AR, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:7078–7083. doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cillo AR, et al. Plasma viremia and cellular HIV-1 DNA persist despite autologous hematopoietic stem cell transplantation for HIV-related lymphoma. Journal of acquired immune deficiency syndromes. 2013;63:438–441. doi: 10.1097/QAI.0b013e31828e6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi RT, et al. No effect of raltegravir intensification on viral replication markers in the blood of HIV-1-infected patients receiving antiretroviral therapy. Journal of acquired immune deficiency syndromes. 2012;59:229–235. doi: 10.1097/QAI.0b013e31823fd1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malnati MS, et al. A universal real-time PCR assay for the quantification of group-M HIV-1 proviral load. Nature protocols. 2008;3:1240–1248. doi: 10.1038/nprot.2008.108. [DOI] [PubMed] [Google Scholar]
- 23.Chargin A, et al. Identification and characterization of HIV-1 latent viral reservoirs in peripheral blood. Journal of clinical microbiology. 2015;53:60–66. doi: 10.1128/JCM.02539-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biswas N, et al. ADAR1 is a novel multi targeted anti-HIV-1 cellular protein. Virology. 2012;422:265–277. doi: 10.1016/j.virol.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbloom DI, et al. Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open forum infectious diseases. 2015;2:ofv123. doi: 10.1093/ofid/ofv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.