Abstract
The incidence and severity of herpes zoster (HZ) increases with age. The live attenuated zoster vaccine (ZV) generates immune responses similar to HZ. We compared the immune responses to ZV in young and older to adults to increase our understanding of the immune characteristics that may contribute to the increased susceptibility to HZ in older adults. Young (25–40 years; N=25) and older (60–80 years; N=33) adults had similar magnitude memory responses to varicella-zoster virus (VZV) ex-vivo restimulation measured by responder cell-frequency (RCF) and flow cytometry, but the responses were delayed in older compared with young adults. Only young adults had an increase in dual-function VZV-specific CD4+ and CD8+ T cell effectors defined by co-expression of IFNγ, IL2 and CD107a after vaccination. In contrast, older adults showed marginal increases in VZV-specific CD8+CD57+ senescent T cells after vaccination, which were already higher than those of young adults before vaccination. An increase in VZV-stimulated CD4+CD69+CD57+PD1+ and CD8+CD69+CD57+PD1+ T cells from baseline to post-vaccination was associated with concurrent decreased VZV-memory and CD8+ effector responses, respectively, in older adults. Blocking the PD1 pathway during ex-vivo VZV restimulation increased the CD4+ and CD8+ proliferation, but not the effector cytokine production, which modestly increased with TIM-3 blockade. We conclude that high proportions of senescent and exhausted VZV-specific T cells in the older adults contribute to their poor effector responses to a VZV challenge. This may underlie their inability to contain VZV reactivation and prevent the development of HZ.
Keywords: Immune senescence, vaccines, varicella zoster-virus, herpes zoster
Introduction
Herpes zoster (HZ) affects more than 1 million Americans each year (1). This occurs disproportionately in older individuals; more than 60% of cases occur in people at least 50 years old, and more than 50% occur in people at least 60 years old (2). Moreover, older adults experience more morbidity from HZ, especially because of the occurrence, duration, and severity of HZ-related pain, which is the most significant complication of HZ (2–4).
HZ is the clinical manifestation of varicella-zoster virus (VZV) reactivation from latently infected dorsal root ganglia. The molecular biology and physiology of VZV latency and reactivation are not well understood (5). However, VZV cell-mediated immunity (CMI) is necessary and sufficient to prevent VZV symptomatic reactivation and the development of HZ (6, 7). VZV CMI typically decreases with age (8, 9), allowing the virus to reactivate/replicate unchecked. In immunologically intact older adults and in individuals with a relatively preserved or reconstituted immune system, the occurrence of HZ typically boosts VZV-specific CMI to levels sufficient to prevent subsequent episodes of HZ. We previously showed that VZV-specific interferon (IFN)γ-secreting effectors increase in number rapidly after HZ to reach a peak at 1 to 2 weeks after onset of symptoms, while memory CD4+ responses peak at 4 to 6 weeks (10). Higher levels of VZV-specific CMI compared with age-matched non-HZ controls are maintained for ≥3 years after HZ develops (11).
The burden of HZ in older people has been mitigated by the licensure of a live, attenuated zoster vaccine (ZV). The pivotal placebo-controlled trial of ZV demonstrated an efficacy of 51% for preventing HZ in participants ≥60 years of age (8). This was associated with a significant immunologic boost in VZV-specific effector and memory T cells (11) with kinetics similar to the immune response to HZ (10). The immune response to ZV measured by responder cell frequency (RCF) and IFNγ-ELISPOT was significantly lower in an older cohort of vaccinees (age ≥70 years), and decreased progressively with advancing age and with the interval after vaccination. However, a CMI surrogate of vaccine-conferred protection against HZ was not found in the pivotal study.
The similarities between the wild type and attenuated vaccine VZV, which differ by 15 non-synonymous mutations out of a genome of 125,000 base-pairs (12), and of the immune responses to HZ and ZV (10) suggest that vaccination with ZV may induce on a smaller scale immune responses that are similar to VZV reactivation in vivo. Thus, ZV might be valuable as a surrogate of VZV reactivation to determine the differences in CMI responses between older and young adults. This may provide important information about the nature of immune protection against HZ and why older adults are more likely to develop HZ, including more severe HZ, after VZV reactivation than young adults (13, 14).
We compared VZV-specific memory and effector responses to ZV in young and older adults with the following objectives: 1) to extend our understanding of the age-related differences in VZV-specific CMI memory responses that may correlate with protection conferred by the vaccine; 2) to determine age-related differences in effector responses that might prevent clinical disease after VZV reactivation; 3) to identify the role of immune senescence and exhaustion as potential contributors to these differences between young and older adults. In addition, we studied the modulatory effect of several pathways, including PD-1, TIM-3 and LAG-3, previously associated with downregulation of effector T cell responses in older adults and immune compromised hosts (15–20).
Methods
Study Design
The study was approved by the Institutional Review Boards of the University of Colorado School of Medicine and Emory University to include 33 young adults (25 to 40 years of age) and 44 older adults (60 to 80 years of age) with previous VZV infection documented by VZV-specific serology, in good health and with no underlying immune suppressive conditions (NCT01331161). After signing informed consent subjects received live, attenuated ZV (Zostavax™; Merck & Co., Inc., Whitehouse Station, NJ). Peripheral blood mononuclear cells (PBMC) were obtained before vaccination [Day 0 (D0)] and on D7, D14, D30, D90 and D180 and cryopreserved for adaptive immunity studies using a validated protocol (21). Cells were stored and/or shipped in liquid nitrogen tanks. All assays were performed at the University of Colorado Denver Anschutz Medical Center.
VZV-specific responder cell frequency (RCF)
VZV-specific CD4 memory T cells were enumerated by adding a limiting dilution step to a conventional lymphocyte proliferation assay as previously described (8, 22). The RCF assay utilized 24 replicate cultures of 6 serial dilutions of PBMC ranging from 100,000 to 3,125 cells per well. PBMC were thawed as previously described (21). Cells with viability ≥70% were stimulated with VZV or mock-infected control antigen for 8 days after which they were pulsed with 3H-thymidine for 6 hours. Incorporated radioactivity was measured with a beta counter, and the RCF calculated as described by Henry et al (23). Responder wells were defined as those in which counts per minute exceeded the mean+ 3 SD counts per minute of the control cultures at the same cell concentration. The percentage of non-responder wells was plotted on a log scale against the number of cells per well plotted on a linear scale, and the RCF was interpolated at the 37% non-responder well frequency. Results were expressed as number of responder cells/105 PBMC. The analytical sensitivity of this assay is limited by the total number of cells used at the highest concentration of cells/well. For this analysis, the lower limit of quantitation of the assay was 0.04 responders/105 PBMC. Values <0.04 responders/105 PBMC were assigned an arbitrary value of 0.02 responders/105 PBMC. All samples from each subject were tested in the same run to avoid potential inter-run variability.
Flow cytometric enumeration of VZV-specific T cell subsets
Thawed PBMC were cultured at 37°C in 5% CO2 at 106 cells/ml in RPMI 1640 (Mediatech; Manassas, VA) with L-glutamine (Gemini Bio-Products; West Sacramento, CA), 10% human AB serum (Gemini Bio-Products;), 2% HEPES (Mediatech) and 1% penicillin-streptomycin (Gemini Bio-Products) in the presence of infectious VZV (60,000 plaque forming units/ml) or medium control for 2 days. Brefeldin A (Sigma) and anti-CD107a (BD Biosciences; San Jose, CA, clone H4A3) were added for the last 16 h (24). At the end of the incubation, PBMC were washed with 2% FBS in PBS and incubated with antibody against surface markers CD3 (BD, clone UCHT1), CD8 (BD, clone SK1), CD57 (BD, clone NK-1), CD69 (BD, clone FN50) and PD1 (BD, clone EH12.1) for 30 min at 4°C. For intracellular staining, PBMC were permeabilized and fixed for 20 min at 4°C, washed and resuspended with permeabilization buffer and stained with antibodies against internal markers: IL2 (BD, clone MQ1-17H12), BCL-2 (BD, clone BCL-2/100) and IFNγ (BD, clone B27) for 30 min at 4°C. Unbound antibodies were removed by washing with buffer, and 150,000–200,000 events were acquired with a Guava EasyCyte Plus (Millipore) or Gallios (Beckman Coulter) instruments and FlowJo (Tristar) or Kaluza (Beckman Coulter) Analysis software. VZV-specific T-cell subsets were expressed as percentages of the parent CD4+ or CD8+ lymphocyte populations. All samples from each subject were tested in the same run to avoid potential inter-run variability.
T-cell proliferation and cytokine production measured by flow cytometry after blocking receptors associated with exhaustion
Thawed PBMC were cultured in the presence of infectious VZV (120,000 pfu/ml) or medium control for 2 days, or stained with Cell Trace Violet (Biolegend) and cultured in the presence of VZV cell lysate or control for 6 days. Blocking experiments were set up with 10ug/ml anti-PDL1 (Biolegend, clone 29E.2A3), 10 µg/ml anti-PD1 (Biolegend clone EH12.2H7), 10 µg/ml anti-TIM-3 (Biolegend clone F38-2E2) and/or LAG-3 Fc chimera (R&D Systems), 2µg/ml. Brefeldin A was added for the last 16 h of virus stimulation. PBMC were washed with PBS and incubated with Zombie Yellow viability stain (Biolegend). Cells were washed, stained and analyzed as above.
Statistical analysis
The sample size was selected to reject the null hypothesis of equal RCF means in older adults and young adults with 80% power at the alpha level of 0.01. The adaptive immunity data were analyzed with Prism 6 (GraphPad) and Spice v5.3 software (NIAID) software. Parametric or nonparametric tests were used as appropriate based on the distribution of the data. Significance was defined by p<0.05.
Results
Demographic characteristics of the study population
This study used PBMC from 25 young and 33 older adults. Table I shows that young (age mean ± S.D.= 32 ± 4 years) and older adults (age = 65 ± 4 years) were similar in gender, race and ethnicity.
Table I.
Demographic Characteristics
| Category | Young | Older |
|---|---|---|
|
| ||
| Number | 25 | 33 |
|
| ||
| Mean years of age (S.D.) | 32 (4) | 65 (4) |
|
| ||
| Number of Females (%) | 16 (64) | 17 (51) |
|
| ||
| Number (%) | ||
| White | 19 (76) | 30 (91) |
| Black | 2 (8) | 2 (6) |
| American Indian | 0 | 1 (3) |
| Asian | 2 (8) | |
| Mixed | 2 (8) | |
|
| ||
| Number of Hispanics (%) | 3 (12) | 2 (6) |
Abbreviations: S.D.= standard deviation
VZV-specific T-cell memory responses to ZV
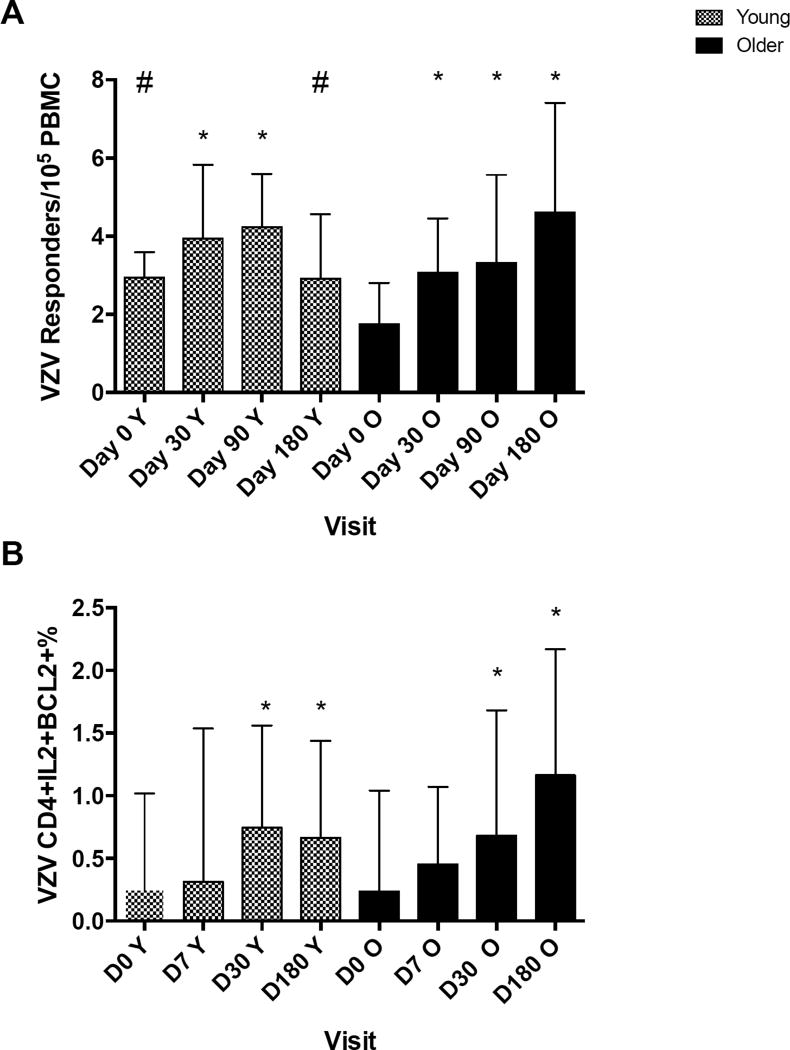
Memory responses to ZV were measured by the VZV-RCF assay that assesses the frequency of VZV-specific CD4+ memory T cells. Before vaccination, the VZV-specific RCF was significantly higher in young compared with older adults (medians of 2.9 and 1.7 responders/105 PBMC, respectively; p=0.01; Fig 1A). After ZV administration both groups had significant RCF increases that did not differ at D30 and D90 between them. However, the kinetics of the RCF response to ZV differed between groups. In young adults the RCF increased up to D90 and was lower at D180 with a negative trend compared to D90 (p=0.08), whereas in the older adults the RCF continued to increase after D90. The flow cytometric analysis of CD4+IL2+Bcl2+ memory T cells measured through ex vivo live virus stimulation of PBMC showed similar kinetics (Fig 1B).
Figure 1. VZV-specific memory responses after ZV administration to young and older adults.
The bars represent medians and upper quartiles of measurements from 25 young and 33 older recipients of ZV. Memory responses were measured by RCF (panel A) and flow cytometry (panel B) at the indicated time points. Asterisks indicate significant and changes from baseline (p < 0.05) within each age group. Hash tags indicate significant differences between groups. The gating strategy used for panel 1B is shown in Supplemental Figure 2A.
VZV-specific CD4+ and CD8+ effector function before and after ZV
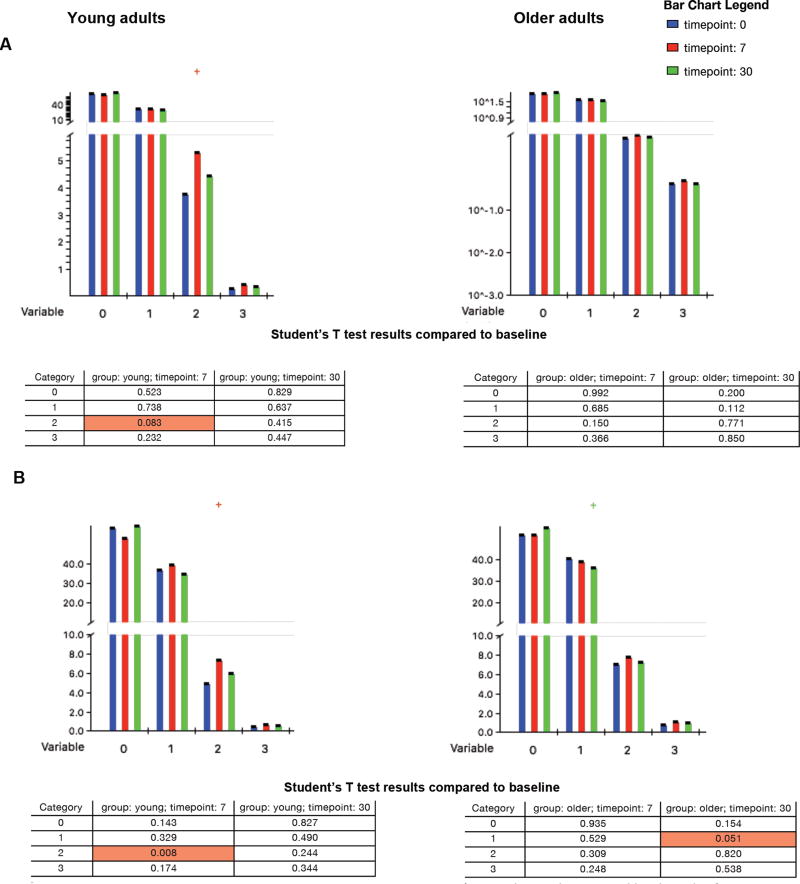
One potential explanation for the lower incidence of HZ in young adults is that they mount faster and/or more robust responses to VZV reactivation compared with older adults, thereby controlling the viral replication before it becomes clinically apparent. To address this hypothesis, we examined VZV-specific CD4+ and CD8+ functionality before and in the first month after vaccination by measuring expression of IL2, IFNγ and CD107a on CD4+ and CD8+ T cells re-stimulated ex vivo with live VZV, which, by virtue of being presented in the context of MHC class I and II, can stimulate both CD8+ and CD4+ effectors. There were no significant differences in the distribution of VZV-specific functional CD4+ T cells at baseline between young and older adults (p=0.12, Fig 2A). At D7, young adults had a marginal increase in dual-function VZV-specific CD4+ T cells compared to baseline (p=0.08; Fig 2A), while older adults had no significant changes over time (Fig 2A). The response of CD8+ T cells differed in young and older adults. At D7 dual function VZV-specific CD8+ T cells significantly increased in young participants (p=0.008; Fig 2B), but not in the older adults (Fig 2B). The older adults had a decrease in single-function VZV-specific CD8+ T cells at D30 post-vaccination (p=0.05, Fig 2B) with an apparent increase in no-function CD8+ T cells, which did not reach statistical significance.
Figure 2. VZV-specific Effector CD4 (A) and CD8 (B) Responses to ZV in Young (Left) and Older Adults (Right).
The data were derived from 25 young and 33 older adults whose PBMC were ex vivo restimulated with live VZV and mock-infected control. The data show expression of IFNγ, IL2 and CD107a measured by flow cytometry after subtraction of background. The bars show the mean% of the T cell subsets expressing the number of markers (variables) indicated on the x axis at the time points indicated in the legend. Marginal and significant differences are highlighted by crosses above the bars. The tables show p values for changes at the times indicated in the column headings compared with D0 in each T cell subsets grouped by the number of expressed markers (category). Young adults showed increases of dual function VZV-effectors at D7, which were marginal for CD4 (p=0.08) and significant for CD8 effectors (p=0.008), whereas older adults showed a marginal decrease in single function CD8 effectors at D30 (p=0.05).
VZV-specific exhausted and senescent T cells
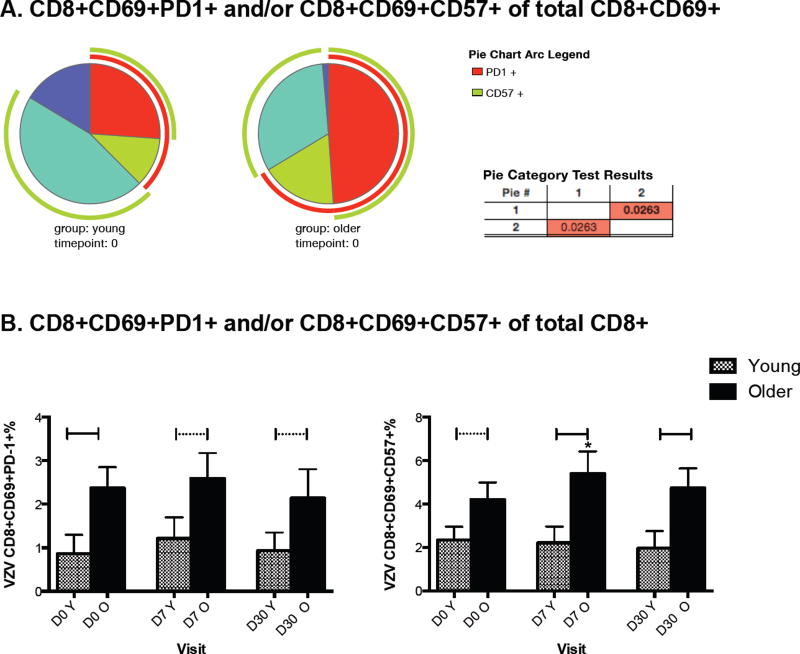
Older individuals have increased proportions of dysfunctional T cells that may contribute to their susceptibility to HZ (19, 25). To determine the contribution of dysfunctional T cells to VZV-specific responses, we identified VZV-specific CD4+ and CD8+ T cells in ex vivo live VZV-restimulated PBMC by the expression of the CD69 activation marker. Among the CD69+ T cells, VZV-specific dysfunctional senescent and exhausted T cells expressing CD57 and PD1, respectively, were further enumerated. There were no differences in VZV-specific dysfunctional CD4+ T cells between young and older adults before or after vaccination (Supplemental Figure 1). However, the distribution of dysfunctional VZV-specific CD8+ T cells significantly differed between groups at D0 (p=0.03, Fig 3A). The VZV-specific CD8+ T cells of the older adults were almost exclusively comprised of exhausted and senescent cells with an average of 99% of the VZV-specific CD8+CD69+ cells expressing CD57 and/or PD1, whereas in young adults, 80% of the VZV-specific CD8+CD69+ cells expressed CD57 and/or PD1 (p=0.03, Fig 3A). Differences in VZV-specific exhausted and senescent CD8+ cells between young and older adults persisted up to D30 after vaccination (Fig 3B). Furthermore, older adults had a marginal increase in VZV-specific senescent CD8+ T cells at D7 from mean ± SEM of 4.2 ± 0.8% to 5.4 ± 1% (p=0.06; Fig 3B), but young adults had no significant changes in either senescent or exhausted VZV-specific CD8+ T cells over time.
Figure 3. VZV-specific Exhausted and Senescent CD8 T cells in Young and Older Adults.
The data were derived from 25 young and 33 older adults whose PBMC were ex vivo restimulated with live VZV and mock-infected control. The data show expression of PD1 and CD57 on CD69+ T cells measured by flow cytometry in VZV-restimulated PBMC after subtraction of background control. Panel A: Pies show the distribution of CD8+ T cells expressing both PD1 and CD57 (red slices), only CD57 (teal slices), only PD1 (green slices), neither PD1 nor CD57 (blue slices) at D0 in young (left) and older adults (right). The light green arc indicates total CD57 and the red arc total PD1. The table shows that the distributions were significantly different (p=0.03) between young and older adults. Panel B: Bars show mean and SEM of the VZV-specific CD8+CD69+PD1+% (left graph) and CD8+CD69+CD57+% (right graph) at each visit in young and older adults. The horizontal continuous lines indicate significant differences between young and older adults; dotted lines indicate marginal differences. Asterisk indicates a marginal increase compared to D0 in senescent VZV-specific CD8+ T cells in older adults (p=0.06).
Association of VZV-specific exhausted and senescent T cells with memory and effector responses to ZV
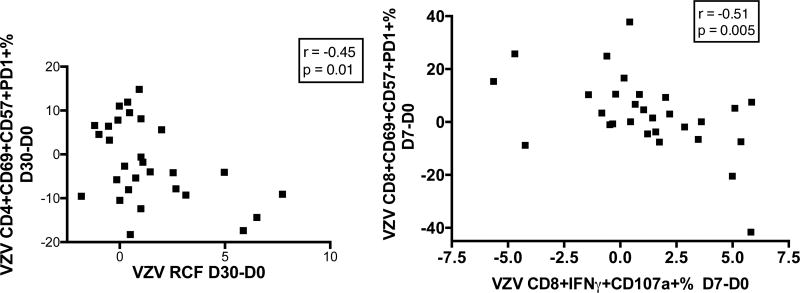
To determine the potential interaction of exhausted and senescent CD4+ and CD8+ T cells with memory and effector responses to ZV in older adults, we performed correlation analyses of the change in VZV RCF between baseline and Day 30 with the concomitant change in VZV-specific CD4+ T cells expressing exhaustion and/or senescence markers. Of note, VZV-specific effectors and senescent/exhausted T cells were measured in different cell preparations from the same donor at the same time point, which rules out the possibility of an assay-imposed bias (an assay-imposed bias has to be considered when all the T cell subsets add up to 100%, because there is a chance that when a single subset has an absolute and real increase, the percentages of the other subsets will show a relative decrease, which may not reflect any absolute changes in these other subsets). Young adults did not show significant associations between changes in exhausted/senescent T cells and VZV-specific responses, but in older adults the gain in VZV-specific RCF after vaccination was significantly negatively correlated with the gain in VZV CD4+CD69+CD57+PD1+% (r = −0.45, p=0.01; Fig 4). Similar correlation analyses of the VZV-specific CD8+CD107a+IFNγ+ cytotoxic T lymphocytes (CTL) showed that the CTL increase from D0 to D7 was higher in older participants who had a decrease in VZV-specific CD8+CD69+CD57+PD1+% from D0 to D7 (r=−0.51, p=0.005; Fig 4). No associations were observed in young adults.
Figure 4. Increases of VZV-Specific CD4+ or CD8+ T Cells Expressing both CD57 and PD1 Correlate with Decreases in RCF (Left) and CD8+ CTL (Right), Respectively, in Older Adults.
Data were derived from 33 older adults. Peak response for RCF memory was D30 and for CD8+IFNγ+CD107a effector was D7. P values and coefficients of correlation calculated by Pearson correlation analyses are shown on each graph. The gating strategy used for the flow cytometry data is shown in Supplemental Figure 2B.
The role of exhausted and senescent T cells in the attenuation of VZV-specific immune responses
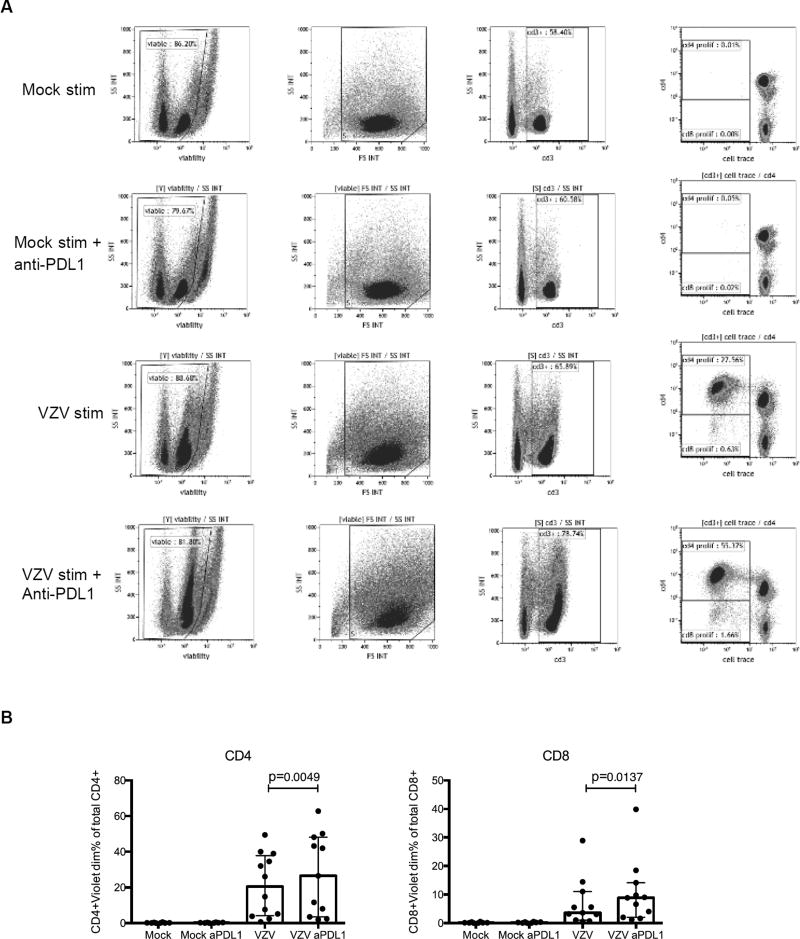
To gain additional insight into the role of PD1 and other markers of immune senescence, including TIM-3 and LAG-3, in the attenuation of VZV-specific immune responses in the older adults, we blocked the interaction of these molecules with their ligands during ex vivo VZV restimulation. PBMC from 16 older adults were treated ex vivo with a neutralizing anti-PDL1 mAb and/or anti-TIM-3 mAb and/or human LAG-3 Fc chimera blocking agents. The addition of anti-PDL1 increased CD4+% and CD8+% proliferating T cells measured by dye dilution after VZV ex vivo restimulation [median (IQR) for CD4+: 15%(4;34) vs. 27%(3;48), p=0.005; and for CD8+: 3%(1;6) vs. 9%(2;14), p=0.01; Fig 5]. There was no additional benefit from adding to the anti-PDL1 neutralizing mAb either TIM-3 or LAG-3 blocking agents (data not shown). Blocking TIM-3 or LAG-3 alone or in combination did not increase proliferation. It is important to note in Fig. 5B that the proliferative response to ex vivo PD1 pathway blockade varied in magnitude across participants with 6 of 11 showing increases of 1.2- to 3-fold and the remaining 5 participants showing differences within the error of the test.
Figure 5. Increased ex-vivo VZV-specific proliferation of PBMC from older adults by blockade of the PD1 pathway.
Panel A shows the gating strategy in a sample with a response to anti-PDL1. Data in panel B used PBMC collected from 11 older adults at 7 to 14 days after vaccination. Dots represent results of each participant. Bars and whiskers indicate medians and lower and upper quartiles of the composite results, which did not have a normal distribution. Stimulation conditions are listed under each bar: mock=mock-stimulated background control; aPDL1= anti-PDL1; VZV= VZV-stimulated. P values calculated by Wilcoxon matched-pairs signed rank test showed significant increases both in CD4+ and CD8+ VZV-specific T cell proliferation after addition of anti-PDL1.
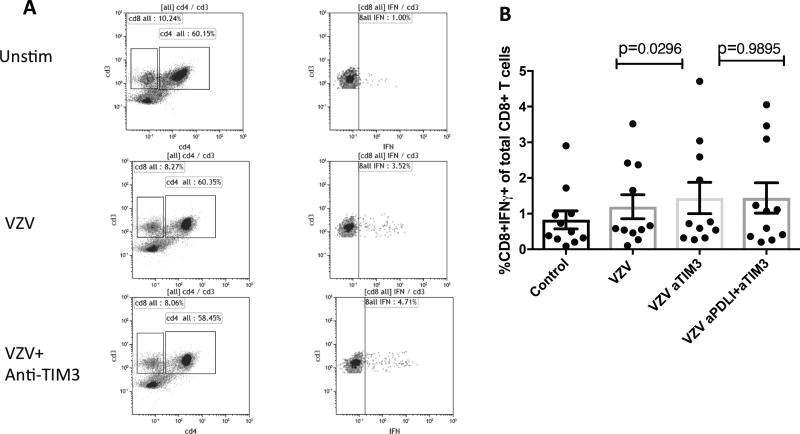
The blockade of the TIM-3 pathway ex vivo resulted in small increases of CD8+IFNγ+% after ex vivo restimulation with VZV live virus (mean ± SEM of 1.2±0.3% vs. 1.4±0.4%; p=0.02; Fig 6B). Blocking PD-1 or LAG-3 pathways in addition to TIM-3 did not have an additive or synergistic effect.
Figure 6. Effect of Inhibitory Pathway Blockades on VZV-Specific Effector T-Cell Responses in Older Adults.
Data were derived from PBMC collected from 11 older adults at 14 days after ZV administration. Panel A shows the gating strategy for CD8+IFNγ+ T cells. In Panel B the dots indicate the values of each of the 11 participants and the bars indicate means and SEM of the composite results, which had a normal distribution. P values were calculated by paired Student’s T-test
Discussion
We focused this study on effector responses to ZV because the administration of this high titer live attenuated VZV vaccine may be a model that recapitulates the immune responses to wild type VZV reactivation from latency (8). Among the effector responses to ZV, dual function VZV-specific CD8+ T cells significantly increased and VZV-specific effector CD4+ T cells moderately increased in young adults at 1 week after vaccination, suggesting that brisk CD8+ and CD4+ responses to VZV replication are available to young adults when exposed to VZV (26). In contrast, dual function effector responses in older adults did not significantly rise above baseline in the first 30 days after vaccination. Rapid and/or polyfunctional effector responses may contribute to control of viral replication and prevent HZ. This is in agreement with the immune mechanism that controls other herpesviral infections (27, 28) and with previous reports showing that adults with recent and/or frequent exposures to VZV, who did not develop symptomatic VZV infection, had robust VZV-specific CD8+ and CD4+ T cell responses detected by ex vivo restimulation (29–31). The important role of CD8+ T cells in the control of VZV replication could also be inferred from the accumulation of this T cell subset in the ganglia of individuals with HZ, where VZV is actively replicating (32). Furthermore, we showed that high frequencies of VZV-specific CD8+ CTL detected by ex vivo restimulation in HIV-infected children correlated with protection against HZ (33). Our current report complements the previously published data and indicates that the magnitude and the speed with which VZV-specific CTL are generated decreases with age. Our data suggest that the lower and slower VZV-specific CD4+ and CD8+ effector T cell responses to a VZV challenge in older compared with young adults may partially account for the increased risk and morbidity of HZ in the older adults.
We also found a difference between the kinetics of memory T cell responses of young and older adults after ZV. Although both groups had significant increases of their VZV-specific memory T cells after vaccination young adults reached peak response faster than older adults and quenched the expansion of memory cells before 180 days post-immunization. In contrast, VZV-specific memory CD4+ T cells continued to increase beyond 90 days after immunization in the older adults. This delayed and prolonged expansion of VZV-specific memory T cells was demonstrated in the RCF assay and confirmed by flow cytometric enumeration of the proportions of CD4+IL2+Bcl2+ T cells. In our previous studies the VZV-specific memory responses to ZV were assessed at 6 weeks post-immunization, when memory responses were high, and subsequently only at 1 year post-immunization, when the memory responses had already decreased. The increase in memory cells over a prolonged period of time in older adults may reflect prolonged vaccine viral replication after immunization, likely due to inefficient CD8+ effectors, and persistent stimulation of the T cell memory responses. Alternatively, the continuous increase of VZV-specific memory CD4+ T cells beyond 90 days after immunization may reflect memory inflation, which has been previously demonstrated for CMV-specific T cells in older adults (34). It is probable that VZV and CMV memory inflations occur through similar mechanisms of prolonged homeostatic proliferation made possible by the decrease of the naïve T-cell compartment in older adults (34). An alternative explanation, is that regulatory responses are less efficient in older adults compared with young adults allowing T cells to continue to proliferate and acquire memory features. Regardless of the mechanism, it seems that peak memory response in older adults occurs between 3 and 6 months after ZV administration or even later.
The kinetics of the memory responses to ZV in older adults has important clinical implications in addition to the pathogenesis of HZ. The protective efficacy of ZV against HZ decreases over time (35, 36) and there is interest in designing booster regimens to maintain its efficacy (37). Since peak vaccine-induced memory responses in older adults occur 3 to 6 months or later after ZV administration, any strategy using a second dose of ZV to boost immune protection against HZ should be attempted ≥ 12 months after primary immunization, when there is good evidence that the immune response to the first dose of vaccine has quenched. This observation is supported by the results of a previous study in which the administration of a booster dose of ZV 6 weeks after the initial dose did not appreciably affect VZV-specific effector T cells (38). At the other extreme, we recently showed that VZV-specific effector and memory CD4+ T cells significantly increased after administration of a booster dose of ZV ≥10 years after the initial dose (37). It remains to be determined if there is an optimal interval between 12 months and 10 years that may elicit a maximum boost of the VZV-specific T cell responses.
Dysfunctional CD8+ T cells expressing markers of exhaustion, such as PD1, or senescence, such as CD57, increase host susceptibility to infections and tumors (39, 40). We found that in older adults virtually all VZV-specific CD8+ T cells expressed PD1 and/or CD57 before vaccination, which differed significantly from young adults. After vaccination, older adults continued to exhibit a higher proportion of VZV-specific dysfunctional CD8+ T cells compared with young adults. In fact, in older adults there was an increase in senescent CD8+CD69+CD57+% T cells at D7 after vaccination. At D7 after vaccination young adults had a significant increase in VZV-specific CD8+ dual-function effectors, but older adults failed to exhibit a significant increase in VZV effectors. Taken together, these data suggest that older adults would be less able to mount a rapid effector response to VZV reactivation. The model that emerges is that older adults respond to a VZV challenge predominately with senescent and exhausted cells and/or their VZV CD8+ T cell responders rapidly acquire markers of senescence and exhaustion, thereby limiting the potential of the VZV-specific T cells to effectively clear the viral infection.
This model is further supported by the observations that in older adults the frequency of dysfunctional VZV-specific CD8+ T cells is negatively correlated with the increase in VZV-specific CD8+CD107a+ CTL at D7 after ZV, and that the proportions of dysfunctional VZV-specific CD4+ T cells is negatively correlated with the VZV-specific CD4+ memory responses to ZV. This is in accordance with observations in humans and in mice demonstrating that exhausted and senescent cells have limited replicative capacity and limited ability to upregulate effector mechanisms (41, 42). Similar correlations, however, were absent in young adults. Potential explanations for the absence of an inverse relationship between dysfunctional T cells and effector or memory responses in young adults include the following: 1) PD1 expression on CD8+ T cells of young adults is a marker of activation, as previously described in the context of acute infections (43), and, therefore, has different functional implications for young compared with older adults; 2) young adults have a low number of dysfunctional T cells and a larger number of functional memory T cells that can contribute to the response; 3) young adults have a higher number of naïve T cells than older adults that can serve as a reservoir for de novo CTL.
One of the markers of T cell dysfunction in this study, PD1, has been extensively studied in tumor and viral infections, including HZ, both in humans and in animal models (18, 19, 39, 40). Blocking the PD1 pathway in vivo significantly improves the cure rates in individuals with PDL1+ tumors. Furthermore, PDL1 expression on antigen presenting cells can induce regulatory T cells that block effector T cell function. The expression of PDL1 on endothelial cells seems to inhibit T cell motility, which is necessary for T cell migration to the site of viral replication (39). We showed that blocking the PD1 pathway during VZV ex vivo restimulation of PBMC from older adults increased VZV-specific proliferative responses, but gain of function was relatively modest and varied among participants. TIM3 and LAG3 pathways blockade increased IFNγ production by CD8+ T cells. However, the gain in IFNγ production by CD8+ T cells was counterbalanced by a loss of IFNγ production by CD4+ T cells such that the analysis of total CD3+ T cells did not show any changes. Although previous studies showed that T cells generally express multiple inhibitory receptors that may synergize to decrease their proliferative and functional capacity (16), we could not elicit additive or synergistic activity by combined blockade of TIM3, LAG3 and PD1 in all possible combinations. An alternative explanation for the decreased VZV-specific immune responses of older adults consists of exacerbated regulatory T cell responses as suggested by previous studies (20, 44).
This study had several limitations. It had a relatively small number of participants and our evaluation was limited to effector, senescence and exhaustion markers and did not include regulatory T cell responses. We also did not quantitate the number of VZV-specific effector T cells due to a paucity of known epitopes needed for the manufacture of tetramers or other multimers. Although several VZV epitopes recognized in the context of MHC class I and class II have been described (17, 45–54), they are still limited to a few HLA types and more developmental work needs to be done in this area.
An important outcome of this study was to identify characteristics of the VZV-specific CMI responses in older adults that may contribute to their increased susceptibility to HZ. This included finding that senescent and exhausted T cells made up a significant proportion of the CMI responses of older adults, and that in older adults CTL responses predominately included dysfunctional CD8+ T cells. These observations, as well as the demonstration of the lack of early effector responses to the viral challenge in older adults, identify new targets for development of new vaccines and prevention strategies against HZ.
Supplementary Material
Acknowledgments
This study was supported by funding from NIAID U19 AI090023.
The authors thank the Vaccine Research Clinic and Hope Clinic staff for clinical research support; Dr. Sai Druaisinghan and Ms. Alice Cho, Julie Patterson Bartlett and Megan McCausland for technical support.
Bibliography
- 1.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 3.Kost RG, Straus SE. Postherpetic neuralgia--pathogenesis, treatment, and prevention. N Engl J Med. 1996;335:32–42. doi: 10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 4.Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med. 1997;157:1217–1224. [PubMed] [Google Scholar]
- 5.Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J Neurovirol. 2003;9:194–204. doi: 10.1080/13550280390194000. [DOI] [PubMed] [Google Scholar]
- 6.Vermont CL, Jol-van der Zijde EC, Hissink Muller P, Ball LM, Bredius RG, Vossen AC, Lankester AC. Varicella zoster reactivation after hematopoietic stem cell transplant in children is strongly correlated with leukemia treatment and suppression of host T-lymphocyte immunity. Transplant infectious disease : an official journal of the Transplantation Society. 2014;16:188–194. doi: 10.1111/tid.12180. [DOI] [PubMed] [Google Scholar]
- 7.Vossen MT, Biezeveld MH, de Jong MD, Gent MR, Baars PA, von Rosenstiel IA, van Lier RA, Kuijpers TW. Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella. The Journal of infectious diseases. 2005;191:198–206. doi: 10.1086/426866. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, Silber JL, MacGregor RR, Chan K, Gershon AA, Levin MJ. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201:1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Besouw NM, Verjans GM, Zuijderwijk JM, Litjens NH, Osterhaus AD, Weimar W. Systemic varicella zoster virus reactive effector memory T-cells impaired in the elderly and in kidney transplant recipients. Journal of medical virology. 2012;84:2018–2025. doi: 10.1002/jmv.23427. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ U. S. D. o. V. A. C. S. P. S. P. S. Investigators. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinlivan M, Breuer J, Schmid DS. Molecular studies of the Oka varicella vaccine. Expert Rev Vaccines. 2011;10:1321–1336. doi: 10.1586/erv.11.93. [DOI] [PubMed] [Google Scholar]
- 13.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 14.Toi CS, Lay ML, Lucas R, Chew CB, Taylor J, Ponsonby AL, Dwyer DE G. Ausimmune Investigator. Varicella zoster virus quantitation in blood from symptomatic and asymptomatic individuals. J Med Virol. 2013;85:1491–1497. doi: 10.1002/jmv.23605. [DOI] [PubMed] [Google Scholar]
- 15.Araki K, Youngblood B, Ahmed R. Programmed Cell Death 1-Directed Immunotherapy for Enhancing T-Cell Function. Cold Spring Harb Symp Quant Biol. 2014 doi: 10.1101/sqb.2013.78.019869. [DOI] [PubMed] [Google Scholar]
- 16.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu C, McCausland M, Sidney J, Duh FM, Rouphael N, Mehta A, Mulligan M, Carrington M, Wieland A, Sullivan NL, Weinberg A, Levin MJ, Pulendran B, Peters B, Sette A, Ahmed R. Broadly reactive human CD8 T cells that recognize an epitope conserved between VZV, HSV and EBV. PLoS Pathog. 2014;10:e1004008. doi: 10.1371/journal.ppat.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James SF, Traina-Dorge V, Deharo E, Wellish M, Palmer BE, Gilden D, Mahalingam R. T cells increase before zoster and PD-1 expression increases at the time of zoster in immunosuppressed nonhuman primates latently infected with simian varicella virus. Journal of neurovirology. 2014;20:309–313. doi: 10.1007/s13365-014-0237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schub D, Janssen E, Leyking S, Sester U, Assmann G, Hennes P, Smola S, Vogt T, Rohrer T, Sester M, Schmidt T. Altered phenotype and functionality of varicella zoster virus-specific cellular immunity in individuals with active infection. The Journal of infectious diseases. 2015;211:600–612. doi: 10.1093/infdis/jiu500. [DOI] [PubMed] [Google Scholar]
- 20.Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, Jackson SE, Fuentes-Duculan J, Suarez-Farinas M, Mabbott NA, Lacy KE, Ogg G, Nestle FO, Krueger JG, Rustin MH, Akbar AN. The Characterization of Varicella Zoster Virus-Specific T Cells in Skin and Blood during Aging. J Invest Dermatol. 2015;135:1752–1762. doi: 10.1038/jid.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, Ferrari G, Etter PE, Berrong M, Canniff JD, Carter D, Defawe OD, Garcia A, Garrelts TL, Gelman R, Lambrecht LK, Pahwa S, Pilakka-Kanthikeel S, Shugarts DL, Tustin NB. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J Immunol Methods. 2010;363:42–50. doi: 10.1016/j.jim.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 23.Henry C, Marbrook J, Vann DC, Kodlin DC, Wojsy C. Limiting dilution analysis. In: Mishell BB, Shigii SM, editors. Selected methods in cell mediated immunity. Freeman Press; San Francisco: 1980. pp. 138–152. [Google Scholar]
- 24.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 25.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinlivan ML, Ayres K, Ran H, McElwaine S, Leedham-Green M, Scott FT, Johnson RW, Breuer J. Effect of viral load on the outcome of herpes zoster. J Clin Microbiol. 2007;45:3909–3914. doi: 10.1128/JCM.00874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenberg PD, Reusser P, Goodrich JM, Riddell SR. Development of a treatment regimen for human cytomegalovirus (CMV) infection in bone marrow transplantation recipients by adoptive transfer of donor-derived CMV-specific T cell clones expanded in vitro. Ann N Y Acad Sci. 1991;636:184–195. doi: 10.1111/j.1749-6632.1991.tb33450.x. [DOI] [PubMed] [Google Scholar]
- 28.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogunjimi B, Smits E, Heynderickx S, Van den Bergh J, Bilcke J, Jansens H, Malfait R, Ramet J, Maecker HT, Cools N, Beutels P, Van Damme P. Influence of frequent infectious exposures on general and varicella-zoster virus-specific immune responses in pediatricians. Clinical and vaccine immunology : CVI. 2014;21:417–426. doi: 10.1128/CVI.00818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vossen MT, Gent MR, Weel JF, de Jong MD, van Lier RA, Kuijpers TW. Development of virus-specific CD4+ T cells on reexposure to Varicella-Zoster virus. The Journal of infectious diseases. 2004;190:72–82. doi: 10.1086/421277. [DOI] [PubMed] [Google Scholar]
- 31.Malavige GN, Rohanachandra LT, Jones L, Crack L, Perera M, Fernando N, Guruge D, Ogg GS. IE63-specific T-cell responses associate with control of subclinical varicella zoster virus reactivation in individuals with malignancies. British journal of cancer. 2010;102:727–730. doi: 10.1038/sj.bjc.6605542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steain M, Sutherland JP, Rodriguez M, Cunningham AL, Slobedman B, Abendroth A. Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia. Journal of virology. 2014;88:2704–2716. doi: 10.1128/JVI.03445-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg A, Huang S, Song LY, Fenton T, Williams P, Patterson J, Tovar-Salazar A, Levin MJ. Immune Correlates of Herpes Zoster in HIV-Infected Children and Youth. J Virol. 2012;86:2878–2881. doi: 10.1128/JVI.06623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman CA, Marques A, Toney J, Boardman K, Su SC, Li X, Chan IS, Parrino J, Annunziato P, Oxman MN G. Shingles Prevention Study. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–909. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, Pachucki C, Keay S, Menzies B, Griffin MR, Kauffman C, Marques A, Toney J, Keller PM, Li X, Chan IS, Annunziato P G. Shingles Prevention Study. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–1328. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin MJ, Schmader KE, Pang L, Williams-Diaz A, Zerbe G, Canniff J, Johnson MJ, Caldas Y, Cho A, Lang N, Su SC, Parrino J, Popmihajlov Z, Weinberg A. Cellular and Humoral Responses to a Second Dose of Herpes Zoster Vaccine Administered 10 Years After the First Dose Among Older Adults. J Infect Dis. 2016;213:14–22. doi: 10.1093/infdis/jiv480. [DOI] [PubMed] [Google Scholar]
- 38.Vermeulen JN, Lange JM, Tyring SK, Peters PH, Nunez M, Poland G, Levin MJ, Freeman C, Chalikonda I, Li J, Smith JG, Caulfield MJ, Stek JE, Chan IS, Vessey R, Schodel FP, Annunziato PW, Schlienger K, Silber JL. Safety, tolerability, and immunogenicity after 1 and 2 doses of zoster vaccine in healthy adults >/=60 years of age. Vaccine. 2012;30:904–910. doi: 10.1016/j.vaccine.2011.11.096. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3--potential mechanisms of action. Nature reviews. Immunology. 2015;15:45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 40.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. 2014;35:51–60. doi: 10.1016/j.it.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 43.Brooks DG, McGavern DB, Oldstone MB. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J Clin Invest. 2006;116:1675–1685. doi: 10.1172/JCI26856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vukmanovic-Stejic M, Sandhu D, Sobande TO, Agius E, Lacy KE, Riddell N, Montez S, Dintwe OB, Scriba TJ, Breuer J, Nikolich-Zugich J, Ogg G, Rustin MH, Akbar AN. Varicella zoster-specific CD4+Foxp3+ T cells accumulate after cutaneous antigen challenge in humans. J Immunol. 2013;190:977–986. doi: 10.4049/jimmunol.1201331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frey CR, Sharp MA, Min AS, Schmid DS, Loparev V, Arvin AM. Identification of CD8+ T cell epitopes in the immediate early 62 protein (IE62) of varicella-zoster virus, and evaluation of frequency of CD8+ T cell response to IE62, by use of IE62 peptides after varicella vaccination. The Journal of infectious diseases. 2003;188:40–52. doi: 10.1086/375828. [DOI] [PubMed] [Google Scholar]
- 46.Jones L, Black AP, Malavige GN, Ogg GS. Persistent high frequencies of varicella-zoster virus ORF4 protein-specific CD4+ T cells after primary infection. Journal of virology. 2006;80:9772–9778. doi: 10.1128/JVI.00564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones L, Black AP, Malavige GN, Ogg GS. Phenotypic analysis of human CD4+ T cells specific for immediate-early 63 protein of varicella-zoster virus. European journal of immunology. 2007;37:3393–3403. doi: 10.1002/eji.200737648. [DOI] [PubMed] [Google Scholar]
- 48.Jones L, Malavige G, Jeffery K, Kemp E, Breuer J, Klenerman P, Ogg GS. Tracking epitope-specific antiviral CD4+ T cell responses to a live attenuated vaccine reveals ongoing functional responses. Vaccine. 2009;27:7398–7401. doi: 10.1016/j.vaccine.2009.08.081. [DOI] [PubMed] [Google Scholar]
- 49.Kleemann P, Distler E, Wagner EM, Thomas S, Klobuch S, Aue S, Schnurer E, Schild H, Theobald M, Plachter B, Tenzer S, Meyer RG, Herr W. Varicella-zoster virus glycoproteins B and E are major targets of CD4+ and CD8+ T cells reconstituting during zoster after allogeneic transplantation. Haematologica. 2012;97:874–882. doi: 10.3324/haematol.2011.052597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malavige GN, Jones L, Black AP, Ogg GS. Rapid effector function of varicella-zoster virus glycoprotein I-specific CD4+ T cells many decades after primary infection. The Journal of infectious diseases. 2007;195:660–664. doi: 10.1086/511274. [DOI] [PubMed] [Google Scholar]
- 51.Malavige GN, Jones L, Black AP, Ogg GS. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clinical and experimental immunology. 2008;152:522–531. doi: 10.1111/j.1365-2249.2008.03633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milikan JC, Kinchington PR, Baarsma GS, Kuijpers RW, Osterhaus AD, Verjans GM. Identification of viral antigens recognized by ocular infiltrating T cells from patients with varicella zoster virus-induced uveitis. Investigative ophthalmology & visual science. 2007;48:3689–3697. doi: 10.1167/iovs.07-0020. [DOI] [PubMed] [Google Scholar]
- 53.van der Heiden PL, de Boer R, van der Steen DM, Kester MG, van der Hoorn MW, Haarman WM, Barnby-Porritt HE, Fry JW, Napper CE, Marijt EW, Willemze R, Falkenburg JH, Heemskerk MH. Identification of varicella-zoster virus-specific CD8 T cells in patients after T-cell-depleted allogeneic stem cell transplantation. Journal of virology. 2009;83:7361–7364. doi: 10.1128/JVI.02662-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laing KJ, Russell RM, Dong L, Schmid DS, Stern M, Magaret A, Haas JG, Johnston C, Wald A, Koelle DM. Zoster Vaccination Increases the Breadth of CD4+ T Cells Responsive to Varicella Zoster Virus. J Infect Dis. 2015 doi: 10.1093/infdis/jiv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.