Abstract
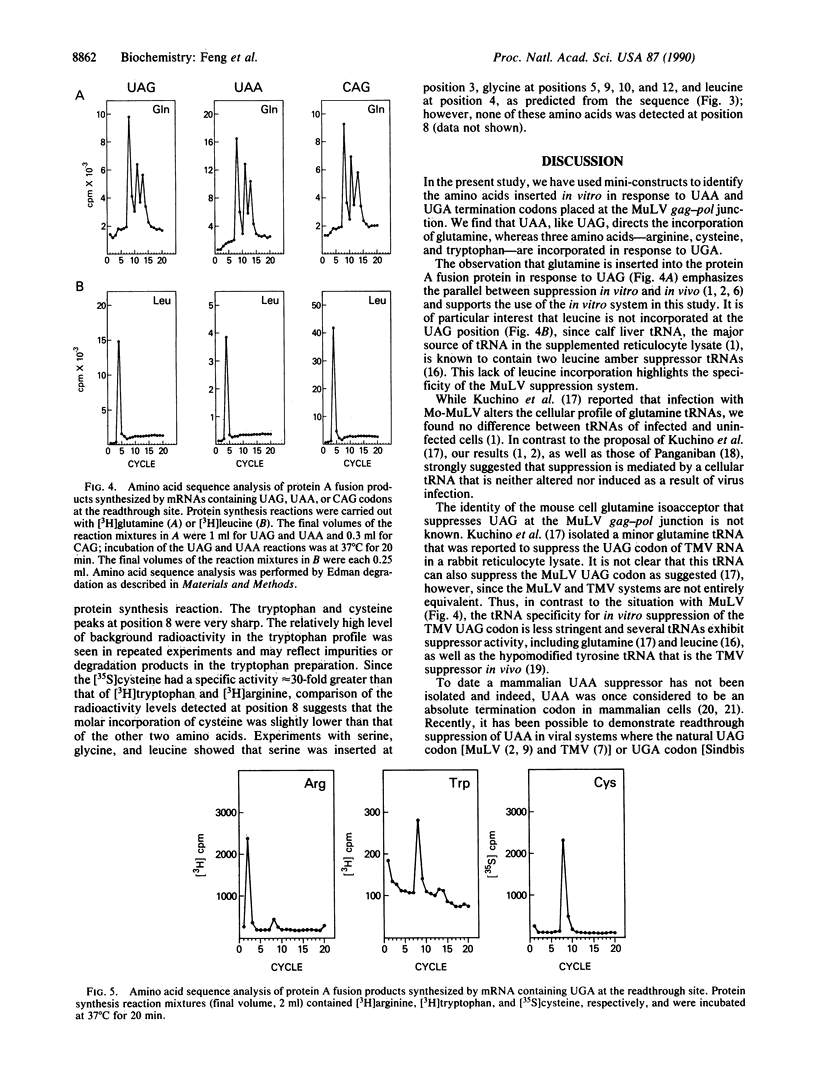
Expression of the murine leukemia virus pol gene occurs by translational readthrough of an in-frame UAG codon between the gag and pol coding regions. In a previous study, we mutated the UAG codon to UAA or UGA and demonstrated that both of these termination codons could be suppressed in reticulocyte lysates and in infected cells with the same efficiency as UAG. We now report the identity of the amino acids inserted in vitro in response to UAA and UGA in fusion products containing the gag-pol junction region. The results show that UAA, like UAG, directs the incorporation of glutamine, whereas UGA directs the incorporation of three amino acids, arginine, cysteine, and tryptophan. To our knowledge, this is the first report indicating misreading of UAA as glutamine and UGA as arginine and cysteine in higher eukaryotes. Interestingly, although our protein synthesis system presumably contains other known UAG and UGA suppressors, these tRNAs did not suppress the termination codons in our experiments. Thus, it seems possible that the sequence surrounding the gag-pol junction not only promotes suppression but also helps determine which tRNAs function in suppression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckingham R. H., Kurland C. G. Codon specificity of UGA suppressor tRNATrp from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5496–5498. doi: 10.1073/pnas.74.12.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Beaudet A., Nirenberg M. RNA codons and protein synthesis. 15. Dissimilar responses of mammalian and bacterial transfer RNA fractions to messenger RNA codons. J Mol Biol. 1968 Oct 14;37(1):99–118. doi: 10.1016/0022-2836(68)90076-4. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H. UGA suppression by normal tRNA Trp in Escherichia coli: codon context effects. Nucleic Acids Res. 1981 Feb 25;9(4):983–991. doi: 10.1093/nar/9.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. X., Hatfield D. L., Rein A., Levin J. G. Translational readthrough of the murine leukemia virus gag gene amber codon does not require virus-induced alteration of tRNA. J Virol. 1989 May;63(5):2405–2410. doi: 10.1128/jvi.63.5.2405-2410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. X., Levin J. G., Hatfield D. L., Schaefer T. S., Gorelick R. J., Rein A. Suppression of UAA and UGA termination codons in mutant murine leukemia viruses. J Virol. 1989 Jun;63(6):2870–2873. doi: 10.1128/jvi.63.6.2870-2873.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D. Recognition of nonsense codons in mammalian cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3014–3018. doi: 10.1073/pnas.69.10.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Motoyoshi F., Takamatsu N., Okada Y. In vitro mutagenesis of the putative replicase genes of tobacco mosaic virus. Nucleic Acids Res. 1986 Nov 11;14(21):8291–8305. doi: 10.1093/nar/14.21.8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Power M. D., Masiarz F. R., Luciw P. A., Barr P. J., Varmus H. E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988 Jan 21;331(6153):280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- Jones D. S., Nemoto F., Kuchino Y., Masuda M., Yoshikura H., Nishimura S. The effect of specific mutations at and around the gag-pol gene junction of Moloney murine leukaemia virus. Nucleic Acids Res. 1989 Aug 11;17(15):5933–5945. doi: 10.1093/nar/17.15.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Beier H., Akita N., Nishimura S. Natural UAG suppressor glutamine tRNA is elevated in mouse cells infected with Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1987 May;84(9):2668–2672. doi: 10.1073/pnas.84.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Li G. P., Rice C. M. Mutagenesis of the in-frame opal termination codon preceding nsP4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J Virol. 1989 Mar;63(3):1326–1337. doi: 10.1128/jvi.63.3.1326-1337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. P., Aker M., Sitney K. C., Mortimer R. K. First position wobble in codon-anticodon pairing: amber suppression by a yeast glutamine tRNA. Gene. 1986;49(3):383–388. doi: 10.1016/0378-1119(86)90375-6. [DOI] [PubMed] [Google Scholar]
- Marshall R. E., Caskey C. T., Nirenberg M. Fine structure of RNA codewords recognized by bacterial, amphibian, and mammalian transfer RNA. Science. 1967 Feb 17;155(3764):820–826. doi: 10.1126/science.155.3764.820. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Wills N., Arlinghaus R. B. Suppression of murine retrovirus polypeptide termination: effect of amber suppressor tRNA on the cell-free translation of Rauscher murine leukemia virus, Moloney murine leukemia virus, and Moloney murine sarcoma virus 124 RNA. J Virol. 1980 May;34(2):464–473. doi: 10.1128/jvi.34.2.464-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Panganiban A. T. Retroviral gag gene amber codon suppression is caused by an intrinsic cis-acting component of the viral mRNA. J Virol. 1988 Oct;62(10):3574–3580. doi: 10.1128/jvi.62.10.3574-3580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Pure G. A., Robinson G. W., Naumovski L., Friedberg E. C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J Mol Biol. 1985 May 5;183(1):31–42. doi: 10.1016/0022-2836(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Raftery L. A., Egan J. B., Cline S. W., Yarus M. Defined set of cloned termination suppressors: in vivo activity of isogenetic UAG, UAA, and UGA suppressor tRNAs. J Bacteriol. 1984 Jun;158(3):849–859. doi: 10.1128/jb.158.3.849-859.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Stokes P. E., Smythers G. W., Dhar R., Oroszlan S. Characterization of the phosphorylation sites and the surrounding amino acid sequences of the p21 transforming proteins coded for by the Harvey and Kirsten strains of murine sarcoma viruses. J Biol Chem. 1982 Oct 10;257(19):11767–11773. [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Uhlén M., Nilsson B., Guss B., Lindberg M., Gatenbeck S., Philipson L. Gene fusion vectors based on the gene for staphylococcal protein A. Gene. 1983 Sep;23(3):369–378. doi: 10.1016/0378-1119(83)90025-2. [DOI] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D., Haenni A. L. Novel amber suppressor tRNAs of mammalian origin. EMBO J. 1987 Oct;6(10):3049–3055. doi: 10.1002/j.1460-2075.1987.tb02611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Morch M. D. Stop making sense: or Regulation at the level of termination in eukaryotic protein synthesis. FEBS Lett. 1988 Aug 1;235(1-2):1–15. doi: 10.1016/0014-5793(88)81225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss W. A., Friedberg E. C. Normal yeast tRNA(CAGGln) can suppress amber codons and is encoded by an essential gene. J Mol Biol. 1986 Dec 20;192(4):725–735. doi: 10.1016/0022-2836(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]