Abstract
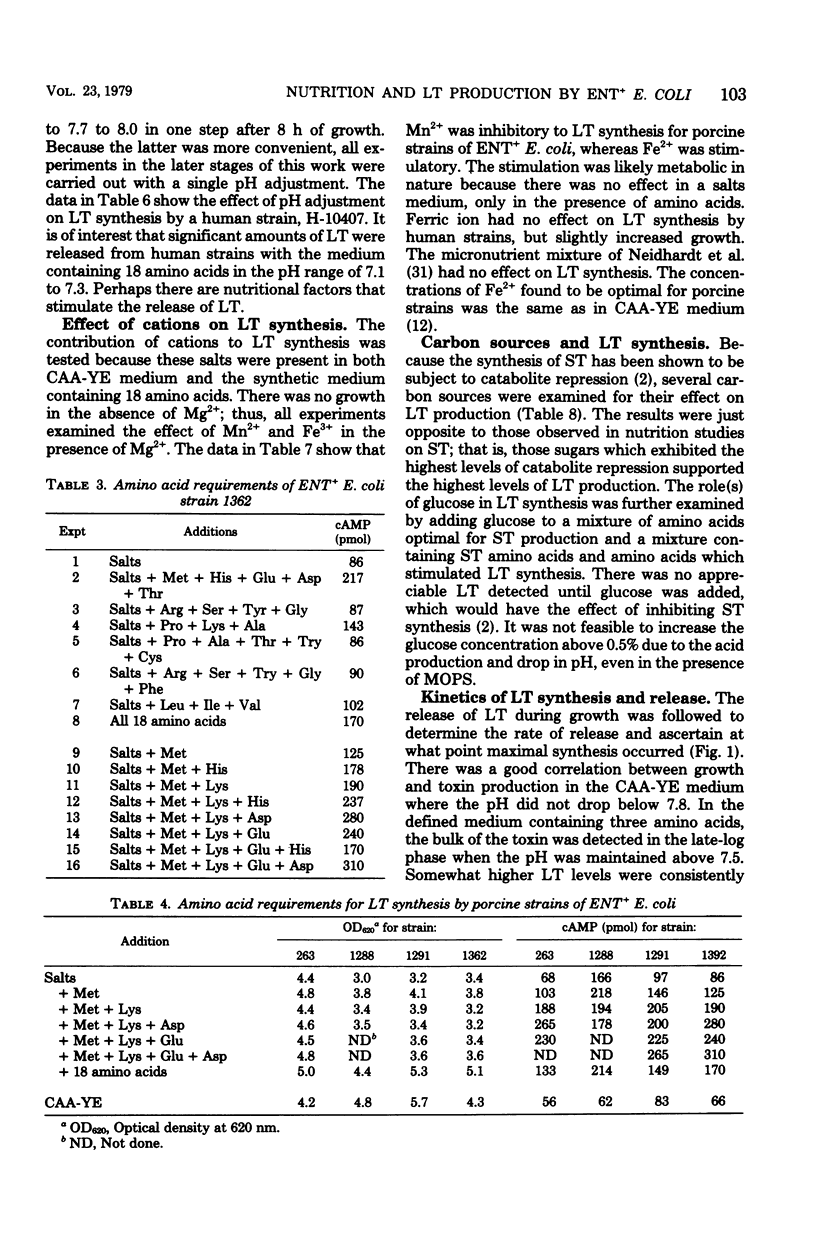
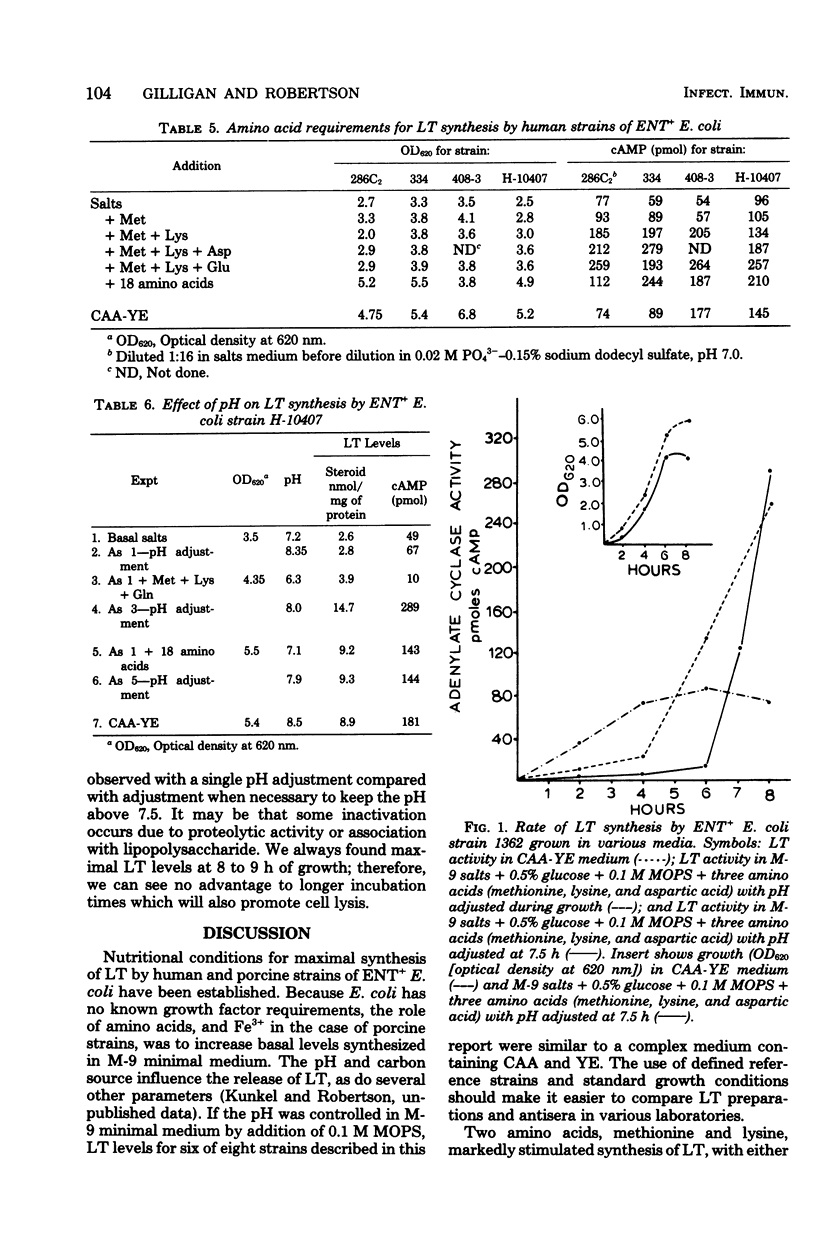
Optimal growth conditions have been established for production of heat-labile enterotoxin (LT) by both porcine and human strains of enterotoxigenic (ENT+) Escherichia coli. There were no unusual growth factor requirements, and some strains produced fairly high levels of LT in a basal salts medium containing 0.5% glucose if the pH was carefully controlled. Several amino acids markedly stimulated LT synthesis when added to the basal salts-glucose medium. Methionine and lysine were the most stimulatory for both human and porcine strains. Either aspartic acid or glutamic acid further enhanced LT synthesis in the presence of methionine and lysine, with aspartic acid being more stimulatory for porcine strains and glutamic acid more stimulatory for human strains. There were no apparent vitamin requirements and no unusual cations needed for toxin synthesis except that Fe3+ was slightly stimulatory for porcine strains. The stimulation by Fe3+ was observed only in the presence of the three amino acids, suggesting that the effect was indirect rather than on toxin synthesis. The carbon source also influenced the yield of LT. Glucose supported maximal synthesis, but other carbon sources which exhibit a high degree of catabolite repression also supported high levels of synthesis. Little or no LT was released below pH 7.0; therefore, because the pH drops during growth from 7.5 to 6.8, even in highly buffered media, it was necessary to adjust the pH to 8.0 to effect complete release of cell-associated toxin. The defined medium containing three amino acids reduced the amount of UV-absorbing material in culture supernatants about fivefold and increased LT activity for various strains from two- to fivefold over a complex Casamino Acids-yeast extract medium. Conditions found to be optimal for synthesis of LT were inhibitory for the heat-stable enterotoxin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Nutrition and enterotoxin synthesis by enterotoxigenic strains of Escherichia coli: defined medium for production of heat-stable enterotoxin. Infect Immun. 1977 Mar;15(3):781–788. doi: 10.1128/iai.15.3.781-788.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Robertson D. C. Repression of heat-stable enterotoxin synthesis in enterotoxigenic Escherichia coli. Infect Immun. 1977 Sep;17(3):629–633. doi: 10.1128/iai.17.3.629-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom C. O., Kon C. An improved protein binding assay for cyclic AMP. Anal Biochem. 1974 Apr;58(2):459–468. doi: 10.1016/0003-2697(74)90214-0. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Richardson S. H. Biochemistry of Vibrio cholerae virulence. 3. Nutritional requirements for toxin production and the effects of pH on toxin elaboration in chemically defined media. Infect Immun. 1973 Apr;7(4):567–572. doi: 10.1128/iai.7.4.567-572.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd, Ryder R. C., Richardson S. H. Biochemistry of vibrio cholerae virulence. II. Skin permeability factor-cholera enterotoxin production in a chemically defined medium. Infect Immun. 1971 Nov;4(5):611–618. doi: 10.1128/iai.4.5.611-618.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Donta S. T. Interactions of choleragenoid and GM1 ganglioside with enterotoxins of Vibrio cholerae and Escherichia coli in cultured adrenal cells. J Infect Dis. 1976 Mar;133 (Suppl):115–119. doi: 10.1093/infdis/133.supplement_1.s115. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Smith D. M. Stimulation of steroidogenesis in tissue culture by enterotoxigenic Escherichia coli and its neutralization by specific antiserum. Infect Immun. 1974 Mar;9(3):500–505. doi: 10.1128/iai.9.3.500-505.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J. Comparison of the tissue receptors for Vibrio cholerae and Escherichia coli enterotoxins by means of gangliosides and natural cholera toxoid. Infect Immun. 1973 Dec;8(6):851–859. doi: 10.1128/iai.8.6.851-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J., Braemer A. C., Bidlack D. E. Properties of the enterotoxic component in Escherichia coli enteropathogenic for swine. Infect Immun. 1973 Feb;7(2):178–189. doi: 10.1128/iai.7.2.178-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor H. S., Tao P., Gorbach S. L. Stimulation of intestinal adenyl cyclase by Escherichia coli enterotoxin: comparison of strains from an infant and an adult with diarrhea. J Infect Dis. 1974 Jan;129(1):1–9. doi: 10.1093/infdis/129.1.1. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J., Fiedler R. P. Adrenal cells in tissue culture. II. Steroidogenic responses to nucleosides and nucleotides. Endocrinology. 1969 May;84(5):1113–1117. doi: 10.1210/endo-84-5-1113. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampen J. O. Release of penicillinase by Bacillus licheniformis. J Gen Microbiol. 1967 Aug;48(2):261–268. doi: 10.1099/00221287-48-2-261. [DOI] [PubMed] [Google Scholar]
- Lariviére S., Gyles C. L., Barnum D. A. Preliminary characterization of the heat-labile enterotoxin of Escherichia coli F11(P155). J Infect Dis. 1973 Sep;128(3):312–320. doi: 10.1093/infdis/128.3.312. [DOI] [PubMed] [Google Scholar]
- Lust W. D., Dye E., Deaton A. V., Passonneau J. V. A modified cyclic AMP binding assay. Anal Biochem. 1976 May 7;72:8–15. doi: 10.1016/0003-2697(76)90500-5. [DOI] [PubMed] [Google Scholar]
- MacAlister T. J., Irvin R. T., Costerton J. W. Cell envelope protection of alkaline phosphatase against acid denaturation in Escherichia coli. J Bacteriol. 1977 Apr;130(1):339–346. doi: 10.1128/jb.130.1.339-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell D. H., Anselmo C. R., Wishnow R. M. Factors influencing heat-labile Escherichia coli enterotoxin activity. Infect Immun. 1976 Aug;14(2):383–388. doi: 10.1128/iai.14.2.383-388.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllby R., Hjalmarsson S. G., Wadström T. Separation of E. coli heat-labile enterotoxin by preparative isotachophoresis. FEBS Lett. 1975 Aug 1;56(1):30–33. doi: 10.1016/0014-5793(75)80104-9. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S. H. Factors influencing in vitro skin permeability factor production by Vibrio cholerae. J Bacteriol. 1969 Oct;100(1):27–34. doi: 10.1128/jb.100.1.27-34.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein I., Green R. F., Santos D. S., Maas W. K. Partial purification and characterization of a heat-labile enterotoxin of Escherichia coli. Infect Immun. 1976 Jun;13(6):1710–1720. doi: 10.1128/iai.13.6.1710-1720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J. I., Stavric S., Konowalchuk J. Assay of Escherichia coli heat-labile enterotoxin with vero cells. Infect Immun. 1977 May;16(2):617–622. doi: 10.1128/iai.16.2.617-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


