Abstract
Natural antibodies (NAbs) play an important role in early host defense, autophagy and tissue remodeling, and in immune regulation. They arise spontaneously (without specific immunization), and are already present at birth. NAbs are produced by B1 B cells, MZ B cells and other B cell types. They include all major Ig subclasses but IgM antibodies are prevalent, especially early in development. NAbs may be poly-specific, recognize particular auto-antigens, or detect neo-determinants such as those exposed during apoptosis or generated by oxidation. NAbs do not require cognate T cell help but depend on soluble mediators produced by T cells. Our recent studies suggest that γδ T cells may have a special relationship with NAbs, and play a prominent role in their regulation, in part through the fine-tuning of IL-4-levels. The spontaneously activated state of these cells likely enables their cytokine production and other functions in the absence of external stimulation. Ontogenetically, the earlier arising γδ T cells are better positioned than αβ T cells to shape the developing repertoire of NAbs. Intriguingly, ligand specificities of NAbs and γδ T cell receptors appear to be overlapping, perhaps allowing γδ cognate help for certain NAb specificities. Via NAbs, γδ T cells could exert a regulatory influence on numerous processes in health and disease.
Introduction
Prior to any immunization, circulating antibodies already exist in normal healthy humans and mice (Avrameas, 1991; Lutz et al., 2008). They are present at birth, and in mice have been shown to arise under germ-free conditions. These natural antibodies (NAbs) include all Ig subclasses. Natural IgM is prevalent particularly early in ontogeny and has been studied most extensively. B1 B cells appear to be the primary source of NAbs in mice (Savage and Baumgarth, 2015), but other B cell-types, most notably marginal zone B cells (Durand et al., 2009), contribute as well (Avrameas and Selmi, 2013; Avrameas, 2016). Although sometimes referred to as “non-specific”, NAbs in fact may be mono- or poly-specific, and recognize auto-antigens, neo-antigens and certain foreign antigens. Importantly, recent studies revealed that NAbs play a critical role in the early host defense against pathogens, protection against malignancy, tissue homeostasis and immune regulation. Available information about NAbs and treatment opportunities with intravenous immunoglobulin (IVIG) has been expertly reviewed by others (Ehrenstein and Notley, 2010; Gronwall and Silvermann, 2014; Madi et al., 2012; McCoy et al., 2006; Panda and Ding, 2015; Rahyab et al., 2011; Schwartz-Albiez et al., 2009; Vas et al., 2013). It is only summarized here, followed by a discussion of new data suggesting that γδ T cells become involved in regulating NAbs and their functional activity.
Specificity and function of natural antibodies
The primary B cell repertoire is not random (Perlmutter et al., 1985). Evidence for recurrent VH gene rearrangements in mice, humans and other vertebrates, at a time when the repertoire is not yet affected by antigenic selection, has been reported (reviewed in (Vas et al., 2013)). Use of microarray chips allowing the simultaneous detection of antibodies specific for up to 300 defined self antigens revealed that IgM repertoires in cord blood were very similar between individuals indicating that different humans are born with the same autoantibodies produced in utero regardless of variances in IgG autoantibodies found in their mothers (Madi et al., 2009). The mechanisms responsible for this consistency are not yet fully understood.
The early IgM antibodies in mice and humans are mostly germline-encoded and are produced by CD5+ B1a B cells (Savage and Baumgarth, 2015). They carry specificities for common bacterial Ags, auto-antigens, certain phospholipids, DNA and several cell membrane proteins. Table 1 lists some of the specificities of these NAbs (Air, 2015; Basnet et al., 2010; Bohn et al., 1994; Bovin, 2013; Buneva et al., 2013; Chen et al., 2009; Chikazawa et al., 2013; Chou et al., 2009; Durrbach et al., 2007; Fukuda et al., 2004; Hamanova et al., 2014; Hardy and Hayakawa, 2005; Kalyanaraman et al., 1982; Kulik et al., 2009; Lebon et al., 2011; Li et al., 2009; Llorente et al., 1999; Morales-Buenrostro et al., 2008; Posner et al., 1981; Robert-Guroff et al., 1982; Sauerborn et al., 2011; Shilova et al., 2011; Silvermann, 2015; Skurnik et al., 2012; Toth et al., 1984; Tsiantoulas et al., 2013; Tuominen et al., 2006; Turunen et al., 2012; Wang et al., 2013; Xu et al., 2013). Other important targets of NAbs include the Thomsen-Friedenreich tumor antigen (CD176) (Ulsemer et al., 2013), neural gangliosides relevant in Guillain-Barre syndrome (Boffey et al., 2004) and amyloid in Alzheimer’s disease (Kayed et al., 2011). Intriguingly, many of the autoreactive specificities are similarly found throughout evolution (Flajnik and Rumfelt, 2000; Gonzalez et al., 1988; Marchalonis et al., 1993). The non-random nature of the natural repertoire suggests that these early antibodies might be programmed to enable normal development, ensure essential functions and protect against common pathogens. Indeed, natural IgM has been characterized as protective in infections with Influenza virus, Pseudomonas aeruginosa and Streptococcus pneumoniae (reviewed in (Ehrenstein and Notley, 2010)). Natural IgM also affects B cell development. Mice genetically deficient in surface (s) IgM exhibit changes in B cell subsets including B1a B cells and MZ B cells along with increased splenic and impaired peritoneal B cell survival (Baker and Ehrenstein, 2002; Notley et al., 2010). Deficiency in sIgM has also been linked to increases in autoimmunity and atherosclerosis in both humans and mouse models. Self-reactive IgE exacerbates interferon responses in autoimmunity (Henault et al., 2016). Figure 1 lists some of the known functions of NAbs (Benatuil et al., 2005; Chen et al., 2009; Elluru et al., 2014; Matter and Ochsenbein, 2008; Panda et al., 2013; Pires et al., 2010; Rapaka et al., 2010; Stager et al., 2003; Veljkovic et al., 2010; Xu et al., 2008; Zabel et al., 2013), (McCoy et al., 2006), (Britschgi et al., 2009; Dimayuga et al., 2009; Elvington et al., 2012; Frostegard, 2010; Galkina and Ley, 2009; Gounopoulos et al., 2007; Gronwall et al., 2012; Shimomura et al., 2008; Sjoberg et al., 2009; Warrington and Rodrigues, 2010), (Aksentijevich et al., 1991; Lobo et al., 2014; Nogueira-Martins and Mariano, 2010; Warrington and Lewis, 2011).
Table 1. Broad Target Range of Natural Antibodies.
Reference numbers in the table refer to list of references following the main body of the text. Marked in red: NAbs with pathogenic effect.
| Category | Antigens recognized | species | References |
|---|---|---|---|
| Pathogen-derived | Poly-N-acetyl glucosamine (Staph. aureus capsule) | human | Skurnik et al. 2012 |
| Porphyromonas gingivalis gingipain | Turunen et al. 2012 | ||
| Pneumococcal virulence proteins | Lebon et al. 2011 | ||
| Adenovirus type 5 | mouse | Xu et al. 2013 | |
| HIV | human | Llorente et al. 1999 | |
| HTLV, simian type C virus p24 structural core protein p19 |
Toth et al. 1984 Kalyanaraman et al. 1982 Robert-Guroff et al. 1982 Posner et al. 1981 |
||
| Influenza | Air 2015 | ||
| Measles virus | Durrbach et al. 2007 | ||
| autologous | Apoptotic cells including thymocytes and senescent eythrocytes and their antigens phosphorylcholine |
mouse, human |
Chou et al. 2009 Silverman et al. 2015 Chikazawa et al. 2013 Hardy et al. 2005 Chen et al. 2009 |
| Tumor cells, activated T cells | human | Bohn et al. 1994 | |
| Proliferation-related self peptides | Fukuda et al. 2004 | ||
| Annexin IV | mouse, human | Kulik et al. 2009 | |
| Nucleic acids | human |
Buneva et al. 2013 Henault et al. 2016 |
|
| Glycans, sialoglycans | chicken, human |
Shilova et al. 2011 Bovin et al. 2013 |
|
| Bone morphogenic protein | human | Sauerborn et al. 2011 | |
| IFN-β, IFN-γ | Sauerborn et al. 2011 | ||
| α(1,3)-galactosyl | Hamanova et al. 2014 | ||
| CD40 | Elluru et al. 2014 | ||
| allogeneic | HLA | Morales-Buenrostro et al. 2008 | |
| xenogenic | αGal | Li et al. 2009 | |
| N-glycolylneuraminic acid | mouse | Basnet et al. 2010 | |
| Oxidation-induced | Apoptotic cells | mouse, human | Chou et al. 2009 |
| Oxidized lipids, cardiolipin | mouse |
Tsiantoulas et al. 2013 Tuominen et al. 2006 |
|
| Malondialdehyde acetaldehyde adducts | human | Wang et al. 2013 |
Figure 1. Diverse functions of natural antibodies.
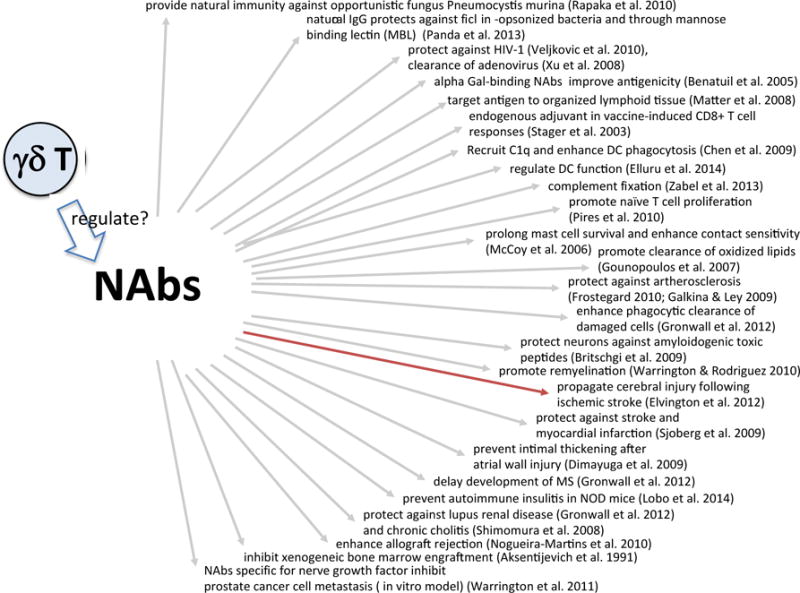
Marked in red: NAbs with pathogenic effect.
Influence of T cells and cytokines
B cells are quite capable of producing antibodies in the absence of any T cell help, including isotypes that require class switch recombination. However, in our mouse facility, mice lacking all T cells (B6.TCR-β−/−/δ −/−) had serum total Ig levels that reached only about ¼ of those in wt mice. Levels of IgM and surprisingly, IgE antibodies were nearly normal, whereas IgA and IgG antibodies were significantly reduced, especially IgG1 (Huang et al., 2015). Cytokines produced by non-B cells can determine development and steady state levels of NAbs. For example, IL-18 can boost production of protective natural IgM (reviewed in (Ehrenstein and Notley, 2010)). Natural IgE is also produced in the absence of MHCII cognate help but its production depends on IL-4 (McCoy et al., 2006). Their cytokine dependence subjects NAbs to the non-specific regulatory influence of other cell types.
A connection between γδ T cells and IgE production in non-immunized mice
Lymphocytes expressing γδ TCRs (γδ T cells) represent a lineage whose biological role remains poorly defined. Functionally, they resemble αβ T cells (Bonneville et al., 2010) but in terms of specificity and mechanism of TCR-ligand-recognition, they share properties with B cells (Chien et al., 1996; Zeng et al., 2012). In several studies with mice carrying distinct mutations affecting TCR-signaling, increased non-immune IgE levels could be correlated with increases in γδ T cells (Nunez-Cruz et al., 2003). In particular, this was observed in mice deficient in the Tec family tyrosine kinase Itk, which are unable to mount conventional IL-4- or IL-4 plus IL-13 producing T helper 2 (Th2) responses (Felices et al., 2009; Qi et al., 2009). Nevertheless, such mice had elevated levels of serum IgE and increased numbers of germinal center B cells, a phenotype found to be dependent on the presence of γδ T cells. γδ T cells in Itk−/− mice produced more Th2 cytokines and expressed at elevated levels co-stimulatory molecules important for B cell help, which suggested that they directly promote B cell activation and Ig class switching. In the same year, data generated in our lab with TCR-signaling competent mice revealed that IgE levels (both pre- and post-immunization) are determined by the composition of γδ T cells. Although mice deficient in all γδ T cells had slightly reduced levels of serum IgE, others lacking only Vγ4+ and Vγ6+ subsets had vastly increased background IgE levels as well as exacerbated IgE responses following immunization (Huang et al., 2009). In these mice, Vγ1+ and especially the NKT-like IL-4-producing Vγ1/Vδ6.3+ γδ T cells (Gerber et al., 1999) were much increased, and so were serum levels of IL-4 (Huang et al., 2015). In fact, the genetically γδ-deficient mice (B6.TCR-Vγ4/6−/−) closely resembled Itk−/− mice. Together, these observations implied that the composition of γδ T cells can play a critical role in setting steady state levels of IgE antibodies in non-immunized mice.
Effect of γδ T cells on serum Ig levels in non-immunized mice
After these findings, we extended the analysis to other Ig classes (Huang et al., 2015). Our data suggest that the net effect of γδ T cells on total serum Ig in normal non-immunized C57BL/6 mice is slightly enhancing as adult animals genetically deficient in all γδ T cells (B6.TCR-δ −/−) had approximately 2-fold lower Ig levels. Net total Ig levels in these mice are lower mainly due to significantly reduced levels of IgG1 and IgG2b. Further insight came from the analysis of partially γδ T cell-deficient mouse strains. Thus, mice lacking Vγ1+ γδ T cells exhibit total Ig levels that are even further diminished than those in B6.TCR-δ−/− mice, and show much reduced levels of IgM, IgG3, IgG1 and IgG2b. IgG2c and IgA were diminished also but to a lesser degree. Hence, Vγ1+ γδ T cells appear to enhance the production of several Ig subclasses in non-immunized mice. On the other hand, mice deficient in Vγ4+ and Vγ6+ γδ T cells exhibit much increased total Ig levels, mainly due to increases in IgM, IgG1, IgG2b and IgG2c. IgE antibodies are even more elevated but, due to their low concentration, contribute only little to overall increases in Ig levels. IgG3 and IgA are not affected. Hence, Vγ4+ and perhaps Vγ6+ γδ T cells, two subsets in mice distinguished by their ability to produce IL-17 (O’Brien et al., 2009), appear to exert an inhibitory effect on most Ig production under steady state conditions. Our data suggest that this inhibition in non-immunized mice is mediated in large part through regulating Vγ1+ γδ T cells, their IL-4 production, and IL-4-dependent IL-4 production by αβ T cells (Huang et al., 2015). Consistently, for some Ig subclasses, most obviously IgM, but also IgG2b and IgG2c, this regulation is still evident even in the absence of αβ T cells. Finally, it has been shown that Vγ1+ γδ T cells kill tissue macrophages and thus resolve inflammatory immune responses (Carding and Egan, 2000; Dalton et al., 2003). As macrophages can be a source of IL-18, this cytotoxic activity might enable indirect control of IL-18-dependent antibody-types, at least in inflammation.
Comparing serum Ig levels in non-immunized adult wt C57BL/6 mice with mice lacking all γδ T cells (B6.TCR-δ −/−) or all αβ T cells (B6.TCR-β−/−) initially showed that the absence of αβ T cells affects levels of total serum Ig and most Ig subclasses more than does the absence of γδ T cells. IgE antibodies in non-immunized mice were a notable exception: They were diminished in the absence of all γδ T cells but not in the absence of all αβ T cells (Huang et al., 2015). However, mice lacking only subsets of γδ T cells revealed far greater changes in Ig levels, suggestive of potent enhancing and suppressive regulation by these cells (Huang et al., 2015). This powerful regulation is all the more noteworthy considering that γδ T cells in circulation and in the lymphoid tissues represent a much smaller cell population than αβ T cells.
γδ T cells affect the repertoire of antibodies in non-immunized mice
The observation that changes in γδ T cells differentially affect levels of Ig subclasses in non-immunized mice suggests an influence on the repertoire and specificities of NAbs. To explore this possibility, we examined antibodies specific for chromatin, DNA and nuclear antigens in non-immunized mice (Huang et al., 2015). We found that the same change in γδ T cells that increased all Ig subclasses except IgG3 and IgA - a genetic deficiency in Vγ4+ and Vγ6+ γδ T cells, also led to substantial increases in anti chromatin antibodies. However, the anti chromatin antibodies were still further increased relative to the elevated total Ig levels, indicating a change in the composition of the elevated antibodies, and in their specificity. We also found a clear increase in antibodies detecting dsDNA/histone complexes and smaller increases in antibodies specific for ssDNA, and anti-nuclear autoantibodies. Finally, cell transfer and depletion experiments showed the change in antibody composition/specificity to be driven by the dys-regulated Vγ1+ γδ T cells derived from non-immunized B6.TCR-Vγ4/6−/− mice. Hence, γδ T cells affect the antibody repertoire in non-immunized mice (Huang et al., 2015).
Do γδ T cells have a special connection with natural antibodies?
The above-mentioned observations clearly implicate γδ T cells in the regulation of antibodies independently of immunization, and thus establish their involvement with NAbs. However, it remains to be determined if γδ T cells have a unique functional connection with NAbs. Do they play a role distinct from that of other T cells, in the development and maintenance of these antibodies? Such a role might even help to explain the evolutionary conservation of γδ T cells. We suggest that such a special connection is probable, for the following reasons: Firstly, unlike the majority of unstimulated αβ T cells, which are antigenically naïve and metabolically inactive, many peripheral γδ T cells in non-immunized mice already exist in a state of moderate activation (Tough and Sprent, 1998). This activated/memory phenotype could support background levels of cytokine production capable of sustaining some B cell differentiation and spontaneous antibody production. Secondly, in mouse ontogeny, γδ T cells develop roughly at the same time as the earliest B cells, and for a short period of time, the fetal liver gives rise to precursors of both cell-types (Havran and Allison, 1988; Whitlock et al., 1985). αβ T helper cells, on the other hand, arise several days later (Marrack et al., 1988). Moreover, γδ thymus-emigrants are functionally more mature than αβ thymus-emigrants (Jin et al., 2009; Narayan et al., 2012). In particular, γδ thymocytes are already sufficiently differentiated to produce polarized cytokines including IL-4 and IL-13 (Jin et al., 2009). Thus, γδ T cells are enabled to support and modulate B cell development and antibody production from the start, by supplying cytokines prior to any immunization. In addition, γδ T cells might interact with B cells via ligands expressed on B cells such as members of the CD1 family of molecules (Delia et al., 1988; Duan et al., 2008; Sonoda and Stein-Streilein, 2002), which are recognized by γδ T cells (Dieude et al., 2011; Porcelli et al., 1992; Russano et al., 2006). Lastly, one might consider the early idea that B1 B cells and γδ T cells occupy equivalent positions in a “layered immune system” (Herzenberg and Herzenberg, 1989). We actually suspect that there is resemblance or overlap between antigen specificities of NAbs and γδ T cells. Thus, both recognize poly-anionic ligands including certain synthetic polypeptides (Cady et al., 2000) but also DNA/protein (unpublished data) and phospholipid/protein complexes (Born et al., 2003; Dieude et al., 2011; Lafer et al., 1981), and recent studies suggest a shared specificity for insulin (Aydintug et al., 2014; Liu et al., 2002; Zhang et al., 2010). Thus, in addition to non-specific cytokine support, at least some γδ T cells conceivably could provide help to B cells via cognate interactions between γδ TCR, antigen and BCR. Hence, he extent to which antigen specificities of γδ TCRs and NAbs might be related in non-immunized mice would be revealing with regard to the biological role of γδ T cells.
Modulating NAbs and their contribution to immune responsiveness via γδ T cells?
Although B cells can spontaneously produce antibodies in the absence of all T cells, T cells affect antibody production when they are present. The assumption that γδ T cells modulate levels and repertoire of NAbs invites additional points: First, γδ T cells in humans undergo large changes in population size and composition during ontogeny (Parker et al., 1990), due to genetic variation, in the course of hematopoietic transplantation (Airoldi et al., 2015), and as a consequence of diseases (Bank and Marcu-Malina, 2013; Pauza et al., 2015). Thus, one might expect based on the findings in mice that such changes in human γδ T cells have an effect on human B cells and their natural production of immunoglobulins: Large variation of NAbs might occur as a consequence of the changes in human γδ T cells. Furthermore, similar to the mice, specific targeting of the relevant human γδ T cells, directly or through their regulators, might effectively change human NAbs, complement IVIG in certain cases, and at times might be preferable over targeting all B cells (e.g. using antibody drugs against CD19) or entire antibody classes (e.g. antibody drugs against IgE).
Acknowledgments
We would like to thank Drs. Lawrence Wysocki and Thiago Detanico for helpful discussions.
Abbreviations
- NAb
natural antibody
- TCR
T cell receptor
- IL-4
interleukin 4
References
- Air GM. Influenza virus antigenicity and broadly neutralizing epitopes. Curr Opin Virol. 2015;11:113–121. doi: 10.1016/j.coviro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi I, Bertaina A, Prigione I, et al. Gammadelta T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125:2349–2358. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksentijevich I, Sachs DH, Sykes M. Natural antibodies can inhibit bone marrow engraftment in the rat — mouse species combination. J Immunol. 1991;147:4140–4146. [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodies: from “horror autotoxicus” to “gnoti seauton”. Immunology Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Avrameas S, Selmi C. Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun. 2013;41:46–49. doi: 10.1016/j.jaut.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Avrameas SC. Autopolyreactivity confers a holistic role in the immune system. Scand J Immunol. 2016 doi: 10.1111/sji12414. [DOI] [PubMed] [Google Scholar]
- Aydintug MK, Zhang L, Wang C, et al. Gammadelta T cells recognize the insulin B:9-23 peptide antigen when it is dimerized through thiol oxidation. Molecular Immunology. 2014;60:116–128. doi: 10.1016/j.molimm.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N, Ehrenstein MR. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J Immunol. 2002;169:6686–6690. doi: 10.4049/jimmunol.169.12.6686. [DOI] [PubMed] [Google Scholar]
- Bank I, Marcu-Malina V. Quantitative peripheral blood perturbations of gammadelta T cells in human disease and their clinical implications. Clinic Rev Allerg Immunol. 2013 doi: 10.1007/s12016-013-8391-x. [DOI] [PubMed] [Google Scholar]
- Basnet NB, Ide K, Tahara H, et al. Deficiency of N-glycolylneuraminic acid and Galalpha1-3Galbeta1-4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural natibodies. Xenotransplantation. 2010;17:440–448. doi: 10.1111/j.1399-3089.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- Benatuil L, Kaye JA, Rich RF, et al. The influence of natural antibody specificity on antigen immunogenicity. European Journal of Immunology. 2005;35:2638–2647. doi: 10.1002/eji.200526146. [DOI] [PubMed] [Google Scholar]
- Boffey J, Nicholl D, Wagner ER, et al. Innate murine B cells produce anti-disialosyl antibodies reactive with Campylobacter jejuni LPS and gangliosides that are polyreactive and encoded by a restricted set of unmutated V genes. J Neuroimmunol. 2004;152:98–111. doi: 10.1016/j.jneuroim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Bohn J, Roggenbuck D, Settmacher U, et al. Binding of natural human IgM auto-antibodies to human tumor cell lines and stimulated normal T lymphocytes. Immunol Lett. 1994;39:187–194. doi: 10.1016/0165-2478(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nature Reviews Immunology. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- Born WK, Vollmer M, Reardon C, et al. Hybridomas expressing gammadelta T-cell receptors respond to cardiolipin and beta2-glycoprotein 1 (apolipoprotein H) Scand J Immunol. 2003;58:374–381. doi: 10.1046/j.1365-3083.2003.01315.x. [DOI] [PubMed] [Google Scholar]
- Bovin NV. Natural antibodies to glycans. Biochemistry (Mosc) 2013;78:786–797. doi: 10.1134/S0006297913070109. [DOI] [PubMed] [Google Scholar]
- Britschgi M, Olin CE, Johns HT, et al. Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12145–12150. doi: 10.1073/pnas.0904866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneva VN, Krasnorutskii MA, Nevinsky GA. Natural antibodies to nucleic acids. Biochemistry (Mosc) 2013;78:127–143. doi: 10.1134/S0006297913020028. [DOI] [PubMed] [Google Scholar]
- Cady CT, Lahn M, Vollmer M, et al. Response of murine γδ T cells to the synthetic polypeptide poly-Glu50Tyr50. J Immunology. 2000;165:1790–1798. doi: 10.4049/jimmunol.165.4.1790. [DOI] [PubMed] [Google Scholar]
- Carding SR, Egan PJ. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunological Reviews. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Park Y-B, Patel E, et al. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y-H, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- Chikazawa M, Otaki N, Shibata T, et al. An apoptosis-associated mammary protein deficiency leads to an enhanced production of IgM antibodies against multiple damage-associated molecules. PLoS One. 2013;8:e68468. doi: 10.61371/journal.pone.0068468. Print 0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JE, Pearson J, Scott P, et al. The interaction of gamma delta T cells with activated macrophages is a property of the Vg1 subset. J Immunol. 2003;171:6488–6494. doi: 10.4049/jimmunol.171.12.6488. [DOI] [PubMed] [Google Scholar]
- Delia D, Cattoretti G, Fontanella E, et al. CD1c but neither CD1a nor CD1b molecules are expressed on normal, activated, and malignant human B cells: idneification of a new B-cell subset. Blood. 1988;72:241–247. [PubMed] [Google Scholar]
- Dieude M, Striegl H, Tyznik AJ, et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted gammadelta T cells in the normal murine repertoire. J Immunol. 2011;186:4771–4781. doi: 10.4049/jimmunol.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimayuga PC, Cesena FH, Chyu KY, et al. Natural antibodies and complement modulate intimal thickening after arterial injury. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1593–15600. doi: 10.1152/ajpregu.00114.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B, Niu H, Xu Z, et al. Intrafollicular location of marginal zone/CD1d(hi) B cells is associated with autoimmune pathology in a mouse model of lupus. Lab Invest. 2008;88:1008–1020. doi: 10.1038/labinvest.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CA, Hartvigsen K, Fogelstrand L, et al. Phosphoinositide 3-kinase p110 delta regulates natural antibody production, marginal zone and B-1 B cell function, and autoantibody responses. J Immunol. 2009;183:5673–5684. doi: 10.4049/jimmunol.0900432. [DOI] [PubMed] [Google Scholar]
- Durrbach A, Baple E, Preece AF, et al. Virus recognition by specific natural antibodies and complement results in MHC I cross-presentation. European Journal of Immunology. 2007;37:1254–1265. doi: 10.1002/eji.200636129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nature Reviews Immunology Epub ahead of print. 2010 doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- Elluru SR, Kaveri SV, Bayry J. Regulation of human dendritic cell function by natural anti-CD40 antibodies. Methods Mol Biol. 2014;1155:47–54. doi: 10.1007/978-1-4939-0669-7_5. [DOI] [PubMed] [Google Scholar]
- Elvington A, Atkinson C, Kulik L, et al. Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J Immunol. 2012;188:1460–1468. doi: 10.4049/jimmunol.1102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felices M, Yin CC, Kosaka Y, et al. Tec kinase Itk in gammadelta T cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci (USA) 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Rumfelt LL. Early and natural antibodies in non-mammalian vertebrates. Current Topics in Microbiology and Immunology. 2000;252:233–240. doi: 10.1007/978-3-642-57284-5_24. [DOI] [PubMed] [Google Scholar]
- Frostegard J. Low level natural antibodies against phosphorylcholine: a novel risk marker and potential mechanism in artherosclerosis and cardiovascular disease. Clin Immunol. 2010;134:47–54. doi: 10.1016/j.clim.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Takao Y, Miyazaki Y, et al. New type of natural antibodies reactive to cytotoxic T lymphocyte-directed cancer vaccine peptides. Immunobiology. 2004;209:245–253. doi: 10.1016/j.imbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*) Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber DJ, Azuara V, Levraud J-P, et al. IL-4-producing γδ T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- Gonzalez R, Charlemagne J, Mahana W, et al. Specificity of natural serum antibodies present in phylogenetically distinct fish species. Immunology. 1988;63:31–36. [PMC free article] [PubMed] [Google Scholar]
- Gounopoulos P, Merki E, Hansen LF, et al. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–837. [PubMed] [Google Scholar]
- Gronwall C, Silvermann GJ. Natural IgM: Beneficial autoantibodies for the control of inflammatory and autoimmune disease. J Clin Immunol. 2014;34(Suppl 1):S12–S21. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall C, Vas J, Silverman GJ. Protective roles of natural IgM antibodies. Front Immunol. 2012 doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanova M, Zdrazilova Dubska L, Valik D, et al. Natural antibodies against alpha(1,3) galactosyl epitope in the serum of cancer patients (article in Czech) Epidemiol Mikrobiol Imunol. 2014;63:130–133. [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. Development of B cells producing natural antibodies to thymocytes and senescent erythrocytes. Springer Semin Immunopathol. 2005;26:363–375. doi: 10.1007/s00281-004-0183-1. [DOI] [PubMed] [Google Scholar]
- Havran W, Allison JP. Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature. 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- Henault J, Riggs JM, Karnell JL, et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nature Immunol. 2016;17:196–203. doi: 10.1038/ni.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Huang Y, Heiser RA, Detanico TO, et al. Gammadelta T cells affect IL-4 production and B-cell tolerance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E39–E48. doi: 10.1073/pnas.1415107111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Jin N, Roark CL, et al. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol. 2009;183:849–855. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Roark CL, Miyahara N, et al. Allergic airway hyperresponsiveness-enhancing gammadelta T cells develop in normal untreated mice and fail to produce IL-4/13, unlike Th2 and NKT cells. J Immunol. 2009;182:2002–2010. doi: 10.4049/jimmunol.0803280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman VS, Sarngadharan MG, Nakao Y, et al. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Jackson GR, Estes DM, et al. Alzheimer’s disease: review of emerging treatment role for intravenous immunoglobulins. J Cent Nerv Syst Dis. 2011;3:67–73. doi: 10.4137/JCNSD.S5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik L, Fleming SD, Moratz C, et al. Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J Immunol. 2009;182:5363–5373. doi: 10.4049/jimmunol.0803980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer EM, Rauch J, Andrzejewski C, Jr, et al. Polyspecific monoclonal lupus autoantibodies reactive with both polynucleotides and phospholipids. Journal of Experimental Medicine. 1981;153:897–909. doi: 10.1084/jem.153.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon A, Verkaik NJ, Labout JA, et al. Natural antibodies against several pneumococcal virulence proteins in children during the pre-pneumococcal-vaccine era: the generation R study. Infect Immun. 2011;79:1680–1687. doi: 10.1128/IAI.01379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009;21:70–74. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Liu E, Moriyama H, Abiru N, et al. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J Clin Invest. 2002;110:1021–1027. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente M, Sanchez-Palomino S, Manes S, et al. Natural human antibodies retrieved by phage display libraries from healthy donors: polyreactivity and recognition of human immunodeficiency virus 1 gp120 epitopes. Scand J Immunol. 1999;50:270–279. doi: 10.1046/j.1365-3083.1999.00516.x. [DOI] [PubMed] [Google Scholar]
- Lobo PI, Brayman KL, Okusa MD. Natural IgM anti-leukocyte autoantibodies (IgM-ALA) regulate inflammation induced by innate and adaptive immune mechanisms. J Clin Immunol. 2014;34(Suppl 1):S22–29. doi: 10.1007/s10875-014-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends in Immunol. 2008;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Madi A, Bransburg-Zabary S, Kenett DY, et al. The natural autoantibody repertoire in newborns and adults: a current overview. Adv Exp Med Biol. 2012;750:198–212. doi: 10.1007/978-1-4614-3461-0_15. [DOI] [PubMed] [Google Scholar]
- Madi A, Hecht I, Bransburg-Zabary S, et al. Organization of the autoantibody repertoire in healthy newborns and adults revealed by system level informatics of antigen microarray data. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14484–14489. doi: 10.1073/pnas.0901528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis JJ, Hohman VS, Thomas C, et al. Antibody production in sharks and humans: a role for natural antibodies. Dev Comp Immunol. 1993;17:41–53. doi: 10.1016/0145-305x(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kushnir E, Born W, et al. The development of helper T cell precursors in mouse thymus. J Immunol. 1988;140:2508–2514. [PubMed] [Google Scholar]
- Matter MS, Ochsenbein AF. Natural antibodies target virus-antibody complexes to organized lymphoid tissue. Autoimmun Rev. 2008;7:480–486. doi: 10.1016/j.autrev.2008.03.018. [DOI] [PubMed] [Google Scholar]
- McCoy KD, Harris NL, Diener P, et al. Natural IgE production in the absence of MHC class II cognate help. Immunity. 2006;24:329–339. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, et al. “Natural” human leukocyte antige antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- Narayan K, Sylvia KE, Malhotra N, et al. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nature Immunol published online April. 2012;1:2012. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Martins MF, Mariano M. B-1 cell participation in T-cell-mediated alloimmune response. Immunobiology. 2010;215:264–274. doi: 10.1016/j.imbio.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Notley CA, Baker N, Ehrenstein MR. Secreted IgM enhances B cell receptor signaling and promotes splenic but impairs peritoneal B cell survival. J Immunol. 2010;184:3386–3393. doi: 10.4049/jimmunol.0902640. [DOI] [PubMed] [Google Scholar]
- Nunez-Cruz S, Aguado E, Richelme S, et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nature Immunology. 2003;4:999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- O’Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. European Journal of Immunology. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. 2015;194:13–20. doi: 10.4049/jimmunol.1400844. [DOI] [PubMed] [Google Scholar]
- Panda S, Zhang J, Tan NS, et al. Natural IgG antibodies provide innate protection against ficolin-opsonized bacteria. EMBO Journal. 2013;32:2905–2919. doi: 10.1038/emboj.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza CD, Poonia B, Li H, et al. GammaDelta T cells in HIV disease: past, present and future. Frontiers in Immunology. 2015;5:1–11. doi: 10.3389/fimmu.2014.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter RM, Kearney JF, Chang SP, et al. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985;227:1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Pires AE, Afonso AF, Queiros A, et al. Treatment with polyclonal immunoglobulin during T-cell reconstitution promotes naive T-cell proliferation. J Immunother. 2010;33:618–625. doi: 10.1097/CJI.0b013e3181d3cb19. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4-8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–597. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- Posner LE, Robert-Guroff M, Kalyanaraman VS, et al. Natural antibodies to the human T cell lymphoma virus in patients with cutaneous T cell lymphomas. Journal of Experimental Medicine. 1981;154:333–346. doi: 10.1084/jem.154.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Xia M, Hu J, et al. Enhanced development of CD4+ gammadelta T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahyab AS, Alam A, Kapoor A, et al. Natural antibody - Biochemistry and functions. Glob J Biochem. 2011;2:283–288. [PMC free article] [PubMed] [Google Scholar]
- Rapaka RR, Ricks DM, Alcorn JF, et al. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. Journal of Experimental Medicine. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M, Fahey KA, Maeda M, et al. Identification of HTLV p19 specific natural human antibodies by competition with monoclonal antibody. Virology. 1982;122:297–305. doi: 10.1016/0042-6822(82)90229-x. [DOI] [PubMed] [Google Scholar]
- Russano AM, Agea E, Corazzi L, et al. Recognition of pollen-derived phosphatidyl-ethanolamine by human CD1d-restricted gamma delta T cells. J Allergy Clin Immunol. 2006;117:1178–1184. doi: 10.1016/j.jaci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Sauerborn M, van de Vosse E, Delawi D, et al. Natural antibodies against bone morphogenic proteins and interferons in healthy donors and in patients with infections linked to type-1 cytokine responses. J Interferon Cytokine Res. 2011;31:661–669. doi: 10.1089/jir.2010.0075. [DOI] [PubMed] [Google Scholar]
- Savage HP, Baumgarth N. Characteristics of natural antibody-secreting cells. Ann N Y Acad Sci. 2015;1362:132–142. doi: 10.1111/nyas.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Albiez R, Monteiro RC, Rodriguez M, et al. Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clin Exp Immunol. 2009;158(Suppl 1):43–50. doi: 10.1111/j.1365-2249.2009.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilova NV, Navakouski MJ, Huflejt M, et al. Changes in the repertoire of natural antibodies caused by immunization with bacterial antigens. Biochemistry (Mosc) 2011;76:862–866. doi: 10.1134/S0006297911070170. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Mizoguchi E, Sugimoto K, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- Silvermann GJ. Protective natural antibodies to apoptotic cells: evidence of convergent selection of recurrent innate-like clones. Ann N Y Acad Sci. 2015;1362:164–175. doi: 10.1111/nyas.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg BG, Su J, Dahlbom I, et al. Low levels of IgM antibodies against phosphorylcholine-A potential risk marker for ischemic stroke in men. Atherosclerosis. 2009;203:528–532. doi: 10.1016/j.atherosclerosis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Skurnik D, Kropec A, Roux D, et al. Natural antibodies in normal juman serum inhibit Stapylococcus aureus capsular polysaccharide vaccine efficacy. Clin Infect Dis. 2012;55:1188–1197. doi: 10.1093/cid/cis624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda KH, Stein-Streilein J. CD1d on antigen-transporting APC and splenic marginal zone B cells promotes NKT cell-dependent tolerance. European Journal of Immunology. 2002;32:848–857. doi: 10.1002/1521-4141(200203)32:3<848::AID-IMMU848>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Stager S, Alexander J, Kirby AC, et al. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nature Medicine. 2003;9:1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- Toth FD, Szabo B, Vaczi L, et al. Natural antibodies to simian type-C viruses and human retrovirus HTLV in patients with lymphoid malignancies. Acta Microbiol Hung. 1984;31:387–392. [PubMed] [Google Scholar]
- Tough DF, Sprent J. Lifespan of γ/δ T cells. Journal of Experimental Medicine. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiantoulas D, Gruber S, Binder CJ. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Front Immunol. 2013;3:415. doi: 10.3389/fimmu.2012.00415. eCollection 02012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen A, Miller YI, Hansen LF, et al. A natural antibody to oxidized cardiolipin binds to oxidixed loe-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- Turunen SP, Kummu O, Harila K, et al. Recognition of Porphyromonas gingivalis gingipain epitopes by natural IgM binding to malondialdehyde modified low-density lipoprotein. PLoS One. 2012;7:e34910. doi: 10.31371/journal.pone.0034919. Epub 0032012 Apr 0034915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulsemer P, Henderson G, Toutounian K, et al. Specific humoral immune response to the Thomsen-Friedenreich tumor antigen (CD176) in mice after vaccination with the commensal bacterium Bacteroides ovatus D-6. Cancer Immunol Immunother. 2013;62:875–887. doi: 10.1007/s00262-013-1394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vas J, Gronwall C, Silverman GJ. Fundamental roles of the innate-like repertoire of natural antibodies in immune homeostasis. Frontiers in Immunology. 2013 Feb 5;4(4):4. doi: 10.3389/fimmu.2013.00004. eCollection 02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljkovic M, Dopsaj V, Stringer WW, et al. Aerobic exercise training as a potential source of natural antibodies protective against human immunodeficiency virus-1. Scand J Med Sci Sports. 2010;20:469–474. doi: 10.1111/j.1600-0838.2009.00962.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Turunen SP, Kummu O, et al. Natural antibodies of newborns recognize oxidative stress-related malondialdehyde acetaldehyde adducts on apoptotic cells and atherosclerotic plaques. International Immunology. 2013;25:575–587. doi: 10.1093/intimm/dxt022. [DOI] [PubMed] [Google Scholar]
- Warrington AE, Rodrigues M. Method of identifying natural antibodies for remyelination. J Clin Immunol. 2010;30(Suppl 1):S50–S55. doi: 10.1007/s10875-010-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington RJ, Lewis KE. Natural antibodies against nerve growth factor inhibit in vitro prostate cancer cell metastasis. Cancer Immunol Immunother. 2011;60:187–195. doi: 10.1007/s00262-010-0934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C, Denis K, Robertson D, et al. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]
- Xu Z, Qiu Q, Tian J, et al. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nature Medicine. 2013;19:452–457. doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- Xu Z, Tian J, Smith JS, et al. Clearance of adenovirus by Kupffer cells is mediated by scanvenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel F, Kundig TM, Bachmann MF. Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol. 2013;3:367–362. doi: 10.1016/j.coviro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Zeng X, Wei YL, Huang J, et al. Gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jin N, Nakayama M, et al. Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9–23. J Autoimmun. 2010;34:478–484. doi: 10.1016/j.jaut.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


