Abstract
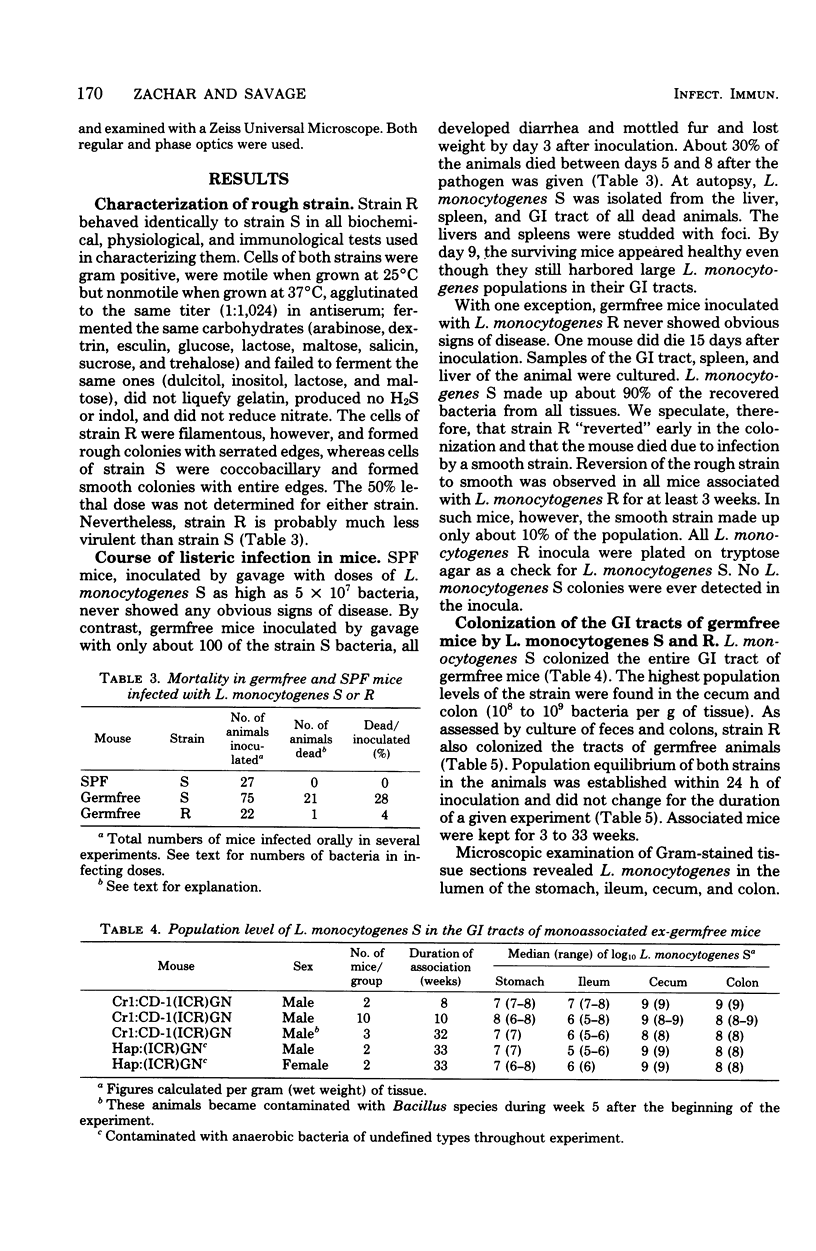
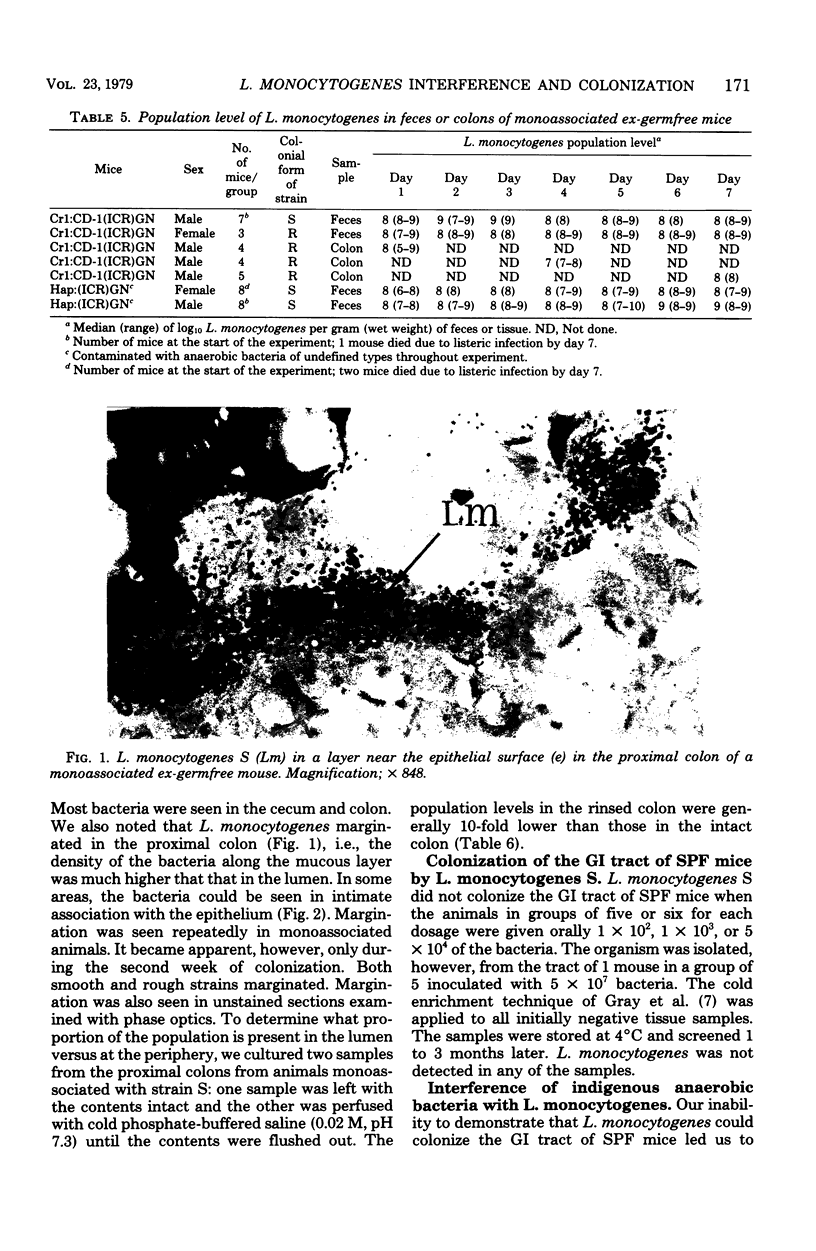
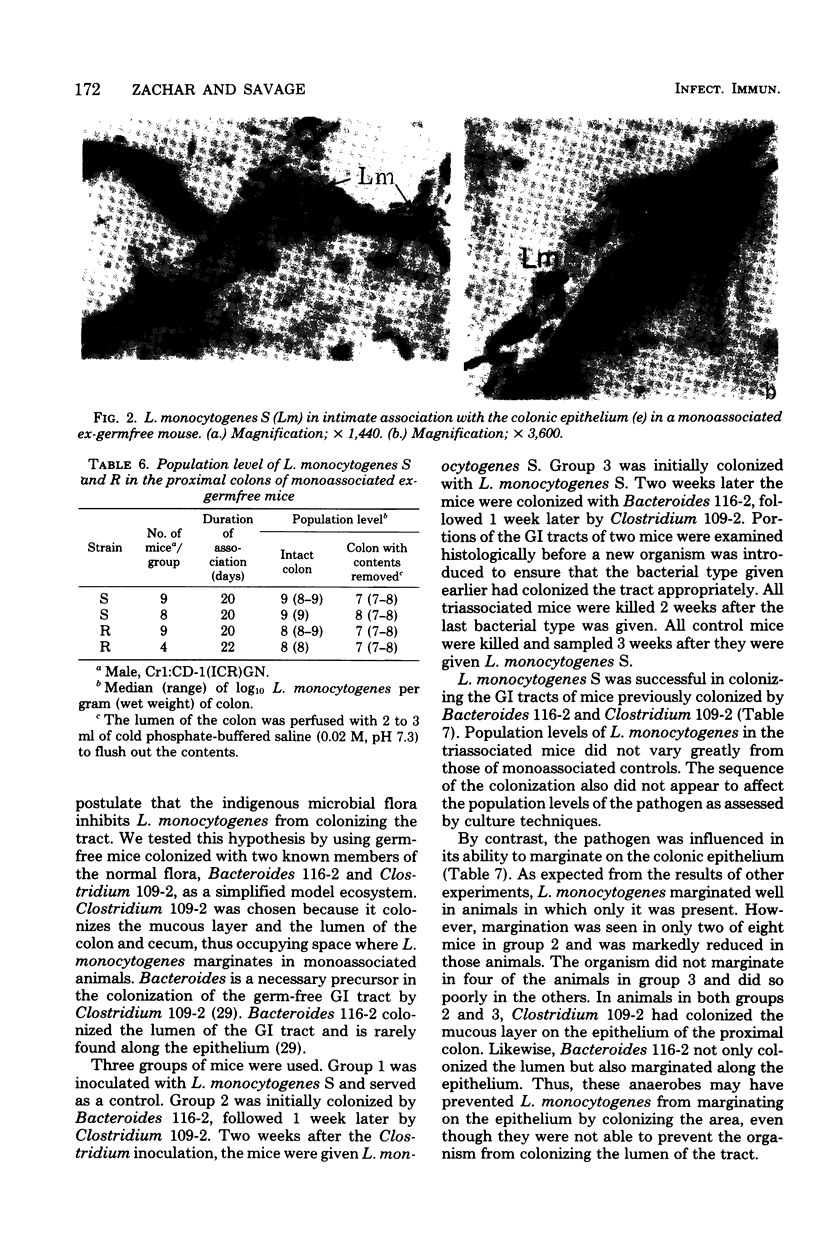
Two strains of Listeria monocytogenes, one that formed smooth colonies on agar surfaces and a varient of it that formed rough colonies, colonized the gastrointestinal tracts of germfree mice. Within 24 h after mice were inoculated orally with about 100 bacteria, the population levels per gram (wet weight) of tissue of both strains were 10(5) to 10(7) in the stomach and ileum and 10(8) to 10(9) in the cecum and colon, respectively. As detected in Gram-stained histological sections, in such gnotobiotes, the bacteria colonized the lumen in all areas of the tract and much of the mucus layer on the epithelial surface in the proximal colon. The strain that formed smooth colonies did not colonize the tracts of specific-pathogen-free mice, but did colonize, to the same levels as in germfree mice, the stomachs and bowels of ex-germfree mice previously associated with two members of the indigenous flora (Bacteroides and Clostridium). In the latter animals, however, the listeria did not form layers on the colonic epithelium as efficiently as they did in monoassociated gnotobiotes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967 Oct;126(1):301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- Beerens H., Tahon-Castel M. M. Milieu à l'acide nalidixique pour l'isolement des streptocoques, D. pneumoniae, listeria, erysipelothrix. Ann Inst Pasteur (Paris) 1966 Jul;111(1):90–93. [PubMed] [Google Scholar]
- GRAY M. L. Epidermiological aspects of listeriosis. Am J Public Health Nations Health. 1963 Apr;53:554–563. doi: 10.2105/ajph.53.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Killinger A. H. Listeria monocytogenes and listeric infections. Bacteriol Rev. 1966 Jun;30(2):309–382. doi: 10.1128/br.30.2.309-382.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. L., Stafseth H. J., Thorp F., Sholl L. B., Riley W. F. A New Technique for Isolating Listerellae from the Bovine Brain. J Bacteriol. 1948 Apr;55(4):471–476. [PMC free article] [PubMed] [Google Scholar]
- Kampelmacher E. H., Huysinga W. T., van Noorle Jansen L. M. The presence of Listeria monocytogenes in feces of pregnant women and neonates. Zentralbl Bakteriol Orig A. 1972 Nov;222(2):258–262. [PubMed] [Google Scholar]
- Kampelmacher E. H., Maas D. E., van Noorle Jansen L. M. Occurrence of Listeria monocytogenes in feces of pregnant women with and without direct animal contact. Zentralbl Bakteriol Orig A. 1976 Mar;234(2):238–242. [PubMed] [Google Scholar]
- Kampelmacher E. H., van Noorle Jansen L. M. Uber die Isolierung von L. monocytogenes bei klinisch gesunden Personen. Zentralbl Bakteriol Orig A. 1972 Jul;221(1):70–77. [PubMed] [Google Scholar]
- Krämer J. Inhibition of different serotypes of Listeria monocytogenes by enterocins in solid and liquid media. J Med Microbiol. 1977 Aug;10(3):367–372. doi: 10.1099/00222615-10-3-367. [DOI] [PubMed] [Google Scholar]
- Miller J. K., Burns J. Histopathology of Listeria monocytogenes after oral feeding to mice. Appl Microbiol. 1970 May;19(5):772–775. doi: 10.1128/am.19.5.772-775.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBS R., COSTELLO R. ASSOCIATION OF GERMFREE MICE WITH BACTERIA ISOLATED FROM NORMAL MICE. J Exp Med. 1965 Jul 1;122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEELIGER H., LINZENMEIER G. Die Listeriose und ihre Erreger (List. monocytogenes). Z Hyg Infektionskr. 1953;136(4):335–378. [PubMed] [Google Scholar]
- Savage D. C., Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968 Jul 1;128(1):97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S. Cecal enlargement and microbial flora in suckling mice given antibacterial drugs. Infect Immun. 1971 Feb;3(2):342–349. doi: 10.1128/iai.3.2.342-349.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial interference between indigenous yeast and lactobacilli in the rodent stomach. J Bacteriol. 1969 Jun;98(3):1278–1283. doi: 10.1128/jb.98.3.1278-1283.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Indigenous microorganisms prevent reduction in cecal size induced by Salmonella typhimurium in vaccinated gnotobiotic mice. Infect Immun. 1976 Jan;13(1):172–179. doi: 10.1128/iai.13.1.172-179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974 Mar;9(3):591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis J., Seeliger H. P. Incidence of Listeria monocytogenes in nature. Appl Microbiol. 1975 Jul;30(1):29–32. doi: 10.1128/am.30.1.29-32.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




