Abstract
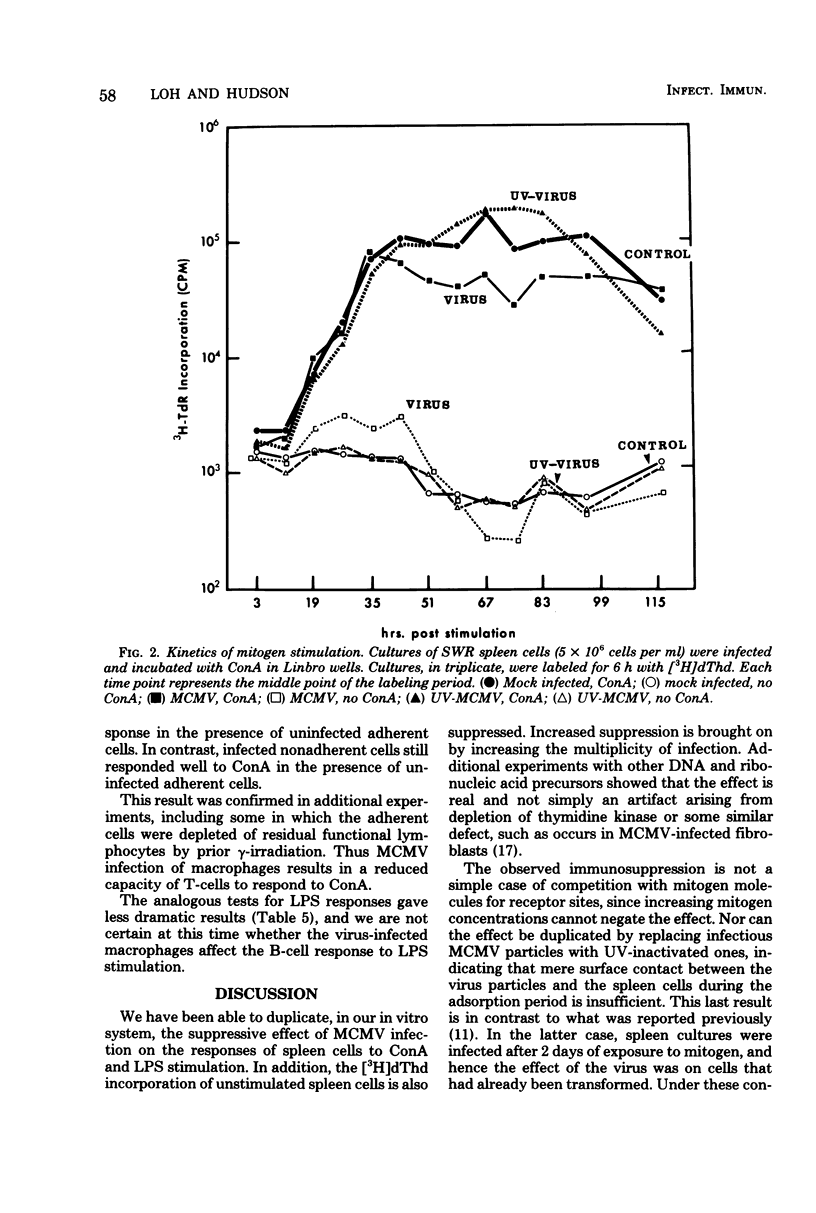
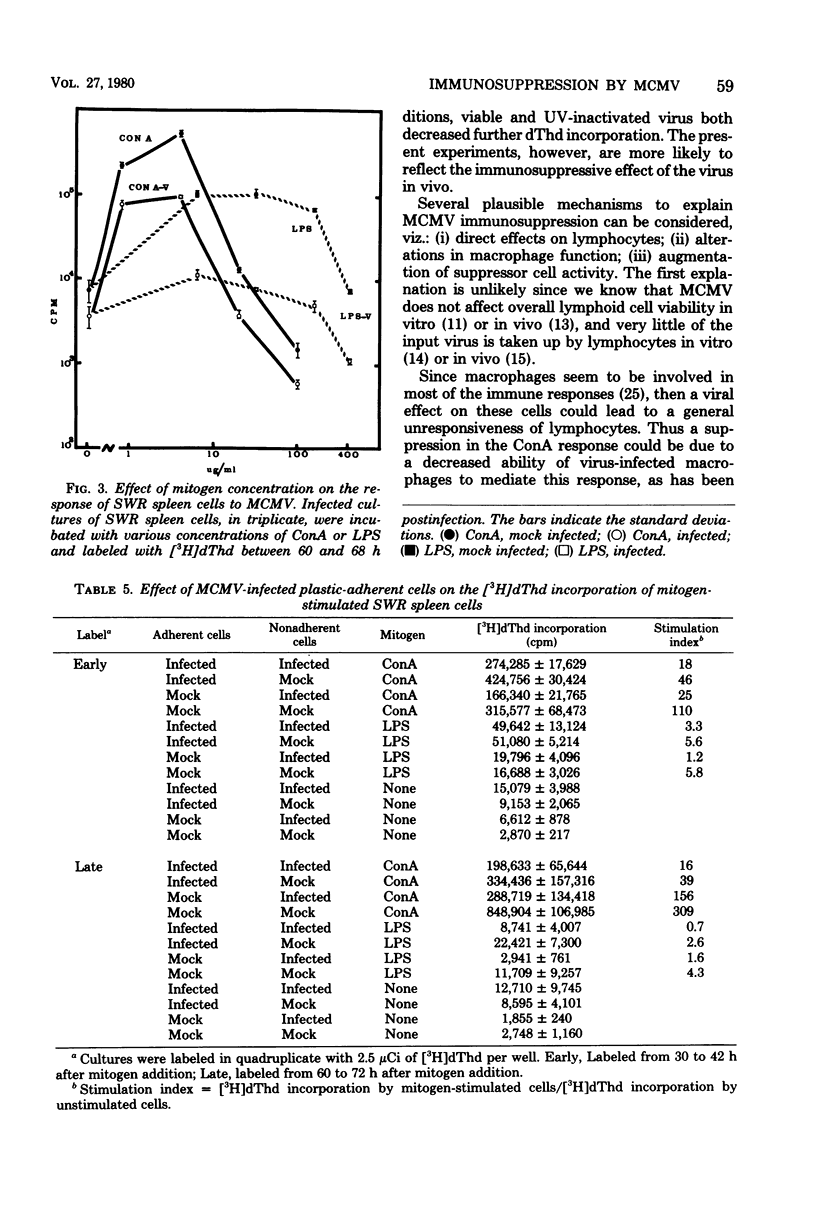
Murine cytomegalovirus suppressed the ability of spleen cells to respond to mitogens in vitro. The degree of suppression was proportional to the multiplicity of infection. This effect could not be explained by cytolysis of lymphocytes, an alteration in the kinetics of the response to mitogen, or a direct competition between virions and mitogen molecules for cell-surface receptors. Nor was it due to simple contact between cell and virus, since ultraviolet-inactivated murine cytomegalovirus failed to suppress the response to mitogens. Reconstitution experiments were performed which involved mixing various combinations of infected and uninfected macrophages and lymphocytes. Under these conditions, it was found that the infected macrophages and lymphocytes. Under these conditions, it was found that the infected macrophages had an impaired capacity to mediate the response ot T lymphocytes to concanavalin A. This suggests that murine cytomegalovirus may cause immunosuppression indirectly by interfering with macrophage function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Booss J., Wheelock E. F. Correlation of survival from murine cytomegalovirus infection with spleen cell responsiveness to Concanavallin A. Proc Soc Exp Biol Med. 1975 Jun;149(2):443–445. doi: 10.3181/00379727-149-38824. [DOI] [PubMed] [Google Scholar]
- Booss J., Wheelock E. F. Progressive inhibition of T-cell function preceding clinical signs of cytomegalovirus infection in mice. J Infect Dis. 1977 Mar;135(3):478–481. doi: 10.1093/infdis/135.3.478. [DOI] [PubMed] [Google Scholar]
- Calderon J., Kiely J. M., Lefko J. L., Unanue E. R. The modulation of lymphocyte functions by molecules secreted by macrophages. I. Description and partial biochemical analysis. J Exp Med. 1975 Jul 1;142(1):151–164. doi: 10.1084/jem.142.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Parker J., Estes J. D., Rongey R. W. Induction of disseminated virulent cytomegalovirus infection by immunosuppression of naturally chronically infected wild mice. Infect Immun. 1974 Oct;10(4):966–969. doi: 10.1128/iai.10.4.966-969.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. R., Overall J. C., Glasgow L. A. Synergistic effect on mortality in mice with murine cytomegalovirus and Pseudomonas aeruginosa, Staphylococcus aureus, or Candida albicans infections. Infect Immun. 1976 Oct;14(4):982–989. doi: 10.1128/iai.14.4.982-989.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Miller J., Najarian J. S. Cytomegalovirus-induced immune suppression. II. Cell-mediated immunity. Clin Exp Immunol. 1974 Sep;18(1):119–126. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Loh L., Misra V., Judd B., Suzuki J. Multiple interactions between murine cytomegalovirus and lymphoid cells in vitro. J Gen Virol. 1978 Jan;38(1):149–159. doi: 10.1099/0022-1317-38-1-149. [DOI] [PubMed] [Google Scholar]
- Jacobs R. P., Cole G. A. Lymphocytic choriomeningitis virus-induced immunosuppression: a virus-induced macrophage defect. J Immunol. 1976 Sep;117(3):1004–1009. [PubMed] [Google Scholar]
- Kelsey D. K., Olsen G. A., Overall J. C., Jr, Glasgow L. A. Alteration of host defense mechanisms by murine cytomegalovirus infection. Infect Immun. 1977 Dec;18(3):754–760. doi: 10.1128/iai.18.3.754-760.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh L., Hudson J. B. Interaction of murine cytomegalovirus with separated populations of spleen cells. Infect Immun. 1979 Dec;26(3):853–860. doi: 10.1128/iai.26.3.853-860.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Gould J. The role of macrophages in mice infected with murine cytomegalovirus. J Gen Virol. 1978 Oct;41(1):143–153. doi: 10.1099/0022-1317-41-1-143. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Thymidine kinase activity in mouse 3T3 cells infected by murine cytomegalovirus (MCV). Virology. 1977 Jul 15;80(2):430–433. doi: 10.1016/s0042-6822(77)80019-6. [DOI] [PubMed] [Google Scholar]
- Opitz H. G., Niethammer D., Jackson R. C., Lemke H., Huget R., Flad H. D. Biochemical characterization of a factor released by macrophages. Cell Immunol. 1975 Jul;18(1):70–75. doi: 10.1016/0008-8749(75)90037-4. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E., Ennis F. A. Cytotoxic T lymphocyte response to murine cytomegalovirus infection. Nature. 1978 Jun 15;273(5663):541–543. doi: 10.1038/273541a0. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Steigbigel R. T. Effect of in vitro virus infection on response of human monocytes and lymphocytes to mitogen stimulation. J Immunol. 1978 Sep;121(3):1052–1058. [PubMed] [Google Scholar]
- Rosenstreich D. L., Farrar J. J., Dougherty S. Absolute macrophage dependency of T lymphocyte activation by mitogens. J Immunol. 1976 Jan;116(1):131–139. [PubMed] [Google Scholar]
- Selgrade M. K., Ahmed A., Sell K. W., Gershwin M. E., Steinberg A. D. Effect of murine cytomegalovirus on the in vitro responses of T and B cells to mitogens. J Immunol. 1976 May;116(5):1459–1465. [PubMed] [Google Scholar]
- Stadecker M. J., Calderon J., Karnovsky M. L., Unanue E. R. Synthesis and release of thymidine by macrophages. J Immunol. 1977 Nov;119(5):1738–1743. [PubMed] [Google Scholar]
- Starr S. E., Allison A. C. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977 Aug;17(2):458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Webb D. R., Jr, Jamieson T. Control of mitogen-induced transformation: characterization of a splenic suppressor cell and its mode of action. Cell Immunol. 1976 Jun 1;24(1):45–57. doi: 10.1016/0008-8749(76)90130-1. [DOI] [PubMed] [Google Scholar]


