Abstract
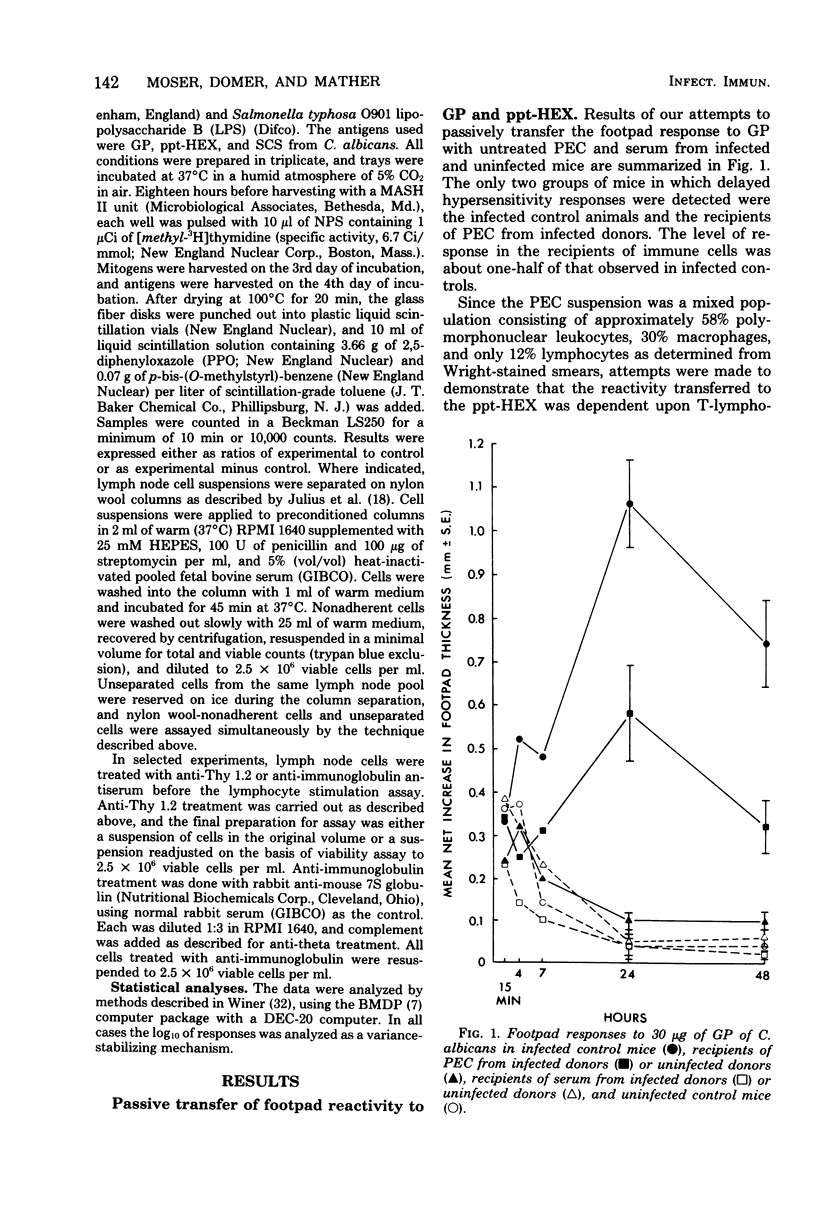
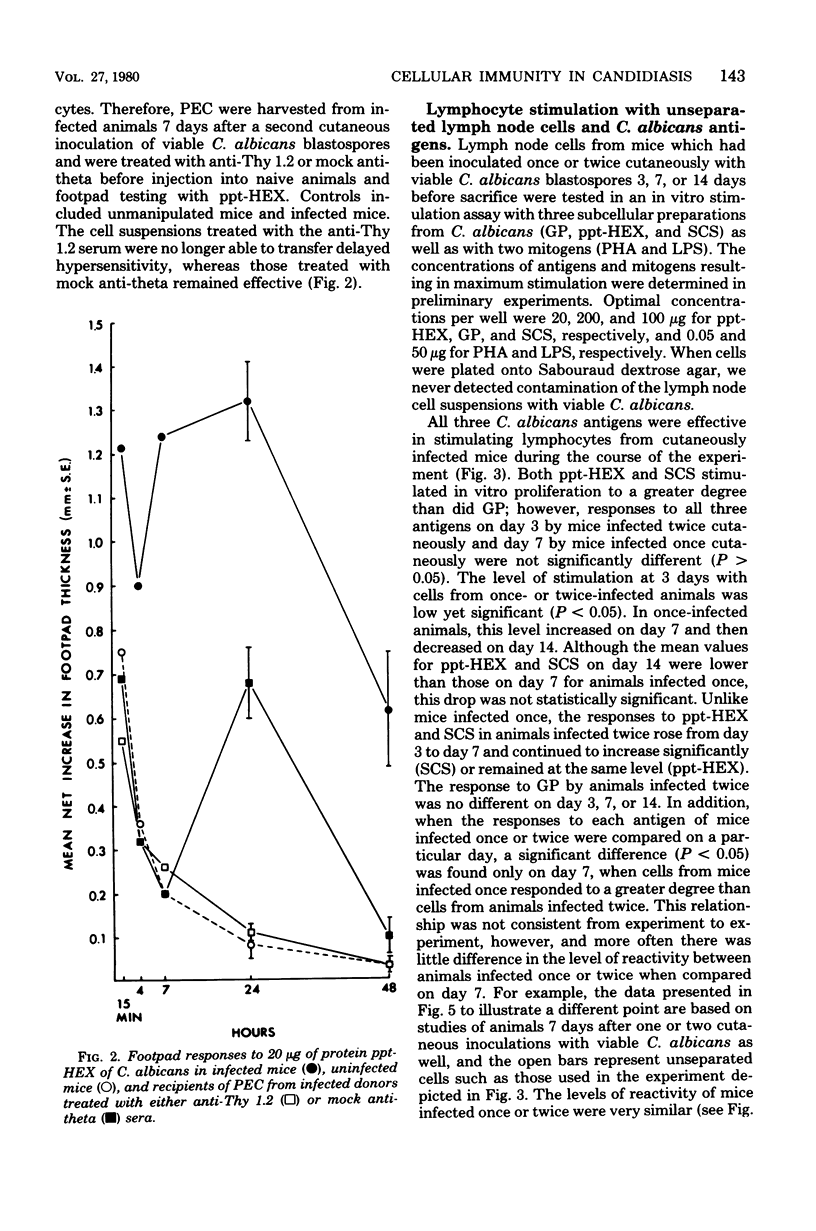
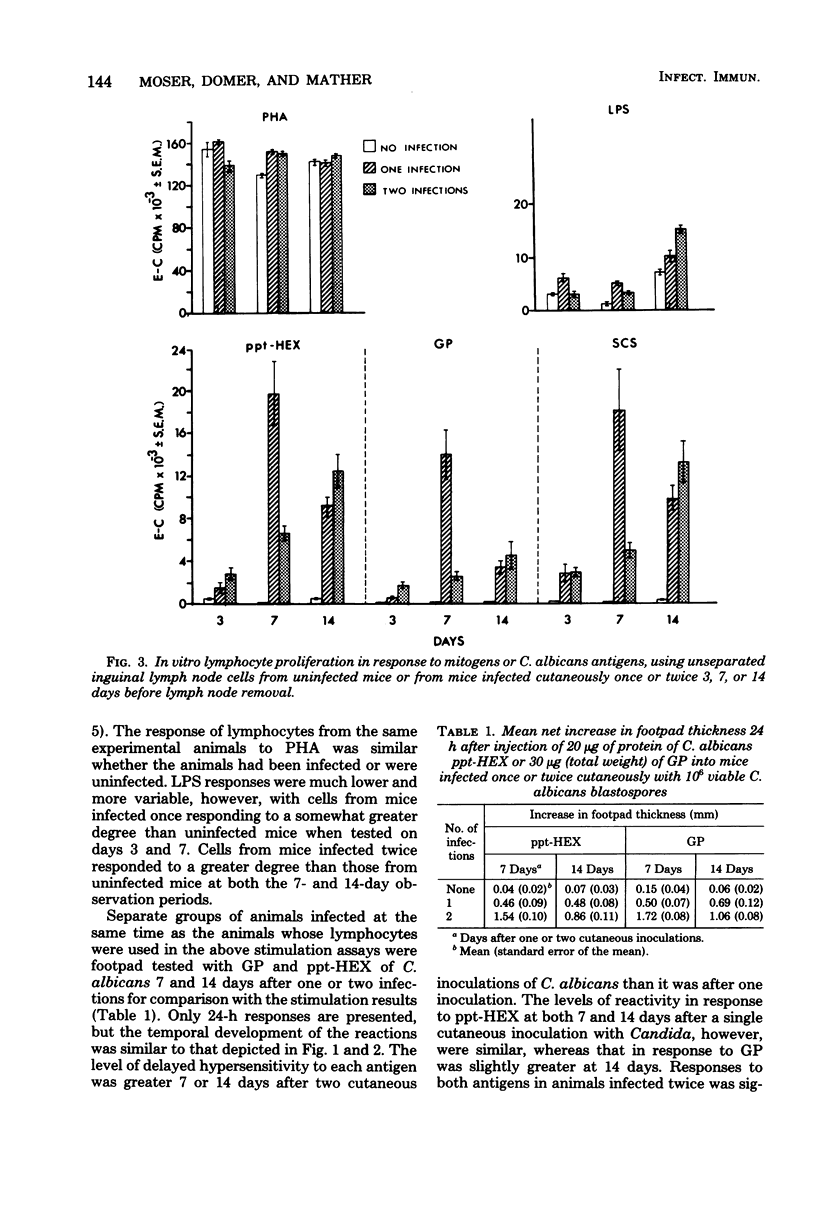
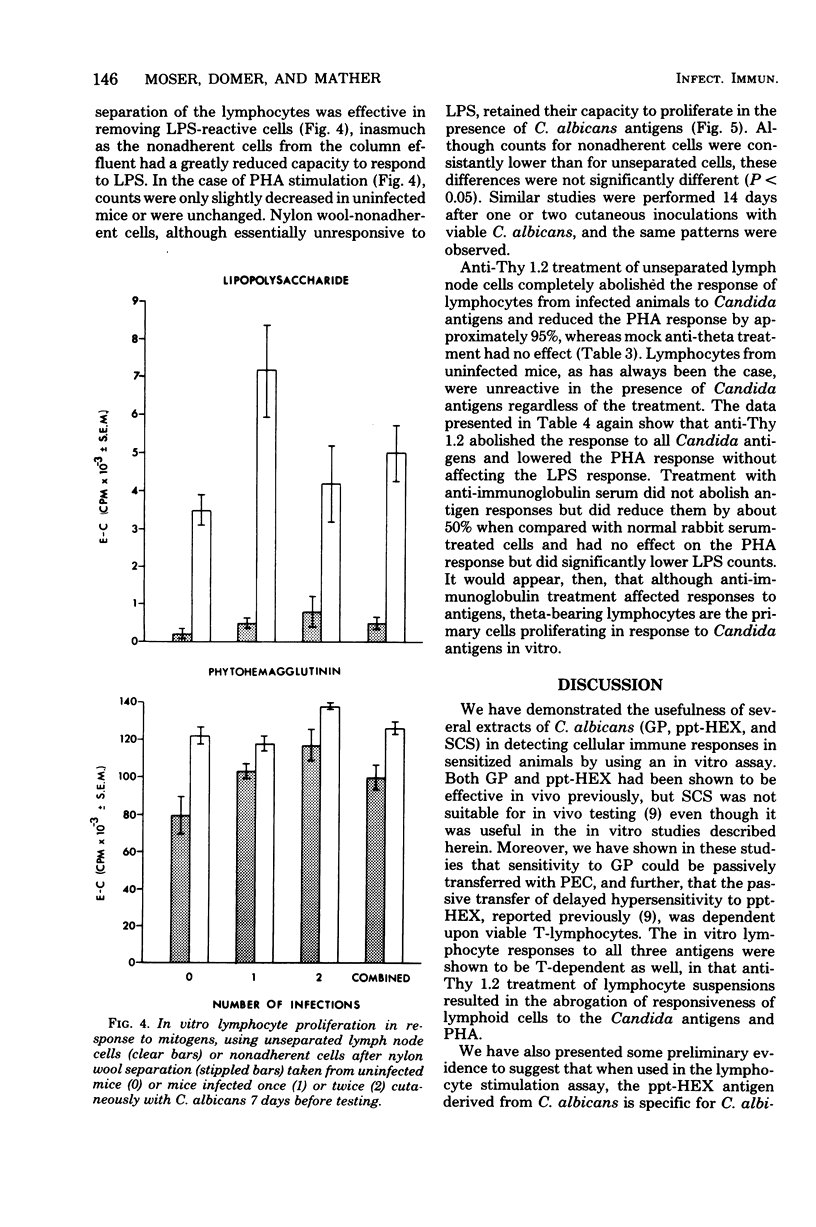
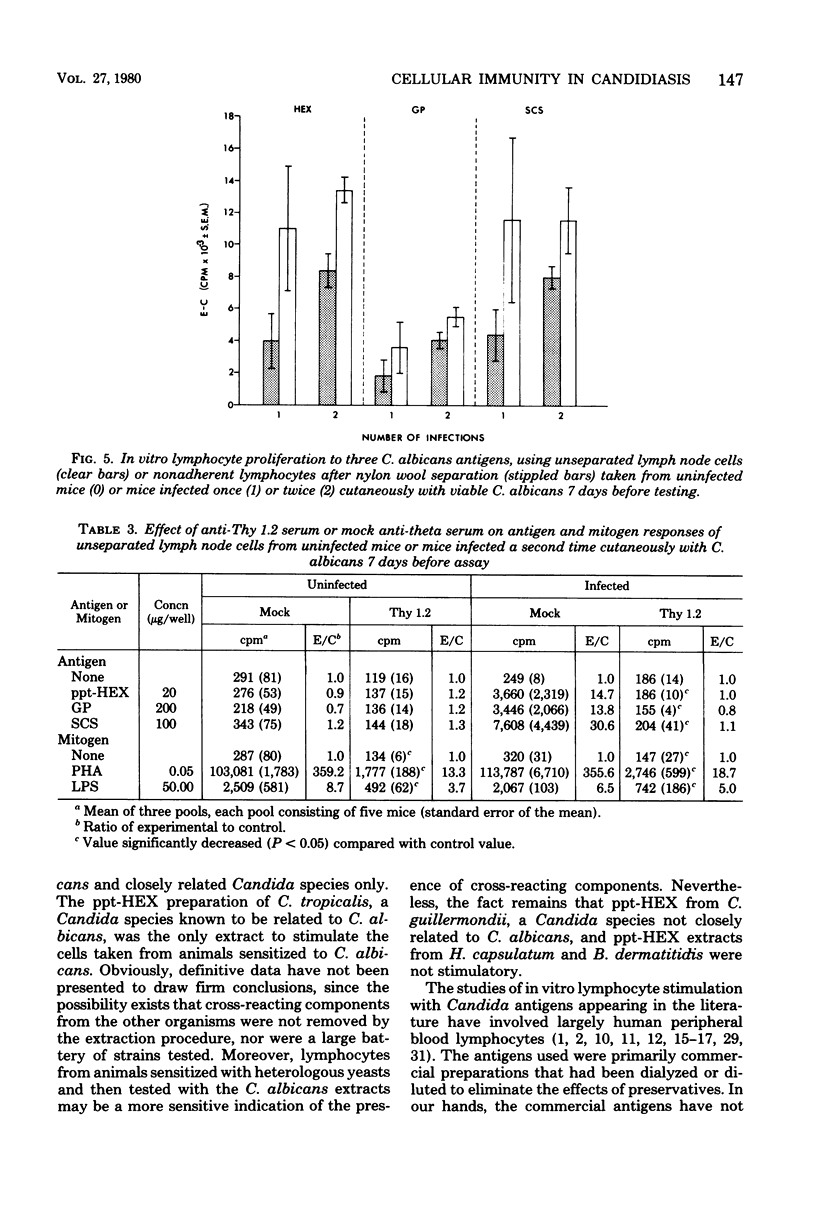
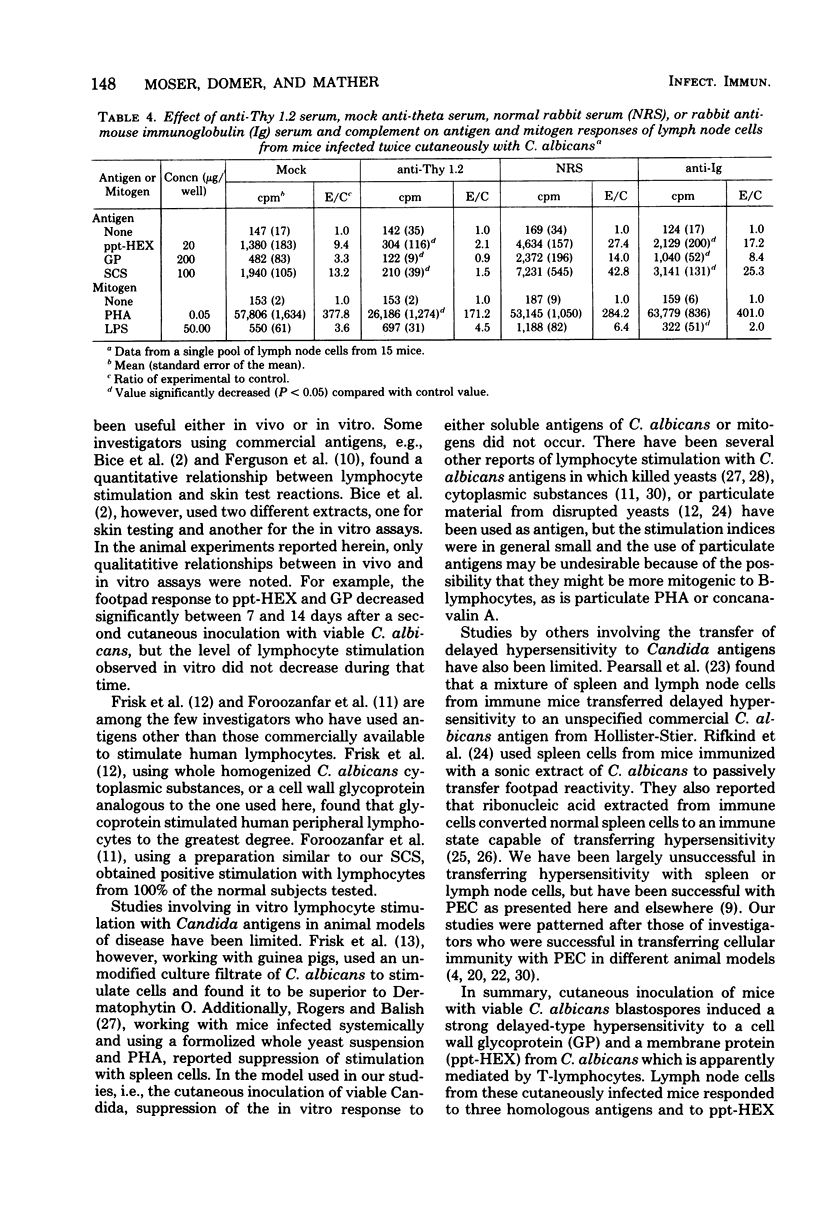
Male CBA/J mice, sensitized by cutaneous inoculation with viable Candida albicans blastospores, were used to study in vivo and in vitro cellular immune responses. Three antigens of C. albicans, viz., a cell wall glycoprotein (GP), a membrane extract (ppt-HEX), and soluble cytoplasmic substances (SCS), were used in vitro in a lymphocyte stimulation assay, whereas the GP and ppt-HEX were used in vivo to detect delayed hypersensitivity by the footpad assay. Delayed hypersensitivity to GP and ppt-HEX was transferred from sensitized donors to naive recipients with peritoneal exudate cells and not with serum. Moreover, the transfer of the reactivity to ppt-HEX was abrogated by the prior treatment of the transfer suspension with anti-theta 1.2 serum and complement. The in vitro lymphocyte response to GP and ppt-HEX correlated qualitatively with the in vivo responses. SCS, a preparation shown to be ineffective in vivo previously, did stimulate lymphocytes from sensitized animals in vitro. The in vitro response to Candida antigens, as well as phytohemagglutinin, was abolished by treatment of the lymphocyte suspension with anti-thymocyte 1.2 serum before assay, whereas anti-immunoglobulin serum had less effect on these responses. The in vivo and in vitro reactivity to the Candida antigens, therefore, was dependent upon viable T-lymphocytes. Preliminary specificity studies were carried out in the lymphocyte stimulation assay, using lymphocytes from mice infected with C. albicans tested against ppt-HEX preparations extracted from two other species of Candida, C. tropicalis and C. guillermondii, and from two other pathogenic yeast forms, Histoplasma capsulatum and Blastomyces dermatitidis. Significant cross-reactivity was observed with C. tropicalis only, a species which is known to be serologically related to C. albicans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H., Des Prez R. M. Tranformation of lymphocytes of normal and hospitalized adults by Candida albicans extract. Proc Soc Exp Biol Med. 1973 Dec;144(3):826–829. doi: 10.3181/00379727-144-37691. [DOI] [PubMed] [Google Scholar]
- Bice D. E., Lopez M., Rothschild H., Salvaggio J. Comparison of Candida-delayed hypersensitivity skin test size with lymphocyte transformation, migration inhibitory factor production and antibody titer. Int Arch Allergy Appl Immunol. 1974;47(1):54–62. doi: 10.1159/000231200. [DOI] [PubMed] [Google Scholar]
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Cohen R., Roth F. J., Delgado E., Ahearn D. G., Kalser M. H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969 Mar 20;280(12):638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- Domer J. E. In vivo and in virto cellular responses to cytoplasmic and cell wall antigens of Histoplasma capsulatum in artificially immunized or infected guinea pigs. Infect Immun. 1976 Mar;13(3):790–799. doi: 10.1128/iai.13.3.790-799.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. C., Kershnar H. E., Collin W. K., Stiehm E. R. Correlation of cutaneous hypersensitivity with lymphocytic response to Candida albicans. Am J Clin Pathol. 1977 Oct;68(4):499–504. doi: 10.1093/ajcp/68.4.499. [DOI] [PubMed] [Google Scholar]
- Foroozanfar N., Yamamura Y., Hobbs J. R. Standardization of lymphocyte transformation to Candida immunogen. Clin Exp Immunol. 1974 Feb;16(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Frisk A., Wasserman J. Lymphocyte stimulation with Candida albicans antigens. Antonie Van Leeuwenhoek. 1969 Jun;35:E13–E13. [PubMed] [Google Scholar]
- Frisk A., von Stedingk L. V., Wasserman J. Lymphocyte stimulation in Candida albicans infections. Sabouraudia. 1974 Mar;12(1):87–94. [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett A. M., Woods R. J., Temperley I. J., Mullins G. M. Cell mediated immunity to recall antigens in vivo and in vitro. Ir J Med Sci. 1977 Jun;146(6):167–174. doi: 10.1007/BF03030954. [DOI] [PubMed] [Google Scholar]
- Helander I. The lymphocyte transformation test in dermatophytosis. Mykosen. 1978 Mar;21(3):71–80. doi: 10.1111/j.1439-0507.1978.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Davis J. R., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. I. Use of the intradermal skin and footpad swelling tests as assays of active and passive sensitization. J Infect Dis. 1976 Jan;133(1):50–56. doi: 10.1093/infdis/133.1.50. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. II. Characterization of the active component in immunogenic RNA extracts. J Infect Dis. 1976 May;133(5):533–537. doi: 10.1093/infdis/133.5.533. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. II. Molecular classes in immunogenic RNA extracts that transfer delayed hypersensitivity. J Infect Dis. 1976 May;133(5):523–532. doi: 10.1093/infdis/133.5.523. [DOI] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Effect of systemic candidiasis on blastogenesis of lymphocytes from germfree and conventional rats. Infect Immun. 1978 Apr;20(1):142–150. doi: 10.1128/iai.20.1.142-150.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Suppression of lymphocyte blastogenesis by Candida albicans. Clin Immunol Immunopathol. 1978 Jul;10(3):298–305. doi: 10.1016/0090-1229(78)90185-x. [DOI] [PubMed] [Google Scholar]
- Shannon D. C., Johnson G., Rosen F. S., Austen K. F. Cellular reactivity to Candida albicans antigen. N Engl J Med. 1966 Sep 29;275(13):690–693. doi: 10.1056/NEJM196609292751302. [DOI] [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Steele R. W., Cannady P. B., Jr, Moore W. L., Jr, Gentry L. O. Skin test and blastogenic responses to Sporotrichun schenckii. J Clin Invest. 1976 Jan;57(1):156–160. doi: 10.1172/JCI108255. [DOI] [PMC free article] [PubMed] [Google Scholar]


