Abstract
Infectious diseases are the leading cause of mortality worldwide, with viruses in particular making global impact on healthcare and socioeconomic development. In addition, the rapid development of drug resistance to currently available therapies and adverse side effects due to prolonged use is a serious public health concern. The development of novel treatment strategies is therefore required. The interaction of nanostructures with microorganisms is fast-revolutionizing the biomedical field by offering advantages in both diagnostic and therapeutic applications. Nanoparticles offer unique physical properties that have associated benefits for drug delivery. These are predominantly due to the particle size (which affects bioavailability and circulation time), large surface area to volume ratio (enhanced solubility compared to larger particles), tunable surface charge of the particle with the possibility of encapsulation, and large drug payloads that can be accommodated. These properties, which are unlike bulk materials of the same compositions, make nanoparticulate drug delivery systems ideal candidates to explore in order to achieve and/or improve therapeutic effects. This review presents a broad overview of the application of nanosized materials for the treatment of common viral infections.
Keywords: advances, hepatitis, HIV, influenza, nanotechnology, vaccine, virus
Introduction
Infectious disease agents such as bacteria, viruses, fungi1 and parasites account for approximately 15 million deaths worldwide, with acute respiratory infections and human immunodeficiency virus (HIV) being the leading causes.2 Viral infections alone pose significant global health challenges by affecting millions of people worldwide, with a negative impact on both health and socioeconomic development.3 Efficient treatment of viral infection is hindered by the development of drug resistance, especially those associated with HIV4–7 and influenza.8 This phenomenon constitutes a public health threat, which includes increased morbidity and mortality,9 added costs associated with the use of more expensive drugs and a greater burden on public health systems.2 Consequently, there is an obvious requirement for the development of novel methods to treat viral infections.
Nanotechnology refers to the development or application of particles with dimension(s) that fall into the nanometer range (10−9 or one billionth of a meter).10,11 The interaction between nanoscience and biological systems is known as ‘nanobiotechnology’,12,13 while the associated area known as ‘nanomedicine’ deals with the application of nanostructured materials to diagnose, treat and prevent diseases.14
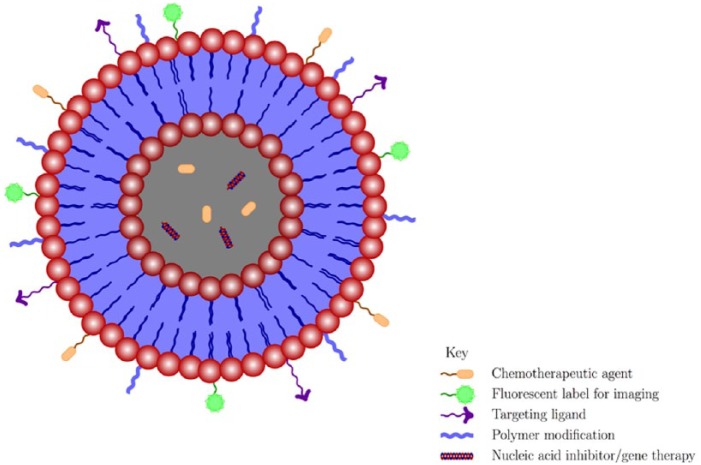
The first nanosystems applied in medicine were introduced to increase the efficacy of current, yet dose-limiting and poorly bioavailable drugs.15 Currently, nanoparticles are known to exert their antiviral activities by various mechanisms. First, the unique properties of nanoparticles such as (1) small particle size (which can facilitate drug delivery into anatomically privileged sites),11,16 (2) large surface area to volume ratios (which ensures that large drug payloads can be accommodated),17 and (3) tunable surface charge (to facilitate cellular entry across the negatively charged cellular membrane),18,19 make nanoparticles attractive tools for viral treatment. Second, it has been demonstrated that nanoparticles can contain biomimetic properties,20–22 which result in intrinsic antiviral properties. Popular examples of these include silver nanoparticles23,24 and dendrimers.25,26 Third, the possibility of drug encapsulation,16,27 functionalization by the formation of stable structures,28 or modifications (with polymers such as poly(ethylene glycol) (PEG))17,29 can all lead to optimized drug dosing and improved delivery by increasing stability30 and drug retention times.31 Finally, it is believed that drug delivery can be vastly improved by engineering nanoparticles with targeting moieties to increase specificity to desired cell types, target tissues or sub-cellular compartments.17,19,32 A condensed summary of the mechanistic approaches to engineer nanoparticles with improved treatment benefits is shown in the schematic in Figure 1.
Figure 1.
Multifunctional mechanisms for engineering nanoparticles with benefits in drug delivery.
Certain challenges exist for the treatment and subsequent eradication of viruses in the infected host. One major example is the establishment of reservoirs in cellular and anatomically privileged sites such as the blood-brain barrier (BBB) and blood-testis barrier.33 This leads to low-level replication34 in these compartments, which are inaccessible to conventional therapeutics. Nanoparticulate drug carriers are, however, able to traverse these membranes and are therefore promising tools to be investigated for circumventing this obstacle.35 Other challenges in viral treatment include the use of RNA interference (RNAi) technology – a popular molecular approach for the treatment of many infectious diseases.36 The inability of RNA to cross the cell membrane, due to the large molecular weight and anionic charge,37 rapid renal clearance, uptake by phagocytes, and toxicity due to stimulated immune response,38 all present limitations which prohibits their clinical utility. The incorporation of siRNA onto nanocarriers, however, can also overcome this limitation39 to achieve successful inhibition of viral replication.
This review will provide an overview of the most recent (past 5 years) and relevant literature, which describes the application of nanotechnology for the treatment of common viral infections. Examples of nanosystems with applications in both drug and vaccine delivery for prevention of these viral infections are also reviewed. Finally, important considerations for nanoparticle antigenicity as well as the requirements for the design of nanomaterials, which are unique to viruses, are discussed.
Examples of biocompatible systems
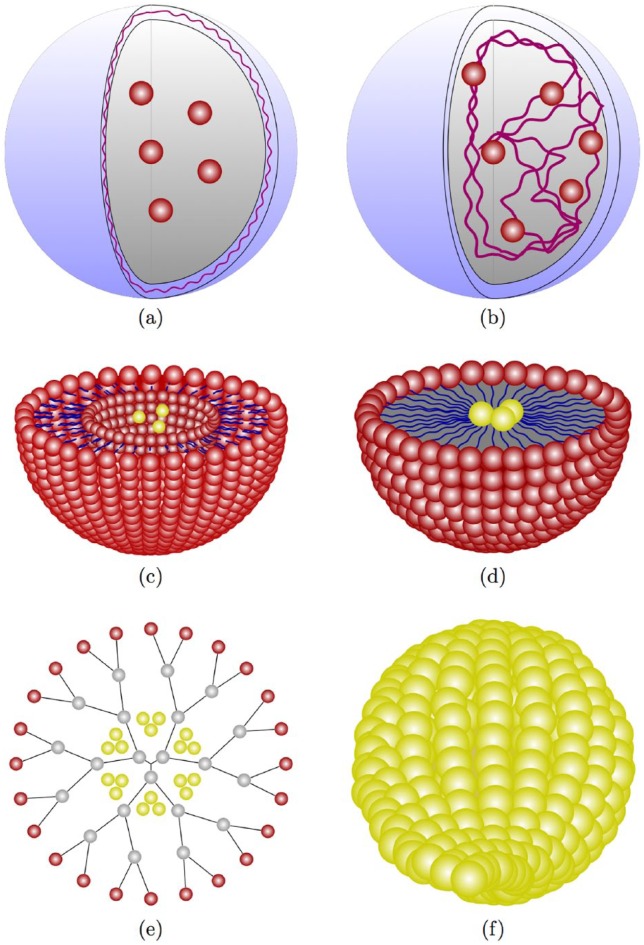
A nanopharmaceutical refers to any nanomaterial with therapeutic potential, for example, dendrimers, liposomes, micelles and nanocapsules.11,40 These can function as therapeutic agents, whereby the drug is either dissolved, entrapped, encapsulated, adsorbed or chemically attached.41 Nanoparticles can have various shapes and chemical compositions42 and can be classified according to the way drugs are delivered or by the characteristics of the matrix of which it is composed.43 Here, we describe the most common types of nanocarriers (Figure 2) that are used for drug delivery, based on their composition.
Figure 2.
Examples of common nanocarriers used for antiviral drug delivery: (a) nanocapsules,44 (b) nanosphere,45 (c) liposome,46 (d) micelle,27 (e) dendrimers,47 and (f) gold nanoparticle.48
Organic nanoparticles
Organic nanoparticles are the most extensively researched type of nanoparticle for drug delivery and the most widely approved system for therapeutic use in humans.43 The most common types of organic nanoparticles are presented as follows.
Polymeric nanoparticles
Polymeric nanoparticles are colloidal solids with sizes ranging from 10 to 1000 nm. The small size can facilitate capillary penetration and uptake by cells resulting in increased concentrations at target sites.49 Polymers approved by the World Health Organization (WHO) and the Food and Drug Administration (FDA) for use in medicine and pharmaceuticals include polylactides (PLA), polyglycolides (PGA) and poly(lactide-co-glycolides) (PLGA).50 Poly(D,L-lactide-co-glycolide) (PLG) and PLGA-based nanoparticles are most widely used due to their superior biocompatibility and biodegradability profiles.51 Surface modifications with hydrophilic polymers such as PEG are essential to reduce non-specific interactions with serum proteins, decrease susceptibility to opsonization52,53 and to defer uptake by phagocytosis, thereby prolonging the drug half-life and further altering the biodistribution and pharmacokinetic profile of the drug,54 and has thus been considered as the ‘gold-standard’ of cloaking agent systems.55 Polymeric nanoparticles can be classified as nanocapsules or nanospheres.
Nanocapsules
Nanocapsules are hollow spheres, in which the drug is confined to an inner cavity, surrounded by a polymer coating.56 The size can range from 50 to 300 nm, and they are characterized by their low density and high loading capacities.49
An example of the use of nanocapsules in enhancing drug distribution is described;33 limited antiviral distribution to brain tissue may be due to the permeability glycoprotein (P-gp) efflux transporter. Solutol® HS15 is an excipient that is able to inhibit P-gp, thereby improving drug distribution across the BBB.11 Results from this study demonstrated that Solutol® HS15 nanocapsules loaded with the HIV protease inhibitor, indinavir, showed significantly increased uptake in the brain and testes of mice, compared to control mice where only indinavir solution was administered.33
Nanospheres
These are matrix systems where the drug is physically or uniformly dispersed, with sizes ranging from 100 to 200 nm in diameter.56,57 Several research studies have been done using nanospheres for the treatment of hepatitis B virus (HBV),58 herpes simplex virus (HSV),45 and influenza,59,60 while comprehensive review articles on the application of these agents in viral treatment are also available.61,62
Liposomes
Liposomes are spherical63 carriers ranging from 20 to 30 nm in size. They are composed of a phospholipid bilayer (which can mimic cell membranes and directly fuse with microbial membranes),64 containing an aqueous core.2 Hydrophilic and lipophilic drugs (or other biologically active compounds) can be incorporated into the inner aqueous cavity or the phospholipid bilayer, respectively.65 Additional advantages of liposomes are that they are relatively non-toxic and biodegradable.63 Liposomal formulations have been extensively studied in vaccine studies due to their ability to act as immunological adjuvants.66
Micelles
Micelles range in size from 10 to 100 nm.57 These are composed of an inner hydrophobic core (which can incorporate poorly water soluble drugs) and surrounded by an outer hydrophilic polymer (such as PEG, which can increase circulation time and consequently improve accumulation).49 Examples of these include polymeric micelles, which have attracted much attention as drug delivery agents with significant therapeutic potential.67–69 Drug encapsulation with polymeric micelles is one of the most attractive nanotechnologies used to improve both the water solubility and stability of otherwise technologically limited (poorly water soluble and unstable) drugs.70 An additional advantage of using micelles in therapeutics is that they display a slower rate of dissociation, thereby enabling a longer drug retention time, and eventually a higher accumulation of the drug at the target site.35
Dendrimers
Dendrimers are symmetrical, macromolecular, and hyper-branched structures radiating from a central core via connectors and branching units, where interaction with its target environment is controlled by the terminal groups.71 These are globular in nature and comprised of three distinct domains (central core, branches, and terminal functional groups).72 They have increased functionality because they can encapsulate several chemical moieties, interior layers and have the ability to display multiple surface groups (multivalent surface).35,71
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) represent an alternative drug delivery system to the conventional colloidal nanoparticles, described above. The use of SLNs also aims to combine the advantages of conventional nanocarriers, while avoiding some of their limitations. For example, large-scale production of polymeric nanoparticles is a major challenge, which limits their utility in drug delivery, whereas the production of SLNs can be achieved in both cost-effective and relatively simple ways (e.g. by high pressure homogenization and micro emulsion techniques).73 Additional advantages of using SLNs include increased stability, safety and availability, and decreased toxicity, with improved drug-release profiles, compared to synthetic polymer nanoparticles.74–76
Inorganic nanoparticles
Metallic nanoparticles can be smaller than organic nanoparticles, ranging between 1 nm and 100 nm in size, while their loading efficacy is much higher.35 There are two main approaches for the synthesis of metallic nanoparticles: the ‘bottom-up’ (or self-assembly) approach refers to the construction of the nanoparticle, level by level (e.g. atom by atom or cluster by cluster), and the ‘top-down’ approach uses chemical or physical methods to reduce the inorganic material to its nanosized form.77 The reaction conditions (pH, temperature, time, or concentration) can be used to modify the nanoparticle characteristics (size and shape), while the choice of reducing agent can influence properties such as loading capacity, release, and aggregation profiles.43
Gold nanoparticles
Gold nanoparticles (GNPs) are widely researched as nanocarriers due to their excellent conductivity, flexibility of surface modification, biocompatibility, and simplistic preparation methods.78 Other advantages afforded by their unique physical and chemical properties include the gold core (which is inert and non-toxic),79 photophysical properties (which can facilitate efficient drug release at remote sites),80 and versatility of functionalization via thiol linkages.81 There are basic GNP preparation methods which exist and can produce nanoparticles of varying diameters (1–2 nm,82 1.5–5 nm,83,84 or 10–150 nm,85–87 depending on the application).
Silver nanoparticles
Silver nanoparticles are the most effective of the metallic nanoparticles against bacteria, viruses and other eukaryotic microorganisms,88 particularly due to the inherent inhibitory and bactericidal potential of silver,89 but also because of their good conductivity, catalytic properties, and chemical stability.64 The key mechanisms of action of silver nanoparticles are the release of silver ions (which enhances antimicrobial activity),90 cell membrane disruption, and DNA damage.91 The reader is referred to a detailed review on the application of silver nanoparticles as virucidal agents.92
Other metallic nanoparticles
Various other metallic nanoparticles such as titanium,93 zinc,94 and copper,95 as well as metal oxide nanoparticles such as iron oxide, zinc oxide, and titanium dioxide96 have demonstrated specific antiviral activities. Others, like platinum nanoparticles, which are used for the detection of influenza virus,97 are yet to be evaluated.
Core-shell nanoparticles contain a simple spherical core particle, which is completely surrounded by a shell of a different material,98 which can be monometallic or bimetallic in nature.99 Several types of core-shell nanoparticles have been demonstrated to have biomedical applications.100–103
The reader is referred to recently published literature giving a comprehensive account of the application of metal and metal oxide nanoparticles in the treatment of viral infections.96
Antiviral nanotherapeutics
Several nanomedicines have been approved or are currently undergoing investigation for the treatment of viral infections (Table 1). Examples of studies investigating the antiviral activities of potential nanotherapeutics in development are presented in the sections that follow.
Table 1.
Nanomedicines which are approved or under evaluation for the treatment of viral infections.
| Name | Company | Description | Mechanism of action | Indication | Approval year/stage of development | Reference |
|---|---|---|---|---|---|---|
| Inflexal V® | Crucell, Berna Biotech | Virosomal (150 nm liposomes) vaccine | Mimicking native antigen presentation: Liposomes mimic the native virus structure, thus allowing for cellular entry and membrane fusion Retention of the natural presentation of antigens on liposomal surface provides for high immunogenicity. |
Influenza | 1997 | Herzog et al.,104 Mischler and Metcalfe,105 Bachmann and Jennings106 |
| Epaxal® | Crucell, Berna Biotech | Inactivated virosomal (liposome) vaccine | Unique mechanism of action which mimics the natural process | HAV | 1999 | Bovier107 |
| PegIntron® | Merck | PEGylated interferon alfa-2b | Improved stability of protein through PEGylation | HCV | 2001 | Alconcel et al.108 |
| Pegasys® | Genentech | PEGylated interferon alfa-2b | Improved stability of protein through PEGylation | HBV, HCV | 2002 | Alconcel et al.108 |
| Influvac® Plus | Solvay Pharma/Abbott | Virosome vaccine | Containing influenza surface proteins neuraminidase and hemagglutinin | Influenza | 2005 | Waknine109 |
| FluquitTM
(STP 702) |
Sirnaomics Inc. | Short interfering RNA (SiRNA) therapeutic |
Gene silencing | H5N1 and H1N1 influenza | Preclinical evaluation | Sirnaomics110 |
| Cervisil®
(STP 909) |
Sirnaomics Inc. | Short interfering RNA (SiRNA) therapeutic |
Gene silencing | HPV | Preclinical evaluation | Sirnaomics110 |
| VivaGel®
(SPL 7013) |
Starpharma | Dendrimer | Lysine-based dendrimer with naphthalene disulfonic acid surface groups | HIV, HSV | Clinical trial (number: NCT00740584) (approved for used against bacterial vaginosis) |
Starpharma111 |
| DermaVir | Genetic Immunity | Therapeutic vaccine | Synthetic plasmid DNA immunogen expressing 15 antigens, inducing significant expansions of the HIV-specific precursor/memory T cell pool. | HIV | Clinical trial (number NCT00270205 |
Rodriguez et al.112 |
| Doravirine (MK-1439) |
Merck | Solid drug nanoparticle formulation | Non-nucleoside reverse transcriptase inhibitor | HIV | Clinical trial (number: NCT02549040) |
Molina et al.113 |
| ARB-001467 TKM-HBV | Arbutus Biopharma | Wet lipid nanoparticle | Lipid particle containing three RNAi therapeutics that target three sites on the HBV genome |
HBV | Clinical trial (number: NCT02631096) |
Seto et al.114 |
HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSV, herpes simplex virus.
HIV
A cure or vaccine for HIV/AIDS remains elusive. Treatment is based on the use of drugs that target the various stages in the life cycle of the virus. The current antiretroviral (ARV) armamentarium includes six classes of drugs, that is, nucleoside/nucleotide reverse transcriptase inhibitors (N(t) RTIs),115 non-nucleoside inhibitors (NNRTIs),116 protease inhibitors (PIs),117 entry/fusion inhibitors (FIs),118 CCR5 antagonists,119 and integrase inhibitors.120,121
The combination of three or more drugs, known as highly active ARV therapy (HAART) has significantly improved the expectancy and quality of life of HIV-infected individuals.122 This type of therapy, however, is not devoid of unwanted occurrences; suboptimal adherence, heavy pill burdens, toxicity and other negative side effects, are all limitations of currently available therapeutics. Moreover, the chronic nature of HIV/AIDS infection requires that life-long treatment be taken, which can result in the development of drug resistance. It is therefore essential that novel methods to enhance the inhibition of HIV infection be investigated and developed.
Nanotechnology-based drug systems for HIV treatment represent an important option that requires ongoing investigation. Modern drug design, which can incorporate ARV drug delivery with nanosystems can decrease the dosage requirements and toxic side effects associated with current heavy pill burdens (which reduces the possibility of drug resistance), thereby improving the safety and efficacy profiles of the drug.35
Different reviews have been published that focus specifically on HIV/AIDS vaccine development123–125 and delivery of siRNA for the treatment of HIV.37 The reader is also referred to comprehensive accounts on conventional methods for HIV treatment and the recent advances using different types of nanoformulations with their respective applications in HIV treatment.11,126,127
In a study performed by Chiodo et al.,128 the NRTI drugs abacavir (ABC) and lamivudine (3TC) were attached to glucose-coated GNPs and evaluated for their anti-HIV activity, in vitro. Smart functionalization was achieved via the primary hydroxyl groups of the drugs, through an ester bond that can be cleaved off in acidic conditions (e.g. in the vagina to inhibit viral replication), to render the hydroxyl group available to facilitate chain termination – a fundamental mechanism of action of the NRTI class of drugs. These results illustrate a new level of multi-functionalization of GNPs as multivalent drug delivery systems for the treatment of HIV.128
Regulatory T (Treg) cells are a specialized subpopulation of T-cells129 that are important components of the immune system130 and are also susceptible to HIV infection.131 Infection with HIV can lead to immune hyperactivation, which can subsequently result in erosion, depletion, or exhaustion of T-cells. Treg cells are therefore of significant importance during HIV infection because of the potential to suppress immune hyperactivation and inflammation,132 thereby preventing HIV disease progression. Jaramillo-Ruiz et al.,133 demonstrated for the first time that carbosilane dendrimers can be used for the prevention of Treg cell infection with HIV, in vitro. The negative phenotypic effects and decreased functionality of these cells due to HIV infection were also decreased with the application of these dendrimers. In addition, high biocompatibility and significant reduction in p24 antigen production was observed in cell culture and intracellularly.133
In a study by Parboosing et al.,134 RNA decoys in the form of a 16-mer oligoribonucleotide originating from the stem loop 3 of the HIV packaging signal, were attached to dendrimers in an effort to disrupt the packaging process of the HIV life cycle. The results of this study demonstrated efficient delivery into lymphocytes and modest cytoprotective effect against HIV infection.134
Jayant et al.,135 demonstrated that an ARV (tenofovir) and an investigational latency-reversing drug136 (vorinostat) can be co-encapsulated on ultrasmall (10 ± 3 nm) iron oxide nanoparticles. This research achieved a sustained drug release period (increased by 30%) showing absolute drug release profiles over a 5-day period with simultaneous activation of latent HIV in cultured human astrocytes. Improved transmigration ability across the BBB and in vitro antiviral efficacy was also demonstrated.135
Similarly, there has been a multitude of other studies that have investigated nanoparticles as novel agents in ARV drug delivery,137–139 other small molecule HIV inhibitors,140,141 and in vaccine development.142
HBV
HBV causes inflammation of the liver and is the cause of chronic infection in approximately 240 million people. Complications of HBV infection include cirrhosis and liver cancer and accounts for more than 780,000 deaths per year.143 Current anti-HBV nano-therapy includes interferon (IFN)-α, pegylated IFN (Pegasys®), lamivudine (Epivir®), adefovir (Hepsera®), entecavir (Baraclude®), telvivudine (Tyzeka®), and tenofovir (Viread®).144 Limitations of anti-HBV treatment include high costs, undesirable side effects, the risk of liver failure during hepatic flares, and development of drug resistance.145
New developments for HBV treatment using nanotechnology are being investigated. In an in vitro study done by Wang et al.,146 different types of cationic nanoparticles composed of biodegradable polymers were prepared by nanoprecipitation and solvent evaporation methods. These nanoparticles were evaluated for their transfection efficiencies in delivering siRNA and DNA to finally achieve inhibition of hepatitis B surface antigen (HBsAg) production. The results demonstrated that methoxy poly (ethyleneglycol)–poly(lactide) (mPEG–PLA) nanoparticles, containing a polyethyleneimine (PEI) layer, achieved the highest ant-HBV effect, and that successful delivery of siRNA is dependent on both size and surface charge.146
Hepatitis C virus
Hepatitis C virus (HCV) infects approximately 130–150 million people globally, with progression to liver cirrhosis or liver cancer being a common occurrence. Approximately 500,000 people die each year as a result of HCV-related liver disease.147 Standard nano-treatment for HCV infection is based on the use of PEGylated IFN and ribavirin.148
Peginterferon α-2a (Pegasys®) was approved by the FDA for the treatment of HCV in 2002, while Peginterferon α-2b (PegIntron ®) was available in 2001. The latter drug has a molecular mass of 31 kDa showing superior results in clinical studies (versus the un-PEGylated form IFN-α2b of 19 kDa).149
It has been demonstrated that IFN-α can be efficiently coupled to GNPs (physical binding), complexed with hyaluronic acid (HA) (via a thiolated interaction) for the target-specific and long-acting delivery in mouse models. These nano-complexes remained in the liver for 7 days post-injection (when compared to native IFN-α and PEG-Intron), thus offering great potential for the enhanced and prolonged treatment of HCV infection.150
Notable results showing >99% HCV inhibition were reported by Wang et al. where nanozymes were constructed using GNPs functionalized with RNAse A and anti-HCV oligonucleotides, for active cleavage of sequence-specific HCV RNA in both cell culture and mouse models. These nanozymes also displayed excellent stability against proteinase degradation, effective internalization, and good toxicity profiles.151
In a separate study152 cross-linked polymeric micelles (CLPM) were used to target HCV, in vitro. The micelles were loaded with the recently identified potent anti-HCV compound, camptothecin (CPT),153 which is also associated with limitations such as poor water solubility and chemical instability. The CLPMs used in this study enabled the formation of suitable amphiphilic micelles containing a hydrophobic core and hydrophilic shell, which demonstrated high loading capacity for CPT while maintaining HCV antiviral activity and reducing cytotoxicity.
In a study done by Moon et al.,154 siRNA targeting the proviral host factor required for HCV replication, was attached to lipidoid nanoparticles (lipid-like delivery molecules)155 and investigated for its antiviral properties in mouse models. The results showed potent anti-HCV activity over several days, and could have important implications for treatment in patients who are non-responsive to current HCV drug regimens.154
Cationic liposomes, particularly cholesterol-based types, are well suited for clinical application due to the decreased toxicity. Vitamin E (α-tocopherol) is rich with lipid-soluble antioxidants, with physiological pathways that can facilitate targeted delivery from the serum to the liver.36 Vitamin E was attached to cholesterol-based cationic liposomes and used to effectively deliver inhibitory siRNA specifically to the liver in mouse models. Both HCV core antigen production and firefly luciferase activity (used as a reporter gene to determine extent of HCV replication)156 were suppressed.36
Influenza
Influenza is a highly infectious respiratory disease157 with epidemics which are associated with morbidity worldwide,158 while annual epidemics and sporadic pandemics results in the deaths of millions of people. Antigenic shifts and mutations of the genome between different species of influenza results in the high degree of variation, thereby enabling the emergence of novel influenza strains and drug resistance.159 The emergence of new strains continues to pose a public health threat.160
STP702 (FluquitTM) from Sirnaomics is a polymer-based nanotherapeutic which is currently under preclinical investigation. This incorporates siRNA targeting the conserved regions of influenza for effective antiviral activity against H5N1 (avian flu), H1N1 (swine flu), and newly emerging H7N9.161
‘Nanotrap’ particles are thermoresponsive hydrogels which are capable of capturing live infectious virus, viral RNA, and viral proteins.162 This type of novel technology can be extended to treatment of infectious diseases such as the influenza virus. Hendricks et al.,163 used liposomes for the delivery of glycan sialylneolacto-N-tetraose c (LSTc)-sialoside – a synthetic decoy receptor for influenza binding. The results showed that these liposomes are highly effective at competitively binding and capturing influenza A viruses, and can inhibit infection of target cells in a dose-dependent manner.163
Hemagglutinin (HA) and neuraminidase (NA) are influenza glycoproteins, which function in viral attachment (to sialic-acid containing receptors on the cell surface) and release, respectively.164 Oseltamivir is a NA inhibitor that inhibits cell–cell spread and ongoing influenza transmission from occurring.165 In a study by Li et al.,166 oseltamivir-modified silver nanoparticles were shown to efficiently decrease H1N1 infection by inhibiting both HA and NA activities, in vitro. It was shown that prevention of DNA fragmentation, chromatin condensation, and caspase-3 activity also contributed to the antiviral properties of these nano-constructs. The toxicity profiles of these oseltamivir-modified silver nanoparticles, evaluated by cytopathic effect, transmission electron microscopy, and cell viability assays, were also demonstrated to be enhanced in MDCK cells, when compared to oseltamivir controls.166
In another study, titanium dioxide (TiO2) nanoparticles functionalized with DNA fragments targeting the 3′ non-coding region of influenza A virus were synthesized using a polylysine linker. These nanocomposites were able to enter cells without transfection agents and were demonstrated to be efficient inhibitors of influenza A virus, in vitro. Control samples containing random DNA sequences, unbound DNA fragments in the presence of nanoparticles, and naked nanoparticles showed minor antiviral effects.167
HSV
HSV is the causative agent of orofacial lesions, encephalitis (HSV-1),168 genital (HSV-2) infections,169 or disseminated disease.170 The standard treatment for HSV infections is acyclovir,171 with valacyclovir and famciclovir being precursor drugs with better bioavailability.172
Acyclovir is used for the management of HSV with treatment modalities including oral, parenteral, or topical application.173 There are, however, limitations associated with these treatment modes, which include poor oral bioavailability (15–30%), poor patient compliance, and low skin permeability, respectively.174 Buccal administration of drugs provides an alternative route to improve efficiency and absorption of otherwise poorly absorbed drugs.175 Nanospheres were evaluated as delivery agents for the buccal delivery of acyclovir in an effort to increase bioavailability. In vivo studies in rabbits showed a marked increase in the absorption of acyclovir-loaded nanospheres with peak plasma concentrations three fold higher than the free drug using oral dosing. The results also showed that the maximum drug concentration was prolonged (6 h versus 2 h), and this can reduce the frequency of drug administration.176
Several studies have demonstrated increased HSV inhibition using acyclovir-loaded nanoparticles,170,177 and the inherent antiviral action of silver nanoparticles.178–180 Increased bioavailability was also demonstrated in nanoparticles loaded with anti-herpetic siRNA in mice181 and acyclovir in rabbits.182 Another recent study conducted in rat models, showed that hybrid polymeric nanoparticles loaded with acyclovir effectively improved permeability through vaginal membranes, and can increase the tissue distribution and bioavailability compared to the free drug. This will have important implications for the clinical therapy of HSV in the female population.183 Other in vivo studies demonstrated similar results with increased drug retention times184,185 as well as enhanced dermal delivery.186
Human papillomavirus
Epithelial cells are the target cells for human papillomavirus (HPV) infection and can result in a range of symptoms, varying from common warts to cervical neoplasia and cancer. There are more than 100 types of HPV that have been classified, with only a subset being identified as high-risk.187
STP909 (Cervisil®) is a nanobased drug candidate, which incorporates siRNA for the treatment of HPV16 and HPV18 – two of the high-risk genotypes accounting for approximately 70% of cervical cancer cases. The results of in vitro studies show that strong duplexes are formed with the mRNA from the E7 genes in both HPV16 and HPV18, while in vivo rabbit studies demonstrate that these nanoparticles exert their antiviral activity by knock-down technology of the E7 gene.110 The reader is referred to several other gene silencing studies targeting the E7 gene in mice models188 and mammalian cells,189 as well as vaccine studies190,191 investigating nanoparticles in vaccine formulations.
Other viruses
SPL7013, marketed as VivaGel® (Starpharma, Melbourne, Australia), is a poly-L-lysine dendrimer-based pharmaceutical that has potent antiviral action against the sexually transmitted HSV, HIV69,192, and HPV.193,194 Safety studies in clinical trials, however, demonstrated mild irritation and inflammation in the participants from the VivaGel® arm of the study.195,196
Zika virus (ZIKV) is a mosquito-borne and sexually transmitted infection, which the WHO declared as a global emergency outbreak in 2016,197 affecting more than 20 countries in the Americas alone.198 The unavailability of effective drugs or a vaccine hinders the efforts to control ZIKV infection globally.199 VivaGel® has also been shown to have potent antiviral activity against ZIKV.111
Respiratory syncytial virus (RSV) is the leading cause of severe lower tract respiratory disease, including bronchiolitis and pneumonia,200,201 and is the leading cause of hospitalization of infants.202 It can also cause severe respiratory illness in the elderly and immunocompromised populations. ALN-RSV01 is a lipid-based nanoparticulate system containing siRNA and targets the nucleocapsid ‘N’ gene, a key viral protein of RSV. This was the first RNAi-based therapy approved for clinical trials and has since entered human phase II clinical trials, which demonstrate both safe and promising antiviral effects.203,204
Human parainfluenza 3 (HPIV-3) is an airborne virus, which infects human epithelial cells,205 and it is the causative agent of respiratory tract disease in infants and children.206 A recent study demonstrated inhibition of HPIV-3 replication, probably due to a cell-virus blocking mechanism using silver nanoparticles. The results of this study demonstrate that the inhibitory activity is dependent on both the size and zeta potential of the nanoparticles.178
Ebola virus disease (EVD) is an often fatal and highly contagious disease in humans and non-human primates and is responsible for sporadic outbreaks of Ebola hemorrhagic fever.207,208 TKM-130803, developed by Tekmira Pharmaceuticals, is a lipid-based nanosystem containing siRNA directed against EVD. Results from a recent clinical trial showed that the drug was well tolerated, however, no significant protection was achieved in infected patients. This may have been attributable to the high viral loads and advanced stage of disease of the enrolled patients.209 These results are in contrast to studies using non-human primates,210,211 where TKM-130803 was protective following administration of lethal doses Ebola virus. Further investigation into the drug formulation and dosage requirements of TKM-130803 are therefore warranted.
Human norovirus (HuNoV) has emerged as a leading cause of gastroenteritis outbreaks worldwide. The lack of effective antiviral treatment212 against HuNoV is due to the absence of an appropriate animal model and inability to propagate the virus in cell culture, required for antiviral research. Gold/copper sulfide (AuCuS) core-shell nanoparticles have demonstrated rapid antiviral activity against GI.1 (Norovirus) virus-like particles. The AuCuS nanoparticles interact with the capsid proteins causing denaturizing (causing disruption and ultimately inactivation), thereby resulting in its virucidal activity.213 Virus inactivation by metallic nanoparticles is a promising alternative to other physical and chemical methods.
Nanovaccines
Nanovaccinology has applications in both prophylactic and therapeutic approaches and can be used to either increase antigen processing or presentation and/or as an immunostimulatory adjuvant.51 This approach offers many advantages over traditional vaccine design; it has the potential to overcome the limitations associated with conventional vaccines (weak immunogenicity, intrinsic in vivo instability, toxicity and the requirement of multiple administrations).214
The enhanced humoral and cellular immune response that is elicited by nano based vaccines is due to the smaller size – which increases uptake by phagocytic cells, the gut-associated lymphoid tissue, and the mucosa-associated lymphoid tissue. This subsequently leads to enhanced antigen recognition and presentation.214
Surface modification of these nanocarriers with targeting moieties (peptides, carbohydrates, or antibodies) can facilitate specific and selective immune responses by targeting specific receptors on the surface of various immune cells.215–217 An additional benefit of incorporating nanoparticles in vaccine formulations is accomplishing slow and sustained release of antigens or adjuvants.51,214 Nanovaccines can also eliminate the requirement for cold-chain transport or storage as the formulation can be lyophilized, thereby prolonging shelf-life over an increased range of temperatures (from 0°C to 4°C).2 Another major advantage of using nanoparticles in vaccine delivery is that the sizes of these particles are approximately the same as viruses and bacteria, which the immune system readily identifies.91 Examples of key vaccine studies incorporating nanotechnology are presented in the following section.
The occurrence of hepatitis A virus (HAV) is sporadic and epidemic in nature and is most closely associated with food-borne infections, transmitted via the fecal–oral route.218 No specific treatment for HAV exists; however, it has been shown that cyclosporine A and silibinin can inhibit viral replication.219 Epaxal® is an approved, liposomal-based vaccine for the prevention of HAV15 and can be applied as an adjuvant with immuno-potentiating reconstituted influenza virus (IRIV); containing purified influenza antigens (neuraminidase and haemagglutinin).220 Liposomes (virosomes) have been used to prepare these aluminum-free vaccines based on formalin-inactivated HAV (strain RG-SB).107 Exapal® demonstrates good immunogenicity, efficacy, and tolerability in both adults and children.221
Limitations of parenteral vaccines include requirements such as trained medical personnel, cold-chain maintenance, danger of reusing needles, high-dose regimens, and possibility of non-responsive immune response.222 Mucosally administered vaccines are therefore an alternative approach that requires investigation. Chitosan, with its non-toxic, biodegradable and good biological profile, was investigated for the ability to form positively charged nanoparticles to facilitate the incorporation of other negatively charged therapeutic proteins or antigens by electrostatic interactions.223 Both humoral and mucosal immune responses were elicited in mouse models, making this approach a valuable gene delivery system for nasal vaccination against HBV.
HepaXen is a liposome-based vaccine, which was initially intended to have antiviral action against hepatitis A, C, and E. Preclinical studies with this vaccine, which incorporates recombinant hepatitis B surface antigen and plasmid DNA encoding the protein, elicited an immune response which was 20 times greater than that of a leading prophylactic vaccine.224 It is, however, uncertain whether a clinically valid vaccine candidate will become available,225 as the last update from Lipoxen (the bio-pharmaceutical company involved in HepaXen’s development) was from a report in 2008, which stated that a partner organization (Serum Institute of India) would be responsible for the vaccines co-development.226
Inflexal® V is a licensed virosomal adjuvant-based influenza vaccine, which has been on the market since 1995. The virosomes consist of reconstituted influenza virus envelope proteins, lacking the inner core and nucleic acid. Inflexal® V is extremely biocompatible and efficacious as it mimics natural infection. This vaccine also represents a good immunogenicity profile and is effective in immunocompromised and immunocompetent adults, children, and the elderly.104 Influvac® is another licensed surface antigen inactivated subunit vaccine against influenza infection, showing good immunogenicity and safety profiles.109,227
In a study done by Tao et al.,228 the highly conserved extracellular matrix 2 protein (M2e) of influenza A virus was attached to 12 nm GNPs via thiol-gold interactions. Vaccination in mouse models provided complete protection following exposure to lethal PR8-H1N1 influenza, when the adjuvant CpG (a cysteine-guanine rich oligonucleotide) was added to the M2e-gold conjugates.228
A number of other reports have been published that report results on influenza vaccines and the success in eliciting protective immunity.228–233 The reader is also referred to literature on recent vaccine research done on other viruses such as Ebola virus,234 RSV,235–237 HPV,190 norovirus, and rotavirus.229
Nanoparticle uptake
Uptake is an important consideration in the design of nanotherapeutics, because this will have a direct influence on the therapeutic load, and hence the appropriate dose, entering the cells. Variations in the physical properties of the nanoparticles, as well as differences in the cellular membrane characteristics, can affect the efficacy of the uptake process.238,239
Accordingly, nanoparticle size is a major determinant of cellular uptake with approximately 50 nm in diameter being optimum for non-phagocytic cells.239 Various ligands (proteins or peptides) can be used to enhance cellular uptake.240 For example, the HIV-derived TAT peptide is a well-recognized cell penetrating peptide, which can be used to facilitate cellular entry.241,242 The surface charge of the nanoparticle has influence on whether or not it can traverse the negatively charged cell membrane,18 whereby increasing the overall surface charge of the nanoparticle can result in increased uptake across cellular membranes.239
Mechanisms of cellular internalization of nanoparticles include phagocytosis, macropinocytosis, caveolar-mediated endocytosis, or clathrin-mediated endocytosis.19 The size of the nanoparticle also determines the mechanism by which nanoparticles enter the cells and where it subsequently localizes intracellularly.11 It has recently been demonstrated that the shape of nanoparticles is also a determining factor of the mechanism of uptake.243–247 Therefore, knowledge of both of these aspects is invaluable in the engineering of nanoparticles targeted to specific micro-environments.
Antigenicity
The complement system forms part of the immune system and can be classed into four pathways (classical, alternate, lectin, and lytic pathways), which can be stimulated by synthetic materials.248 The classical complement pathway is driven by antigen/antibody complex formation, while the others are antibody independent.249 Certain nanocarriers, such as immunoliposomes and carbon nanotubes, are able to activate the complement system,250 and can therefore promote opsonization or clearance of foreign nanomaterials,251 thereby limiting its in vivo utility.
As described earlier, PEG is an important polymer for incorporation into nanoparticles (and drug carriers in general), mainly to facilitate enhanced bioavailability and therapeutic efficacy. The presence of anti-PEG antibodies has been demonstrated in patients receiving PEGylated drugs, but also in healthy individuals who remain unexposed to PEGylated therapeutics.252 In additional, PEG polymers and PEG-like structures may be present in various consumer products,253 cosmetics,254 laxatives, and other pharmaceutical applications.51,255 In this regard, antibodies that are specifically directed at PEG may compromise the safety, efficacy256, and tolerance257 of PEGylated nanocarriers.
Nanoparticle biodegradation and elimination
As the range of nanoparticles and their respective applications in medicine increases, it also becomes increasingly necessary to better understand the biodegradation processes. Biodegradation processes are also a critical determinant of sustained drug release and biodistribution profiles.258 A systematic and complete analysis of the absorption, distribution, metabolism, and excretion pharmacokinetics of nanoparticles will result in improved and rational drug design.259 Several factors, such as polymer composition, tacticity, hydrophobicity/hydrophilicity profiles, particle size, and molecular weight, can affect the rate of degradation.260 Nanoparticle degradation has, however, been poorly studied at the cellular level,261 and there is a paucity of information from in vivo studies.
Eventually, nanoparticles must exit the cell (via exocytosis) if biodegradation did not occur. The rate of exocytosis depends largely on nanoparticle composition and surface properties. For instance, cationic particles that tend to agglomerate intracellularly262 have a slower rate of elimination compared to PEGylated particles that avoid protein interaction and subsequent agglomeration.263 Subsequently, nanoparticles are excreted from the body. Nanoparticles <5 nm may be excreted in the urine while larger particles are often reabsorbed into systemic circulation and excreted mainly via the liver, kidneys, or colon.264,265
Some nanoparticles may be too large to undergo renal clearance and can accumulate in the body since they cannot be degraded.11,49 Uptake by macrophages of the mononuclear phagocytic system (MPS) can then modify/increase blood circulation time.266 This also has important implications for viruses such as HIV, which infect and reside in these cells.
Limitations of nanoparticles as therapeutics
The poor permeability of biological membranes can limit the use of many important therapeutic agents.267 Furthermore, not all cell types have the required machinery to conduct any one of the endocytotic pathways (macropinocytosis, phagocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, and clathrin- and caveolin-independent endocytosis),268 thereby limiting uptake and utility of nanoparticles in medicine.
Non-specific cellular uptake can be defined as the internalization of extraneous materials and is characterized by poor material selectivity.269 Non-specific uptake of nanoparticles by macrophages (which are the main component of the immune system) and organs of the reticuloendothelial system (e.g. liver and spleen) presents a significant limitation for its use as therapeutic agents. This phenomenon results in removal of the nanoparticles from circulation before reaching the target sites, thereby reducing treatment efficacy.270,271 A common approach to overcome non-specific interactions is to introduce PEG molecules with an optimum molecular weight onto the nanoparticle surface272 and the use of active targeting ligands.269
Certain physical processes that enable contact between nanoparticle surfaces can cause aggregation of nanoparticles, thereby resulting in clusters, which renders the particles larger than the nanometer range. This has important implications for the uptake,273 persistence,274 toxicity,275,276 fate, and mobility277 of nanoparticles. The use of polymers, which act as steric stabilizers on the nanoparticle surface, however, can decrease aggregation in aqueous suspensions.278 Other technical limitations of systemically administered nanoparticle-based therapeutics include uptake by the reticuloendothelial system and macrophages (discussed before), renal and biliary clearance, and enzymatic degradation of any proteins that may be present.279,280
As the range of nanoparticles and their utility in biomedical applications increases, so too does the requirement for toxicity studies, to evaluate the corresponding safety concerns in humans. Important parameters to consider include type of nanoparticle and its surface modification, dosage administered and the biodistribution at both cellular and organismal levels.281 Examples of toxicity concerns include the effects of nanoparticle accumulation, circulation time,282 and subsequent slow elimination or clearance.259 Nanoparticle toxicity can potentially result in pulmonary toxicity,283 renal and hepatotoxicity,284 neurotoxicity,285 and spermatoxicity.286
Nanoparticle requirements that are unique to viral infections
Viruses are obligate intracellular parasites whose interactions with host cells often comprise a variety of receptor-ligand interactions. The intrinsic characteristics of viral disease, which include complexities in life cycles, different stages of replication in different sub-cellular compartments or organelles, differences in replication dynamics, the possibility of latent infection in inaccessible biological compartments and the development of drug resistance, all result in unique requirements for drug design.
Nanotechnology has been shown to be highly effective for biomedical applications such as cancer therapy,287 with several marketed compounds such as Caelyx® and Doxil®.288 A major limitation of chemotherapeutic agents, however, is the lack of specificity to the tumorous site, thereby necessitating large doses of toxic drugs to be delivered in order to achieve sufficient concentrations.287
An important requirement for any effective therapeutic agent is delivery to the appropriate place at the appropriate concentrations for the appropriate period of time.289 Two types of targeting mechanisms are possible. Passive targeting can occur due to increased permeability or leakiness (which can be caused by malignancy or inflammation) of the local vasculature. This results in the diseased area becoming more permissive to the accumulation of the nanotherapeutic agent. On the other hand, active targeting requires ligand (peptide, antibody, etc.) attachment to direct the nanotherapeutic to specific receptors, epitopes, or sites.11 Active targeting is an important requirement for the treatment of virus infection because many antiviral drugs are required to localize at specific sub-cellular regions or organelles, which is dependent on the stage of replication and the mode of action of the drug. For example, integrase inhibitors preventing the strand transfer reaction of the HIV life cycle requires activity specifically in the nucleus of the cell where this process occurs.290 Active targeting by incorporating a nuclear localization signal on the nanocarrier, for example, is therefore desirable to enhance specificity.
As previously mentioned, the size of the therapeutic is another critical consideration for infectious disease drug development, where entry to biologically inaccessible compartments (example viruses traversing the BBB and blood-testis barrier)33 is necessary to prevent the establishment of latent infection with ongoing low-level replication. The BBB is a compactly controlled and selectively permeable barrier,291 which restricts the passage of many substances, thereby hindering drug delivery (in efficient concentrations) from the blood to the brain.292 HIV is able to establish latency in the brain where there is no exposure to ARV drugs.291 Replication in this compartment can lead to several neurological disorders.293 Nanocarriers have been successfully developed, which are capable of overcoming these barriers to achieve both targeted and specific delivery.291,294 The increased ability of ARV-conjugated nanoparticles to cross the BBB, while therapeutic efflux is reduced, is necessary to control and limit viral replication in this anatomically privileged site.295 The use of nanoparticles to facilitate entry into these compartments is therefore an excellent option due to the small size. The reader is referred to recent literature on the challenges and recent advances in the treatment of HIV across the BBB.296,297
The drug payload to nanocarrier size ratio is an important consideration. High drug-loading and entrapment efficiency298 is necessary to ensure that sufficient concentrations at the target site is available, thereby decreasing the possibility of the development of drug resistance. Targeted delivery of nanoformulations directly to the target site will also increase drug efficacy.299
Premature drug-release has important implications for the treatment of systemic and intracellular infections.64 Nanoparticles are retained for much longer periods than conventional therapeutics in the body91 and therefore slow and sustained release is achievable. Controlled and sustained release is also an important consideration to ensure that the drug concentration is maintained within the therapeutic window,81 thereby also decreasing the possibility of drug resistance.
Conclusion and future perspective
Nanoparticle-based delivery systems present new opportunities to overcome challenges associated with conventional drug therapies and have therefore attracted enormous interest in the treatment of viral infections. Nanomaterials can be engineered to incorporate conventional antiviral properties with those modifications that are unique to nanosystems (ultra small and controllable size, large surface area to volume ratio, and the ability to tailor the surface with the possibility of multi-functionalization). This is undoubtedly a promising tool for biomedical research and clinical use.
The recent advances in nanomedicine [ability to encapsulate or incorporate drugs with surface modification, targeted drug delivery (intracellularly or to specific cell populations), biocompatibility, and the ability to achieve slow and sustained drug release] offers superior therapeutic potential, compared to conventional approaches. These modifications can overcome common limitations associated with nanoparticles for biomedical applications, including increased permeability of biological membranes with associated specific uptake, and decreased toxicity profiles. Similarly, poorly water soluble and unstable drugs can be modified and complexed with nanocarriers to achieve improved solubility and stability under physiological conditions.
Future research should explore the possibility of (1) multi-functionalization to achieve concurrent drug delivery and imaging (via a fluorescent signal, for example), to determine in vitro localization, and specific cell/tissue/compartment targeting (using targeting ligands like peptides and proteins or molecular recognition strategies, for example) and (2) multiplexing, in order to increase the spectrum of disease that can be treated in heterogeneous populations, by simple, reliable and cost-effective methods. Improvements (increasing bioavailability and reducing toxicity) of currently available conventional antivirals should also be explored using advances in nanotechnology. As previously discussed, ‘nanotraps’ have illustrated effective inhibition of influenza viruses. This can be extended to other viruses such as HIV, hepatitis, and so on by specifically modifying the attachment carbohydrates of the defined host receptors. To this end, further research and development of these particles are required.
There is a paucity of information on the interaction between the immune system and nanomaterials.250 When engineered to enable immune response modulation, as in the case of nanovaccines, two modes of action are possible: (1) to enhance antigen presentation and processing or (2) to function as an immunostimulatory adjuvant, both of which have important implications in drug design. Studies investigating the immunological characterization of nanocarriers are necessary to advance these systems closer to the reality of pharmaceutical application. In addition, studies relating to the pre-existence and induction of anti-PEG antibodies, and the impact of PEGylated nanotherapeutics, require careful attention.
The incorporation of nanotechnology for the treatment of infectious disease offers enormous potential for enhanced mechanisms of action of currently available therapeutics, or the development of novel therapeutics, both of which are desperately required in an era of drug resistance. Despite the various advantages that these nanoparticles have compared to conventional therapies, investigation into the toxicities and potential deleterious effects of certain nanosystems are still required.
Microbial evolution, resulting in the development of drug resistance, remains to be a major public health concern. Similarly, the evolution of technology, particularly exploiting the dynamic and versatile nature of nanomedicine, is necessary for the effective combating of infectious disease agents.
Acknowledgments
The authors would like to thank Dr Andrew Swanson for the images in Figures 1 and 2.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We acknowledge the National Health Laboratory Service Research Trust (grant number 94503) and the National Research Foundation (grant number 99294) for the financial support. The Medical Research Council and Aspen Pharmacare are also acknowledged.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Lavanya Singh, Department of Virology, National Health Laboratory Service, University of KwaZulu-Natal, Durban, South Africa.
Hendrik G. Kruger, Catalysis and Peptide Research Unit, University of KwaZulu-Natal, Durban, South Africa
Glenn E.M. Maguire, Catalysis and Peptide Research Unit, University of KwaZulu-Natal, Durban, South Africa
Thavendran Govender, Catalysis and Peptide Research Unit, University of KwaZulu-Natal, Durban, South Africa.
Raveen Parboosing, Department of Virology, National Health Laboratory Service, University of KwaZulu-Natal, Durban, South Africa.
References
- 1. Panáček A, Kolář M, Večeřová R, et al. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials 2009; 30: 6333–6340. [DOI] [PubMed] [Google Scholar]
- 2. Qasim M, Lim D-J, Park H, et al. Nanotechnology for diagnosis and treatment of infectious diseases. J Nanosci Nanotechnol 2014; 14: 7374–7387. [DOI] [PubMed] [Google Scholar]
- 3. Mehendale R, Joshi M, Patravale VB. Nanomedicines for treatment of viral diseases. Crit Rev Ther Drug Carrier Syst 2013; 30: 1–49. [DOI] [PubMed] [Google Scholar]
- 4. Little SJ, Holte S, Routy J-P, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347: 385–394. [DOI] [PubMed] [Google Scholar]
- 5. Shafer R, Rhee S-Y, Pillay D, et al. HIV-1 protease and reverse transcriptase mutations for drug resistance surveillance. AIDS 2007; 21: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lockhat HA, Silva JR, Alves CN, et al. Binding free energy calculations of nine FDA-approved protease inhibitors against HIV-1 subtype C I36T↑ T containing 100 amino acids per monomer. Chem Biol Drug Des 2016; 87: 487–498. [DOI] [PubMed] [Google Scholar]
- 7. Maseko SB, Natarajan S, Sharma V, et al. Purification and characterization of naturally occurring HIV-1 (South African subtype C) protease mutants from inclusion bodies. Protein Expr Purif 2016; 122: 90–96. [DOI] [PubMed] [Google Scholar]
- 8. Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis 2009; 48: S3–S13. [DOI] [PubMed] [Google Scholar]
- 9. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis 2006; 42: S82–S89. [DOI] [PubMed] [Google Scholar]
- 10. Nalwa HS. Handbook of nanostructured materials and nanotechnology, vol. 1 San Diego, CA: Academic Press, 1999, pp. 1–3. [Google Scholar]
- 11. Parboosing R, Maguire GE, Govender P, et al. Nanotechnology and the treatment of HIV infection. Viruses 2012; 4: 488–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scheller FW, Bier FF, Pfeiffer D. Biosensoren: grundlagen und anwendungen. TM-Tech Mess 1995; 62: 213–219. [Google Scholar]
- 13. Niemeyer CM, Mirkin CA. Nanobiotechnology: concepts, applications and perspectives, vol. 1 Weinheim: John Wiley & Sons, 2004. [Google Scholar]
- 14. Medepalli KK. Advanced nanomaterials for biomedical applications. Cambridge: ProQuest, 2008. [Google Scholar]
- 15. Schütz CA, Juillerat-Jeanneret L, Mueller H, et al. Therapeutic nanoparticles in clinics and under clinical evaluation. Nanomedicine (Lond) 2013; 8: 449–467. [DOI] [PubMed] [Google Scholar]
- 16. Kumar A, Ma H, Zhang X, et al. Gold Nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment. Biomaterials 2012; 33: 1180–1189. [DOI] [PubMed] [Google Scholar]
- 17. McNeil SE. Unique benefits of nanotechnology to drug delivery and diagnostics. Methods Mol Biol 2011; 697: 3–8. [DOI] [PubMed] [Google Scholar]
- 18. Caron J, Reddy LH, Lepêtre-Mouelhi S, et al. Squalenoyl nucleoside monophosphate nanoassemblies: new prodrug strategy for the delivery of nucleotide analogues. Bioorganic Med Chem Lett 2010; 20: 2761–2764. [DOI] [PubMed] [Google Scholar]
- 19. Petros RA, Desimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 2010; 9: 615–627. [DOI] [PubMed] [Google Scholar]
- 20. Gagliardi M. Biomimetic and bioinspired nanoparticles for targeted drug delivery. Ther Deliv 2017; 8: 289–299. [DOI] [PubMed] [Google Scholar]
- 21. Sanvicens N, Marco MP. Multifunctional nanoparticles – properties and prospects for their use in human medicine. Trends Biotechnol 2008; 26: 425–433. [DOI] [PubMed] [Google Scholar]
- 22. Bowman MC, Ballard TE, Ackerson CJ, et al. Inhibition of HIV fusion with multivalent gold nanoparticles. J Am Chem Soc 2008; 130: 6896–6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun RW, Chen R, Chung NP, et al. Silver nanoparticles fabricated in Hepes buffer exhibit cytoprotective activities toward HIV-1 infected cells. Chem Commun (Camb) 2005; 5059–5061. [DOI] [PubMed] [Google Scholar]
- 24. Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, et al. Mode of antiviral action of silver nanoparticles against HIV-1. J Nanobiotechnology 2010; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mallipeddi R, Rohan LS. Progress in antiretroviral drug delivery using nanotechnology. Int J Nanomedicine 2010; 5: 533–547. [PMC free article] [PubMed] [Google Scholar]
- 26. Wang W, Guo Z, Chen Y, et al. Influence of generation 2-5 of PAMAM dendrimer on the inhibition of Tat peptide/TAR RNA binding in HIV-1 transcription. Chem Biol Drug Des 2006; 68: 314–318. [DOI] [PubMed] [Google Scholar]
- 27. Chiappetta DA, Facorro G, De Celis ER, et al. Synergistic encapsulation of the anti-HIV agent efavirenz within mixed poloxamine/poloxamer polymeric micelles. Nanomedicine 2011; 7: 624–637. [DOI] [PubMed] [Google Scholar]
- 28. Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed 2007; 18: 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm 2008; 5: 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santos-Martinez MJ, Rahme K, Corbalan JJ, et al. Pegylation increases platelet biocompatibility of gold nanoparticles. J Biomed Nanotechnol 2014; 10: 1004–1015. [DOI] [PubMed] [Google Scholar]
- 31. Gabizon A, Catane R, Uziely B, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 1994; 54: 987–992. [PubMed] [Google Scholar]
- 32. Muthu MS, Singh S. Targeted nanomedicines: effective treatment modalities for cancer, AIDS and brain disorders. Nanomedicine (Lond) 2009; 4: 105–118. [DOI] [PubMed] [Google Scholar]
- 33. De Oliveira MP, Garcion E, Venisse N, et al. Tissue distribution of indinavir administered as solid lipid nanocapsule formulation in mdr1a (+/+) and mdr1a (−/−) CF-1 mice. Pharm Res 2005; 22: 1898–1905. [DOI] [PubMed] [Google Scholar]
- 34. Hellmuth J, Valcour V, Spudich S. CNS reservoirs for HIV: implications for eradication. J Virus Erad 2015; 1: 67–71. [PMC free article] [PubMed] [Google Scholar]
- 35. Mahajan SD, Aalinkeel R, Law W-C, et al. Anti-HIV-1 nanotherapeutics: promises and challenges for the future. Int J Nanomedicine 2012; 7: 5301–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duan L, Yan Y, Liu J, et al. Target delivery of small interfering RNAs with vitamin E-coupled nanoparticles for treating hepatitis C. Sci Rep 2016; 6: 24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adesina SK, Akala EO. Nanotechnology approaches for the delivery of exogenous siRNA for HIV therapy. Mol Pharm 2015; 12: 4175–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen BM, Su YC, Chang CJ, et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem 2016; 88: 10661–10666. [DOI] [PubMed] [Google Scholar]
- 39. Toub N, Malvy C, Fattal E, et al. Innovative nanotechnologies for the delivery of oligonucleotides and siRNA. Biomed Pharmacother 2006; 60: 607–620. [DOI] [PubMed] [Google Scholar]
- 40. Bawa R. Nanopharmaceuticals: nanopharmaceuticals. Eur J Nanomed 2010; 3: 34–40. [Google Scholar]
- 41. Walsh T, Viviani M-A, Arathoon E, et al. New targets and delivery systems for antifungal therapy. Med Mycol 2010; 38: 335–347. [PubMed] [Google Scholar]
- 42. Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol 2007; 2: 469–478. [DOI] [PubMed] [Google Scholar]
- 43. Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release 2016; 224: 86–102. [DOI] [PubMed] [Google Scholar]
- 44. Mora-Huertas C, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm 2010; 385: 113–142. [DOI] [PubMed] [Google Scholar]
- 45. Baram-Pinto D, Shukla S, Richman M, et al. Surface-modified protein nanospheres as potential antiviral agents. Chem Commun 2012; 48: 8359–8361. [DOI] [PubMed] [Google Scholar]
- 46. Bergeron MG, Desormeaux A. Liposomes encapsulating antiviral drugs, www.google.ch/patents/US5773027 (1998, accessed 12 December 2016).
- 47. Dutta T, Agashe HB, Garg M, et al. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro: research paper. J Drug Target 2007; 15: 89–98. [DOI] [PubMed] [Google Scholar]
- 48. Giljohann DA, Seferos DS, Daniel WL, et al. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl 2010; 49: 3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ochekpe NA, Olorunfemi PO, Ngwuluka NC. Nanotechnology and drug delivery part 2: nanostructures for drug delivery. Trop J Pharm Res 2009; 8: 275–287. [Google Scholar]
- 50. Stevanovic M, Uskokovic D. Poly (lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci 2009; 5: 1–14. [Google Scholar]
- 51. Zhao L, Seth A, Wibowo N, et al. Nanoparticle vaccines. Vaccine 2014; 32: 327–337. [DOI] [PubMed] [Google Scholar]
- 52. Aggarwal P, Hall JB, McCleland CB, et al. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev 2009; 61: 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kovochich M, Marsden MD, Zack JA. Activation of latent HIV using drug-loaded nanoparticles. PLoS ONE 2011; 6: e18270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Santos-Magalhães NS, Mosqueira VCF. Nanotechnology applied to the treatment of malaria. Adv Drug Deliv Rev 2010; 62: 560–575. [DOI] [PubMed] [Google Scholar]
- 55. Mignani S, Majoral J-P. Dendrimers as macromolecular tools to tackle from colon to brain tumor types: a concise overview. New J Chem 2013; 37: 3337–3357. [Google Scholar]
- 56. Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001; 70: 1–20. [DOI] [PubMed] [Google Scholar]
- 57. Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 2007; 65: 259–269. [DOI] [PubMed] [Google Scholar]
- 58. Zeng P, Xu Y, Zeng C, et al. Chitosan-modified poly (D, L-lactide-co-glycolide) nanospheres for plasmid DNA delivery and HBV gene-silencing. Int J Pharm 2011; 415: 259–266. [DOI] [PubMed] [Google Scholar]
- 59. Dehghan S, Kheiri MT, Tabatabaiean M, et al. Dry-powder form of chitosan nanospheres containing influenza virus and adjuvants for nasal immunization. Arch Pharm Res 2013; 36: 981–992. [DOI] [PubMed] [Google Scholar]
- 60. Mohajer M, Khameneh B, Tafaghodi M. Preparation and characterization of PLGA nanospheres loaded with inactivated influenza virus, CpG-ODN and Quillaja saponin. Iran J Basic Med Sci 2014; 17: 722–726. [PMC free article] [PubMed] [Google Scholar]
- 61. Gregory AE, Williamson D, Titball R. Vaccine delivery using nanoparticles. Front Cell Infect Microbiol 2013; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thorley AJ, Tetley TD. New perspectives in nanomedicine. Pharmacol Ther 2013; 140: 176–185. [DOI] [PubMed] [Google Scholar]
- 63. Kuntworbe N, Martini N, Shaw J, et al. Malaria intervention policies and pharmaceutical nanotechnology as a potential tool for malaria management. Drug Dev Res 2012; 73: 167–184. [Google Scholar]
- 64. Amin R. Nanotechnology in controlling infectious disease. In: Hunter RJ, Preedy VR. (eds) Nanomedicine in health and disease. Enfield, NH: Science Publishers, 2011, pp. 167–183. [Google Scholar]
- 65. Garnett MC. Targeted drug conjugates: principles and progress. Adv Drug Deliv Rev 2001; 53: 171–216. [DOI] [PubMed] [Google Scholar]
- 66. Perrie Y, Mohammed AR, Kirby DJ, et al. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm 2008; 364: 272–280. [DOI] [PubMed] [Google Scholar]
- 67. Kabanov AV, Batrakova EV, Alakhov VY. Pluronic ® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 2002; 82: 189–212. [DOI] [PubMed] [Google Scholar]
- 68. Arifin DR, Palmer AF. Polymersome encapsulated hemoglobin: a novel type of oxygen carrier. Biomacromolecules 2005; 6: 21720–22181. [DOI] [PubMed] [Google Scholar]
- 69. Zhang L, Gu F, Chan J, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 2008; 83: 761–769. [DOI] [PubMed] [Google Scholar]
- 70. Moretton MA, Glisoni RJ, Chiappetta DA, et al. Molecular implications in the nanoencapsulation of the anti-tuberculosis drug rifampicin within flower-like polymeric micelles. Colloids Surf B Interfaces 2010; 79: 467–479. [DOI] [PubMed] [Google Scholar]
- 71. Sahoo SK, Labhasetwar V. Nanotech approaches to drug delivery and imaging. Drug Discov Today 2003; 8: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 72. Caminade A-M, Laurent R, Majoral J-P. Characterization of dendrimers. Adv Drug Deliv Rev 2005; 57: 2130–2146. [DOI] [PubMed] [Google Scholar]
- 73. Muèller RH, Maèder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 2000; 50: 161–177. [DOI] [PubMed] [Google Scholar]
- 74. Gupta U, Jain NK. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv Drug Deliv Rev 2010; 62: 478–490. [DOI] [PubMed] [Google Scholar]
- 75. Patel PA, Patravale VB. AmbiOnp: solid lipid nanoparticles of amphotericin B for oral administration. J Biomed Nanotechnol 2011; 7: 632–639. [DOI] [PubMed] [Google Scholar]
- 76. Xie S, Tao Y, Pan Y, et al. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J Control Release 2014; 187: 101–117. [DOI] [PubMed] [Google Scholar]
- 77. Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine 2010; 6: 257–262. [DOI] [PubMed] [Google Scholar]
- 78. Cui Y, Zhao Y, Tian Y, et al. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012; 33: 2327–2333. [DOI] [PubMed] [Google Scholar]
- 79. Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005; 1: 325–327. [DOI] [PubMed] [Google Scholar]
- 80. Skirtach AG, Muñoz Javier A, Kreft O, et al. Laser-induced release of encapsulated materials inside living cells. Angew Chem Int Ed Engl 2006; 45: 4612–4617. [DOI] [PubMed] [Google Scholar]
- 81. Ghosh P, Han G, De M, et al. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev 2008; 60: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 82. Schmid G. Large clusters and colloids. Metals in the embryonic state. Chem Rev 1992; 92: 1709–1727. [Google Scholar]
- 83. Brust M, Walker M, Bethell D, et al. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc, Chem Commun 1994; 7: 801–802. [Google Scholar]
- 84. Templeton AC, Wuelfing WP, Murray RW. Monolayer-protected cluster molecules. Acc Chem Res 2000; 33: 27–36. [DOI] [PubMed] [Google Scholar]
- 85. Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 1951; 11: 55–75. [Google Scholar]
- 86. Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973; 241: 20–22. [Google Scholar]
- 87. Grabar KC, Freeman RG, Hommer MB, et al. Preparation and characterization of Au colloid monolayers. Anal Chem 1995; 67: 735–743. [Google Scholar]
- 88. Gong P, Li H, He X, et al. Preparation and antibacterial activity of Fe3o4@Ag nanoparticles. Nanotechnology 2007; 18: 285604. [Google Scholar]
- 89. Cho K-H, Park J-E, Osaka T, et al. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 2005; 51: 956–960. [Google Scholar]
- 90. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009; 27: 76–83. [DOI] [PubMed] [Google Scholar]
- 91. Huh AJ, Kwon YJ. ‘Nanoantibiotics’: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 2011; 156: 128–145. [DOI] [PubMed] [Google Scholar]
- 92. Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, et al. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnology 2011; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gerrity D, Ryu H, Crittenden J, et al. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J Environ Sci Health A Tox Hazard Subst Environ Eng 2008; 43: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 94. Antoine TE, Mishra YK, Trigilio J, et al. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antiviral Res 2012; 96: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ingle AP, Duran N, Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol 2014; 98: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 96. Yadavalli T, Shukla D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomedicine 2017; 13: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang Z-H, Zhuo Y, Yuan R, et al. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens Bioelectron 2015; 69: 321–327. [DOI] [PubMed] [Google Scholar]
- 98. Chaudhuri RG, Paria S. Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 2011; 112: 2373–2433. [DOI] [PubMed] [Google Scholar]
- 99. Tojo C, Buceta D, López-Quintela MA. Core-shell nanocatalysts obtained in reverse micelles: structural and kinetic aspects. J Nanomater 2015; 2015: 1–10. [Google Scholar]
- 100. Chatterjee K, Sarkar S, Rao K, et al. Core/shell nanoparticles in biomedical applications. Adv Colloid Interface Sci 2014; 209: 8–39. [DOI] [PubMed] [Google Scholar]
- 101. Cheong S, Ferguson P, Hermans IF, et al. Synthesis and stability of highly crystalline and stable iron/iron oxide core/shell nanoparticles for biomedical applications. Chem Plus Chem 2012; 77: 135–140. [Google Scholar]
- 102. Varanda LC, Imaizumi M, Santos FJ, et al. Iron oxide versus Fe55Pt45/Fe3O4: improved magnetic properties of core/shell nanoparticles for biomedical applications. IEEE Trans Magn 2008; 44: 4448–4451. [Google Scholar]
- 103. Arias JL, López-Viota M, Ruiz MA, et al. Development of carbonyl iron/ethylcellulose core/shell nanoparticles for biomedical applications. Int J Pharm 2007; 339: 237–245. [DOI] [PubMed] [Google Scholar]
- 104. Herzog C, Hartmann K, Künzi V, et al. Eleven years of inflexal® V – a virosomal adjuvanted influenza vaccine. Vaccine 2009; 27: 4381–4387. [DOI] [PubMed] [Google Scholar]
- 105. Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine 2002; 20: B17–B23. [DOI] [PubMed] [Google Scholar]
- 106. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010; 10: 787–796. [DOI] [PubMed] [Google Scholar]
- 107. Bovier PA. Epaxal®: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines 2008; 7: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 108. Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polym Chem 2011; 2: 1442–1448. [Google Scholar]
- 109. Waknine Y. International Approvals: Avalox IV, Pegasys, Influvac – Medscape, 2005, http://www.medscape.com/viewarticle/508903
- 110. Sirnaomics. Advancing RNAi therapeutics, vol. 2016, http://new.sirnaomics.com (2016, accessed 24 November 2016). [Google Scholar]
- 111. Starpharma. VivaGel active against Zika virus, www.starpharma.com/news/281 (2016, accessed 10 October 2016).
- 112. Rodriguez B, Asmuth DM, Matining RM, et al. Safety, tolerability and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J Acquir Immune Defic Syndr 2013; 64: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Molina J, Squires K, Sax PE, et al. Doravirine is non-inferior to darunavir/r in phase 3 treatment-naive trial at week 48. In: Program and abstracts of the 2017 conference on retroviruses and opportunistic infections (CROI 2017), Seattle, WA, 13–16 February 2017, abstract 45LB. [Google Scholar]
- 114. Seto W-K, Yuen M-F. New pharmacological approaches to a functional cure of hepatitis B. Clin Liver Dis 2016; 8: 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res 2010; 85: 39–58. [DOI] [PubMed] [Google Scholar]
- 116. De Béthune MP. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years. Antiviral Res 1989–2009; 2010; 85: 75–90. [DOI] [PubMed] [Google Scholar]
- 117. Flexner C. HIV-protease inhibitors. N Engl J Med 1998; 338: 1281–1293. [DOI] [PubMed] [Google Scholar]
- 118. Münch J, Ständker L, Adermann K, et al. Discovery and optimization of a natural HIV-1 entry inhibitor targeting the gp41 fusion peptide. Cell 2007; 129: 263–275. [DOI] [PubMed] [Google Scholar]
- 119. Fätkenheuer G, Pozniak AL, Johnson MA, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med 2005; 11: 1170–1172. [DOI] [PubMed] [Google Scholar]
- 120. Barbaro G, Scozzafava A, Mastrolorenzo A, et al. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des 2005; 11: 1805–1843. [DOI] [PubMed] [Google Scholar]
- 121. Esté JA, Cihlar T. Current status and challenges of antiretroviral research and therapy. Antiviral Res 2010; 85: 25–33. [DOI] [PubMed] [Google Scholar]
- 122. Mamo T, Moseman EA, Kolishetti N, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine 2010; 5: 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Boyapalle S, Mohapatra S, Mohapatra S. Nanotechnology applications to HIV vaccines and microbicides. J Glob Infect Dis 2012; 4: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Glass JJ, Kent SJ, De Rose R. Enhancing dendritic cell activation and HIV vaccine effectiveness through nanoparticle vaccination. Expert Rev Vaccines 2016; 15: 719–729. [DOI] [PubMed] [Google Scholar]
- 125. Liu Y, Chen C. Role of nanotechnology in HIV/AIDS vaccine development. Adv Drug Deliv Rev 2016; 103: 76–89. [DOI] [PubMed] [Google Scholar]
- 126. Kumar L, Verma S, Prasad DN, et al. Nanotechnology: a magic bullet for HIV AIDS treatment. Artif Cells Nanomed Biotechnol 2015; 43: 71–86. [DOI] [PubMed] [Google Scholar]
- 127. Vashist A, Kaushik A, Vashist A, et al. Recent trends on hydrogel based drug delivery systems for infectious diseases. Biomater Sci 2016; 4: 1535–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chiodo F, Marradi M, Calvo J, et al. Glycosystems in nanotechnology: gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein J Org Chem 2014; 10: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Oswald-Richter K, Grill SM, Shariat N, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol 2004; 2: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008; 133: 775–787. [DOI] [PubMed] [Google Scholar]
- 131. Moreno-Fernandez ME, Zapata W, Blackard JT, et al. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol 2009; 83: 12925–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Méndez-Lagares G, Jaramillo-Ruiz D, Pion M, et al. HIV infection deregulates the balance between regulatory T cells and IL-2-producing CD4 T cells by decreasing the expression of the IL-2 receptor in Treg. J Acquir Immune Defic Syndr 2014; 65: 278–282. [DOI] [PubMed] [Google Scholar]
- 133. Jaramillo-Ruiz D, De La, Mata FJ, Gómez R, et al. Nanotechnology as a new therapeutic approach to prevent the HIV-infection of Treg cells. PLoS ONE 2016; 11: e0145760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Parboosing R, Chonco L, De La, Mata FJ, et al. Potential inhibition of HIV-1 encapsidation by oligoribonucleotide–dendrimer nanoparticle complexes. Int J Nanomedicine 2017; 12: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jayant RD, Atluri VSR, Agudelo M, et al. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int J Nanomedicine 2015; 10: 1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Elliot JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10: e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chaowanachan T, Krogstad E, Ball C, et al. Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis. PLoS ONE 2013; 8: e61416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jiang Y, Cao S, Bright DK, et al. Nanoparticle-based ARV drug combinations for synergistic inhibition of cell-free and cell-cell HIV transmission. Mol Pharm 2015; 12: 4363–4374. [DOI] [PubMed] [Google Scholar]
- 139. Li W, Wang Q, Li Y, et al. A nanoparticle-encapsulated non-nucleoside reverse-transcriptase inhibitor with enhanced anti-HIV-1 activity and prolonged circulation time in plasma. Curr Pharm Des 2015; 21: 925–935. [DOI] [PubMed] [Google Scholar]
- 140. Bastian AR, Nangarlia A, Bailey LD, et al. Mechanism of multivalent nanoparticle encounter with HIV-1 for potency enhancement of peptide triazole virus inactivation. J Biol Chem 2015; 290: 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Smith KA, Lin X, Bolshakov O, et al. Activation of HIV-1 with nanoparticle-packaged small-molecule protein phosphatase-1-targeting compound. Sci Pharm 2015; 83: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Caucheteux SM, Mitchell JP, Ivory MO, et al. Polypropylene sulfide nanoparticle p24 vaccine promotes dendritic cell-mediated specific immune responses against HIV-1. J Invest Dermatol 2016; 136: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 143. World Health Organization. Hepatitis B Fact Sheet no. 204, www.who.int/mediacentre/factsheets/fs204/en/ (2001, accessed 10 October 2016).
- 144. Manzoor S, Saalim M, Imran M, et al. Hepatitis B virus therapy: what’s the future holding for us? World J Gastroenterol 2015; 21: 12558–12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liaw Y-F, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351: 1521–1531. [DOI] [PubMed] [Google Scholar]
- 146. Wang J, Feng S-S, Wang S, et al. Evaluation of cationic nanoparticles of biodegradable copolymers as siRNA delivery system for hepatitis B treatment. Int J Pharm 2010; 400: 194–200. [DOI] [PubMed] [Google Scholar]
- 147. World Health Organization. Hepatitis C Fact Sheet no. 164, www.who.int/mediacentre/factsheets/fs164/en/ (2000, accessed 10 October 2016).
- 148. Szunerits S, Barras A, Khanal M, et al. Nanostructures for the inhibition of viral infections. Molecules 2015; 20: 14051–14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2003; 2: 214–221. [DOI] [PubMed] [Google Scholar]
- 150. Lee M-Y, Yang J-A, Jung HS, et al. Hyaluronic acid–gold nanoparticle/interferon α complex for targeted treatment of hepatitis C virus infection. ACS Nano 2012; 6: 9522–9531. [DOI] [PubMed] [Google Scholar]
- 151. Wang Z, Liu H, Yang SH, et al. Nanoparticle-based artificial RNA silencing machinery for antiviral therapy. Proc Natl Acad Sci U S A 2012; 109: 12387–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Jiménez-Pardo I, González-Pastor R, Lancelot A, et al. Shell cross-linked polymeric micelles as camptothecin nanocarriers for anti-HCV therapy. Macromol Biosci 2015; 15: 1381–1391. [DOI] [PubMed] [Google Scholar]
- 153. Abian O, Vega S, Sancho J, et al. Allosteric inhibitors of the NS3 protease from the hepatitis C virus. PLoS ONE 2013; 8: e69773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Moon J-S, Lee S-H, Han S-H, et al. Inhibition of hepatitis C virus in mouse models by lipidoid nanoparticle-mediated systemic delivery of siRNA against PRK2. Nanomedicine 2016; 12: 1489–1498. [DOI] [PubMed] [Google Scholar]
- 155. Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol 2008; 26: 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 2005; 11: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Guillot L, Le Goffic R, Bloch S, et al. Involvement of toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J Biol Chem 2005; 280: 5571–5580. [DOI] [PubMed] [Google Scholar]
- 158. Bao Y, Bolotov P, Dernovoy D, et al. The influenza virus resource at the National Center for Biotechnology Information. J Virol 2008; 82: 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jung J, Park B, Oh S, et al. Integration of reverse transcriptase loop-mediated isothermal amplification with an immunochromatographic strip on a centrifugal microdevice for influenza A virus identification. Lab Chip 2015; 15: 718–725. [DOI] [PubMed] [Google Scholar]
- 160. Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009; 459: 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Barik S. New treatments for influenza. BMC Med 2012; 10: 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shafagati N, Patanarut A, Luchini A, et al. The use of nanotrap particles for biodefense and emerging infectious disease diagnostics. Pathog Dis 2014; 71: 164–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Hendricks GL, Weirich KL, Viswanathan K, et al. Sialylneolacto-N-tetraose C (LSTc)-bearing liposomal decoys capture influenza A virus. J Biol Chem 2013; 288: 8061–8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wagner R, Matrosovich M, Klenk H-D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 2002; 12: 159–166. [DOI] [PubMed] [Google Scholar]
- 165. Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360: 953–956. [DOI] [PubMed] [Google Scholar]
- 166. Li Y, Lin Z, Zhao M, et al. Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl Mater Interfaces 2016; 8: 24385–24393. [DOI] [PubMed] [Google Scholar]
- 167. Levina AS, Repkova MN, Mazurkova NA, et al. Nanoparticle-mediated nonviral DNA delivery for effective inhibition of influenza A viruses in cells. IEEE Trans Nanotechnol 2016; 15: 248–254. [Google Scholar]
- 168. Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet 2001; 357: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 169. Taylor T, Brockman M, McNamee E, et al. Herpes simplex virus. Front Biosci 2002; 7: d752–d764. [DOI] [PubMed] [Google Scholar]
- 170. Cavalli R, Donalisio M, Bisazza A, et al. Enhanced antiviral activity of acyclovir loaded into nanoparticles. Methods Enzymol 2012; 509: 1–19. [DOI] [PubMed] [Google Scholar]
- 171. Brady RC, Bernstein DI. Treatment of herpes simplex virus infections. Antiviral Res 2004; 61: 73–81. [DOI] [PubMed] [Google Scholar]
- 172. Balfour HH., Jr. Antiviral drugs. New Engl J Med 1999; 340: 1255–1268. [DOI] [PubMed] [Google Scholar]
- 173. Laskin OL. Clinical pharmacokinetics of acyclovir. Clin Pharmacokinet 1983; 8: 187–201. [DOI] [PubMed] [Google Scholar]
- 174. Arnal J, Gonzalez-Alvarez I, Bermejo M, et al. Biowaiver monographs for immediate release solid oral dosage forms: aciclovir. J Pharm Sci 2008; 97: 5061–5073. [DOI] [PubMed] [Google Scholar]
- 175. Sudhakar Y, Kuotsu K, Bandyopadhyay A. Buccal bioadhesive drug delivery – a promising option for orally, less efficient drugs. J Control Release 2006; 114: 15–40. [DOI] [PubMed] [Google Scholar]
- 176. Al-Dhubiab BE, Nair AB, Kumria R, et al. Formulation and evaluation of nano-based drug delivery system for the buccal delivery of acyclovir. Colloids Surf B Biointerfaces 2015; 136: 878–884. [DOI] [PubMed] [Google Scholar]
- 177. Lembo D, Swaminathan S, Donalisio M, et al. Encapsulation of acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int J Pharm 2013; 443: 262–272. [DOI] [PubMed] [Google Scholar]
- 178. Gaikwad S, Ingle A, Gade A, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomedicine 2013; 8: 4303–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Hu R, Li S, Kong F, et al. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res 2014; 13: 7022–7028. [DOI] [PubMed] [Google Scholar]
- 180. Orlowski P, Tomaszewska E, Gniadek M, et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014; 9: e104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Steinbach JM, Weller CE, Booth CJ, et al. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. J Control Release 2012; 162: 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Bhosale UV, Devi K, Choudhary S. Development and in vitro-in vivo evaluation of oral drug delivery system of acyclovir loaded PLGA nanoparticles. Int J Drug Deliv 2013; 5: 331–343. [Google Scholar]
- 183. Ramyadevi D, Rajan K, Vedhahari B, et al. Heterogeneous polymer composite nanoparticles loaded in situ gel for controlled release intra-vaginal therapy of genital herpes. Colloids Surf B Interfaces 2016; 146: 260–270. [DOI] [PubMed] [Google Scholar]
- 184. Ensign LM, Tang BC, Wang Y-Y, et al. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci Transl Med 2012; 4: 138ra179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gide P, Gidwani S, Kothule K. Enhancement of transdermal penetration and bioavailability of poorly soluble acyclovir using solid lipid nanoparticles incorporated in gel cream. Indian J Pharm Sci 2013; 75: 138–142. [PMC free article] [PubMed] [Google Scholar]
- 186. Jain S, Mistry MA, Swarnakar NK. Enhanced dermal delivery of acyclovir using solid lipid nanoparticles. Drug Deliv Transl Res 2011; 1: 395–406. [DOI] [PubMed] [Google Scholar]
- 187. Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110: 525–541. [DOI] [PubMed] [Google Scholar]
- 188. Chapoy-Villanueva H, Martinez-Carlin I, Lopez-Berestein G, et al. Therapeutic silencing of HPV 16 E7 by systemic administration of siRNA-neutral DOPC nanoliposome in a murine cervical cancer model with obesity. J BUON 2014; 20: 1471–1479. [PubMed] [Google Scholar]
- 189. Tahamtan A, Tabarraei A, Moradi A, et al. Chitosan nanoparticles as a potential nonviral gene delivery for HPV-16 E7 into mammalian cells. Artif Cells Nanomed Biotechnol 2015; 43: 366–372. [DOI] [PubMed] [Google Scholar]
- 190. Saleh T, Bolhassani A, Shojaosadati SA, et al. MPG-based nanoparticle: an efficient delivery system for enhancing the potency of DNA vaccine expressing HPV16E7. Vaccine 2015; 33: 3164–3170. [DOI] [PubMed] [Google Scholar]
- 191. Uddin MN, Kouzi SA, Hussain MD. Strategies for developing oral vaccines for human papillomavirus (HPV) induced cancer using nanoparticle mediated delivery system. J Pharm Pharm Sci 2015; 18: 220–234. [DOI] [PubMed] [Google Scholar]
- 192. Rupp R, Rosenthal SL, Stanberry LR. VivaGel™(SPL7013 gel): a candidate dendrimer–microbicide for the prevention of HIV and HSV infection. Int J Nanomedicine 2007; 2: 561–566. [PMC free article] [PubMed] [Google Scholar]
- 193. Eastman P. Progress being made on potential of microbicides to prevent genital HPV infection. Oncol Time 2010; 32: 32–34. [Google Scholar]
- 194. McGowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004). AIDS 2011; 25: 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Cohen CR, Brown J, Moscicki A-B, et al. A phase I randomized placebo controlled trial of the safety of 3% Spl7013 gel (Vivagel®) in healthy young women administered twice daily for 14 days. PLoS ONE 2011; 6: e16258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Moscicki A-B, Rupert K, Yifei M, et al. Measurement of mucosal biomarkers in a phase 1 trial of intravaginal 3% SPL7013 gel (VivaGel®) to assess expanded safety. J Acquir Immune Defic Syndr 2012; 59: 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Gulland A. Zika virus is a global public health emergency, declares WHO. BMJ 2016; 352: i657. [DOI] [PubMed] [Google Scholar]
- 198. Lucey DR, Gostin LO. The emerging Zika pandemic: enhancing preparedness. JAMA 2016; 315: 865–866. [DOI] [PubMed] [Google Scholar]
- 199. Kaushik A, Tiwari S, Jayant RD, et al. Electrochemical biosensors for early stage Zika diagnostics. Trends Biotechnol 2017; 35: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sun L, Singh AK, Vig K, et al. Silver nanoparticles inhibit replication of respiratory syncytial virus. J Biomed Nanotechnol 2008; 4: 149–158. [Google Scholar]
- 201. Blanken MO, Rovers MM, Molenaar JM, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. New Engl J Med 2013; 368: 1791–1799. [DOI] [PubMed] [Google Scholar]
- 202. Devincenzo J, Lambkin-Williams R, Wilkinson T, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A 2010; 107: 8800–8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J 2011; 6: 1130–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zamora MR, Budev M, Rolfe M, et al. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med 2011; 183: 531–538. [DOI] [PubMed] [Google Scholar]
- 205. Bose S, Basu M, Banerjee AK. Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells. J Virol 2004; 78: 8146–8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Skiadopoulos M, Surman S, Riggs J, et al. Evaluation of the replication and immunogenicity of recombinant human parainfluenza virus type 3 vectors expressing up to three foreign glycoproteins. Virology 2002; 297: 136–152. [DOI] [PubMed] [Google Scholar]
- 207. Chepurnov A, Bakulina L, Dadaeva A, et al. Inactivation of Ebola virus with a surfactant nanoemulsion. Acta Trop 2003; 87: 315–320. [DOI] [PubMed] [Google Scholar]
- 208. Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 2004; 78: 4330–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Dunning J, Sahr F, Rojek A, et al. Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med 2016; 13: e1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Geisbert TW, Lee AC, Robbins M, et al. Post-exposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 2010; 375: 1896–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Thi EP, Mire CE, Lee AC, et al. Lipid nanoparticle siRNA treatment of Ebola-virus-Makona-infected nonhuman primates. Nature 2015; 521: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Centers for Disease Control and Prevention. Norovirus, https://www.cdc.gov/norovirus/about/treatment.html (2016, accessed 10 April 2017).
- 213. Broglie JJ, Alston B, Yang C, et al. Antiviral activity of gold/copper sulfide core/shell nanoparticles against human norovirus virus-like particles. PLoS ONE 2015; 10: e0141050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kim M-G, Park JY, Shon Y, et al. Nanotechnology and vaccine development. Asian J Pharm Sci 2014; 9: 227–235. [Google Scholar]
- 215. Misumi S, Masuyama M, Takamune N, et al. Targeted delivery of immunogen to primate M cells with tetragalloyl lysine dendrimer. J Immunol 2009; 182: 6061–6070. [DOI] [PubMed] [Google Scholar]
- 216. Raghuwanshi D, Mishra V, Suresh MR, et al. A simple approach for enhanced immune response using engineered dendritic cell targeted nanoparticles. Vaccine 2012; 30: 7292–7299. [DOI] [PubMed] [Google Scholar]
- 217. Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev 2013; 65: 1271–1281. [DOI] [PubMed] [Google Scholar]
- 218. World Health Organization. Hepatitis A Fact Sheet no. 328, www.who.int/mediacentre/factsheets/fs328/en/ (2016, accessed 10 October 2016).
- 219. Esser-Nobis K, Harak C, Schult P, et al. Novel perspectives for hepatitis A virus therapy revealed by comparative analysis of hepatitis c virus and hepatitis A virus RNA replication. Hepatology 2015; 62: 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Copland MJ, Rades T, Davies NM, et al. Lipid based particulate formulations for the delivery of antigen. Immunol Cell Biol 2005; 83: 97–105. [DOI] [PubMed] [Google Scholar]
- 221. Riedemann S, Reinhardt G, Ibarra H, et al. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal®) in healthy toddlers and children in Chile. Acta Paediatr 2004; 93: 412–414. [PubMed] [Google Scholar]
- 222. Sjogren MH. Prevention of hepatitis B in non-responders to initial hepatitis B virus vaccination. Am J Med 2005; 118: 34–39. [DOI] [PubMed] [Google Scholar]
- 223. Lebre F, Borchard G, Faneca H, et al. Intranasal administration of novel chitosan nanoparticle/DNA complexes induces antibody response to hepatitis B surface antigen in mice. Mol Pharm 2016; 13: 472–482. [DOI] [PubMed] [Google Scholar]
- 224. Laing P, Bacon A, Mccormack B, et al. The ‘co-delivery’ approach to liposomal vaccines: application to the development of influenza-A and hepatitis-B vaccine candidates. J Liposome Res 2006; 16: 229–235. [DOI] [PubMed] [Google Scholar]
- 225. Batdelger D, Dandii D, Dahgwahdorj Y, et al. Clinical experience with therapeutic vaccines designed for patients with hepatitis. Curr Pharm Des 2009; 15: 1159–1171. [DOI] [PubMed] [Google Scholar]
- 226. EvaluateTM Press Release. Lipoxen reports significant progress in the development of its DNA vaccine delivery patent portfolio, www.evaluategroup.com/Universal/View.aspx?type=Story&id=149258 (2008, accessed 22 February 2017).
- 227. Giezeman K, Nauta J, De Bruijn I, et al. Trivalent inactivated subunit influenza vaccine influvac®: 25-year experience of safety and immunogenicity. Vaccine 2009; 27: 2414–2417. [DOI] [PubMed] [Google Scholar]
- 228. Tao W, Ziemer KS, Gill HS. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine (Lond) 2014; 9: 237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tan M, Jiang X. Norovirus P particle: a subviral nanoparticle for vaccine development against norovirus, rotavirus and influenza virus. Nanomedicine (Lond) 2012; 7: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Galloway AL, Murphy A, Desimone JM, et al. Development of a nanoparticle-based influenza vaccine using the PRINT® technology. Nanomedicine 2013; 9: 523–531. [DOI] [PubMed] [Google Scholar]
- 231. Kanekiyo M, Wei C-J, Yassine HM, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013; 499: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Neuhaus V, Chichester JA, Ebensen T, et al. A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine 2014; 32: 3216–3222. [DOI] [PubMed] [Google Scholar]
- 233. Sawaengsak C, Mori Y, Yamanishi K, et al. Chitosan nanoparticle encapsulated hemagglutinin-split influenza virus mucosal vaccine. AAPS PharmSciTech 2014; 15: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Bengtsson KL, Song H, Stertman L, et al. Matrix-M adjuvant enhances antibody, cellular and protective immune responses of a Zaire Ebola/Makona virus glycoprotein (GP) nanoparticle vaccine in mice. Vaccine 2016; 34: 1927–1935. [DOI] [PubMed] [Google Scholar]
- 235. Dwivedi V, Manickam C, Binjawadagi B, et al. PLGA nanoparticle entrapped killed porcine reproductive and respiratory syndrome virus vaccine helps in viral clearance in pigs. Vet Microbiol 2013; 166: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Binjawadagi B, Dwivedi V, Manickam C, et al. Adjuvanted poly (lactic-co-glycolic) acid nanoparticle-entrapped inactivated porcine reproductive and respiratory syndrome virus vaccine elicits cross-protective immune response in pigs. Int J Nanomedicine 2014; 9: 679–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Glenn GM, Fries LF, Thomas DN, et al. A randomized, blinded, controlled, dose-ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. J Infect Dis 2015; 213: 411–422. [DOI] [PubMed] [Google Scholar]
- 238. Adjei IM, Sharma B, Labhasetwar V. Nanoparticles: cellular uptake and cytotoxicity. Adv Exp Med Biol 2014; 811: 73–91. [DOI] [PubMed] [Google Scholar]
- 239. Kettler K, Veltman K, Van De Meent D, et al. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ Toxicol Chem 2014; 33: 481–492. [DOI] [PubMed] [Google Scholar]
- 240. Jeynes JCG, Jeynes C, Merchant MJ, et al. Measuring and modelling cell-to-cell variation in uptake of gold nanoparticles. Analyst 2013; 138: 7070–7074. [DOI] [PubMed] [Google Scholar]
- 241. Vivès E, Richard J, Rispal C, et al. Tat peptide internalization: seeking the mechanism of entry. Curr Protein Pept Sci 2003; 4: 125–132. [DOI] [PubMed] [Google Scholar]
- 242. Kaplan IM, Wadia JS, Dowdy SF. Cationic tat peptide transduction domain enters cells by macropinocytosis. J Control Release 2005; 102: 247–253. [DOI] [PubMed] [Google Scholar]
- 243. Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A 2008; 105: 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Muro S, Garnacho C, Champion JA, et al. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol Ther 2008; 16: 1450–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 2009; 26: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Decuzzi P, Pasqualini R, Arap W, et al. Intravascular delivery of particulate systems: does geometry really matter?. Pharm Res 2009; 26: 235–243. [DOI] [PubMed] [Google Scholar]
- 247. Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat Mater 2009; 8: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Andersson J, Ekdahl KN, Larsson R, et al. C3 adsorbed to a polymer surface can form an initiating alternative pathway convertase. J Immunol 2002; 168: 5786–5791. [DOI] [PubMed] [Google Scholar]
- 249. Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20: 34–50. [DOI] [PubMed] [Google Scholar]
- 250. Salvador-Morales C, Flahaut E, Sim E, et al. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol 2006; 43: 193–201. [DOI] [PubMed] [Google Scholar]
- 251. Sim R, Tsiftsoglou S. Proteases of the complement System. Biochem Soc Trans 2004; 32: 21–27. [DOI] [PubMed] [Google Scholar]
- 252. Lubich C, Allacher P, De La, Rosa M, et al. The mystery of antibodies against polyethylene glycol (peg)-what do we know? Pharm Res 2016; 33: 2239–2249. [DOI] [PubMed] [Google Scholar]
- 253. Richter A, Åkerblom E. Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol 1984; 74: 36–39. [DOI] [PubMed] [Google Scholar]
- 254. Fruijtier-Pölloth C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005; 214: 1–38. [DOI] [PubMed] [Google Scholar]
- 255. Arora R, Srinivasan R. Is polyethylene glycol safe and effective for chronic constipation in children? Arch Dis Child 2005; 90: 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Chen J, Guo Z, Tian H, et al. Production and clinical development of nanoparticles for gene delivery. Mol Ther Clin Methods Dev 2016; 3: 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Garay RP, El-Gewely R, Armstrong JK, et al. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv 2012; 9: 1319–1323. [DOI] [PubMed] [Google Scholar]
- 258. Mohammad AK, Reineke JJ. Quantitative detection of PLGA nanoparticle degradation in tissues following intravenous administration. Mol Pharm 2013; 10: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 259. Fischer HC, Chan WC. Nanotoxicity: the growing need for in vivo study. Curr Opin Biotechnol 2007; 18: 565–571. [DOI] [PubMed] [Google Scholar]
- 260. Panyam J, Dali MM, Sahoo SK, et al. Polymer degradation and in vitro release of a model protein from poly (D, L-lactide-co-glycolide) nano-and microparticles. J Control Release 2003; 92: 173–187. [DOI] [PubMed] [Google Scholar]
- 261. Sulheim E, Baghirov H, Haartman E, et al. Cellular uptake and intracellular degradation of poly (alkyl cyanoacrylate) nanoparticles. J Nanobiotechnology 2016; 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Goodman CM, Mccusker CD, Yilmaz T, et al. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem 2004; 15: 897–900. [DOI] [PubMed] [Google Scholar]
- 263. Oh N, Park J-H. Surface chemistry of gold nanoparticles mediates their exocytosis in macrophages. ACS Nano 2014; 8: 6232–6241. [DOI] [PubMed] [Google Scholar]
- 264. Bertrand N, Leroux J-C. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J Control Release 2012; 161: 152–163. [DOI] [PubMed] [Google Scholar]
- 265. Borel T, Sabliov C. Nanodelivery of bioactive components for food applications: types of delivery systems, properties, and their effect on ADME profiles and toxicity of nanoparticles. Annu Rev Food Sci Technol 2014; 5: 197–213. [DOI] [PubMed] [Google Scholar]
- 266. Müller RH, Gohla S, Keck CM. State of the art of nanocrystals–special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm 2011; 78: 1–9. [DOI] [PubMed] [Google Scholar]
- 267. Singh L, Parboosing R, Kruger HG, et al. Intracellular localization of gold nanoparticles with targeted delivery in MT-4 lymphocytes. Adv Nat Sci: Nanosci Nanotechnol 2016; 7: 045013. [Google Scholar]
- 268. Kuhn DA, Vanhecke D, Michen B, et al. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J Nanotechnol 2014; 5: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Kelf T, Sreenivasan V, Sun J, et al. Non-specific cellular uptake of surface-functionalized quantum dots. Nanotechnology 2010; 21: 285105. [DOI] [PubMed] [Google Scholar]
- 270. Chen H, Wang L, Yeh J, et al. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PgammaMPS copolymer coating. Biomaterials 2010; 31: 5397–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Yu SS, Lau CM, Thomas SN, et al. Size-and charge-dependent non-specific uptake of pegylated nanoparticles by Macrophages. Int J Nanomedicine 2012; 7: 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Van Vlerken LE, Vyas TK, Amiji MM. Poly (ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res 2007; 24: 1405–1414. [DOI] [PubMed] [Google Scholar]
- 273. Albanese A, Chan WC. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011; 5: 5478–5489. [DOI] [PubMed] [Google Scholar]
- 274. Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol 2010; 44: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 275. Nel A, Xia T, Mädler L, et al. Toxic potential of materials at the nanolevel. Science 2006; 311: 622–627. [DOI] [PubMed] [Google Scholar]
- 276. Zook JM, Maccuspie RI, Locascio LE, et al. Stable nanoparticle aggregates/agglomerates of different sizes and the effect of their size on hemolytic cytotoxicity. Nanotoxicology 2011; 5: 517–530. [DOI] [PubMed] [Google Scholar]
- 277. Saleh NB, Pfefferle LD, Elimelech M. Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: measurements and environmental implications. Environ Sci Technol 2008; 42: 7963–7969. [DOI] [PubMed] [Google Scholar]
- 278. Xu H, Yan F, Monson EE, et al. Room-temperature preparation and characterization of poly (ethylene glycol)-coated silica nanoparticles for biomedical applications. J Biomed Mater Res A 2003; 66: 870–879. [DOI] [PubMed] [Google Scholar]
- 279. Huang L, Liu Y. In vivo delivery of RNAi with lipid-based nanoparticles. Annu Rev Biomed Eng 2011; 13: 507–530. [DOI] [PubMed] [Google Scholar]
- 280. Poon Z, Lee JB, Morton SW, et al. Controlling in vivo stability and biodistribution in electrostatically assembled nanoparticles for systemic delivery. Nano Lett 2011; 11: 2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev 2011; 40: 1647–1671. [DOI] [PubMed] [Google Scholar]
- 282. Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 2001; 53: 283–318. [PubMed] [Google Scholar]
- 283. De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine 2008; 3: 133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Lei R, Wu C, Yang B, et al. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid invivo screening method for nanotoxicity. Toxicol Appl Pharmacol 2008; 232: 292–301. [DOI] [PubMed] [Google Scholar]
- 285. Hu Y-L, Gao J-Q. Potential neurotoxicity of nanoparticles. Int J Pharm 2010; 394: 115–121. [DOI] [PubMed] [Google Scholar]
- 286. Yoshida S, Hiyoshi K, Ichinose T, et al. Effect of nanoparticles on the male reproductive system of mice. Int J Androl 2009; 32: 337–342. [DOI] [PubMed] [Google Scholar]
- 287. Yang H-W, Hua M-Y, Liu H-L, et al. Potential of magnetic nanoparticles for targeted drug delivery. Nanotechnol Sci Appl 2012; 5: 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res 2006; 23: 1417–1450. [DOI] [PubMed] [Google Scholar]
- 289. Kingsley JD, Dou H, Morehead J, et al. Nanotechnology: a focus on nanoparticles as a drug delivery system. J Neuroimmune Pharmacol 2006; 1: 340–350. [DOI] [PubMed] [Google Scholar]
- 290. Poeschla EM. Integrase. LEDGF/P75 and HIV replication. Cell Mol Life Sci 2008; 65: 1403–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Demarino C, Schwab A, Pleet M, et al. Biodegradable danoparticles for delivery of therapeutics in CNS infection. J Neuroimmune Pharmacol 2016; 12: 31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res 2007; 24: 1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Kuo Y-C, Su F-L. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm 2007; 340: 143–152. [DOI] [PubMed] [Google Scholar]
- 294. Kaushik A, Jayant RD, Nair M. Advancements in nano-enabled therapeutics for neuroHIV management. Int J Nanomedicine 2016; 11: 4317–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Gomes MJ, Neves JD, Sarmento B. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. Int J Nanomedicine 2014; 9: 1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Kaushik A, Jayant RD, Nikkhah-Moshaie R, et al. Magnetically guided central nervous system delivery and toxicity evaluation of magnetoelectric nanocarriers. Sci Rep 2016; 6: e25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Nair M, Jayant RD, Kaushik A, et al. Getting into the brain: potential of nanotechnology in the management of NeuroAIDS. Adv Drug Deliv Rev 2016; 103: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol 2009; 86: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Cauchetier E, Paul M, Rivollet D, et al. Therapeutic evaluation of free and nanocapsule-encapsulated atovaquone in the treatment of murine visceral leishmaniasis. Ann Trop Med Parasitol 2003; 97: 259–268. [DOI] [PubMed] [Google Scholar]