Abstract
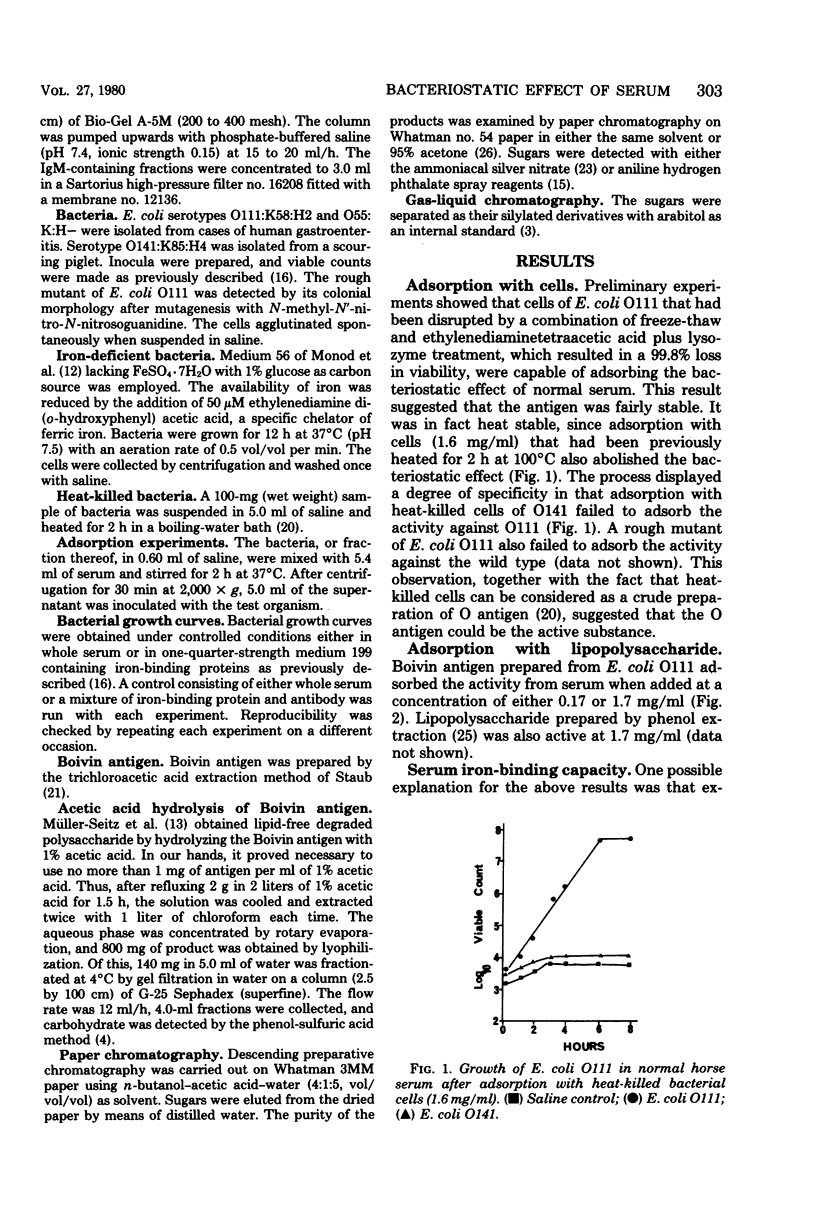
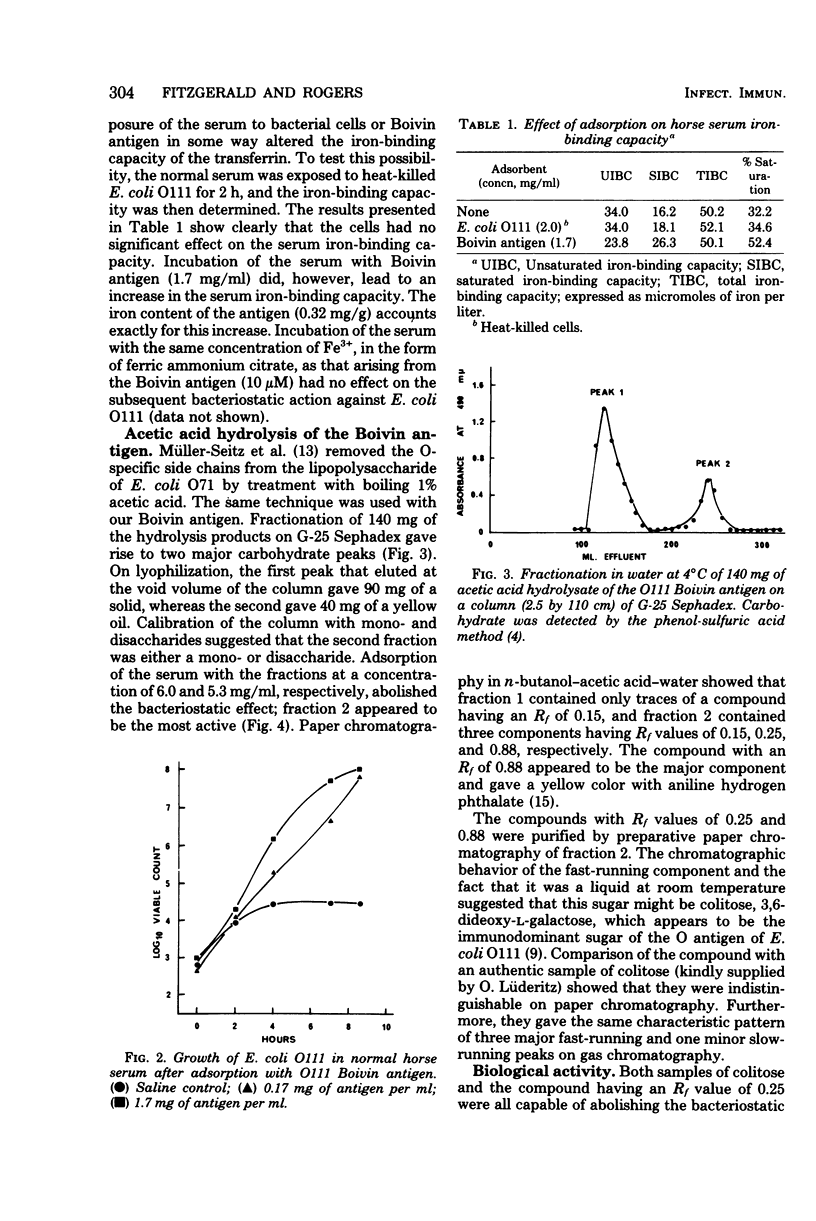
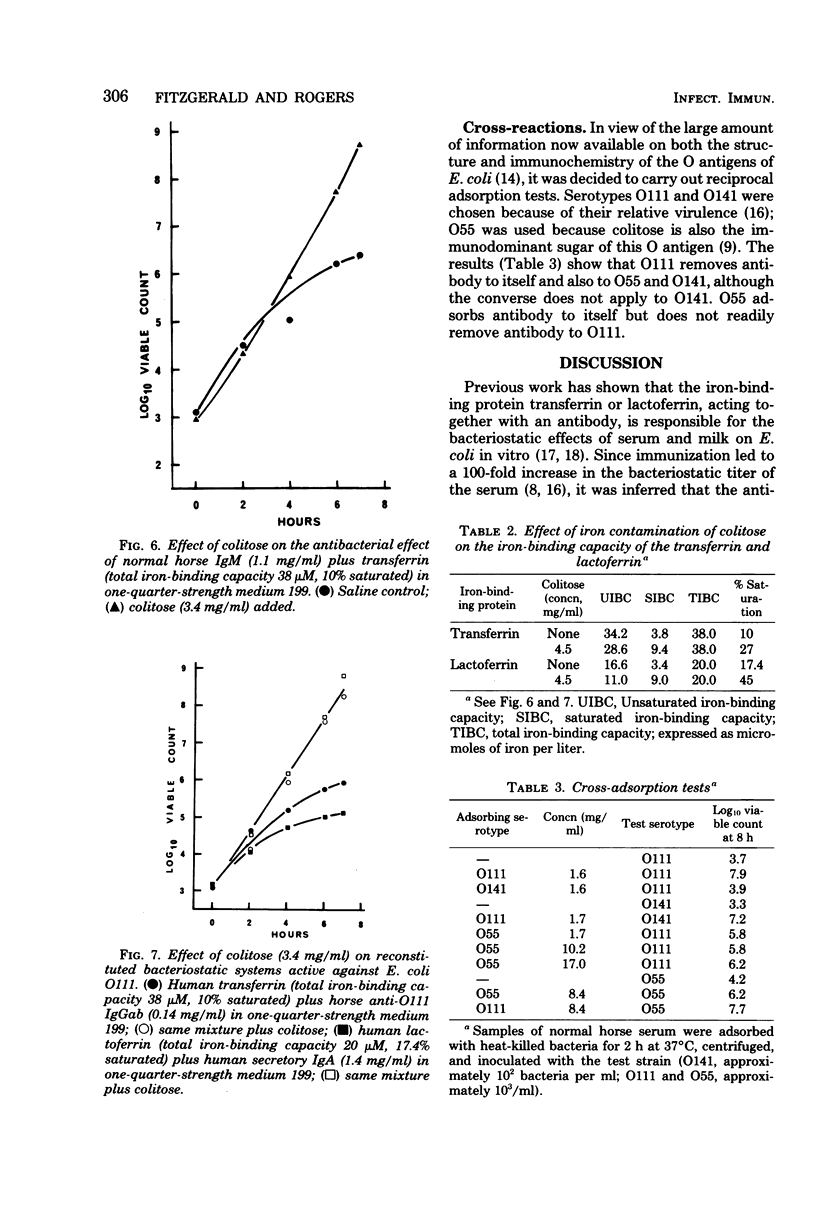
Previous work has shown that antibody and transferrin, acting together, exert a bacteriostatic effect on certain pathogenic Escherichia coli. This effect may be due to the ability of the antibody to interfere with the release of the iron chelator, enterochelin, from the bacterial cell. Enterochelin is essential for the transport of iron from transferrin to the bacterial cell. The nature of the bacterial antigen against which the antibody is directed has now been determined by means of adsorption experiments. It was found that absorption of serum either with hear-killed cells of E. coli O111 or with Boivin antigen abolished the bacteriostatic effect. A monosaccharide, which proved to be colitose (3,6-dideoxy-L-galactose), was isolated after acetic acid hydrolysis of the Boivin antigen. Colitose is the terminal monosaccharide of the O-specific side chain of the lipopolysaccharide from E. coli O111. This monosaccharide abolished the bacteriostatic effect of both whole serum and mixtures of antibody and iron-binding proteins. When administered by the intraperitoneal route, it reduced the resistance of mice to subsequent infection with E. coli O111. This ability of colitose to interfere with antibacterial mechanisms is in accord with published immunochemical studies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Clamp J. R. An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem J. 1971 Dec;125(4):1009–1018. doi: 10.1042/bj1251009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. The biosynthesis of cell wall lipopolysaccharide in Escherichia coli. VII. Studies on the structure of the O-antigenic polysaccharide. J Biol Chem. 1967 Sep 25;242(18):4125–4133. [PubMed] [Google Scholar]
- Kochan I., Kvach J. T., Wiles T. I. Virulence-associated acquisition of iron in mammalian serum by Escherichia coli. J Infect Dis. 1977 Apr;135(4):623–632. doi: 10.1093/infdis/135.4.623. [DOI] [PubMed] [Google Scholar]
- LUDERITZ O., WESTPHAL O., STAUB A. M., LE MINOR L. Preparation and immunological properties of an artificial antigen with colitose (3-deoxy-1-fucose) as the determinant group. Nature. 1960 Nov 12;188:556–558. doi: 10.1038/188556a0. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Medearis D. N., Jr, Camitta B. M., Heath E. C. Cell wall composition and virulence in Escherichia coli. J Exp Med. 1968 Sep 1;128(3):399–414. doi: 10.1084/jem.128.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Seitz E., Jann B., Jann K. Degradation studies on the lipopolysaccharide from E. coli 071:K?:H12. Separation and investigation of O-specific and core polysaccharides. FEBS Lett. 1968 Oct;1(5):311–314. doi: 10.1016/0014-5793(68)80141-3. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Ferric iron and the antibacterial effects of horse 7S antibodies to Escherichia coli O111. Immunology. 1976 Mar;30(3):425–433. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Synge C. Bacteriostatic effect of human milk on Escherichia coli: the role of IgA. Immunology. 1978 Jan;34(1):19–28. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Synge C., Kimber B., Bayley P. M. Production of enterochelin by Escherichia coli 0111. Biochim Biophys Acta. 1977 Apr 27;497(2):548–557. doi: 10.1016/0304-4165(77)90211-2. [DOI] [PubMed] [Google Scholar]
- STAUB A. M., TINELLI R., LUDERITZ O., WESTPHAL O. Etude immunochimique sur les Salmonella. V. Rôle de quelques sucres, et en particulier des 3-6 didésoxyhexoses, dans la spécificité des antigènes O du tableau de Kauffmann-White. Ann Inst Pasteur (Paris) 1959 Mar;96(3):303–332. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


