Abstract
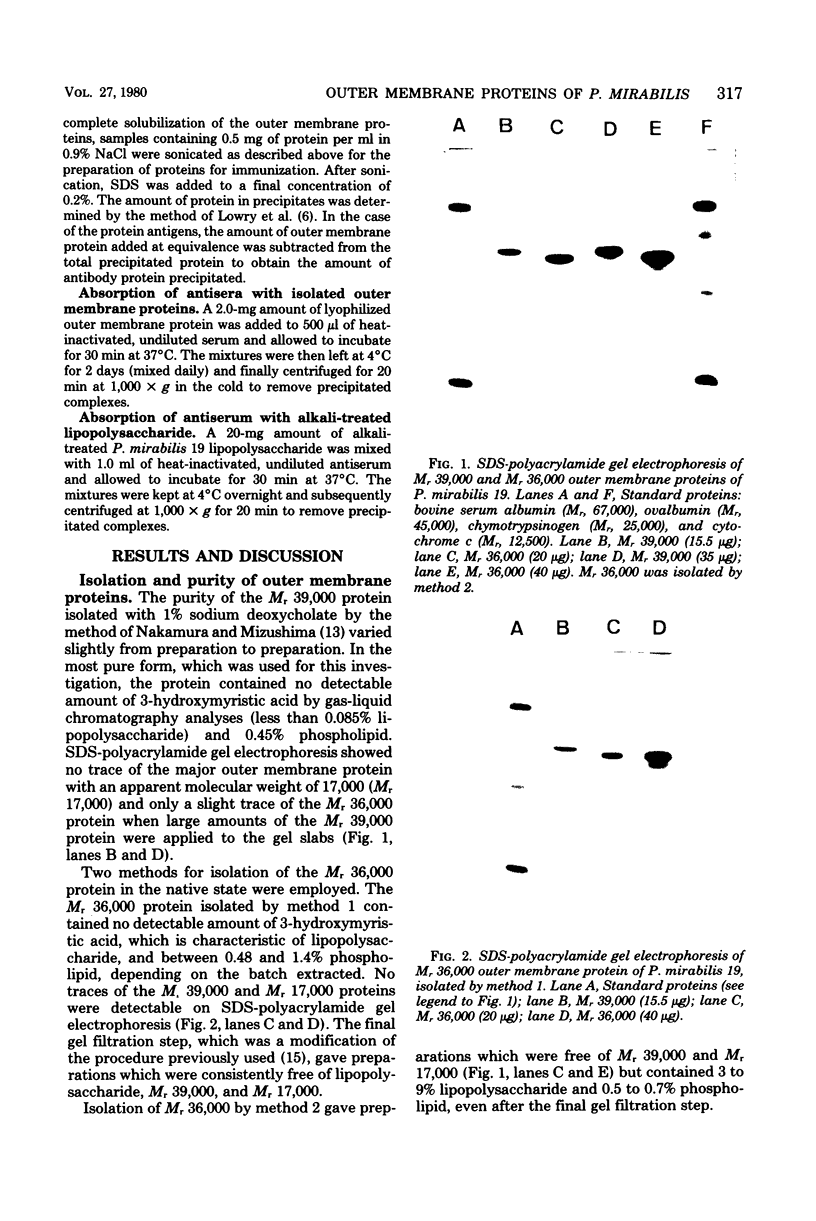
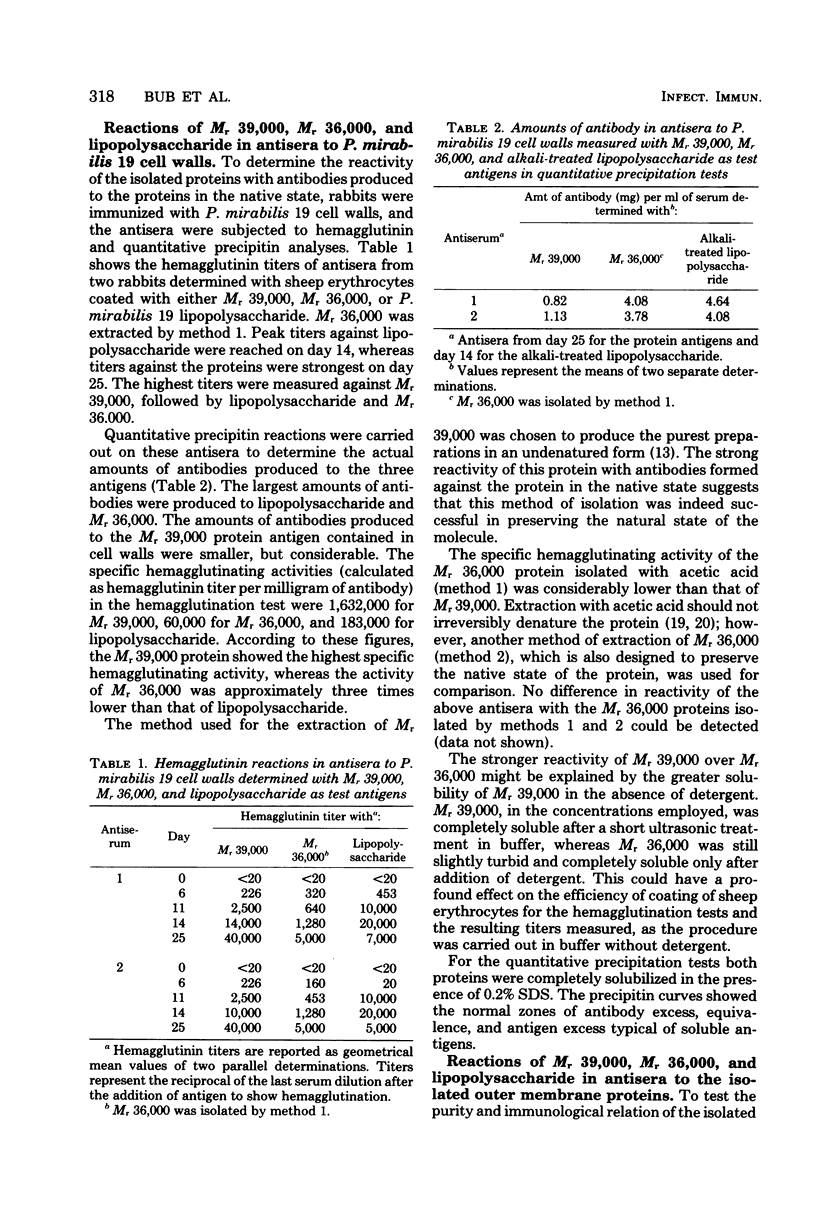
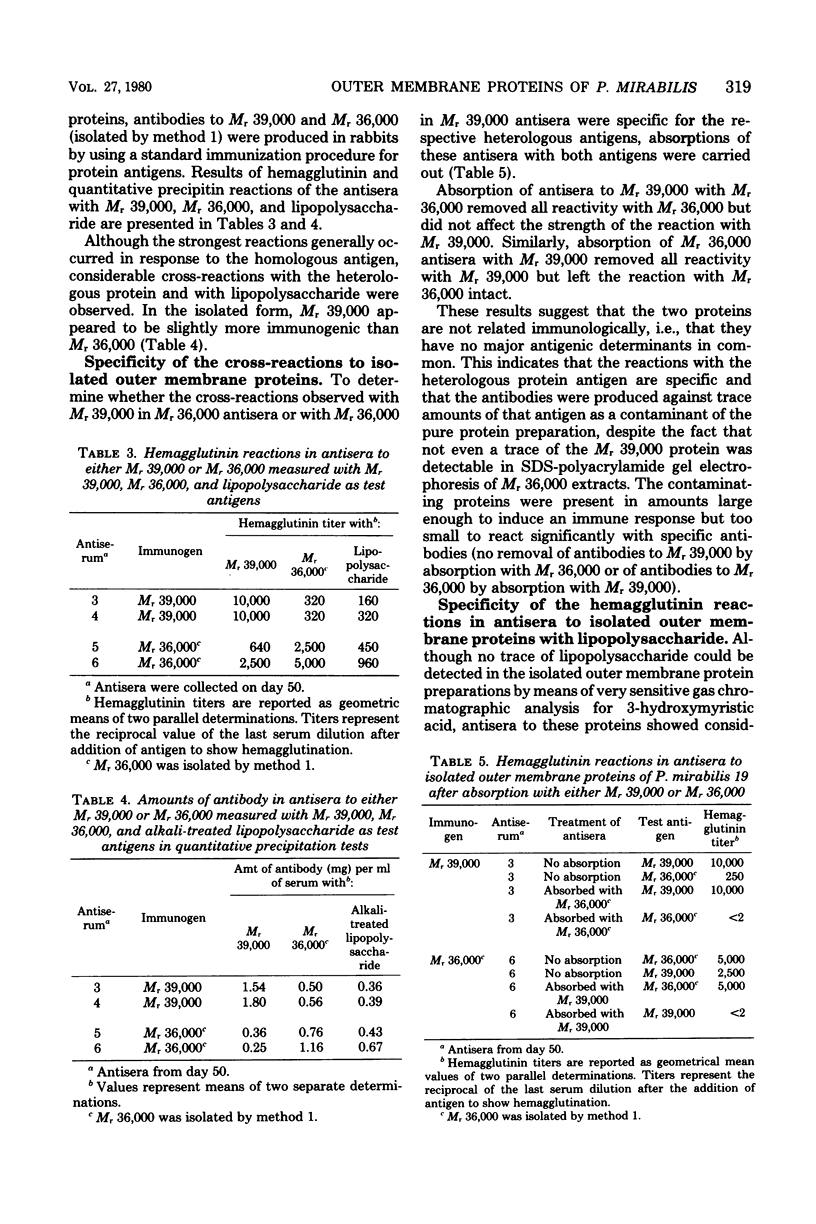
Two proteins with apparent molecular weights of 39,000 and 36,000 (Mr 39,000 and Mr 36,000, respectively) were isolated from the outer membrane of Proteus mirabilis 19. Mr 36,000 was shown to be free of detectable amounts of the Mr 39,000 protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and free of lipopolysaccharide according to gas chromatographic analyses of 3-hydroxymyristic acid content. The Mr 39,000 protein contained no detectable amount of lipopolysaccharide and only a trace of Mr 36,000. Both isolated proteins gave strong reactions in antisera produced to purified P. mirabilis 19 cell walls (outer membrane proteins in the native state). This suggested that the proteins isolated by our methods essentially retained their native configuration upon resolubilization. Antisera produced in rabbits to the isolated proteins showed strongest reactions with the homologous antigen, but some cross-reactions with the heterologous protein and with P. mirabilis 19 lipopolysaccharide were observed. These cross-reactions could be attributed to specific responses to traces of the heterologous (contaminant) proteins present in the purified proteins used as immunizing antigens. The Mr 39,000 and Mr 36,000 proteins have no major antigenic determinants in common. Reactions with P. mirabilis 19 lipopolysaccharide in antisera to the outer membrane proteins could be completely removed by absorption of the antisera with the Mr 36,000 protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Finland M. Excursions into epidemiology: selected studies during the past four decades at Boston City Hospital. J Infect Dis. 1973 Jul;128(1):76–124. doi: 10.1093/infdis/128.1.76. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Martin H. H. Phospholipid and lipopolysaccharide in Proteus mirabilis and its stable protoplast L-form. Difference in content and fatty acid composition. Eur J Biochem. 1976 Aug 16;67(2):487–494. doi: 10.1111/j.1432-1033.1976.tb10714.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Mayer H., Fromme I., Kotelko K., Zych K. Ribitol-containing lipopolysaccharides from Proteus mirabilis and their serological relationship. Eur J Biochem. 1977 Jan 3;72(1):35–40. doi: 10.1111/j.1432-1033.1977.tb11221.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J. The isolation of two different lipopolysaccharide fractions from various Proteus mirabilis strains. Eur J Biochem. 1975 Oct 15;58(2):621–626. doi: 10.1111/j.1432-1033.1975.tb02413.x. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUEDERITZ O., RISSE H. J., SCHULTE-HOLTHAUSEN H., STROMINGER J. L., SUTHERLAND I. W., WESTPHAL O. BIOCHEMICAL STUDIES OF THE SMOOTH-ROUGH MUTATION IN SALMONELLA MINNESOTA. J Bacteriol. 1965 Feb;89:343–354. doi: 10.1128/jb.89.2.343-354.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. In vitro reassembly of the membranous vesicle from Escherichia coli outer membrane components. Role of individual components and magnesium ions in reassembly. Biochim Biophys Acta. 1975 Dec 16;413(3):371–393. doi: 10.1016/0005-2736(75)90122-4. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Schubert D. Association of protein fractions and lipids from human erythrocyte membranes. II. Studies on a loosely bound protein fraction. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):781–790. doi: 10.1515/bchm2.1973.354.2.781. [DOI] [PubMed] [Google Scholar]
- Schubert D., Poensgen J., Werner G. Association of protein fractions and lipids from human erythrocyte membranes. I. Studies on a strongly bound protein fraction. Hoppe Seylers Z Physiol Chem. 1972 Jul;353(7):1034–1042. doi: 10.1515/bchm2.1972.353.2.1034. [DOI] [PubMed] [Google Scholar]