Abstract
A 1,2-dioxolane (FINO2) was identified as a lead compound from a screen of organic peroxides. FINO2 does not induce apoptosis, but instead initiates ferroptosis, an iron-dependent, oxidative cell death pathway. Few compounds are known to induce primarily ferroptosis. In contrast to the perceived instability of peroxides, FINO2 was found to be thermally stable to at least 150 °C. FINO2 was more potent in cancer cells than nonmalignant cells of the same type. One of the enantiomers was found to be more responsible for the observed activity.
Graphical Abstract

In the United States, cancer is the primary cause of death in ages 40–79 and the second leading cause of death overall, making the need for the development of new drugs a necessity.1,2 Conventional agents used in cancer therapy operate by a variety of mechanisms, but the vast majority induce cell death by initiating the mitochondrial pathway of apoptosis.3,4 This common pathway of cell death, while effective for many tumors, is associated with resistance mechanisms, such as modulation of the expression levels of the BCL-2 family of proteins or mutation of the p53 protein, that limit therapeutic efficacy.3–6
Screening libraries of compounds can identify drugs with new mechanisms for the treatment of cancer. A potential problem with this method is that restrictions on the types of compounds included in libraries may limit the possible agents identified. To reduce the risk of mistaking generally reactive and unselective compounds as leads for further development, functional groups that are deemed undesirable are often excluded from these screens.7,8 A class of compounds largely excluded in screening platforms due to perceived instability are the organic peroxides.7–9 Peroxides continue to be excluded even though the World Health Organization recommends derivatives of the peroxide artemisinin (Figure 1a), such as artesunate, in combination therapies for the treatment of uncomplicated malaria.10 Artemisinin, as well as several derivatives including artesunate, are under current investigation as potential anticancer agents.11 A clinical trial in which colorectal cancer patients were treated with artesunate prior to curative resection showed that artesunate was well tolerated and had antiproliferative effects.12 An active phase I clinical trial has the goal of establishing safety and maximum tolerated dose of artesunate for treatment of patients with solid tumors (NCT02353026).
Figure 1.
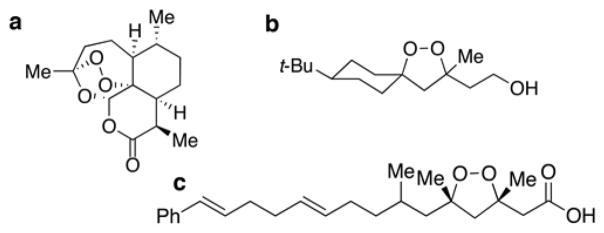
Chemical structures of peroxides. (a) Artemisinin. (b) Lead 1,2-dioxolane (FINO2). (c) Plakinic acid D.
To determine if organic peroxides with structures dissimilar from the artemisinin family (Figure 1a) could be anticancer agents,13,14 we investigated peroxides containing the 1,2-dioxolane scaffold. This scaffold is found in only a few compounds in nature, such as the plakinic acids14 (Figure 1c). The plakinic acids exhibit cytotoxicity toward a few cancer cell lines, but their mechanism of action is unknown.14 The minimal availability of the plakinic acids from the source from which they were isolated, a sponge of the genus Plakortis, limits the quantity of these compounds. Consequently, chemical synthesis was necessary to study the anticancer activity of compounds containing the 1,2-dioxolane scaffold. In this article, we report the first 1,2-dioxolane-containing compound subjected to extensive mechanism of action studies in cancer. This 1,2-dioxolane targets cancer cells by a mechanism distinct from standard chemotherapy drugs. Using a B-lymphoblastic cell leukemia line, RS4;11,15 as a model system, we provide evidence indicating that the 1,2-dioxolane under investigation induces ferroptosis, a nonapoptotic, iron- and reactive oxygen species-dependent form of cell death.16 Furthermore, in vitro this compound is capable of bypassing common chemoresistance pathways such as mutation of p53 and modulation of the expression levels of the BCL-2 proteins.
RESULTS AND DISCUSSION
Identification of a 1,2-Dioxolane As a Lead Compound
Several 1,2-dioxolanes with structures resembling the plakinic acids were prepared17,18 and submitted for testing at the Developmental Therapeutics Program of the National Cancer Institute (NCI) through the In vitro Cell Line Screening Project. Examination of the cytotoxicity profiles of the 1,2-dioxolanes identified a lead compound for further investigation. Keeping with the nomenclature for ferroptosis-inducing compounds as “FIN” compounds,19 the 1,2-dioxolane in our studies will be referred to as FINO2, for ferroptosis-inducing peroxide. FINO2’s cytotoxicity is not limited to one tissue of origin (see Supporting Information Figure 1 for sensitivity across the NCI cancer cell line panel). The lowest concentration of FINO2 to cause 50% growth inhibition (GI50) was observed in an ovarian cancer line, IGROV-1 (GI50 435 nM), and the highest in a lung cancer line, NCI-H322 M (GI50 42 μM). Eighteen out of the 59 cell lines tested showed a GI50 below 2.5 μM, and the average GI50 across all cell lines was 5.8 μM. By comparison, many successful chemotherapeutic agents display average activity across the NCI60 in the micromolar range.20,21 For example, out of 27 Federal Drug Administration (FDA)-approved DNA-damaging agents, 24 possess mean GI50 potency in the micromolar range.20,21
Metrics other than GI50 potency are important when determining the suitability of a lead compound, such as the concentration required to achieve complete cell death and the steepness of the dose–response curve.22 Because many FDA-approved chemotherapy agents have been tested in the NCI60 and the data are publicly available,21,23 the average cytotoxicity of these compounds can be compared to potential lead compounds.24,25 We performed this analysis with respect to FINO2 (Figure 2). When comparing the average concentration needed to induce 50% lethality (LC50) instead of GI50 (Figure 2a), FINO2 has similar potency (average LC50 46 μM) to conventional chemotherapeutic agents such as paclitaxel (75 μM), doxorubicin (13 μM), and vorinostat (70 μM).21 The small difference in concentrations of FINO2 required to cause growth inhibition versus that required to kill the cells suggests FINO2 has a steep dose–response curve. Even though the traditional chemotherapy agents shown have more potent growth inhibition numbers, the distribution of growth inhibition activity across all cell lines is comparable to FINO2.
Figure 2.
Comparison of FINO2 to FDA-approved anticancer agents. (a) GI50, TGI (total growth inhibition), and LC50 values for FINO2 compared to three other anticancer agents. (b) Average (AVG), standard deviation (σ), and coefficient of variation (CV) of FDA-approved chemotherapeutic agents compared to FINO2 to demonstrate similarity in the amount of variation between cell lines.
The CellMiner program23,26 was used to compare FINO2 to other agents tested in the NCI60. Using the GI50 data of several FDA-approved drugs, the averages, standard deviations (σ), and coefficient of variation (CV, standard deviation divided by the mean) were calculated (Figure 2b). The coefficient of variation, a representation of the relative variation within a data set, shows that, out of the compounds analyzed, doxorubicin displays the most variability in its effect on different cell lines and sorafenib displays the least variability, with FINO2 falling in the middle of this list (Figure 2b). Additional analysis using the CellMiner program indicated no significant correlations between FINO2 and the activity of other compounds available through the database or between the activity of FINO2 and the available gene transcript levels.
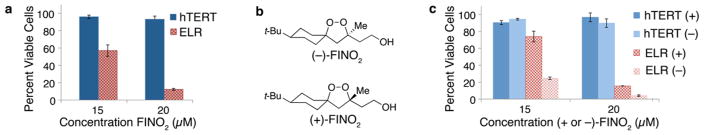
Having identified FINO2 as a candidate for further study, it was necessary to establish its safety and selectivity. The previously reported synthesis17 was optimized to obtain gram quantities of FINO2 (Supporting Information Figure 2a). To address potential stability concerns on the use of organic peroxides as drugs, FINO2 was subjected to thermal stress and demonstrated stability to at least 150 °C (Supporting Information Figure 2b). One system to assess the selectivity index of drug candidates as anticancer agents compares the effect of compounds in cancerous vs noncancerous cells of the same type.27,28 This approach uses a defined model of oncogenic transformation to allow for direct comparison of a noncancerous, immortalized fibroblast cell line (BJ-hTERT) with the same cell line transformed to possess a cancerous phenotype (BJ-ELR).29 This transformation was accomplished by the introduction of Simian Virus 40 large and small T oncoproteins and an oncogenic allele of HRAS.29 Despite the observation that the potency of FINO2 against tumor line BJ-ELR was higher than the average GI50 for the NCI60, treatment with FINO2 had a larger effect on cell viability of the tumor vs the nontumor cell lines (Figure 3a). The steep dose–response curve suggested by the NCI data is clearly indicated by the dramatic decrease in cancer cell viability over only a few micromolar differences. Viability continues to decrease over time in the cancerous cells, but not in the noncancerous cells (Supporting Information Figure 3). This and all experiments were performed in triplicate, and error bars reflect the standard deviation of one experiment. A representative graph of at least three independent experiments is shown.
Figure 3.
Selectivity of FINO2 measured by Promega CellTiter–Glo Luminescence Assay. (a) Comparison of noncancerous (BJ-hTERT; blue) and oncogenically transformed (BJ-ELR; red) fibroblast cells treated with the racemic form of FINO2. (b) Structures of enantiomers of FINO2. (c) Comparison of noncancerous (BJ-hTERT; blue) and oncogenically transformed (BJ-ELR; red) cells treated with individual enantiomers of FINO2. All experiments were performed in triplicate, and error bars reflect the standard deviation of one experiment. A representative graph of at least three independent experiments is shown.
The synthesis of FINO2 produces a racemic mixture,17 so we devised a route to separate the enantiomers so they could be tested individually in the selectivity assay. Oxidation of the pendant hydroxyl group on FINO2 to the carboxylate allowed for coupling to a chiral auxiliary.30 The resulting diastereomers could then be separated. Because one of the diastereomers was crystalline, X-ray crystallography was employed to establish the absolute stereochemistry of the two isomers. Reductive removal of the auxiliary provided the two enantiomerically pure forms of FINO2 (Figure 3b). The (S) enantiomer, (−)-FINO2, was found to be more responsible for the observed activity and selectivity of FINO2 for cancerous fibroblasts (Figure 3c). The steep dose–response curve was again observed with both enantiomers of FINO2. The increased activity of one enantiomer suggests that FINO2 is not simply degrading in an unregulated fashion to form hydrogen peroxide or some other reactive oxygen species (ROS) but instead might have a specific protein interaction necessary for its activity.31 This observation is in contrast to the report that enantiomers of compounds possessing the same core as artemisnin (a 1,2,4-trioxane) display equivalent in vitro activity against malaria parasites.32
FINO2 Induces a Nonapoptotic Form of Cell Death with Features Characteristic of Ferroptosis
Several experiments were performed to discount the mitochondrial apoptotic pathway as a mechanism of cell death induced by FINO2. No increase in Annexin V staining was observed prior to 7-aminoactinomycin D (7AAD) incorporation (Figure 4a), indicating that phosphatidylserine is not exposed to the outer leaflet of the cell membrane upon treatment with FINO2, as would be expected if apoptosis occurred (Supporting Information Figure 4).33 The BioVision MitoCapture Mitochondrial Apoptosis Detection Kit was used to measure the occurrence of mitochondrial outer membrane permeabilization (MOMP). While a shift in fluorescence indicated that MOMP had occurred, this shift occurred only after the majority of cells had died, not before (Supporting Information Figure 5), suggesting MOMP is not necessary for FINO2 to induce cell death. This result was confirmed by overexpression of the Bcl-2 protein to prevent MOMP. Unlike many conventional chemotherapy drugs,5 such as etoposide (Figure 4b), overexpression of the Bcl-2 protein had no effect on FINO2’s ability to induce cell death (Figure 4b and Supporting Information Figure 6). Further experiments showed that caspase activation is not involved in the cell death pathway induced by FINO2, as would be expected for apoptosis (Supporting Information Figure 7).34 The steep dose–response curve observed in the fibroblast cells was even more pronounced in these mechanistic studies (performed in a leukemia cell line), which were quantified by cell death, as opposed to cell viability.
Figure 4.
A nonapoptotic form of cell death induced by FINO2. (a) Annexin V staining is not observed prior to 7AAD incorporation with 6 μM FINO2 treatment at indicated time points. (b) RS4;11 cells overexpressing either GFP or Bcl-2 treated with and FINO2 (left) and etoposide (right). Percent dead cells indicate the Annexin V and 7AAD positive cell population. The nature of the steep dose–response curve induced by FINO2 causes a slight difference in concentrations necessary to induce cell death depending on the preparation of the stock solutions of FINO2 and the passage number of the cells. All experiments were performed at a large concentration range, and the appropriate data are reported.
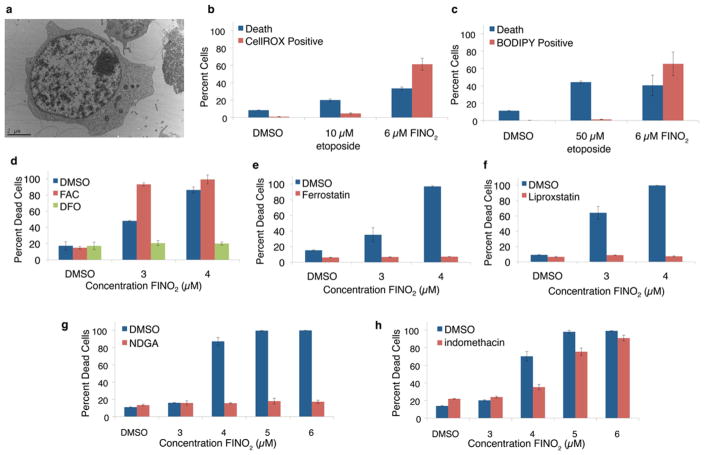
The nomenclature committee on cell death suggests that apoptosis remain a morphological description, as it was first defined.35–37 Therefore, electron microscopy was performed to elucidate the cell death pathway caused by FINO2. Electron microscopy images showed no morphological features characteristic of apoptosis (chromatin condensation, fragmentation of the nucleus, and plasma membrane blebbing), necrosis (plasma membrane rupture), or autophagy (formation of double membrane vacuoles in the cytoplasm, Figure 5a and Supporting Information Figure 8).37 The absence of these features indicates FINO2 is initiating an alternative form of cell death. Having ruled out the apoptotic pathway, it was then possible to consider alternative forms of cell death.
Figure 5.
Many features of ferroptosis exhibited by FINO2-induced cell death. (a) Electron microscopy image of typical RS4;11 cell treated with FINO2 showing a lack of hallmarks of apoptosis, necrosis, and autophagy (performed by the Microscopy Core at New York University Langone Medical Center). Detection of oxidative stress 6 h after indicated treatment by (b) CellROX Green Reagent, Molecular Probes, and (c) BODIPY 581/591 C11. (d) Dependence on iron is shown by iron chelation with 20 μM deferoxamine (DFO) or iron addition with 38 μM ferric ammonium citrate (FAC). (e) Pretreatment with lipophilic antioxidants 500 nM ferrostatin and (f) 200 nM liproxstatin prevent cell death. (g) Pretreatment with 5 μM nordihydroguaiaretic acid (NDGA) prevents cell death. (h) Pretreatment with 100 μM indomethacin partially inhibits cell death. Percent dead cells indicate the Annexin V and 7AAD positive cell population. The nature of the steep dose–response curve induced by FINO2 causes a slight difference in concentrations necessary to induce cell death depending on the preparation of the stock solutions of FINO2 and the passage number of the cells. All experiments were performed at a large concentration range, and the appropriate data are reported.
Additional experiments suggest that cell death is due to ferroptosis. Ferroptosis is a nonapoptotic form of cell death that is induced by the accumulation of lipid peroxides.16 This form of cell death is known to occur in response to a limited number of compounds that inactivate the essential selenium-dependent antioxidant enzyme, glutathione peroxidase IV (GPX4).19,38–41 General oxidative stress upon treatment with FINO2 was indicated by use of a fluorogenic probe, CellROX Green Reagent, that emits at 520 nm upon oxidation and binding to DNA (Figure 5b). This effect was observed about 6 h after the addition of FINO2 but before significant cell death had occurred. To detect for the formation of lipid peroxides in response to FINO2, the membrane-targeted lipid peroxidation detection reagent, C11-BODIPY, was utilized.42 An increase in lipid peroxidation upon treatment with FINO2 (Figure 5c) indicated that oxidative stress in the lipid membrane occurred before death. A time-course experiment with FINO2 using both the CellROX and C11-BODIPY probes ensured that FINO2 did not interact directly with the probes, but that FINO2 did initiate an increase in cellular oxidative stress (Supporting Information Figure 9). In contrast, no oxidative stress occurred upon treatment with the control compound, etoposide. As observed for other ferroptosis-inducing compounds, iron is necessary for FINO2’s mechanism of action. After pretreatment with an iron chelator, deferoxamine (DFO), FINO2 did not cause cell death. By contrast, the addition of exogenous iron, as ferric ammonium citrate (FAC), accelerated cell death by FINO2 beyond what was observed in control experiments (Figure 5d).
Potent inhibition of cell death was observed upon pretreatment with lipophilic antioxidants that inhibit ferroptosis, ferrostatin16,43 and liproxstatin41 (Figure 5e and f). While water-soluble antioxidants do not inhibit all forms of ferroptosis,19 these compounds prevent cell death by eliminating lipid peroxides.43 Because the lipid peroxides implicated specifically in ferroptotic cell death are produced by the oxidation of arachidonic acid,40 the involvement of arachidonic acid metabolism in response to FINO2 treatment was examined. The addition of arachidonic acid increased the potency of FINO2 (Supporting Information Figure 10). The broad-spectrum lipoxygenase inhibitor, nordihydroguaiaretic acid (NDGA), completely inhibited FINO2-induced cell death (Figure 5g), just as it inhibits the induction of ferroptosis by a knockout of glutathione peroxidase IV (GPX4).40 Partial inhibition of cell death induced by FINO2 was observed by treatment with indomethacin, a broad-spectrum cyclooxygenase inhibitor (Figure 5h). By contrast, a protective effect by indomethacin treatment was not observed with previously reported inducers of ferroptosis.19,40
Because the mechanism of cell death induced by FINO2,ferroptosis, bypasses Bcl-2 expression levels, and the mitochondrial pathway as a whole, this mechanism could be used as a platform for identifying efficacious agents when patients are refractory to other treatments due to modulation of the mitochondrial pathway of apoptosis.4,5 The BCL2 gene was identified as one of six genes that can be used to predict overall survival in patients with B-cell lymphoma.44 Additionally, Bcl-2 protein expression was found to be an independent prognostic marker in triple negative breast cancer.45 Several compounds that aim to inhibit Bcl-2 are in clinical trials,46,47 but other BCL-2 proteins such as Mcl-2 can compensate for Bcl-2 inhibition, limiting the effectiveness of these therapies.48
Resistance to chemotherapeutic agents can also occur because many agents depend on the utilization of p53 in the apoptotic process.49 Wild type p53 cells respond more effectively than mutant p53 cells to many chemotherapies and radiation therapy.50 About 50% of cancers, however, harbor a mutation in the TP53 gene,51 leading to the transcription of a nonfunctional, mutant p53 protein. The absence of functional p53 makes establishing a successful treatment regimen for these patients more difficult.6 Upon further examination of the screening data from the NCI, it was noted that FINO2 was cytotoxic toward several cell lines with altered p53 status.52 For example, the GI50’s for mutant p53-expressing nonsmall cell lung cancer cells, NCI-H522 and HOP-92, and the p53-null leukemia cells,52 HL-60, are 1.2 μM, 1.4 μM, and 2.2 μM, respectively. These observations are consistent with the fact that treatment with FINO2 did not increase p53 protein expression even in cells expressing wild type p53 (Supporting Information Figure 11).
Compounds that induce ferroptosis are proposed to be selective because many cancer cells have an increased concentration of iron relative to healthy cells.16,53,54 Ferroptosis was identified by screening compounds selective for engineered tumor cells but not their nonmalignant counterparts.28 The tumor cells were created using serial introduction of genetic elements necessary to induce a cancerous phenotype.29 Compounds that were selective due to an increased rate of proliferation exhibited an incremental increase in cytotoxicity with the introduction of each genetic mutation. The compound that induced ferroptosis, however, only caused an increase in cytotoxicity upon introduction of all genetic elements necessary for malignant transformation.28 Therefore, it was hypothesized that a factor other than the increased proliferation rate of the BJ-ELR cells was causing the observed selectivity of ferroptosis-inducing agents. Upon examination of the iron levels of each cell type, it was discovered that the cancerous BJ-ELR cells have an increased iron content relative to not only the noncancerous BJ-hTERT cells but also the noncancerous cells lacking only the final mutation necessary to induce a cancerous phenotype (cells possessing Simian Virus 40 large and small T oncoproteins but not an oncogenic allele of HRAS).54 These observations suggest the relative levels of iron in different cell types could be the cause of the observed selectivity of FINO2 and other ferroptosis-inducing compounds.
Ferroptosis is dependent on the oxidation of arachidonic acid by the iron-dependent lipoxygenase enzymes.41,55 The products of the lipoxygenase reactions can be further processed to produce the leukotrienes,55,56 while excess hydroperoxide accumulation is controlled by glutathione peroxidase IV (GPX4). GPX4 is responsible for reducing these peroxides to keep their concentration at an appropriate level for cell homeostasis.57 Direct inhibition of GPX4 by agents such as RSL319,54 leads to a toxic accumulation of lipid peroxides, resulting in ferroptosis. Another ferroptosis-inducing compound, erastin,19 is proposed to cause depletion in glutathione levels by inhibiting cystine uptake, a pathway that can be sensitized by both wild type and mutant p53.58 Because glutathione is necessary for GPX4’s antioxidant activity, erastin indirectly causes GPX4 inhibition, resulting in the enzyme’s inability to reduce lipid peroxides effectively.59 As would be expected for a compound that induced ferroptosis, cell death induced by FINO2 was inhibited by an iron chelator and a broad-spectrum lipoxygenase inhibitor, nordihydroguaiaretic acid. These results suggest the lipoxgenase enzymes play a key role in cell death caused by FINO2.
None of the ferroptosis-inducing compounds identified from a high-throughput screen contained the peroxide functionality;28,54 however, in two recent reports, the authors demonstrate that, in some contexts, artemisinin compounds are capable of inducing ferroptosis.60,61 FINO2 and artemisinin share many common characteristics: they both have a requirement of iron for cell death to occur, involve the generation of ROS, and are inhibited by lipophilic antioxidants such as ferrostatin.60–63 There is evidence, however, that artemisinin can be dependent on MOMP and caspase activation.62,64–66 Additionally, the artemisinin family of compounds induce G1 cell cycle arrest.67,68 Cell cycle analysis revealed no change in distribution upon treatment with FINO2, however (Supporting Information Figure 12). Using the CellMiner program to analyze the NCI60 data, it was determined that there was no correlation between the activity of FINO2 and artesunate (−0.478) or dihydroartemisinin (−0.455). In contrast, there is a high level of correlation between artesunate and dihydroartemisinin (0.802). Another noteworthy difference in biological activity of FINO2 and artemisinin compounds is that while FINO2 is more potent in cancer cell lines (AVG GI50 of 5.8 μM) than artemisinin (AVG GI50 of 93 μM) or artesunate (AVG GI50 of 12 μM), artemisinin is more potent (~4 nM) against the malaria parasite than a closely related derivative of FINO2 (~200 nM).69
In conclusion, we have identified a small molecule endoperoxide that warrants further investigation for the potential treatment of cancer. Our results indicate that the peroxide functional group may have broader applications to the treatment of disease than previously suggested. We show that peroxide scaffolds outside that of the artemisinin family may exhibit similar stability profiles, suggesting this class of compounds should not be immediately excluded from investigation due to suspected, but not experimentally verifiable, stability issues.7–9 FINO2 shows selectivity for cancer cells, suggesting that compounds of this kind may be a clinically relevant treatment strategy to pursue. The nonapoptotic mechanism of cell death induced by FINO2 is a desirable feature for the treatment of tumors that have limited response to other chemotherapeutic agents due to either the presence of nonfunctional p53 or inhibition of the mitochondrial pathway of apoptosis.
Supplementary Material
Acknowledgments
We thank J. Beutler and his laboratory (National Cancer Institute, National Institutes of Health) for performing the initial screen of peroxide compounds. We thank the Developmental Therapeutic Program of the National Cancer Institute for performing the in vitro 60-cell line screen with FINO2. We thank D. Morrison (NYU) and J. Meyer (NYU) for helpful discussions and technical assistance. We thank the Cytometry and Cell Sorting Core (NYU) and Q. Jiang (NYU) for technical assistance. We thank H. Samuels (NYU) for helpful discussions. We thank F.-X. Liang and the Microscopy Core at NYU Langone Medical Center for performing electron microscopy experiments. We thank the NYU Molecular Design Institute for the purchase of the Bruker SMART APEXII Diffractometer and C. Hu (NYU) for his assistance with data collection and the determination of the X-ray crystal structure. We thank C. Lin (NYU) for assistance with NMR spectroscopy and mass spectroscopy. We thank W. Hahn and his laboratory (Dana-Farber Cancer Institute) for supplying BJ-ELR cells. We thank B. Stockwell and his laboratory (Columbia University) for helpful discussions. R.P.A. was supported by a Margaret Strauss Kramer Fellowship (Department of Chemistry, NYU). This work was supported by the National Science Foundation (CHE-0848121 to K.A.W.), the National Center for the Advancement of Translational Science, National Institutes of Health (UL1 TR000038), and the National Cancer Institute, National Institutes of Health (R01 CA140729 to W.L.C. and P30 CA016087 to the Laura and Isaac Perlmutter Cancer Center).
Footnotes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschem-bio.5b00900.
Methods, synthetic procedures, and spectra (PDF)
X-ray crystallographic data (CIF)
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. Ca-Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2015. Ca-Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 4.Chonghaile TN, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore VDG, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, DeAngelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A. Pretreatment Mitochondrial Priming Correlates with Clinical Response to Cytotoxic Chemotherapy. Science. 2011;334:1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 6.Gasco M, Crook T. p53 family members and chemoresistance in cancer: what we know and what we need to know. Drug Resist Updates. 2003;6:323–328. doi: 10.1016/j.drup.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bruns RF, Watson IA. Rules for Identifying Potentially Reactive or Promiscuous Compounds. J Med Chem. 2012;55:9763–9772. doi: 10.1021/jm301008n. [DOI] [PubMed] [Google Scholar]
- 8.Baell JB, Holloway GA. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, Reynisson J. Bond stability of the “undesirable” heteroatom–heteroatom molecular moieties for high-throughput screening libraries. Eur J Med Chem. 2011;46:5833–5837. doi: 10.1016/j.ejmech.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Guidelines for the Treatment of Malaria. 2. World Health Organization; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 11.Crespo-Ortiz MP, Wei MQ. Antitumor Activity of Artemisinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. J Biomed Biotechnol. 2012;2012:1–18. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishna S, Ganapathi S, Ster IC, Saeed MEM, Cowan M, Finlayson C, Kovacsevics H, Jansen H, Kremsner PG, Efferth T, Kumar D. A Randomised, Double Blind, Placebo-Controlled Pilot Study of Oral Artesunate Therapy for Colorectal Cancer. EBioMedicine. 2015;2:82–90. doi: 10.1016/j.ebiom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csuk R, Niesen-Barthel A, Barthel A, Kluge R, Ströhl D. Synthesis of an antitumor active endoperoxide from 11-keto-β-boswellic acid. Eur J Med Chem. 2010;45:3840–3843. doi: 10.1016/j.ejmech.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 14.Davidson BS. Cytotoxic Five-Membered Cyclic Peroxides from a Plakortis Sponge. J Org Chem. 1991;56:6722–6724. [Google Scholar]
- 15.Stong RC, Korsmeyer SJ, Parkin JL, Arthur DC, Kersey JH. Human Acute Leukemia Cell Line With the t(4;11) Chromosomal Rearrangement Exhibits B Lineage and Monocytic Characteristics. Blood. 1985;65:21–31. [PubMed] [Google Scholar]
- 16.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, III, Stockwell BR. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez A, Woerpel KA. Synthesis of 1,2-Dioxolanes by Annulation Reactions of Peroxycarbenium Ions with Alkenes. Org Lett. 2005;7:4617–4620. doi: 10.1021/ol051703u. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Hao HD, Zhang Q, Wu Y. A Broadly Applicable Mild Method for the Synthesis of gem-Diperoxides from Corresponding Ketones or 1,3-Dioxolanes. Org Lett. 2009;11:1615–1618. doi: 10.1021/ol900262t. [DOI] [PubMed] [Google Scholar]
- 19.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong CC, Cheng KW, Rigas B. Preclinical Predictors of Anticancer Drug Efficacy: Critical Assessment with Emphasis on Whether Nanomolar Potency Should Be Required of Candidate Agents. J Pharmacol Exp Ther. 2012;341:572–578. doi: 10.1124/jpet.112.191957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holbeck SL, Collins JM, Doroshow JH. Analysis of Food and Drug Administration–Approved Anticancer Agents in the NCI60 Panel of Human Tumor Cell Lines. Mol Cancer Ther. 2010;9:1451–1460. doi: 10.1158/1535-7163.MCT-10-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallahi-Sichani M, Honarnejad S, Heiser LM, Gray JW, Sorger PK. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat Chem Biol. 2013;9:708–714. doi: 10.1038/nchembio.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reinhold WC, Sunshine M, Liu H, Varma S, Kohn KW, Morris J, Doroshow J, Pommier Y. CellMiner: A Web-Based Suite of Genomic and Pharmacologic Tools to Explore Transcript and Drug Patterns in the NCI-60 Cell Line Set. Cancer Res. 2012;72:3499–3511. doi: 10.1158/0008-5472.CAN-12-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hearn JM, Romero-Canelón I, Qamar B, Liu Z, Hands-Portman I, Sadler PJ. Organometallic Iridium(III) Anticancer Complexes with New Mechanisms of Action: NCI-60 Screening, Mitochondrial Targeting, and Apoptosis. ACS Chem Biol. 2013;8:1335–1343. doi: 10.1021/cb400070a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gmeiner WH, Reinhold WC, Pommier Y. Genome-Wide mRNA and microRNA Profiling of the NCI 60 Cell-Line Screen and Comparison of FdUMP[10] with Fluorouracil, Floxuridine, and Topoisomerase 1 Poisons. Mol Cancer Ther. 2010;9:3105–3114. doi: 10.1158/1535-7163.MCT-10-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankavaram U, Varma S, Kane D, Sunshine M, Chary K, Reinhold W, Pommier Y, Weinstein J. CellMiner: a relational database and query tool for the NCI-60 cancer cell lines. BMC Genomics. 2009;10:277. doi: 10.1186/1471-2164-10-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 29.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 30.Dussault PH, Trullinger TK, Cho-Shultz S. Chiral Silyl Ketene Acetals from Thioesters: Reaction with Acetals and Peroxyacetals to form 3-Alkoxy- and 3-Peroxyalkanoates. Tetrahedron. 2000;56:9213–9220. [Google Scholar]
- 31.McConathy J, Owens MJ. Stereochemistry in Drug Action. Prim Care Companion J Clin Psychiatry. 2003;5:70–73. doi: 10.4088/pcc.v05n0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Neill PM, Rawe SL, Borstnik K, Miller A, Ward SA, Bray PG, Davies J, Oh CH, Posner GH. Enantiomeric 1,2,4-Trioxanes Display Equivalent in vitro Antimalarial Activity Versus Plasmodium falciparum Malaria Parasites: Implications for the Molecular Mechanism of Action of the Artemisinins. ChemBioChem. 2005;6:2048–2054. doi: 10.1002/cbic.200500048. [DOI] [PubMed] [Google Scholar]
- 33.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MHJ. Annexin V for Flow Cytometric Detection of Phosphatidylserine Expression on B Cells Undergoing Apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 34.Riedl SJ, Shi Y. Molecular Mechanisms of Caspase Regulation during Apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 35.Kerr JFR, Wyllie AH, Currie AR. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12:1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 37.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 38.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 39.Brigelius-Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta, Gen Subj. 2013;1830:3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Rådmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione Peroxidase 4 Senses and Translates Oxidative Stress into 12/15-Lipoxygenase Dependent- and AIF-Mediated Cell Death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummen GP, van Liebergen LC, Op den Kamp JA, Post JA. C11-BODIPY 581/591, an Oxidation-Sensitive Fluorescent Lipid Peroxidation Probe:(micro) Spectroscopic Characterization and Validation of Methodology. Free Radical Biol Med. 2002;33:473–490. doi: 10.1016/s0891-5849(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 43.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J Am Chem Soc. 2014;136:4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, Levy R. Prediction of Survival in Diffuse Large-B-Cell Lymphoma Based on the Expression of Six Genes. N Engl J Med. 2004;350:1828–1837. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 45.Abdel-Fatah TMA, Perry C, Dickinson P, Ball G, Moseley P, Madhusudan S, Ellis IO, Chan SYT. Bcl2 is an independent prognostic marker of triple negative breast cancer (TNBC) and predicts response to anthracycline combination (ATC) chemotherapy (CT) in adjuvant and neoadjuvant settings. Ann Oncol. 2013;24:2801–2807. doi: 10.1093/annonc/mdt277. [DOI] [PubMed] [Google Scholar]
- 46.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 47.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DCS, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 48.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, Deng X, Zhai D, Shi YX, Sneed T, Verhaegen M, Soengas M, Ruvolo VR, McQueen T, Schober WD, Watt JC, Jiffar T, Ling X, Marini FC, Harris D, Dietrich M, Estrov Z, McCubrey J, May WS, Reed JC, Andreeff M. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Lowe SW, Ruley HE, Jacks T, Housman DE. p53-Dependent Apoptosis Modulates the Cytotoxicity of Anticancer Agents. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein JN, Myers TG, O’Connor PM, Friend SH, Fornace AJ, Kohn KW, Fojo T, Bates SE, Rubinstein LV, Anderson NL, Buolamwini JK, van Osdol WW, Monks AP, Scudiero DA, Sausville EA, Zaharevitz DW, Bunow B, Viswanadhan VN, Johnson GS, Wittes RE, Paull KD. An Information-Intensive Approach to the Molecular Pharmacology of Cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 51.Hollstein M, Sidransky D, Vogelstein B, Harris C. p53 Mutations in Human Cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 52.Berglind H, Pawitan Y, Kato S, Ishioka C, Soussi T. Analysis of p53 mutation status in human cancer cell lines: a paradigm for cell line cross-contamination. Cancer Biol Ther. 2008;7:699–708. doi: 10.4161/cbt.7.5.5712. [DOI] [PubMed] [Google Scholar]
- 53.Pinnix ZK, Miller LD, Wang W, D’Agostino R, Kute T, Willingham MC, Hatcher H, Tesfay L, Sui G, Di X, Torti SV, Torti FM. Ferroportin and Iron Regulation in Breast Cancer Progression and Prognosis. Sci Transl Med. 2010;2:43ra56. doi: 10.1126/scisignal.3001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang WS, Stockwell BR. Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haeggström JZ, Funk CD. Lipoxygenase and Leukotriene Pathways: Biochemistry, Biology, and Roles in Disease. Chem Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu T, Wolfe LS. Arachidonic Acid Cascade and Signal Transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 57.Conrad M, Schneider M, Seiler A, Bornkamm GW. Physiological role of phospholipid hydroperoxide glutathione peroxidase in mammals. Biol Chem. 2007;388:1019–1025. doi: 10.1515/BC.2007.130. [DOI] [PubMed] [Google Scholar]
- 58.Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, Stockwell BR. Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3 doi: 10.7554/eLife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ooko E, Saeed MEM, Kadioglu O, Sarvi S, Colak M, Elmasaoudi K, Janah R, Greten HJ, Efferth T. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22:1045–1054. doi: 10.1016/j.phymed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Eling N, Reuter L, Hazin J, Hamacher-Brady A, Brady NR. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2:517–532. doi: 10.18632/oncoscience.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamacher-Brady A, Stein HA, Turschner S, Toegel I, Mora R, Jennewein N, Efferth T, Eils R, Brady NR. Artesunate Activates Mitochondrial Apoptosis in Breast Cancer Cells via Iron-catalyzed Lysosomal Reactive Oxygen Species Production. J Biol Chem. 2011;286:6587–6601. doi: 10.1074/jbc.M110.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 64.Mercer AE, Maggs JL, Sun XM, Cohen GM, Chadwick J, O’Neill PM, Park BK. Evidence for the Involvement of Carbon-centered Radicals in the Induction of Apoptotic Cell Death by Artemisinin Compounds. J Biol Chem. 2007;282:9372–9382. doi: 10.1074/jbc.M610375200. [DOI] [PubMed] [Google Scholar]
- 65.Lu JJ, Meng LH, Cai YJ, Chen Q, Tong LJ, Lin LP, Ding J. Dihydroartemisinin induces apoptosis in HL-60 leukemia cells dependent of iron and p38 mitogen-activated protein kinase activation but independent of reactive oxygen species. Cancer Biol Ther. 2008;7:1017–1023. doi: 10.4161/cbt.7.7.6035. [DOI] [PubMed] [Google Scholar]
- 66.Gao W, Xiao F, Wang X, Chen T. Artemisinin induces A549 cell apoptosis dominantly via a reactive oxygen species mediated amplification activation loop among caspase-9, -8 and -3. Apoptosis. 2013;18:1201–1213. doi: 10.1007/s10495-013-0857-z. [DOI] [PubMed] [Google Scholar]
- 67.Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate Induces Oxidative DNA Damage, Sustained DNA Double-Strand Breaks, and the ATM/ATR Damage Response in Cancer Cells. Mol Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- 68.Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X, Xue D. Growth inhibitory effects of dihydroartemisinin on pancreatic cancer cells: involvement of cell cycle arrest and inactivation of nuclear factor-κB. J Cancer Res Clin Oncol. 2010;136:897–903. doi: 10.1007/s00432-009-0731-0. [DOI] [PubMed] [Google Scholar]
- 69.Martyn DC, Ramirez AP, Beattie MJ, Cortese JF, Patel V, Rush MA, Woerpel KA, Clardy J. Synthesis of spiro-1,2-dioxolanes and their activity against Plasmodium falciparum. Bioorg Med Chem Lett. 2008;18:6521–6524. doi: 10.1016/j.bmcl.2008.10.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.