Abstract
Predictability is an important characteristic of threat that impacts defensive motivation and attentional engagement. Supporting research has primarily focused on actual threat (e.g., shocks), and it is unclear whether the predictability of less intense threat (e.g., unpleasant pictures) similarly affects motivation and attention. The present study utilized a within-subjects design and examined defensive motivation (startle reflex and self-reported anxiety) and attention (probe N100 and P300) in anticipation of shocks and unpleasant pictures during a no, predictable, and unpredictable threat task. This study also examined the impact of predictability on the P300 to shocks and late positive potential (LPP) to unpleasant pictures. The startle reflex and self-reported anxiety were increased in anticipation of both types of threat relative to no threat. Furthermore, startle potentiation in anticipation of unpredictable threat was greater for shocks compared to unpleasant pictures, but there was no difference for predictable threat. The probe N100 was enhanced in anticipation of unpredictable threat relative to predictable threat and no threat, and the probe P300 was suppressed in anticipation of predictable and unpredictable threat relative to no threat. These effects did not differ between the shock and unpleasant picture trials. Finally, the P300 and early LPP component were increased in response to unpredictable relative to predictable shocks and unpleasant pictures, respectively. The present study suggests that the unpredictability of unpleasant pictures increases defensive motivation, but to a lesser degree relative to actual threat. Moreover, unpredictability enhances attentional engagement in anticipation of, and in reaction to, both types of threat.
Keywords: attention, event-related potentials, pictures, shocks, startle, unpredictability
The identification of potential threat is critical for survival. Predictability is an important characteristic that impacts threat detection (e.g., predictable threat is easier to identify and prepare for relative to unpredictable threat) and has been suggested to differentiate the states of fear and anxiety (Barlow, 2000; Davis et al., 2010; Grillon et al., 2004). Fear is associated with predictable threat and elicits behavioral responses of fight, flight, or immobilization, whereas anxiety is associated with unpredictable threat and elicits hypervigilance and defensive preparedness. The distinction between fear and anxiety has been examined in the laboratory using a no, predictable, and unpredictable threat (NPU-threat) task (Schmitz & Grillon, 2012). In the no threat condition, participants are safe from threat. In the predictable threat condition, threat is signaled by a short duration cue. In the unpredictable threat condition, threat is unsignaled and can happen at any time. Across all three conditions acoustic probes are administered to elicit the startle eye blink reflex as an indicator of defensive motivation. Multiple studies have found that the startle reflex is potentiated (i.e., increased) in anticipation of predictable and unpredictable threat relative to no threat (Grillon et al., 2004; Moberg & Curtin, 2009; Nelson & Shankman, 2011), and startle potentiation is greater for unpredictable threat relative to predictable threat (Gorka et al., 2016; Nelson et al., 2015). These results suggest that the anticipation of threat elicits defensive motivation, which is further augmented when the threat is unpredictable.
The anticipation of threat also impacts measures of attention, and this was recently demonstrated using event-related potentials (ERPs) during the NPU-threat task. Specifically, Nelson et al. (2015) examined the probe N100 and P300 to acoustic startle probes while anticipating no, predictable, and unpredictable electric shocks. The probe N100 is a negative deflection in the ERP that peaks approximately 100 ms after the onset of the probe at frontocentral electrodes and indexes early perceptual processing of auditory stimuli. The probe N100 is enhanced while viewing unpleasant relative to pleasant and neutral pictures (Cuthbert et al., 1998). The probe P300 is a positive deflection in the ERP that peaks approximately 300 ms after the onset of the probe at centroparietal electrodes and indexes attention toward the startle probe. The probe P300 is suppressed when viewing pleasant and unpleasant pictures relative to neutral pictures due to increased attention allocated to the motivationally salient foreground picture (and subsequently decreased attention to the startle probe) (Bradley et al., 2006; Cuthbert et al., 1998; Schupp et al., 1997). In Nelson et al., the probe N100 was enhanced in anticipation of unpredictable shocks relative to predictable shocks and no shocks (which did not differ), and the probe P300 was suppressed to a comparable degree in anticipation of both predictable and unpredictable shocks relative to no shocks. These results suggest that unpredictable threat uniquely enhances early perceptual processing, whereas threat in general (irrespective of predictability) engages later attentional resources.
A key theoretical question is whether there is a threshold for which unpredictability impacts emotional reactivity (Mineka & Kihlstrom, 1978; Staub et al., 1971). In other words, it is important to understand whether unpredictability influences emotional reactivity for even mild to moderate forms of threat. One possibility is that unpredictability is inherently salient and impacts motivation and attention across all threat to a comparable degree. A second possibility is that the impact of unpredictability on motivation and attention varies based on threat intensity. This theoretical issue also has important methodological implications as many studies use different types of threat, but it is rarely known whether they elicit similar or different patterns of emotional reactivity. This issue is particularly important for clinical research where one type of aversive stimulus may not be feasible with certain populations (e.g., shocks cannot be used with children and adolescents) or elicits ceiling level responses that potentially obscure condition or group differences (Bradley et al., 2005).
The NPU-threat task has been employed using a variety of aversive stimuli, including shocks (Bradford et al., 2013; Grillon et al., 2004; Nelson & Shankman, 2011; Shankman et al., 2013), noises (Nelson & Hajcak, 2017; Schmitz et al., 2011), airblasts (Chamberlain et al., 2013; Grillon et al., 2004), and a breathing occlusion (Schroijen et al., 2016). In one of the few investigations that included multiple aversive stimuli, Grillon and colleagues (2004) used a between-subjects design to examine the startle reflex in anticipation of predictable and unpredictable airblasts and shocks. Results indicated that the startle reflex was potentiated during the predictable and unpredictable threat conditions relative to the no threat condition for both types of aversive stimuli, but startle potentiation was greater for shocks relative to airblasts.
An important caveat to Grillon et al. (2004) was that both airblasts and shocks represent actual physical threat. However, not all threat provides immediate danger. For example, emotional pictures are another type of stimuli that have been shown to engage fundamental motivational systems and are commonly used in psychophysiological research. There is robust evidence that viewing unpleasant pictures enhances the startle reflex (Lang, Bradley, & Cuthbert, 1990); although research has indicated that the anticipation of shocks elicits greater startle potentiation relative to viewing unpleasant pictures (Bublatzky et al., 2013; Greenwald et al., 1998; Lissek et al., 2007). The anticipation of unpleasant pictures also potentiates the startle reflex (Dichter et al., 2002; Larson et al., 2007; Lipp et al., 2001; Nitschke et al., 2002; Sabatinelli et al., 2001); however, to date the NPU-threat startle task has not been examined using unpleasant pictures. Pictures are particularly advantageous as they are flexible stimuli, and specific subtypes can be used to examine emotional reactivity to personally relevant phobic or even pleasant content. The present study applied a within-subjects design to examine defensive motivation (startle reflex and self-reported anxiety) and attention (probe N100 and P300) during shock and unpleasant picture versions of the NPU-threat task. In addition, the NPU-threat task has been identified as having several potential strengths, including good psychometric properties (Kaye et al., 2016; Nelson et al., 2015). The present study further tested this notion by examining Cronbach’s alpha, which is an estimate of reliability, across the different versions of the NPU-threat task.
Finally, it is important to note that the NPU-threat task has almost exclusively been used to examine motivation and attention in anticipation of predictable and unpredictable threat. However, predictability has also been shown to impact emotional reactivity to the actual aversive stimulus. For example, one study found that aversive pictures that were preceded by an uncertain valence cue, relative to a certain valence cue, elicited greater skin conductance response and self-reported negative mood (Grupe & Nitschke, 2011). Similarly, a recent study found that neutral and aversive pictures that were preceded by an uncertain valence cue, relative to a certain neutral or certain aversive cue, produced a greater P2 and late positive potential (LPP) response (Dieterich, Endrass, & Kathmann, 2016). To further investigate the impact of predictability on emotional reactivity to the actual aversive stimulus, the current study also measured ERP responses elicited by shocks and unpleasant pictures during the NPU-threat task.
We hypothesized that the startle reflex and self-reported anxiety would be potentiated in anticipation of predictable and unpredictable threat relative to no threat, the probe N100 would be enhanced in anticipation of unpredictable threat relative to predictable threat and no threat, and the probe P300 would be suppressed in anticipation of predictable and unpredictable threat relative to no threat. We also hypothesized that threat-potentiated responding would be greater for the shock relative to unpleasant picture trials. For the ERP responses to the aversive stimuli, we examined the P300 to the shocks (Yamaguchi & Knight, 1991) and the LPP to the unpleasant pictures (Hajcak et al., 2014). The LPP is a sustained positive deflection of the ERP signal that begins as early as 200 ms after stimulus onset and persists throughout stimulus presentation. The LPP is posited to index sustained attention and elaborative processing of salient visual information and is larger while viewing pleasant and unpleasant stimuli relative to neutral stimuli (Cuthbert et al., 2000; Hajcak & Olvet, 2008; Olofsson et al., 2008). We hypothesized that the P300 to shocks and LPP to unpleasant pictures would be greater when the aversive stimuli were delivered with unpredictable relative to predictable timing.
Method
Participants
The sample included 76 introduction to psychology students from Stony Brook University who participated for course credit. Exclusion criteria were an inability to read or write English. The sample was college-aged (M = 19.53, SD = 1.88), 51.3% female, and ethnically/racially diverse, including 21.1% Caucasian, 9.2% Black, 56.6% Asian, 10.5 % Latino, and 2.6% ‘Other’. All participants provided written informed consent, and the research protocol was approved by the Institutional Review Board at Stony Brook University.
Stimuli
Stimuli were administered using PSYLAB (Contact Precision Instruments, London, United Kingdom). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones. Electric shocks were 400-ms in duration and administered to the wrist of the participant’s nondominant hand. Shock intensity was determined ideographically using a work-up procedure for each participant (see below). Unpleasant pictures were from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008) and included threat and mutilation scenes.1 Pictures that were presented during the predictable (valence: M = 2.13, SD = 0.60; arousal: M = 6.67, SD = 0.68) and unpredictable (valence: M = 2.11, SD = 0.46; arousal: M = 6.44, SD = 0.51) threat conditions did not differ in valence or arousal (ps > .48). All pictures were presented for a duration of 1-s, and no picture was ever repeated for a given participant.
Procedure
After electrode placement, participants were seated in a chair approximately 2-ft from a 19-in computer monitor. Participants first completed a 180-s baseline habituation task during which four acoustic startle probes were administered.
The NPU-threat task was a variant of that used by Grillon and colleagues (Schmitz & Grillon, 2012) and has been described elsewhere (Nelson et al., 2015). In the present study, participants completed two versions of the task (counterbalanced), one with shocks and the other with unpleasant pictures as the aversive stimuli. Prior to completing the shock version of the task, shock intensity was determined using a work-up procedure where participants received increasing levels of shock, until they reached a level they described as highly annoying but not painful (maximum shock level was 5-mA). The mean shock level across the entire sample was 2.20 mA (SD = 0.88). The shock electrodes were attached to the participant’s wrist only during the shock version of the task and not during the baseline habituation phase or the unpleasant picture version of the task. Instructions were provided prior to the beginning of both the shock and unpleasant picture versions of the task, regardless of whether it was administered first or second.
For the shock version (see Figure 1), the task included three within-subjects conditions: no shock, predictable shock, and unpredictable shock. Text at the bottom of the screen informed participants of the current condition by displaying “no shock”, “shock at 1”, or “shock at any time”. Each condition lasted 75-s, during which a 5-s visual countdown (i.e., the cue) was presented four times. The interstimulus interval (i.e., time between countdowns during the 75-s condition) ranged from 9 to 15-s during which only the text describing the condition was on the screen. In the no threat condition, no shocks were delivered. In the predictable threat condition, participants received a shock every time the countdown reached 1. In the unpredictable threat condition, shocks were administered at any time. Startle probes were presented both during the countdown (1 to 4-s following countdown onset) and interstimulus interval (5 to 12-s following interstimulus interval onset). The time intervals between shocks and subsequent startle probes were always greater than 10-s to ensure that subsequent probes were not affected by prior shocks.
Figure 1.
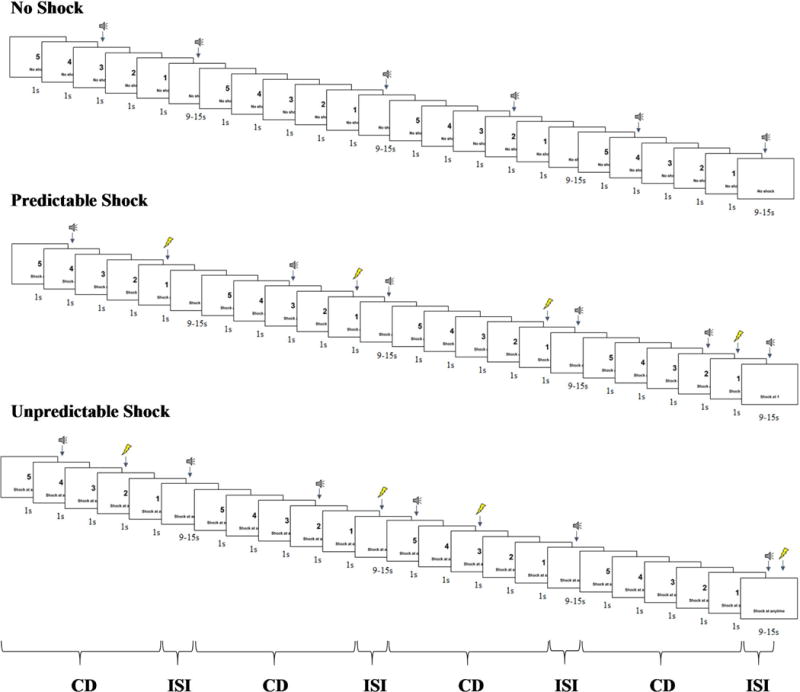
Schematic of the shock version of the no, predictable, and unpredictable threat (NPU-threat) task. Each condition (no threat, predictable threat, and unpredictable threat) was presented twice during the task. The unpleasant picture version of the NPU-threat task was identical expect that an unpleasant picture was presented for 1-s instead of receiving a shock. CD = countdown; ISI = interstimulus interval.
For the unpleasant picture version, the task also included three within-subjects conditions: no unpleasant picture, predictable unpleasant picture, and unpredictable unpleasant picture. Text at the bottom of the screen informed participants of the current condition by displaying “no unpleasant picture”, “unpleasant picture at 1”, or “unpleasant picture at any time”. Timing and duration for the unpleasant picture version were identical to the shock version of the task.
For each version of the task (shocks and unpleasant pictures), there were two presentations of each 75-s condition (no threat, predictable threat, unpredictable threat), during which the countdown appeared four times. Participants received startle probes during three out of four countdowns and interstimulus intervals. Conditions were presented in one of the following orders (counterbalanced): PNUPNU or UNPUNP. Participants completed the opposite condition order for shock and unpleasant picture versions (e.g., PNUPNU for shocks and UNPUNP for unpleasant pictures or UNPUNP for shocks and PNUPNU for unpleasant pictures). All participants received 16 electric shocks (8 during the predictable shock condition and 8 during the unpredictable shock condition), viewed 16 unpleasant pictures (8 during the predictable unpleasant picture condition [4 threat and 4 mutilation] and 8 during the unpredictable unpleasant picture condition [4 threat and 4 mutilation]), and heard 72 startle probes (12 during no shocks, 12 during predictable shocks, 12 during unpredictable shocks, 12 during no unpleasant pictures, 12 during predictable unpleasant pictures, and 12 during unpredictable unpleasant pictures) during the countdown and interstimulus interval (with an equal number of startle probes occurring during the countdown and interstimulus interval).
At the end of each version of the task, participants rated how anxious they felt during the countdown and interstimulus interval of each condition on a scale ranging from 1 (not at all) to 7 (extremely). At the end of the entire experiment, participants reported whether they disliked the shocks or unpleasant pictures the most.
EMG Recording and Processing
Startle eye blink electromyography (EMG) was recorded using PSYLAB and measured from two 4-mm sintered Ag/AgCl electrodes placed over the orbicularis oculi muscle beneath the left eye. EMG activity was sampled at 1000 Hz and filtered between 30 and 500 Hz. Offline, EMG activity was rectified in a 200 ms window, beginning 50 ms before the onset of the startle probe, and a 6-point running average was applied to the rectified data to smooth out sharp peaks. Peak amplitude of the startle reflex was determined in the 20 to 150-ms time frame following the startle probe onset relative to baseline (i.e., average EMG activity in the 50 ms preceding the startle probe onset). Blinks were scored as nonresponses if EMG activity during the 20 to 150-ms post-probe time frame did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the probe-elicited blink response. The present study examined blink magnitude (i.e., averages include values of 0 for nonresponse trials) as it is a more conservative estimate of the startle response (Blumenthal et al., 2005).
EEG Recording and Processing
Continuous electroencephalography (EEG) was recorded using an elastic cap with 34 sintered Ag/AgCl electrodes placed according to the 10/20 system. Electrooculogram (EOG) was recorded using four additional facial electrodes: two placed approximately 1 cm outside of the right and left eyes and two placed approximately 1 cm above and below the right eye. Data were recorded using the Active Two system (BioSemi, Amsterdam, Netherlands). The EEG was digitized with a sampling rate of 1024 Hz using a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz. A common mode sense active electrode producing a monopolar (nondifferential) channel was used as recording reference.
EEG data were analyzed using Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were referenced offline to the average of left and right mastoids, band-pass filtered (0.1 to 30 Hz), and corrected for eye movement artifacts (Gratton et al., 1983). A semiautomatic procedure was employed to detect and reject artifacts. The criteria applied were a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.50 μV within 100-ms intervals. These intervals were rejected from individual channels in each trial. Visual inspection of the data was then conducted to detect and reject remaining artifacts.
Startle probe-locked epochs were extracted with a duration of 1200 ms, including a 200 ms pre-stimulus and 1000 ms post-stimulus interval. The 200 ms pre-stimulus interval was used as the baseline. Separate grand averages were conducted for each level of stimulus (shocks vs. unpleasant pictures), condition (no threat, predictable threat, unpredictable threat), and cue (countdown vs. interstimulus interval), producing 12 different ERP averages. Similar to previous studies measuring startle probe ERPs (Nelson et al., 2015), the probe N100 was scored as the average activity at FCz (where it was maximal) between 90 and 130 ms, and the probe P300 was scored as the average activity at Pz (where it was maximal) between 260 and 320 ms.
Aversive stimulus-locked epochs were also extracted with a duration of 1200 ms, including a 200 ms pre-stimulus and 1000 ms post-stimulus interval. The 200 ms pre-stimulus interval was used as the baseline. Separate grand averages were conducted for predictable and unpredictable aversive stimuli. For shocks, the P300 was scored as the average activity at Pz, (where it was maximal) between 250 and 350 ms. The LPP has been shown to consist of multiple temporal segments that reflect different aspects of emotional processing. For example, the early portion of the LPP (between 300 and 600 ms) has been suggested to index relatively automatic increases in selective attention, while the later portion of the LPP (between 600 and 1000 ms) is associated with more sustained and elaborate processing of the stimulus (Hajcak et al., 2014; Hajcak & Olvet, 2008). Therefore, for unpleasant pictures the LPP was scored as the average activity at Oz and Pz (where it was maximal) and segmented into separate early (300 to 600 ms) and late (600 to 1000 ms) components.
Data Analysis
Five participants were excluded from the startle reflex analyses for having less than 50% useable trials (n = 71). One participant was excluded from the probe N100 and P300 analyses for having less than 50% useable trials (n = 75). One participant was excluded from the self-reported anxiety analyses because they did not complete the ratings (n = 75).
Data were analyzed using a Stimulus (shocks vs. unpleasant pictures) X Condition (no threat, predictable threat, unpredictable threat) X Cue (countdown vs. interstimulus interval) repeated measures analysis of variance (ANOVA). Separate analyses were conducted for each measure of defensive motivation (startle reflex and self-reported anxiety) and attention (probe N100 and P300). Cronbach’s alpha for the startle reflex and probe N100 and P300 was measured as a function of the number of trials. The P300 to the shocks was examined using a one-way repeated measures ANOVA with condition (predictable threat vs. unpredictable threat) as the within-subjects factor. The LPP to the unpleasant pictures was examined using a Condition (predictable threat vs. unpredictable threat) X Time (early LPP vs. late LPP) repeated measures ANOVA. Greenhouse-Geisser epsilons (G-Gε) are reported for repeated measures analyses where assumptions of sphericity were violated. All analyses were conducted in IBM SPSS Statistics, Version 22.0 (Armonk, NY, USA).
Results
Aversive Stimuli
More participants reported disliking the shocks (n = 60, 78.9%) compared to the unpleasant pictures (n = 16, 21.1%), t(75) = 16.77, p < .001.2
Startle Reflex
Figure 2 (top) displays the startle reflex means (and standard errors) across the different aversive stimuli, conditions, and cues. The startle reflex was greater during the shock relative to unpleasant picture trials, F(1, 70) = 25.33, p < .001, ηp2 = .27, and also differed across the threat conditions, F(2, 140) = 62.26, p < .001, G-Gε = .73, ηp2 = .47, such that the startle reflex was greater during the unpredictable threat relative to the predictable threat, F(1, 70) = 54.18, p < .001, ηp2 = .44, and no threat conditions, F(1, 70) = 82.44, p < .001, ηp2 = .54, and the startle reflex was greater during the predictable threat relative to the no threat condition, F(1, 70) = 16.50, p < .001, ηp2 = .19. There was also a main effect of cue, F(1, 70) = 10.18, p < .01, ηp2 = .13, which was qualified by a Stimulus X Cue interaction, F(1, 70) = 24.44, p < .001, ηp2 = .26, and there was a Stimulus X Condition interaction, F(2, 140) = 15.90, p < .001, G-Gε = .80, ηp2 = .19. The Stimulus X Cue interaction was followed-up by conducting separate repeated measures ANOVAs for each level of stimulus. For shocks, the startle reflex was greater during the countdown relative to the interstimulus interval, F(1, 70) = 24.94, p < .001, ηp2 = .26. For unpleasant pictures, the startle reflex did not differ across the countdown or interstimulus interval, F(1, 70) = 0.44, ns.
Figure 2.
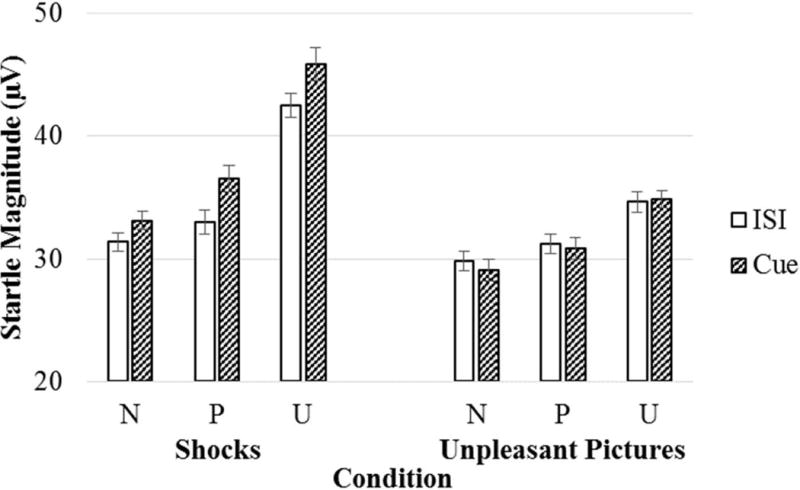
Startle magnitude across different levels of stimulus (shocks vs. unpleasant pictures), condition (no threat, predictable threat, unpredictable threat), and cue (countdown vs. interstimulus interval). Error bars represent standard error. CD = countdown; ISI = interstimulus interval; N = no threat; P = predictable threat; U = unpredictable threat.
To follow-up the Stimulus (shocks vs. unpleasant pictures) X Condition (no threat, predictable threat, unpredictable threat) interaction, separate repeated-measures ANOVAs were conducted for predictable threat vs. no threat (i.e., Stimulus [shocks vs. unpleasant pictures] X Condition [no threat vs. predictable threat]) and unpredictable threat vs. no threat (i.e., Stimulus [shocks vs. unpleasant pictures] X Condition [no threat vs. unpredictable threat]). For the predictable threat analyses, the startle reflex was greater during the predictable threat relative to the no threat condition, F(1, 70) = 16.50, p < .001, ηp2 = .19, but this did not differ between shock and unpleasant picture trials, F(1, 70) = 1.09, ns. For the unpredictable threat analyses, there was a Stimulus X Condition interaction, F(1, 70) = 23.11, p < .001, ηp2 = .25, such that the startle reflex was greater during the unpredictable threat relative to the no threat condition for unpleasant picture trials, F(1, 70) = 31.72, p < .001, ηp2 = .31, but this increase was greater for shock trials, F(1, 70) = 75.90, p < .001, ηp2 = .52.
The follow-up analyses to the Stimulus X Condition interaction collapsed across the different levels of cue (i.e., countdown and interstimulus interval). While there was no Stimulus X Condition X Cue interaction, F(2, 140) = 1.29, ns, we nonetheless conducted additional post-hoc analyses where we separately examined the predictable threat countdown and interstimulus interval. The startle reflex was greater during the predictable threat countdown relative to the no threat countdown during both the shock, F(1, 70) = 10.25, p < .01, ηp2 = .13, and unpleasant picture trials, F(1, 70) = 5.02, p < .05, ηp2 = .07, but the predictable countdown threat-potentiated startle did not differ between the shock and unpleasant picture trials, F(1, 70) = 1.51, ns. Furthermore, the startle reflex was greater during the predictable threat interstimulus interval during the unpleasant picture trials, F(1, 70) = 4.10, p < .05, ηp2 = .06, but not the shock trials, F(1, 70) = 2.60, ns.
Participants were randomly assigned to complete either the shock (n = 37) or unpleasant picture (n = 34) variant of the NPU-threat task first (with the other variant occurring second). To examine whether task order impacted the startle reflex, we conducted a Stimulus (shocks vs. unpleasant pictures) X Condition (no threat, predictable threat, unpredictable threat) X Cue (countdown vs. interstimulus interval) X Task Order (shock trials first vs. unpleasant picture trials first) mixed-measures ANOVA, with stimulus, condition, and cue as within-subjects factors and task order as the between-subjects factor. Results indicated Stimulus X Task Order, F(1, 69) = 15.25, p < .001, ηp2 = .18, and Condition X Task Order interactions, F(2, 138) = 4.18, p < .05, ηp2 = .06, which were qualified by a Stimulus X Condition X Task Order interaction, F(2, 138) = 13.37, p < .001, ηp2 = .16. To follow-up this interaction, separate Stimulus X Condition repeated-measures ANOVAs were conducted for participants who completed the shock trials first and those who completed the unpleasant picture trials first.
As shown in Figure 3, for participants who completed the shock trials first (and unpleasant picture trials second), during the shock trials the startle reflex was greater during the unpredictable threat condition relative to the predictable threat, F(1, 36) = 11.16, p < .01, ηp2 = .24, and no threat conditions, F(1, 36) = 22.96, p < .001, ηp2 = .39, and the startle reflex was greater during the predictable threat relative to the no threat condition, F(1, 36) = 10.69, p < .01, ηp2 = .23. During the unpleasant pictures trials the startle reflex was greater during the unpredictable threat condition relative to the predictable threat, F(1, 36) = 11.01, p < .01, ηp2 = .23, and no threat conditions, F(1, 36) = 16.87, p < .001, ηp2 = .32, but the startle reflex did not differ between the predictable threat and no threat conditions, F(1, 36) = 2.91, ns. For participants who completed the unpleasant picture trials first (and shock trials second), during the unpleasant picture trials the startle reflex was greater during the unpredictable threat condition relative to the predictable threat, F(1, 33) = 4.20, p < .05, ηp2 = .11, and no threat conditions, F(1, 33) = 14.47, p < .001, ηp2 = .31, and the startle reflex was greater during the predictable threat relative to the no threat condition, F(1, 33) = 7.02, p < .05, ηp2 = .18. During the shock trials, the startle reflex was greater during the unpredictable threat condition relative to the predictable threat, F(1, 33) = 57.48, p < .001, ηp2 = .64, and no threat conditions, F(1, 33) = 65.41, p < .001, ηp2 = .67, and but the startle reflex did not differ between the predictable threat and no threat condition, F(1, 33) = 0.86, ns.
Figure 3.
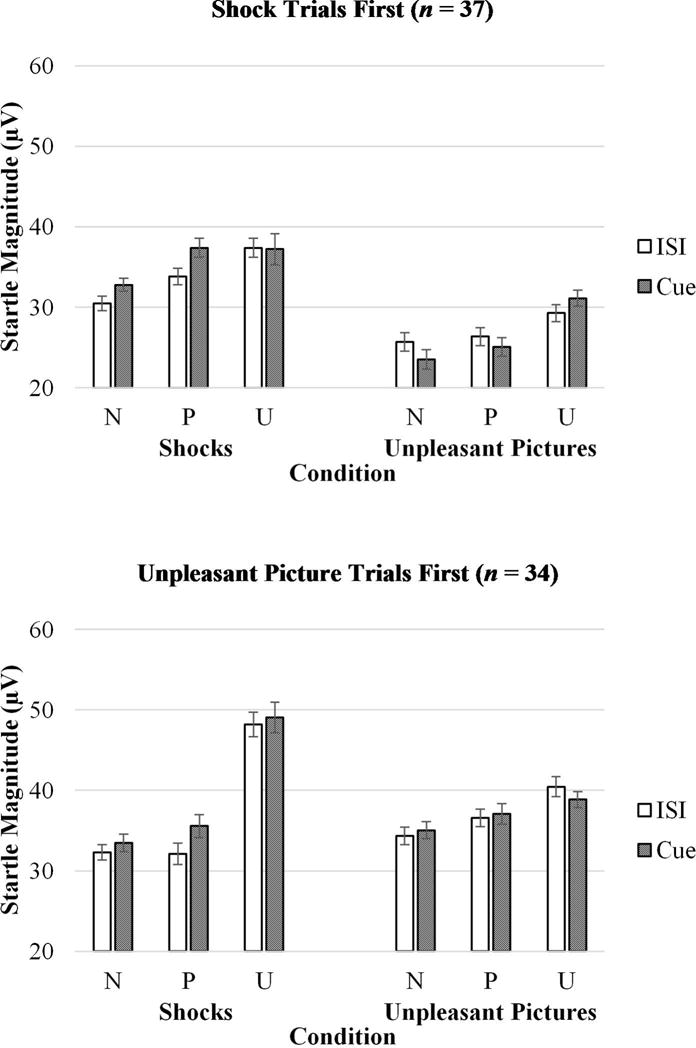
Startle magnitude across different levels of stimulus (shocks vs. unpleasant pictures), condition (no threat, predictable threat, unpredictable threat), and cue (countdown vs. interstimulus interval) for participants who completed shock trials first (top) and unpleasant picture trials first (bottom). Error bars represent standard error. CD = countdown; ISI = interstimulus interval; N = no threat; P = predictable threat; U = unpredictable threat.
Probe N100
Figure 4 displays the startle probe N100 waveforms (left) and three-dimensional rendered scalp distributions (right) across the different conditions. The probe N100 differed across the threat conditions at a trend level, F(2, 148) = 2.66, p < .08, ηp2 = .04. Planned follow-up analyses confirmed that the N100 was enhanced during the unpredictable threat condition relative to the predictable threat, F(1, 74) = 4.46, p < .05, ηp2 = .06, and no threat conditions, F(1, 74) = 3.98, p < .05, ηp2 = .05, but the probe N100 did not differ between the no threat and predictable threat conditions, F(1, 74) = 0.09, ns. The probe N100 enhancement during the unpredictable threat relative to the predictable threat and no threat conditions did not differ between shock and unpleasant picture trials, F(2, 148) = 0.50, ns, and was not moderated by task order, F(2, 146) = 2.68, ns.
Figure 4.
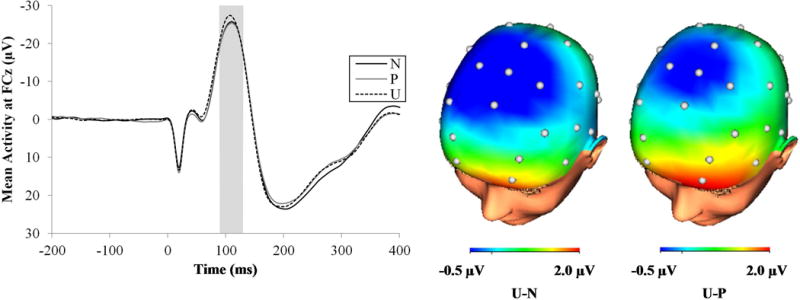
ERP waveforms at FCz (left) and scalp distribution (right) for the probe N100 across the different conditions. Data were collapsed across the countdown and interstimulus interval phases of each condition and the shock and unpleasant picture trials. The shaded regions shows the segment (90 to 130 ms) where the N100 was scored. CD = countdown; ERP = event-related potential; ISI = interstimulus interval; ms = milliseconds; N = no threat; P = predictable threat; U = unpredictable threat.
Probe P300
Figure 5 displays the startle probe P300 waveforms (left) and three-dimensional rendered scalp distributions (right) for the different conditions. The probe P300 was reduced during the shock (M = 14.96 μV, SD = 8.69) relative to unpleasant picture trials (M = 17.49 μV, SD = 7.30), F(1, 75) = 5.66, p < .05, ηp2 = .07. The probe P300 also differed across the threat conditions, F(2, 148) = 6.12, p < .01, such that the probe P300 was suppressed during the predictable threat, F(1, 74) = 10.84, p < .01, ηp2 = .13, and unpredictable threat conditions, F(1, 74) = 7.15, p < .01, ηp2 = .09, relative to the no threat condition, but the probe P300 suppression did not differ between the predictable and unpredictable threat conditions, F(1, 74) = 0.16, ns. The probe P300 suppression during the predictable and unpredictable threat conditions relative to the no threat condition did not differ between the shock and unpleasant picture trials, F(2, 148) = 0.85, ns, and was not moderated by task order, F(2, 146) = 1.96, ns
Figure 5.
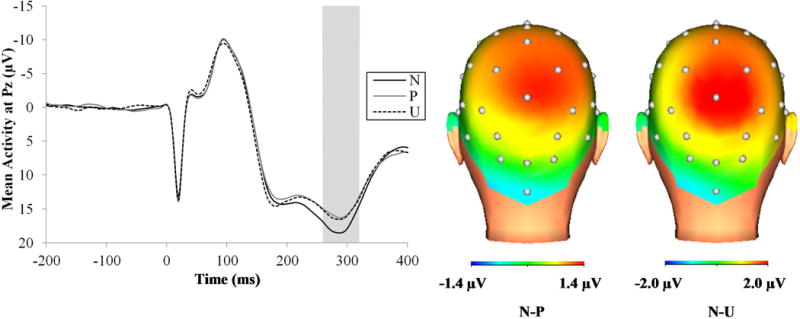
ERP waveforms at Pz (left) and scalp distribution (right) for the probe P300 across the different conditions. Data were collapsed across the countdown and interstimulus interval phases of each condition and the shock and unpleasant picture trials. The shaded regions shows the segment (260 to 320 ms) where the P300 was scored. CD = countdown; ERP = event-related potential; ISI = interstimulus interval; ms = milliseconds; N = no threat; P = predictable threat; U = unpredictable threat.
Self-Reported Anxiety
Table 1 displays self-reported anxiety means and standard deviations across the different aversive stimuli, conditions, and cues. Self-reported anxiety was greater during shock relative to unpleasant picture trials, F(1, 74) = 38.29, p < .001, ηp2 = .34, and also differed as function of threat predictability, F(2, 148) = 191.08, p < .001, G-Gε = .75, ηp2 = .72, such that anxiety was greater during the unpredictable threat condition relative to the predictable threat, F(1, 74) = 104.32, p < .001, ηp2 = .59, and no threat conditions, F(1, 74) = 242.97, p < .001, ηp2 = .77, and anxiety was greater during the predictable threat relative to the no threat condition, F(1, 75) = 157.23, p < .001, ηp2 = .68.
Table 1.
Self-Reported Anxiety during the Shock and Unpleasant Picture Versions of the NPU-Threat Task
| Shocks | Unpleasant Pictures | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Threat | Predictable Threat | Unpredictable Threat | No Threat | Predictable Threat | Unpredictable Threat | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
|
|
|
|||||||||||
| Interstimulus Interval | 1.27 | 0.58 | 3.08 | 1.39 | 4.65 | 1.71 | 1.33 | 0.68 | 2.52 | 1.47 | 3.44 | 1.99 |
| Countdown | 1.44 | 0.68 | 3.69 | 1.39 | 4.71 | 1.77 | 1.49 | 0.86 | 2.61 | 1.41 | 3.49 | 1.88 |
Note. M = mean; SD = standard deviation
There were also Stimulus X Condition, F(2, 148) = 31.15, p < .001, ηp2 = .30, Stimulus X Cue, F(1, 74) = 4.41, p < .05, ηp2 = .06, and Condition X Cue interactions, F(2, 148) = 4.19, p < .05, G-Gε = .91, ηp2 = .05, which were qualified by a Stimulus X Condition X Cue interaction, F(2, 148) = 7.40, p < .001, ηp2 = .09. To follow-up the Stimulus (shocks vs. unpleasant pictures) X Condition (no threat, predictable threat, unpredictable threat) X Cue (countdown vs. interstimulus interval) interaction, separate repeated-measures ANOVAs were conducted for predictable threat vs. no threat (i.e., Stimulus [shocks vs. unpleasant pictures] X Condition [no threat vs. predictable threat] X Cue [countdown vs. interstimulus interval]) and unpredictable threat vs. no threat (i.e., Stimulus [shocks vs. unpleasant pictures] X Condition [no threat vs. unpredictable threat] X Cue [countdown vs. interstimulus interval]). This approach provides separate statistical tests of whether self-reported anxiety potentiation in anticipation of predictable threat and unpredictable threat differed as a function of the aversive stimulus.
For predictable threat, there were main effects of stimulus, F(1, 74) = 20.56, p < .001, ηp2 = .22, condition, F(1, 74) = 157.23, p < .001, ηp2 = .68, and cue, F(1, 74) = 18.56, p < .001, ηp2 = .20, and Stimulus X Condition, F(1, 74) = 35.12, p < .001, ηp2 = .32, and Stimulus X Cue interactions, F(1, 74) = 6.99, p < .01, ηp2 = .09, which were qualified by a Stimulus X Condition X Cue interaction, F(1, 74) = 10.82, p < .01, ηp2 = .13. Follow-up Condition X Cue repeated measures ANOVAs were conducted separately for shock and unpleasant picture trials. For shock trials, there were main effects of condition, F(1, 74) = 186.30, p < .001, ηp2 = .72, and cue, F(1, 74) = 22.81, p < .001, ηp2 = .24, which were qualified by a Condition X Cue interaction, F(1, 74) = 10.09, p < .01, ηp2 = .12. Results indicated that self-reported anxiety was greater during the no threat countdown relative to the no threat interstimulus interval, F(1, 74) = 7.33, p < .01, ηp2 = .09, and this increase was greater during the predictable threat countdown relative to the predictable threat interstimulus interval, F(1, 74) = 19.74, p < .001, ηp2 = .21. For unpleasant picture trials, self-reported anxiety was greater during the predictable threat relative to the no threat condition, F(1, 74) = 63.79, p < .001, ηp2 = .46, but this did not differ as a function of cue, F(1, 74) = 0.39, ns.
For unpredictable threat, there were main effects of stimulus, F(1, 74) = 26.66, p < .001, ηp2 = .27, condition, F(1, 74) = 242.97, p < .001, ηp2 = .77, and cue, F(1, 74) = 4.82, p < .05, ηp2 = .06, which were qualified by a Stimulus X Condition interaction, F(1, 74) = 41.10, p < .001, ηp2 = .36. Follow-up analyses indicated that, for unpleasant picture trials, self-reported anxiety was greater during the unpredictable threat relative to the no threat condition, F(1, 74) = 104.92, p < .001, ηp2 = .59, and this increase was greater for shock trials, F(1, 74) = 285.35, p < .001, ηp2 = .79.
Cronbach’s Alpha
Figure 6 displays Cronbach’s alpha values for the startle reflex (top row), probe N100 (middle row), and probe P300 (bottom row) during shock (left column) and unpleasant picture (right column) trials as a function of the number of trials. Cronbach’s alpha values were interpreted using the following ranges: excellent (> .90), good (.70–.90), moderate (.50–.70), and poor (< .50). The startle reflex was in the excellent range and the probe N100 was in the good range, and this was similar across shock and unpleasant picture trials. The probe P300 was in the good range for shock trials but moderate range for unpleasant picture trials. All three measures across both shock and unpleasant picture trials reached relatively stable Cronbach’s alpha values by the fourth trial. These analyses suggest that the startle reflex and probe N100 and P300 achieved acceptable Cronbach’s alpha values across the shock and unpleasant picture trials.
Figure 6.
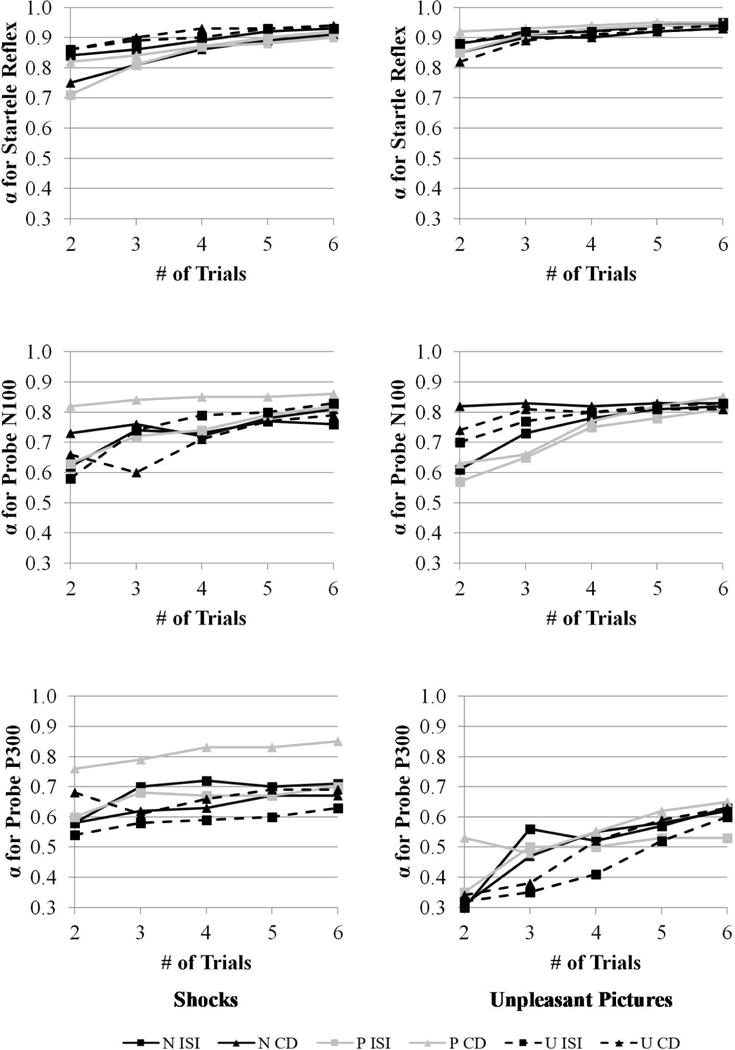
Cronbach’s α for the startle reflex (top row), probe N100 (middle row), and probe P300 (bottom row) for shock (left column) and unpleasant picture (right column) trials as a function of the number of trials across different levels of condition (no threat, predictable threat, unpredictable threat) and cue (countdown vs. interstimulus interval). CD = countdown; ISI = interstimulus interval; N = no threat; P = predictable threat; U = unpredictable threat.
P300 to Shocks
Figure 7 displays the waveforms (left) and three-dimensional rendered scalp distribution (right) for the P300 to predictable and unpredictable shocks. The P300 to shocks was greater for unpredictable relative to predictable shocks F(1, 74) = 45.32, p < .001, ηp2 = .38.
Figure 7.
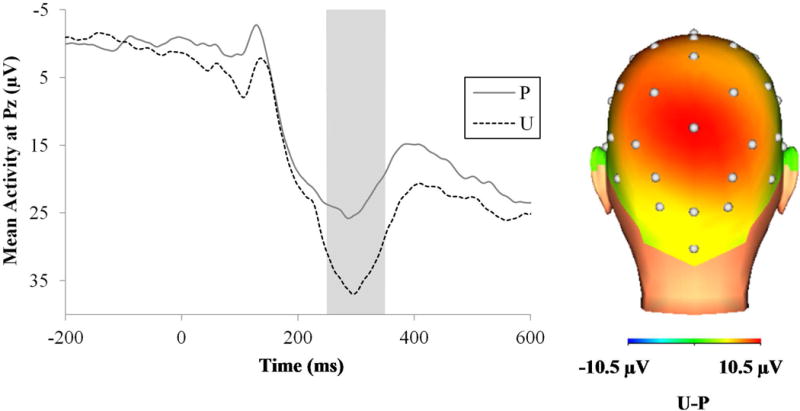
ERP waveforms at Pz (left) and scalp distribution (right) for the P300 to the shocks across the different conditions. The shaded region shows the segment (250 to 350 ms) where the P300 was scored. ERP = event-related potential; ms = milliseconds; P = predictable threat; U = unpredictable threat.
P300 and LPP to Unpleasant Pictures
Figure 8 displays the waveforms (left) and three-dimensional rendered scalp distribution (right) for the LPP to predictable and unpredictable unpleasant pictures. Results indicated a main effect of time, F(1, 74) = 71.11, p < .001, ηp2 = .49, which was qualified by a Condition X Time interaction, F(1, 74) = 9.82, p < .01, ηp2 = .12. The Condition X Time interaction was followed-up by conducting separate repeated measures ANOVAs for each level of time. The early LPP component (i.e., 300 to 600 ms) was greater for unpredictable relative to predictable unpleasant pictures, F(1, 74) = 7.84, p < .01, ηp2 = .10. In contrast, the late LPP component (i.e., 600 to 1000 ms) did not differ between the threat conditions, F(1, 74) = 0.32, ns. These results suggest that the unpredictable, relative to predictable, timing produced a transient increase in the LPP to unpleasant pictures.
Figure 8.
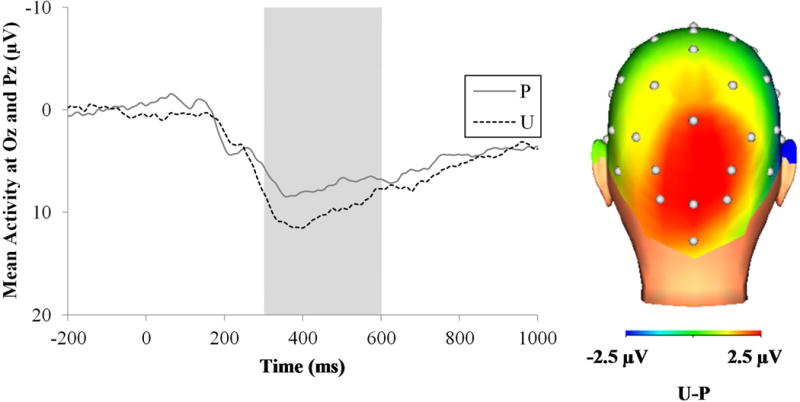
ERP waveforms at pooling of Oz and Pz (left) and scalp distribution (right) for the LPP to the unpleasant pictures across the different conditions. The shaded region shows where the early LPP component was scored (300 to 600 ms). ERP = event-related potential; LPP = late positive potential; ms = milliseconds; P = predictable threat; U = unpredictable threat.
Discussion
The present study examined defensive motivation and attention in anticipation of two different types of predictable and unpredictable threat (i.e., shocks and unpleasant pictures). Overall, the startle reflex and self-reported anxiety were potentiated in anticipation of both types of threat relative to no threat, and this increase was greater for unpredictable compared to predictable threat. In addition, the probe N100 was enhanced in anticipation of unpredictable threat relative to both predictable threat and no threat, and the probe P300 was suppressed in anticipation of predictable and unpredictable threat relative to no threat. The startle reflex and probe N100 and P300 results replicated Nelson et al. (2015) across both shock and unpleasant picture trials.
However, there were also notable differences in defensive motivation and attentional engagement during the shock and unpleasant picture trials. First, shocks elicited a greater overall startle response—and greater unpredictable threat-potentiated startle; there was no difference in predictable threat-potentiated startle between the shock and unpleasant picture trials. Second, shocks also elicited greater overall self-reported anxiety and greater predictable and unpredictable threat-potentiated self-reported anxiety. Third, shocks produced a smaller overall P300 response, but P300 suppression (i.e., the relative decrease in the probe P300 during threat relative to no threat trials) did not differ as a function of aversive stimulus or predictability. Finally, unpredictable, relative to predictable, timing enhanced the P300 to shocks and early LPP to unpleasant pictures.
The present study suggests that unpredictability impacts defensive motivation in anticipation of actual threat and visual threat, but to different degrees. While previous research has reported startle potentiation in anticipation of unpleasant pictures (Dichter et al., 2002; Larson et al., 2007; Lipp et al., 2001; Nitschke et al., 2002; Sabatinelli et al., 2001), the images in these studies were presented with consistent or predictable timing. The current results provide novel evidence that manipulating the temporal predictability of unpleasant pictures further potentiates the startle reflex, and indicates that unpredictability enhances defensive motivation for even lower threshold, less noxious aversive stimuli (Mineka & Kihlstrom, 1978; Staub et al., 1971). However, results indicated an interaction between unpredictability and the type of threat, such that unpredictable shocks were a more potent elicitor of defensive motivation compared to unpredictable unpleasant pictures. There were key differences between shocks and unpleasant pictures that likely contributed to this pattern of results. For example, shocks were a source of actual physical threat, while the unpleasant pictures represented visual or symbolic threat (Lissek et al., 2007). In addition, a larger proportion of participants reported disliking the shocks the most (78.3%) compared to the unpleasant pictures (21.7%), and participants reported greater overall anxiety during the shock relative to unpleasant picture trials. This study suggests that more intense aversive stimuli are associated with increased effects of unpredictability on the startle reflex.
The startle reflex was also potentiated in anticipation of predictable threat (albeit to a lesser degree compared to unpredictable threat) and this did not differ between the shock and unpleasant picture trials. However, there are two important caveats to these findings. First, during the unpleasant picture trials, the startle reflex was potentiated during both the predictable threat countdown and interstimulus interval, although participants were only in danger during the countdown. An important methodological issue might explain this pattern of results. Specifically, a different unpleasant picture was presented during each predictable threat countdown, and this might have introduced an element of uncertainty or unpredictability during the predictable threat interstimulus interval (i.e., participants did not know what specific picture would be presented next). This differs from the shock trials, where the same shock was presented during each predictable threat countdown and there was no startle potentiation during the predictable threat interstimulus interval. Future NPU-threat studies that employ unpleasant pictures should consider using a more homogenous category of pictures to reduce this element of uncertainty during the predictable threat condition. Second, the order in which participants completed the different versions of the NPU-threat task (i.e., shock version first vs. unpleasant picture version first) impacted startle potentiation to predicable threat. Specifically the startle reflex was only potentiated during the predictable threat condition when the NPU-threat task variant was administered first (irrespective of the type of aversive stimulus), but the startle reflex was always potentiated during the unpredictable threat condition (irrespective of the type of aversive stimulus or task order). In a previous investigation of startle habituation during the NPU-threat task, we found that the startle reflex during the predictable threat condition was greater than the no threat condition at the beginning of the task, but did not differ by the end of the task (Nelson et al., 2015). Together, these studies suggest that the startle reflex in anticipation of predicable threat habituates by the end of a single task administration.
The startle probe ERPs provided a neural indicator of attentional engagement during the NPU-threat task that was separate from the startle reflex measure of defensive motivation. In replication of Nelson et al. (2015), the probe N100 was uniquely enhanced while anticipating unpredictable (but not predictable) threat, whereas the P300 was suppressed for both unpredictable and predictable threat. Importantly, these results did not differ as a function of the type of aversive stimulus. To date, a number of different stimuli have been shown to produce probe P300 suppression, including emotional pictures (Bradley et al., 2006; Cuthbert et al., 1998; Schupp et al., 1997), emotional sounds (Keil et al., 2007), cigarette cues (Versace et al., 2010), and a breathing mask during an interoceptive challenge (Alius et al., 2015). Although the current study provides initial evidence that the potential for threat in general, and not necessarily the type of threat, impacts the allocation of attentional resources, only two different aversive stimuli (shocks and unpleasant pictures) were compared. Future studies should continue to examine whether the anticipation of other types of aversive stimuli (e.g., airblasts, noises) similarly elicit comparable attentional engagement.
All three psychophysiological measures demonstrated acceptable Cronbach’s alpha values across both shock and unpleasant picture trials. Cronbach’s alpha for the startle reflex and probe N100 were similar across the different aversive stimuli. In contrast, Cronbach’s alpha for the probe P300 was better during the shock relative to unpleasant picture trials, although both were in the acceptable range. As previously mentioned, shock level was determined ideographically, but participants were presented with the same set of standardized unpleasant pictures. Shocks also elicited a greater overall startle reflex, self-reported anxiety, and P300 suppression. It is possible that this ‘stronger situation’ (Cooper & Withey, 2009; Lissek et al., 2006) elicited a more uniform pattern of attentional engagement across the shock trials, while the ‘weaker situation’ elicited by the unpleasant pictures produced greater variability in the probe P300. Nonetheless, these results add to growing evidence that measures of defensive motivation and attention during the NPU-threat task have good to excellent psychometric properties (Kaye et al., 2016; Nelson et al., 2015).
In addition to the anticipation of threat, predictability also impacted attentional processing of the actual aversive stimuli. Specifically, the P300 to the shocks and unpleasant pictures were enhanced when presented with unpredictable, relative to predictable, timing. This finding is consistent with previous investigations demonstrating that the P300 is larger for unexpected stimuli (Donchin, 1981) and a broader literature on the impact of unpredictability on enhanced sensory and attentional processing of aversive stimuli (Carlsson et al., 2006; Dieterich et al., 2016). Together, the startle probe and aversive stimulus ERP results suggest that unpredictability heightens motivated attention both in anticipation and during processing of threat.
The present study had several limitations that should be taken into consideration. First, the sample consisted of college undergraduates, which may limit the generalizability of the results to other populations (e.g., children, clinical populations). Second, the present study focused on temporal predictability, but there are other characteristics that can be uncertain or unpredictable (e.g., ambiguity of content) that warrant future investigation. Third, the unpleasant pictures consisted of threat and mutilation pictures, some of which are not appropriate for children or adolescents. Future studies should attempt to replicate these findings using other visual stimuli (e.g., angry and scared faces). Finally, the present study focused on the impact of predictability on aversive stimuli; however, appetitive stimuli can also vary in temporal predictability. Future research might leverage the flexibility of emotional pictures and compare the impact of predictability on the anticipation of pleasant and unpleasant pictures.
In conclusion, the present study found that predictable and unpredictable shocks and unpleasant pictures elicited a similar pattern of defensive motivation (startle reflex and self-reported anxiety) and attentional engagement (probe N100 and P300). However, shocks, relative to unpleasant pictures, were a more potent elicitor of defensive motivation, particularly for unpredictable threat. In addition, unpredictability enhanced attentional processing of both aversive stimuli. There has been a growing emphasis in psychological science to establish the psychometric properties of measures used in between-subjects analyses (Hajcak & Patrick, 2015). This study adds to this important effort and supports the utility of the NPU-threat task in measuring different Negative Valence System constructs (i.e., acute threat and potential threat) across multiple units of analysis (i.e., startle reflex, probe N100 and P300, self-reported anxiety). This study provides novel evidence that different types of aversive stimuli produce comparable attention engagement, but more aversive threat elicits greater defensive motivation when it is temporally unpredictable. Future studies should continue to examine these critical issues across additional measures (e.g., fMRI), aversive stimuli (e.g., unpleasant noises), and elements of unpredictability (e.g., intensity).
Acknowledgments
This project was funded by National Institute of Mental Health grant K01MH107808 award to B.D.N.
Footnotes
IAPS pictures included threat (2811, 6242, 6244, 6250, 6350, 6510, 6560, and 9425) and mutilation (3000, 3030, 3051, 3071, 3100, 3110, 3170, and 3266) content.
We also examined whether participants who disliked shocks vs. unpleasant pictures the most differed on the startle reflex, probe N100, or probe P300. To this end, we conducted a Stimulus (shocks vs. unpleasant pictures) X Condition (no threat, predictable threat, and unpredictable threat) X Cue (countdown vs. interstimulus interval) X Group (disliked shocks vs. unpleasant pictures the most) mixed-measures ANOVA with stimulus, condition, and cue as within-subjects factors and group as the between-subjects factor. For the probe N100, there was a Stimulus X Condition X Group interaction, F(2, 146) = 3.68, p > .05, ηp2 = .05; however, follow-up analyses indicated no significant differences between groups. For the probe P300, participants who disliked shocks the most had greater P300 suppression across all stimuli, conditions, and cues relative to those who disliked unpleasant pictures the most, F(1, 73) = 4.81, p < .05, ηp2 = .06. There were no group differences for the startle reflex.
The authors have no conflicts of interest to report.
References
- Alius MG, Pane-Farre CA, Low A, Hamm AO. Modulation of the blink reflex and P3 component of the startle response during an interoceptive challenge. Psychophysiology. 2015;52:140–148. doi: 10.1111/psyp.12295. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037/0003-066X.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychological Science. 2013;24:2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Moulder B, Lang PJ. When good things go bad: The reflex physiology of defense. Psychological Science. 2005;16:468–473. doi: 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Bublatzky F, Guerra PM, Pastor MC, Schupp HT, Vila J. Additive effects of threat-of-shock and picture valence on startle reflex modulation. PLoS ONE. 2013;8:6–11. doi: 10.1371/journal.pone.0054003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Andersson J, Petrovic P, Petersson KM, Ohman A, Ingvar M. Predictability modulates the affective and sensory-discriminative neural processing of pain. NeuroImage. 2006;32:1804–1814. doi: 10.1016/j.neuroimage.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Chamberlain PD, Rodgers J, Crowley MJ, White SE, Freeston MH, South M. A potentiated startle study of uncertainty and contextual anxiety in adolescents diagnosed with autism spectrum disorder. Molecular Autism. 2013;4:31. doi: 10.1186/2040-2392-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WH, Withey MJ. The strong situation hypothesis. Personality and Social Psychology Review. 2009;13:62–72. doi: 10.1177/1088868308329378. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: Attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/S0048577298970536. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Tomarken AJ, Baucom BR. Startle modulation before, during and after exposure to emotional stimuli. International Journal of Psychophysiology. 2002;43:191–196. doi: 10.1016/S0167-8760(01)00170-2. [DOI] [PubMed] [Google Scholar]
- Dieterich R, Endrass T, Kathmann N. Uncertainty is associated with increased selective attention and sustained stimulus processing. Cognitive, Affective, & Behavioral Neuroscience. 2016;16:447–456. doi: 10.3758/s13415-016-0405-8. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise!…Surprise? Psychophysiology. 1981;18:493–514. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Bradley MM, Cuthbert BN, Lang PJ. Startle potentiation: shock sensitization, aversive learning, and affective picture modulation. Behavioral Neuroscience. 1998;112:1069–79. doi: 10.1037/0735-7044.112.5.1069. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D, Weinberg A. Handbook of emotion regulation. 2014. Temporal dynamics of emotion regulation; pp. 43–57. [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Patrick CJ. Situating psychophysiological science within the research domain criteria (RDoC) framework. International Journal of Psychophysiology. 2015;98:223–226. doi: 10.1016/j.ijpsycho.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53:1241–1255. doi: 10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Junghöfer M, Russmann T, Lowenthal W, Lang PJ. Cross-modal attention capture by affective stimuli: Evidence from event-related potentials. Cognitive, Affective & Behavioral Neuroscience. 2007;7:18–24. doi: 10.3758/CABN.7.1.18. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. 2008 doi: 10.1016/j.epsr.2006.03.016. [DOI] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Larson CL, Nitschke JB, Davidson RJ. Common and distinct patterns of affective response in dimensions of anxiety and depression. Emotion. 2007;7:182–191. doi: 10.1037/1528-3542.7.1.182. [DOI] [PubMed] [Google Scholar]
- Lipp OV, Cox D, Siddle DAT. Blink startle modulation during anticipation of pleasant and unpleasant stimuli. Journal of Psychophysiology. 2001;15:155–162. doi: 10.1027//0269-8803.15.3.155. [DOI] [Google Scholar]
- Lissek S, Orme K, Mcdowell DJ, Johnson LL, Luckenbaugh DA, Baas JM, Grillon C. Emotion regulation and potentiated startle across affective picture and threat-of-shock paradigms. Biological Psychology. 2007;76:124–133. doi: 10.1016/j.biopsycho.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: a new perspective on experimental neurosis. Journal of Abnormal Psychology. 1978;87:256–271. doi: 10.1037/0021-843X.87.2.256. [DOI] [PubMed] [Google Scholar]
- Moberg Ca, Curtin JJ. Alcohol selectively reduces anxiety but not fear: startle response during unpredictable versus predictable threat. Journal of Abnormal Psychology. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G. Anxiety and depression symptom dimensions demonstrate unique associations with the startle reflex in anticipation of unpredictable threat in 8 to 14 year-old girls. Journal of Abnormal Child Psychology. 2017;45:397–410. doi: 10.1007/s10802-016-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Hajcak G, Shankman SA. Event-related potentials to acoustic startle probes during the anticipation of predictable and unpredictable threat. Psychophysiology. 2015;52:887–894. doi: 10.1111/psyp.12418. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJC, Ruffalo D, Davidson RJ. Startle potentiation in aversive anticipation: Evidence for state but not trait effects. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. doi: 10.1111/psyp.12244. doi: 10.1111/psyp.12244. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Merikangas K, Swendsen H, Cui L, Heaton L, Grillon C. Measuring anxious responses to predictable and unpredictable threat in children and adolescents. Journal of Experimental Child Psychology. 2011;110:159–170. doi: 10.1016/j.jecp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroijen M, Fantoni S, Rivera C, Vervliet B, Schruers K, van den Bergh O, van Diest I. Defensive activation to (un)predictable interoceptive threat: The NPU respiratory threat test (NPUr) Psychophysiology. 2016;53:905–913. doi: 10.1111/psyp.12621. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Birbaumer N, Lang PJ. Probe P3 and blinks: Two measures of affective startle modulation. Psychophysiology. 1997;34:1–6. doi: 10.1111/j.1469-8986.1997.tb02409.x. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub E, Tursky B, Schwartz GE. Self-control and predictability: their effects on reactions to aversive stimulation. Journal of Personality and Social Psychology. 1971;18:157–162. doi: 10.1037/h0030851. [DOI] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, Cinciripini PM. Cigarette cues capture smokers’ attention: Evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. P300 generation by novel somatosensory stimuli. Electroencephalography and Clinical Neurophysiology. 1991;78:50–5. doi: 10.1109/ICCME.2012.6275616. [DOI] [PubMed] [Google Scholar]


