Abstract
Metal-based drugs have shown early promise as anticancer agents suggesting the potential application of silver(I) complexes as apoptosis-inducing agents. The ability of a silver(I) cyanide containing phosphine complex to induce cell death was evaluated in both a malignant (SNO esophageal cancer) and non-malignant (HDF-a skin and HEK293 kidney) cell lines. A dose-dependent decrease in cell viability was observed in the SNO cells. Light microscopy revealed morphological features indicative of apoptotic cell death. The mode of cell death was confirmed as apoptosis by phosphatidylserine externalization, DNA fragmentation and nuclear condensation. Furthermore, both the non-malignant cell lines showed morphological features indicative of apoptosis when exposed to complex 1. We propose the use of this silver(I) cyanide phosphine complex as an highly effective positive apoptosis control for use in anticancer studies of phosphine complexes.
Keywords: Silver(I) phosphine complex, Apoptosis, Cyanide containing complex, Malignant cells, Anticancer
Introduction
Hanahan and Weinberg (2011) suggested that the diverse range of cancers is owing to six different alterations that occur in their physiological processes. This includes the ability to produce their own growth signals, they are insensitive to antigrowth signals, can evade apoptosis, undergo continuous replication, sustain angiogenesis and can invade tissues contributing to malignant cell growth. The different treatment strategies include; surgery, radiation, chemotherapy, hormonal therapy, immune therapy and drug specific therapy (American Cancer Society 2016). Since cancer is very often drug-resistant, the range of available and effective drugs is limited in spite of efforts to improve therapy and recent advances made in the field of drug discovery. There is consequently a substantial need for the development of new drugs and treatment alternatives.
Chemotherapeutic agents are broadly classified as alkylating agents, anti-metabolites and natural products and derivatives thereof. Metal-based drugs, of which the platinum containing complexes i.e. cisplatin, are classified as alkylating agents (Page and Takimoto 2004). Extensive study of metals in medicine is largely due to the approval, more than three decades ago, of the platinum-based antitumor drug cisplatin as a cancer drug (Rosenberg 1971; Guo and Sadler 1999). The discovery of cisplatin, and the development of its analogues, has allowed for the successful treatment of germ-line cancers including activity against L1210 leukemia in mice and against advanced Sarcoma180 tumors (Rosenberg et al. 1969). Cisplatin and/or analogues are often used in combination with other agents as primary treatment for testicular, cervical, ovarian and head and neck cancers (O’Dwyer et al. 1999). It is also used in secondary treatment of small-cell lung carcinomas (De Pas et al. 2001) and some bladder cancers (Vaughn and Malkowicz 2001). Unfortunately, there are many side-affects associated with the use of platinum-based cancer drugs including gastro-intestinal toxicity, renal toxicity and peripheral neuropathy (Reed et al. 1996), as well as the development of resistance to these platinum-based drugs (Hrubisko et al. 1993; Kartalou and Essigmann 2001; Tagliabue et al. 1993).
The interest in non-platinum metal complexes for cancer chemotherapy has been growing and is motivated by the possibility to develop new agents with a mode of action and clinical profile different from the established platinum metallo-drugs (Meggers 2007; Ott and Gust 2007; Bruijnincx and Sadler 2008). The focus in the development of novel metal-based cancer chemotherapeutic drugs has consequently changed to the use of non-platinum central atoms and to the incorporation of different organic ligands into metal complexes (Meggers 2007; Ott and Gust 2007; Bruijnincx and Sadler 2008; Hartinger and Dyson 2009). These metal based drugs might possibly produce improved anticancer activity in vitro and in vivo. The geometrical flexibility, structural diversity and the ability of silver(I) complexes to adopt a variety of nuclearities makes the study of silver(I) chemistry very attractive (Meijboom et al. 2009). Various Ag(I) complexes have shown promising anticancer activity. These include tetrameric 1:1 and monomeric 1:3 complexes of silver(I) halides with tri(p-tolyl)-phosphine (Zartilas et al. 2009) and Ag(I) complexes comprising aromatic tertiary phosphines and diphosphines (Berners-Price and Sadler 1988; Papathanasiou et al. 1997; McKeage et al. 1998; Liu et al. 2008).
However, literature on the mechanism of the silver(I) complexes as potential anticancer drugs was limited until recently. The apoptotic inducing ability of a number of Ag(I) complexes in malignant cell lines have been reported recently (Ferreira et al. 2015; Human et al. 2015; Potgieter et al. 2015).
Here, we investigated the ability of a silver(I) cyanide containing phosphine complex (referred to herein as complex 1) to induce cell death in an esophageal cancer cell line, and compared its effect to non-tumorigenic cell lines.
Materials and methods
Chemical synthesis and characterization of 1:2 AgCN(PPh3)2 (complex 1)
All the chemicals needed for the synthesis of the silver(I) complex were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received. Complex 1 was prepared according to literature (Muetterties and Alegranti 1972; Romualdo et al. 2002) and is summarized below. The pure complex was first characterized with various analytical methods before being used for biological studies. The Infrared spectra were recorded on a Bruker Tensor 27 FTIR spectrophotometer (Bremen, Germany), using a PIKE miracle Gate ATR accessory. 1H NMR (400 MHz), 13C{H} NMR (101 MHz) and 31P{H} NMR (162 MHz) spectra were recorded on a Bruker Ultrashield Avance III 400 MHz spectrometer. The peaks were referenced to TMS using the residual protio impurities in CDCl3 (1H), or the solvent signal (13C). Melting points were recorded on a Stuart Scientific Melting Point apparatus SMP10 (Stone, Staffordshire, UK), and are uncorrected. Microanalysis was performed on a Thermo Flash 2000 series CHNS/O (Waltham, MA, USA), organic elemental analyzer.
AgCN salt (0.15 g, 1.12 mmol) was added to a solution of triphenylphosphine (L1) (0.6 g, 2.24 mmol) in acetonitrile (50 cm3). The reaction mixture was heated under reflux overnight. The hot solution was filtered and evaporated to ±10 cm3. Thereafter, the solution was left to crystalize at room temperature for 24 h to form small needle like crystals. Yield: 75%. Melting point: 193 °C. IR (ν/cm−1): 3056 (w), 2323 (w), 2119 (m), 1891 (w), 1823 (w), 1670 (w), 1585 (w), 1478 (s), 1433 (s), 1309 (m), 1182 (w), 1182 (w), 1156 (w), 1092 (s), 1069 (m), 1026 (w), 997 (w), 917 (m), 849 (w), 740 (s), 691 (s). 1H NMR (400 MHz, CDCl3): δ (ppm) 7.19 (t, J = 7.0 Hz), 7.18 (m, J = 7.0 Hz), 13C{H} NMR (100 MHz, CDCl3): δ (ppm) 133.8 (d, J(C-P) = 12.5 Hz), 133.4(d, J = 12.0 Hz), 130.58, 129.3 (d, J = 14.9 Hz). 31P{H} NMR (161 MHz, CDCl3): δ (ppm): 25.53. Elemental Analysis: Calculated for C37H30AgNP2: C, 67.49%; H, 4.59%; N, 2.13%. Found: C, 67.07; H, 4.99; N, 2.11.
Preparation of complex 1 for biological studies
A stock solution of complex 1 was prepared in cell culture graded dimethyl sulfoxide (DMSO) (Merck, Darmstadt, Germany). This was followed by heating for 1 h at 70 °C until the crystals solubilized. The stock solution was kept in the dark at 4 °C and heated at 70 °C for 30 min before treatment. Complex 1 is lipophilic in nature and therefore it solubility is limited in aqueous solutions (data not shown). The only safe solvent that could be used for biological studies was DMSO.
Cell culturing of a malignant esophageal (SNO) cell line
A SNO esophageal squamous carcinoma (ATCC, cat no. CCL-185) was used for this study. This cell line was established in 1976 when a tumour specimen was obtained from a 62-year-old zulu male (Bey et al. 1976). The SNO cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Biowest, Riverside, MO, USA), 1.6% penicillin/streptomycin/fungizone (Lonza, Walkersville, MD, USA) and 0.4% gentamicin sulfate (Lonza). The cells were sub-cultured every 48 h in 75 cm3 culture flasks and incubated at 37 °C in a 5% CO2 humidified atmosphere. After 48 h the cells were removed from flasks by means of 0.5% trypsin (Lonza). A total of 6 × 105 cells were plated in a 3.5 cm culture dish and left to adhere for 24 h.
Treatment of SNO cells
Used DMEM medium was removed and a final volume of 1 cm3 medium containing the treatment was added to the 3.5 cm culture dishes. The cells were either left untreated (UC), treated with 1% DMSO (vehicle control) or with 10 µM of complex 1. In addition, the cells were treated with 10 µM of the uncoordinated ligand L1. Cells treated with 100 µM cisplatin (CDDP) (Molekula, Dorset, UK) served as the positive apoptotic control. Cisplatin was prepared in 0.9% sodium chloride (NaCl) followed by heating (20 °C) for 48 h. For dose response studies a concentration range of 2–10 µM and 10–100 µM was used for complex 1 and cisplatin, respectively. Where applicable, a positive necrotic control of 25% hydrogen peroxide (H2O2) (Minema, Gauteng, RSA) was included and was prepared in DMEM before treatment. The concentration of DMSO did not exceed 1% throughout the study for both malignant and non-malignant cells.
Cell culturing of non-malignant HDF-a and HEK293 cell lines
A human dermal fibroblast adult (HDF-a) (ScienCellTM, Carlsbad, CA) and human embryonic kidney (HEK293) (ATCC, cat no. CRL-1573) cell line were used as non-malignant controls. The HDF-a cells were cultured in fibroblast medium (FM) (ScienCell™ , Carlsbad, CA, USA), with additional fibroblast growth stimulants (FGS) (ScienCell), 2% FBS along with 5% penicillin/streptomycin/fungizone and 1% gentamicin sulfate. The HEK293 cells were cultured in the same DMEM as for the SNO cells described in Sect. 2.4. The HDF-a and HEK293 cells were sub-cultured every 72 h and incubated in 75 cm3 CellBIND® culture flasks (Corning Incorporated, New York, NY, USA) at 37 °C in a 5% CO2 humidified atmosphere. After 72 h the cells were removed from the flasks by means of 0.25% trypsin. A total of 2 × 104 cells (in 100 mm3) were seeded in a CellBIND® 96 well plate (Corning Incorporated). This was performed in triplicate and they were left to adhere for 24 (HDF-a) or 48 h (HEK293).
Treatment of HDF-a and HEK293 cells
Used medium was removed and 100 mm3 of medium containing the treatment was added in the triplicate wells. The cells were either left untreated (UC) or treated with 1% DMSO (vehicle control), 10 µM of the uncoordinated ligand L1, 10 µM of complex 1 or 100 µM cisplatin or 25% H2O2.
Cell proliferation assay and morphological studies
The viability of the cells was determined using an alamarBlue® proliferation assay (Serotec, Oxford, UK). Briefly, 10 mm3 of the dye was either added to 100 mm3 of trypsinized SNO cells or directly to the HDF-a and HEK293 cells in the 96 well plate. Wells containing medium only served as a blank. The plate was then covered and incubated for ±2 h at 37 °C in a 5% CO2 humidified atmosphere. After incubation the fluorescence was measured on the Synergy HT Multi-Detection Microplate reader (BioTek, Winooski, VT, USA) at a wavelength (λ) 450 nm (excitation) and 590 nm (emission). It should be noted that the IC50 concentrations for the SNO cells were also determined with the alamarBlue® assay. Morphological changes where observed in all three cell lines using a Zeiss Axiovert 25 inverted microscope (Carl Zeiss, Göttingen, Germany) with Zeiss Axio version 3.1 software (Carl Zeiss) at a magnification of 200×.
Confirming the mode of cell death
The mode of cell death (either apoptosis or necrosis) was studied by using an Annexin-V FITC assay kit (Serotec). The cells were double-labelled with Annexin-V FITC and propidium iodide (PI) fluorochromes according to manufacturer’s instructions with minor adjustments. Briefly, the cells (±3 × 105 cells) were washed twice with cold (4 °C) phosphate buffered saline (PBS), followed by the addition of 100 mm3 1× Binding Buffer™. Two and a half cubic millimetres of Annexin-V along with 5 mm3 PI were added in the dark and incubated for 15 min at room temperature. After incubation, 400 mm3 1 × Binding Buffer™ was added followed by analysis using the FACSAriaTM flow cytometer (BD Biosciences, San Jose, CA, USA) with FACSDivaTM software (BD Biosciences). A total of 10 000 cells were recorded at a wavelength (λ) 492 nm (excitation) and λ 520 nm (emission) for Annexin–V or λ 488 nm (excitation) and λ 575 nm (emission) for PI.
Imaging nuclear changes
The integrity of DNA was determined using a cell permeable Hoechst-33258 dye (Invitrogen, Eugene, OR, USA). The cells were plated onto cover slips in the 3.5 cm culture plates. After treatment, the medium was removed and the attached cells were washed with pre-warmed (37 °C) PBS. The cells were fixed by adding 4% formaldehyde (Sigma) and incubated for 15 min at 37 °C before washing. Thereafter the cells were permeabilized using 0.1% Triton-X (Sigma) for 20 min at 4 °C followed by washing. Hoechst-33258 (1 µg/ml in PBS) was added to the cells and incubated for 20 min at room temperature followed by washing. The coverslip with the fixed cells was mounted on a glass slide containing a drop of buffered glycerol (Rochelle Chemicals, Johannesburg, RSA) and sealed with clear nail varnish. The slides were viewed under an Zeiss Axioplan 2 inverted fluorescent microscope (Carl Zeiss) and images taken using Axio Cam camera (Carl Zeiss) and Axio Vision imaging software (Carl Zeiss) at a excitation of λ 343 and an emission of 483. Images were taken at a magnification of 1000×.
Statistical analyses
The data was analysed using Microsoft Excel and the Students t-Test. All data were analysed for the Standard Error of the Mean (±SEM) that is represented as error bars. The P value of *P < 0.05 and ***P < 0.001 were deemed statistically significant with respect to the vehicle control where n represents the number of biological repeats.
Results and discussion
Biological studies
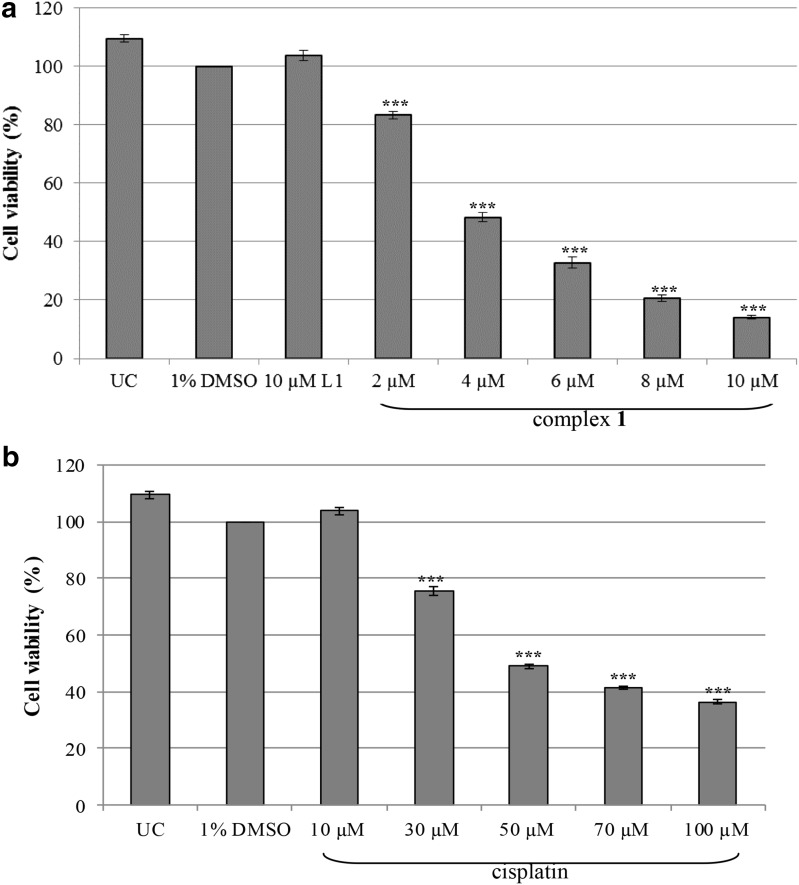
The cytotoxicity of complex 1 was determined in a malignant SNO cell line by using an alamarBlue® assay. Dose-responsive studies were done using increasing concentrations of complex 1 and cisplatin (Fig. 1a, b). When compared to DMSO, the viability of the SNO cells significantly decreased as the concentration increased for both complex 1 and cisplatin. The IC50 inhibitory concentration (concentration of a drug that inhibits 50% of the cellular growth) was calculated using the dose–response curves and is represented in Table 1. Complex 1 had a low IC50 value of 4.02 µM when compared to the IC50 of cisplatin (47.39 µM). This marked degree of toxicity between this class of silver(I) complexes and cisplatin was previously reported (Ferreira et al. 2015; Human et al. 2015; Potgieter et al. 2015). Furthermore, silver(I) saccharinate complexes with monophosphines (Yilmaz et al. 2014) including silver(I) salicylic acid with triphenylphosphine (Poyraz et al. 2011) showed improved cytotoxicity in malignant cells when compared to cisplatin. In addition, silver(I) acetate o-methoxyphenyl diphosphine and dicyclohexylphosphine complexes were significantly cytotoxic to malignant cells when compared to the standard drugs Tamoxifen and 5-Fluorouracil/5-FU (Sulaiman et al. 2015).
Fig. 1.
Dose-response curves of SNO cells treated with varying concentrations of complex 1 (a) and cisplatin (b) for 24 h. Cells that were untreated (UC) or treated with 1% DMSO served as the negative and vehicle control respectively. In addition, cells were treated with the uncoordinated ligand L1. An alamarBlue® assay was used and the percentage viability is expressed with respect to 1% DMSO (100%). Error bars were constructed based on the Standard Error of the mean (±SEM) (n = 9). The P value was calculated using the two-tailed Students t test. The treatments with a P value of *** P < 0.001 were deemed significant with respect to DMSO
Table 1.
Calculated IC50 concentrations for complex 1 and cisplatin in malignant SNO cells. The Standard Error of the Mean (±SEM) is indicated (n = 9)
| Treatment | IC50 concentration (±SEM) |
|---|---|
| Complex 1 | 4.02 µM (±0.94 µM) |
| Cisplatin | 47.39 µM (±7.05 µM) |
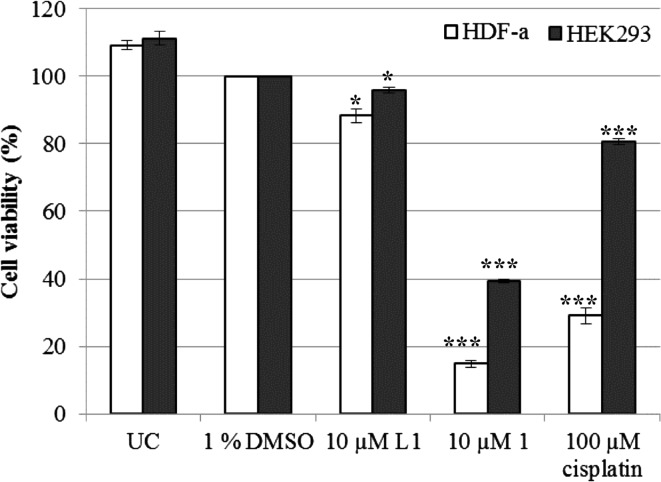
The selectivity of the complex was further determined by using the two non-malignant HDF-a and HEK293 cell lines. This enables one to identify the treatment that will specifically target malignant cells only without harming the non-malignant cells. However, it is evident that complex 1 had a low selectivity by significantly inducing cell death of more than 85% and 60% of HDF-a and HEK293 cells, respectively (Fig. 2). In contrast, other silver(I) phosphine complexes were less, or had comparable, cytotoxic activities for normal cells than for the malignant cells (Poyraz et al. 2011; Yilmaz et al. 2014). In one study, a silver thiocyanate (AgSCN) complex selectivity was determined in the same non-malignant cell lines studied herein (Ferreira et al. 2015). This complex is structurally similar to complex 1 with the only difference being a SCN linked to the silver rather than CN. The thiocyanate complex was less cytotoxic to both non-malignant cell lines when compared to complex 1. Only ±22% of HDF-a cells and 42% of HEK293 cells were non-viable after 24 h of treatment in the above mentioned study.
Fig. 2.
The percentage viability of non-malignant HDF-a and HEK293 cells determined with the alamarBlue® assay. Both cell lines were either untreated (UC) or treated for a 24-h period with 1% DMSO (vehicle), 10 µM L1, 10 µM complex 1 and 100 µM cisplatin (positive control). The percentages were expressed with respect to 1% DMSO (100%). Error bars were constructed based on the Standard Error of the Mean (±SEM) (n = 9). The P value was calculated using the two-tailed Students t test. The treatments with a P value of *P < 0.05 and ***P < 0.001 were deemed significant with respect to DMSO
The effect of the uncoordinated ligand, L1, was evaluated in all three cell lines to determine its cytotoxicity on its own (Figs. 1, 2). Overall the ligand L1 was minimally toxic to all three cell lines with viabilities higher than 88%. The triphenylphosphine (TPP) and o–hydroxy-benzoic acid (o-HbzaH) ligands used by Poyraz and co-workers (2011) were shown to be less toxic than the intact silver(I) phosphine complex. Furthermore, free tertiary phosphines have shown to be toxic to P815 mastocytoma tumour cells, although to a lesser extent than their gold(I), silver(I) and copper(I) phosphine counterparts (McKeage et al. 1998). When comparing the cytotoxicity of the functional silver(I) complex with that of the ligand it appears that the association of these two entities are required to produce the observed anticancer activity. Unfortunately, the AgCN salt was insoluble in DMSO and could not be tested for its cytotoxic activity.
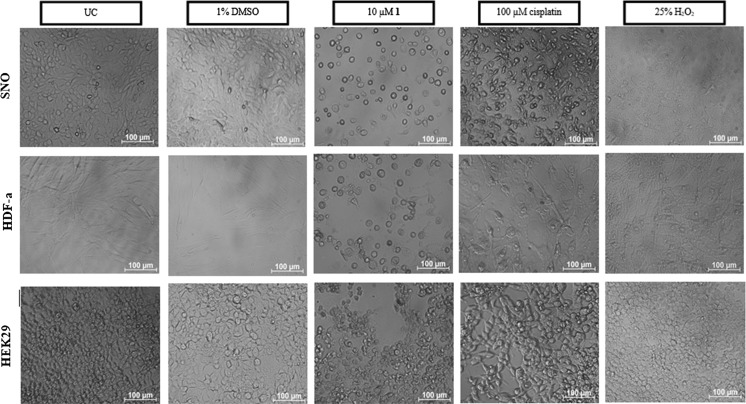
Most studies focus on the role of silver(I) phosphine complexes as anticancer agents and not the cell targeting mechanism thereof. Therefore, morphological changes were monitored in all three cell lines using light microscopy (Fig. 3). Images were taken after 24 h of treatment and structural differences were observed between the three cell lines. The SNO and the HEK293 cells appear to be more circular in shape when compared to the HDF-a cells that appear more spindly. The untreated and DMSO-treated cells appear intact with no indications of cellular stress. When the cells were treated with complex 1, cellular detachment was observed in all three cell lines. The cells that were still attached appeared to be rounded with cellular blebs that are characteristic of apoptotic cell death (Kerr et al. 1972). These features were also observed in the cisplatin (apoptotic control)-treated SNO and HDF-a cells, but was minimal in the HEK293 cells. Necrotic cell death appears to be absent in all the cells when compared to H2O2-treated cells (necrotic control). During cancer chemotherapy, it is important to induce apoptotic cell death due to the lack of an inflammatory response, rather than necrosis, where this response would be evident (Festjens et al. 2006; Kroemer et al. 2009).
Fig. 3.
Light microscope images of malignant SNO, non-malignant HDF-a and HEK293 cells taken 24 h after treatment. All three cell lines were either untreated (UC) or treated with 1% DMSO, 10 µM complex 1, 100 µM cisplatin (apoptotic control) and 25% H2O2 (necrotic control). Images were taken at a magnification of ×200 on a Zeiss Axiovert 25 inverted microscope. Untreated and DMSO treated cells appear to be intact with no signs of cellular stress. Cells treated with complex 1 however show signs of cellular rounding and blebbing which resembles that of cisplatin. Necrotic cell death appears to be absent
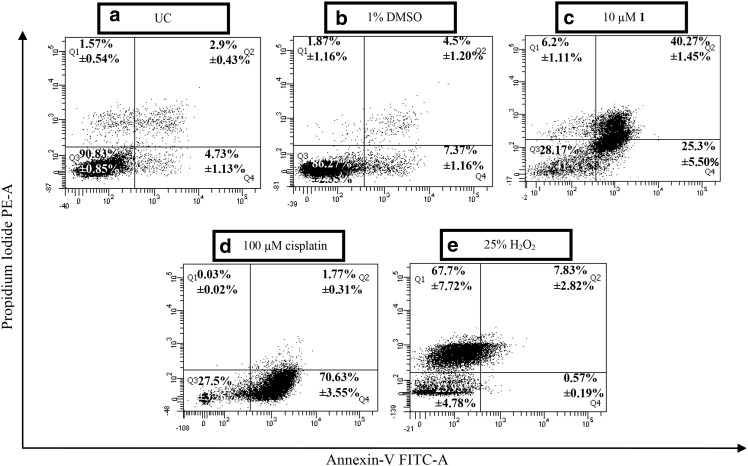
Morphological studies indicate that complex 1 could trigger apoptotic cell death due to the characteristic features observed (Fig. 3). Flow cytometry was used to confirm apoptotic cell death in the SNO cells. The method distinguishes apoptotic cell death from necrosis through phosphatidylserine (PS) externalization. The data are presented as a dot blot that divides the cells in four different quadrants that either represent viable cells (Q3), early (Q4) or late (Q2) apoptotic and finally necrotic cells (Q1) (Fig. 4). Both the untreated (UC) and DMSO-treated cells showed minimal cell death with an average viability of 90.83% for the UC and 86.27% for DMSO in Q3 (Fig. 4a and b). More than 60% of the cells were located in the apoptotic quadrants Q4 and Q2 (early and late stages) after being treated with complex 1 and cisplatin (Fig. 4c, d). The remaining cells were either located in Q3 or minimally in Q1. Cells treated with H2O2 were more than 60% necrotic and are located in Q1 (Fig. 4e).
Fig. 4.
Dot blots representing the mode of cellular death induced in differentially treated malignant SNO cells. Analyses were performed using Annexin-V FITC and PI fluorochromes. Cells were either untreated (UC) (a) or treated with 1% DMSO (b), 10 µM of complexes 1 (c) along with 100 µM cisplatin (d) and 25% H2O2 (e) which served as the apoptotic and necrotic controls, respectively. The average percentage was calculated for all quadrants as represented in each quadrant, followed by the Standard Error of the Mean (±SEM) (n = 3). The four quadrants represent Q3 that is negative for FITC and PI, Q4 that is positive for FITC but negative for PI, Q1 that is positive for PI but negative for FITC and Q2 that is positive for both FITC and PI. Cells undergoing early apoptosis are more likely to be found in Q4 while those of late apoptosis will be found in Q2. Necrotic cells are found in Q1 while Q3 indicates intact viable cells
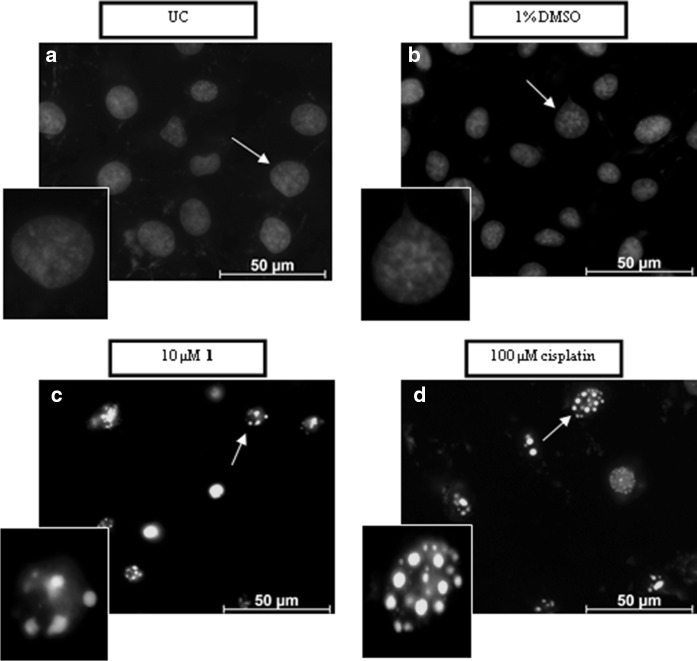
Furthermore, the nuclear integrity was also monitored using a cell permeable Hoechst-33258 dye which also serves as a marker for apoptosis. The nucleus of the cells undergoing apoptosis appears as bright fluorescent structures whereas those with intact nuclei have a weaker blue fluorescence (Fig. 5). The nuclei of the SNO cells that were untreated or treated with DMSO were uniformly stained (Fig. 5a, b). In contrast, SNO cells treated with complex 1 and cisplatin were irregularly stained with small bright circular structures indicating fragmented DNA and nuclear condensation (Fig. 5c, d).
Fig. 5.
Changes in the nuclear morphology of malignant SNO cells taken after 24 h of treatment. The cells were either untreated (a) or treated with 1% DMSO (b), 10 µM complex 1 (c) and 100 µM cisplatin (d). The cells were stained with Hoechst-33258 dye and visualised with a Zeiss Axioplan 2 inverted fluorescence microscope containing an Axio Cam camera (magnification of ×1000). White arrows indicate healthy (uniformly stained) or damaged (irregular stained) nuclei along with the enlargements in the left bottom corner
When comparing the data from Figs. 3, 4 and 5, it is evident that the phosphine complex being studied here results in apoptotic cell death. Similar signs of apoptosis have been reported in SNO cells after being treated with various silver(I) thiocyanate complexes and is summarized in Table 2. Studies reported by Kyros et al. (2010) and Poyraz et al. (2011) showed that specific silver(I) phosphine complexes induce apoptosis in leiomyosarcoma cancer cells (LMS) in a dose-dependent manner due to the observed phosphatidylserine externalisation. Even though silver(I) phosphine complexes were shown to interact with DNA (Kyros et al. 2014; Yilmaz et al. 2014), this study, to our knowledge is the first to report that nuclear condensation and DNA fragmentation occurs after exposure to silver(I) cyanide (Fig. 5).
Table 2.
Apoptotic markers observed in SNO cells after being treated with complex 1 and other related 1:2 silver(I) phosphine complexes
| Silver salt | Phosphine ligand | Apoptotic markers observed |
|---|---|---|
| AgCN | PPh3 | Cellular rounding, membrane blebbing, PS externalization, DNA fragmentation and nuclear condensation |
| AgSCN | PPha3 | Apoptotic bodies, membrane blebbing, PS externalization |
| {P(4-MeC6H4)3}2]a2 | ||
| {P(4-FC6H4)3}2]a2 | ||
| {P(4-ClC6H4)3}2]a2 | ||
| {PPh2(CH2C6H5)}b2 | Apoptotic bodies and membrane blebbing |
Overall, it is suggested that the silver(I) complexes containing a cyanide entity (complex 1) can be used as a highly effective and appropriate positive control when studying how metal-based phosphine complexes induce cell death in cancer cells.
Conclusion
Complex 1 was evaluated for its anticancer activity in malignant SNO esophageal cells. A dose-dependent decrease in viability was observed in the SNO cells and the mode of cell death was confirmed to be apoptosis. However, the complex showed to be cytotoxic to non-malignant HDF-a and HEK293 cells which arguably limits its use as an anticancer agent per se, but its non-selective apoptotic inducing ability makes it a highly effective and appropriate positive control for evaluating Ag-phosphines as potential anti-cancer drugs.
Acknowledgements
The authors gratefully acknowledge Dr. Rehana Malgas-Enus for the synthesis and characterization of complex 1. This work was performed for the partial fulfillment for the requirements for a Ph.D. thesis of Ms Engelbrecht within the graduate program of Biochemistry at the University of Johannesburg. The authors also gratefully acknowledge financial assistance from the University of Johannesburg. This work is based on the research supported in part by the National Research Foundation of South Africa (Grant specific unique reference number (UID) 83863). The Spectrum facility at the University of Johannesburg is acknowledged for the use of the NMR spectrometer and FACSAria flow cytometer.
Contributor Information
Reinout Meijboom, Email: rmeijboom@uj.ac.za.
Marianne J. Cronjé, Email: mariannec@uj.ac.za
References
- American Cancer Society . Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- Berners-Price SJ, Sadler PJ. Phosphines and metal phosphine complexes: relationship of chemistry to anticancer and other biological activity. Struct Bond. 1988;70:27–102. doi: 10.1007/3-540-50130-4_2. [DOI] [Google Scholar]
- Bey E, Alexander J, Whitcutt JM, Hunt JA, Gear JHS. Carcinoma of the esophagus in Africans: establishment of continuously growing cell line from a tumour specimen. In Vitr Plant. 1976;12:107–114. doi: 10.1007/BF02796356. [DOI] [PubMed] [Google Scholar]
- Bruijnincx PCA, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Chem Biol. 2008;12:97–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pas T, de Braud F, Mandala M, Curigliano G, Catania C, Ferretti G, Sozzi P, Solli P, Goldhirsch A. Cisplatin and vinorelbine as second-line chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) resistant to taxol plus gemcitabine. Lung Cancer. 2001;31:267–270. doi: 10.1016/S0169-5002(00)00176-8. [DOI] [PubMed] [Google Scholar]
- Ferreira E, Munyaneza A, Omondi B, Meijboom R, Cronjé MJ. The effect of 1:2 Ag(I) thiocyanate complexes in MCF-7 breast cancer cells. Biometals. 2015;28:765–781. doi: 10.1007/s10534-015-9865-5. [DOI] [PubMed] [Google Scholar]
- Festjens N, van den Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–1387. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Guo Z, Sadler PJ. Medicinal inorganic chemistry. Adv Inorg Chem. 1999;49:183–306. doi: 10.1016/S0898-8838(08)60271-8. [DOI] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hartinger CG, Dyson PJ. Bio-organometallic chemistry from teaching paradigms to medicinal applications. Chem Soc Rev. 2009;38:391–401. doi: 10.1039/B707077M. [DOI] [PubMed] [Google Scholar]
- Hrubisko M, McGown AT, Fox BW. The role of metallothionein, glutathione, glutathione S-transferases and DNA repair in resistance to platinum drugs in a series of L1210 cell lines made resistant to anticancer platinum agents. Biochem Pharmacol. 1993;54:435–442. doi: 10.1016/0006-2952(93)90399-h. [DOI] [PubMed] [Google Scholar]
- Human Z, Munyaneza A, Omondi B, Meijboom R, Cronjé MJ. The induction of cell death by phosphine silver(I) thiocyanate complexes in SNO-esophageal cancer cells. Biometals. 2015;28:219–228. doi: 10.1007/s10534-014-9817-5. [DOI] [PubMed] [Google Scholar]
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/S0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MW, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. Classification of cell death: recommendations of nomenclature committee on cell death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyros L, Kourkoumelis N, Kubick M, Male L, Hursthouse MB, Verginadis II, Gouma E, Karkabounas S, Charalabopoulos K, Hadjikakou SK (2010) Structural properties, cytotoxicity, and anti-inflammatory activity of silver (I) complexes with tris (p-tolyl) phosphine and 5-chloro-2-mercaptobenzothiazole. Bioinorg Chem Appl 386860. doi:10.1155/2010/386860 [DOI] [PMC free article] [PubMed]
- Kyros L, Banti CN, Kourkoumelis N, Kubicki M, Sainis I, Hadjikakou SK. Synthesis, characterization, and binding properties towards CT-DNA and lipoxygenase of mixed-ligand silver(I) complexes with 2-mercaptothiazole and its derivatives and triphenylphosphine. J Biol Inorg Chem. 2014;19:449–464. doi: 10.1007/s00775-014-1089-6. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Galettis P, Farr A, Maharaj L, Samarasinha H, McGechan AC, Baguley BC, Bowen RJ, Berners-Price SJ, McKeage MJ. In vitro antitumour and hepatotoxicity profiles of Au(I) and Ag(I) bidentate pyridyl phosphine complexes and relationships to cellular uptake. J Inorg Biochem. 2008;102:303–310. doi: 10.1016/j.jinorgbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- McKeage MJ, Papathanasiou P, Salem G, Sjaarda A, Swiegers GF, Waring P, Wild SB. Antitumor activity of gold(I), silver(I) and copper(I) complexes containing chiral tertiary phosphines. Met Based Drugs. 1998;5:217–223. doi: 10.1155/MBD.1998.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meggers E. Exploring biologically relevant chemical space with metal complexes. Curr Opin Chem Biol. 2007;11:287–292. doi: 10.1016/j.cbpa.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Meijboom R, Bowen RJ, Berners-Price SJ. Coordination complexes of silver(I) with tertiary phosphine and related ligands. Coord Chem Rev. 2009;253:325–342. doi: 10.1016/j.ccr.2008.03.001. [DOI] [Google Scholar]
- Muetterties EL, Alegranti CW. Solution structure and kinetic study of metal-phosphines and-phosphite complexes. I. The silver(I) system. J Am Chem Soc. 1972;94:6386–6391. doi: 10.1021/ja00773a022. [DOI] [Google Scholar]
- O’Dwyer PJ, Stevenson JP, Johnson SW. Clinical status of cisplatin, carboplatin, and other platinum-based antitumor drugs. Switzerland: Wiley-VCH; 1999. pp. 31–69. [Google Scholar]
- Ott I, Gust R. Non platinum metal complexes as anti-cancer agents. Arch Pharm. 2007;340:117–126. doi: 10.1002/ardp.200600151. [DOI] [PubMed] [Google Scholar]
- Page R, Takimoto C (2004) Cancer management: a multidisciplinary approach: medical, surgical and radiation oncology. Principles of chemotherapy, 8th ed. PPR, New York, pp 21–38
- Papathanasiou P, Salem G, Waring P, Willis AC. Synthesis of gold(I), silver(I) and copper(I) complexes containing substituted (2-aminophenyl)phosphines. Molecular structures of [AuI(2-H2NC6H4PPh2)], [AuI{(±)-2-H2NC6H4PMePh}] and (±)-[Cu(2-H2NC6H4PPh2)2];PF6. J Chem Soc, Dalton Trans. 1997;19:3435–3443. doi: 10.1039/a702757e. [DOI] [Google Scholar]
- Potgieter K, Cronjé MJ, Meijboom R. Synthesis and characterization of silver(I) benzyldihenylphosphine complexes: towards the biological evaluation of SNO cells. Inorg Chim Acta. 2015;437:195–200. doi: 10.1016/j.ica.2015.08.023. [DOI] [Google Scholar]
- Poyraz M, Banti CN, Kourkoumelis N, Dokorou V, Manos MJ, Simčič M, Golič-Grdadolnik S, Mavromoustakos T, Giannoulis AD, Verginadis II, Charalabopoulos K, Hadjikakou SK. Synthesis, structural characterization and biological studies of novel mixed ligand Ag(I) complexes with triphenylphosphine and aspirin or salicylic acid. Inorg Chim Acta. 2011;375:114–121. doi: 10.1016/j.ica.2011.04.032. [DOI] [Google Scholar]
- Reed E, Dabholkar M, Chabner BA. Platinum analogues. In: Chabner BA, Longo DL, editors. Cancer chemotherapy and biotherapy: principles and practice. 2. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 357–378. [Google Scholar]
- Romualdo LL, Bessler KE, Deflon VM. Zum Koordinationsverhalten der phenylhydrazonpropandinitrile: darstellung und strukturelle charakterisierung von Silber(I)-Komplexen. Z Anorg Allg Chem. 2002;628:1098–1103. doi: 10.1002/1521-3749(200206)628:5<1098::AID-ZAAC1098>3.0.CO;2-0. [DOI] [Google Scholar]
- Rosenberg B. Some biological effects of platinum compounds. Platin Met Rev. 1971;15:42–51. [Google Scholar]
- Rosenberg B, Van Camp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Sulaiman NI, Salimin NR, Haque RA, Iqbal MA, Ng SW, Razali MR. Synthesis, spectroscopic characterization, single crystal X-ray determination and cytotoxicity activity against human breast cancer (MCF-7) and colon cancer (HCT 116) cell lines of silver(I) coordination polymer. Polyhedron. 2015;97:188–196. doi: 10.1016/j.poly.2015.05.023. [DOI] [Google Scholar]
- Tagliabue G, Pifferi A, Balconi G, Mascellani E, Geroni C, D’Incali M, Ubezio P. Intracellular glutathione heterogeneity in L1210 murine leukaemia sublines made resistant to DNA-interacting anti-neoplastic agents. Int J Cancer. 1993;54:435–442. doi: 10.1002/ijc.2910540314. [DOI] [PubMed] [Google Scholar]
- Vaughn DJ, Malkowicz SB. Recent development in chemotherapy for bladder cancer. Oncology. 2001;15:763–780. [PubMed] [Google Scholar]
- Yilmaz VT, Gocmen E, Icsel C, Cengiz M, Susluer SY, Buyukgungor O. Synthesis, crystal structures, in vitro DNA binding, antibacterial and cytotoxic activities of new di- and polynuclear silver(I) saccharinate complexes with tertiary monophosphanes. J Photochem Photobiol B: Biol. 2014;131:31–42. doi: 10.1016/j.jphotobiol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Zartilas S, Hadjikakou SK, Hadjiliadis N, Kourkoumelis N, Kyros L, Kubicki M, Baril M, Butler IS, Karkabounas S, Balzarini J. Tetrameric 1:1 and monomeric 1:3 complexes of silver(I) halides with tri(p-tolyl)-phosphine: a structural and biological study. Inorg Chim Acta. 2009;362:1003–1010. doi: 10.1016/j.ica.2007.07.034. [DOI] [Google Scholar]