Abstract
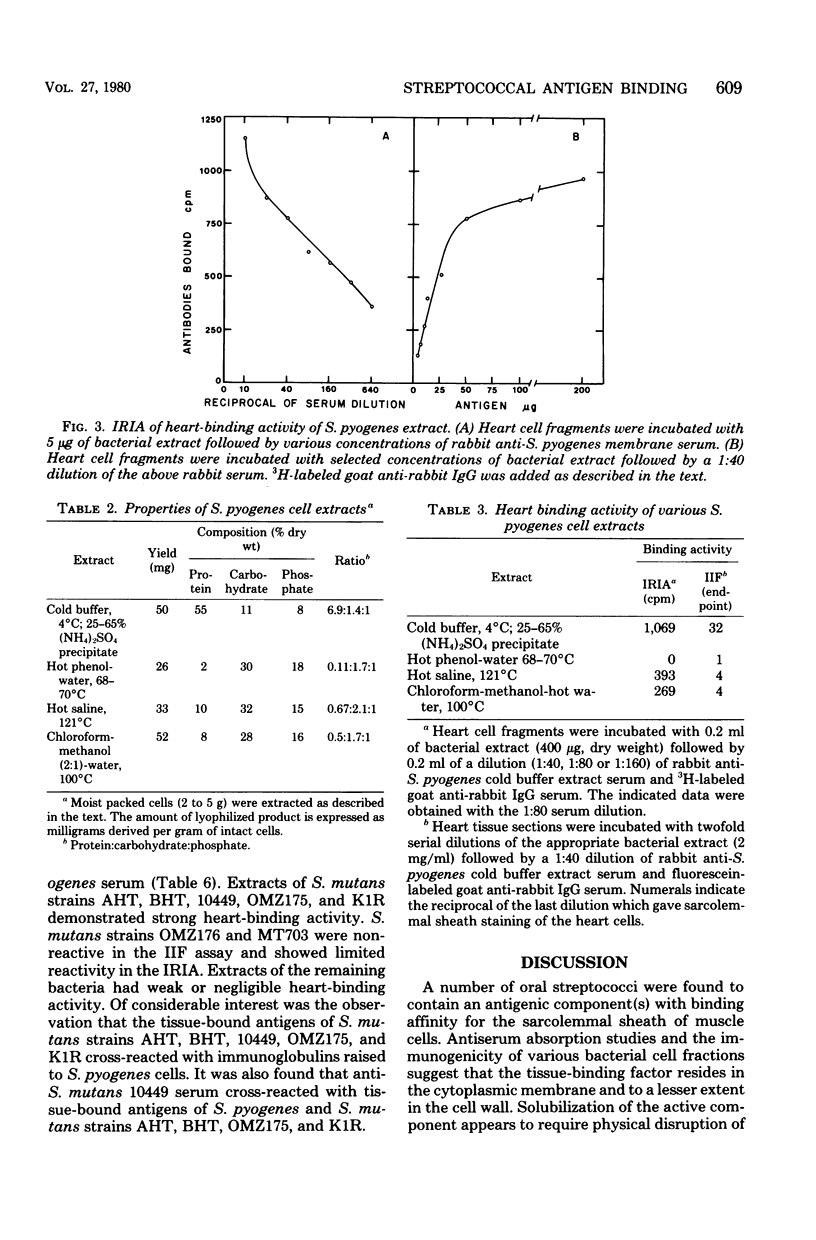
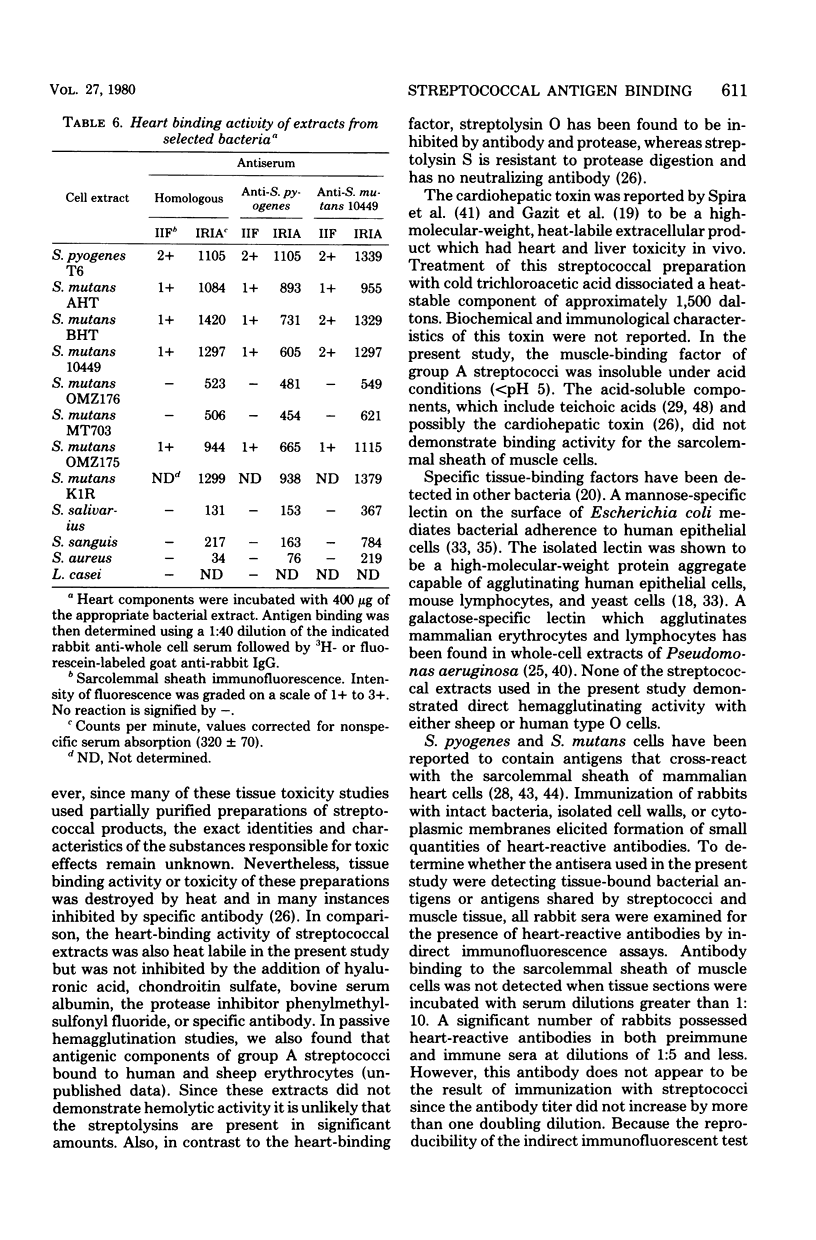
Antigens extracted from cells of Streptococcus pyogenes T6 and Streptococcus mutans strains AHT, BHT, 10449, OMZ175, and K1R adsorbed to the sarcolemmal sheath of cardiac muscle cells in vitro. Similar preparations from S. salivarius, S. sanguis, Staphylococcus aureus, and Lactobacillus casei had weak or negligible tissue-binding activity. Tissue-bound bacterial antigens were detected with homologous rabbit antisera with both indirect immunofluorescence tests and an indirect radioimmunoassay. Serological cross-reactivity was observed between the tissue-binding factors of S. pyogenes and S. mutans cells but not between the bacteria and muscle tissue. In a comparative study of extraction procedures, the greatest yield of tissue-binding factors was obtained from group A streptococci by cell disruption in buffer at 4 degrees C. Hot aqueous phenol and hot water extracts were inactive. Antibodies specific for the tissue-binding factor(s) were readily adsorbed from rabbit anti-S. pyogenes serum by a preparation of isolated cytoplasmic membranes but not by a suspension of cell wall fragments. The heart-binding component of S. pyogenes cell extracts was inactivated by protease digestion and heat treatment and to a lesser extent by periodic acid oxidation. The capacity of heart cell components to adsorb streptococcal antigens was reduced by protease treatment but not by the action of neuraminidase, hyaluronidase, organic solvents, or detergents.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R., Shinefield H. I., Strauss W. G., Maibach H. I. Bacterial adherence to nasal mucosal cells. Infect Immun. 1977 Sep;17(3):546–549. doi: 10.1128/iai.17.3.546-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLEIWEIS A. S., KARAKAWA W. W., KRAUSE R. M. IMPROVED TECHNIQUE FOR THE PREPARATION OF STREPTOCOCCAL CELL WALLS. J Bacteriol. 1964 Oct;88:1198–1200. doi: 10.1128/jb.88.4.1198-1200.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt M. A., Duncan J. L. Adherence of group A streptococci to human epithelial cells. Infect Immun. 1978 Apr;20(1):200–208. doi: 10.1128/iai.20.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Grebe S., Ahmed A., Simpson W. A., Ofek I. Lymphocytes binding and T cell mitogenic properties of group A streptococcal lipoteichoic acid. J Immunol. 1979 Jan;122(1):189–195. [PubMed] [Google Scholar]
- Beachey E. H., Dale J. B., Simpson W. A., Evans J. D., Knox K. W., Ofek I., Wicken A. J. Erythrocyte binding properties of streptococcal lipoteichoic acids. Infect Immun. 1979 Mar;23(3):618–625. doi: 10.1128/iai.23.3.618-625.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner E. H., Sepulveda M. R., Barnett E. V. Quantitative studies of immunofluorescent staining. Relationships of characteristics of unabsorbed antihuman IgG conjugates to their specific and non-specific staining properties in an indirect test for antinuclear factors. Bull World Health Organ. 1968;39(4):587–606. [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H. R., Chorpenning F. W., Roses S. Lipid-free glycerol teichoic acids with potent membrane-binding activity. Infect Immun. 1978 Feb;19(2):462–469. doi: 10.1128/iai.19.2.462-469.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVuono J., Panos C. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect Immun. 1978 Oct;22(1):255–265. doi: 10.1128/iai.22.1.255-265.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. M protein-associated adherence of Streptococcus pyogenes to epithelial surfaces: prerequisite for virulence. Infect Immun. 1972 May;5(5):826–830. doi: 10.1128/iai.5.5.826-830.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Gibbons R. J. Parameters affecting the adherence and tissue tropisms of Streptococcus pyogenes. Infect Immun. 1974 Jan;9(1):85–91. doi: 10.1128/iai.9.1.85-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Gazit E., Ginsburg I., Harris T. N. Dialyzable form of an extracellular streptococcal toxin causing histopathologic and biochemical changes in rabbits. Proc Soc Exp Biol Med. 1972 Jul;140(3):1025–1029. doi: 10.3181/00379727-140-36604. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Gilboa-Garber N. Purification and properties of hemagglutinin from Pseudomonas aeruginosa and its reaction with human blood cells. Biochim Biophys Acta. 1972 Jun 26;273(1):165–173. [PubMed] [Google Scholar]
- Ginsburg I. Mechanisms of cell and tissue injury induced by group A streptococci: relation to poststreptococcal sequelae. J Infect Dis. 1972 Sep;126(3):294–340. doi: 10.1093/infdis/126.3.294. [DOI] [PubMed] [Google Scholar]
- Jackson R. W., Moskowitz M. Nature of a red cell sensitizing substance from streptococci. J Bacteriol. 1966 Jun;91(6):2205–2209. doi: 10.1128/jb.91.6.2205-2209.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN M. H. IMMUNOLOGIC RELATION OF STREPTOCOCCAL AND TISSUE ANTIGENS. I. PROPERTIES OF AN ANTIGEN IN CERTAIN STRAINS OF GROUP A STREPTOCOCCI EXHIBITING AN IMMUNOLOGIC CROSS-REACTION WITH HUMAN HEART TISSUE. J Immunol. 1963 Apr;90:595–606. [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Immunological properties of teichoic acids. Bacteriol Rev. 1973 Jun;37(2):215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. J., Herzberg M. C., Levine M. S., Ellison S. A., Stinson M. W., Li H. C., van Dyke T. Specificity of salivary-bacterial interactions: role of terminal sialic acid residues in the interaction of salivary glycoproteins with Streptococcus sanguis and Streptococcus mutans. Infect Immun. 1978 Jan;19(1):107–115. doi: 10.1128/iai.19.1.107-115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Selinger D. S., Julie N., Reed W. P., Williams R. C., Jr Adherence of group A streptococci to pharyngeal cells: a role in the pathogenesis of rheumatic fever. Science. 1978 Aug 4;201(4354):455–457. doi: 10.1126/science.351810. [DOI] [PubMed] [Google Scholar]
- Spira G., Silberstein Z., Harris T. N., Ginsburg I. Toxic effects induced in rabbits by extracellular products and sonicates of group A streptococci. Proc Soc Exp Biol Med. 1968 Apr;127(4):1196–1201. doi: 10.3181/00379727-127-32908. [DOI] [PubMed] [Google Scholar]
- Triftshauser C., Hayden D. W., Beutner E. H. Procedures for the immunization of goats with human immunoglobulins and complement. Int Arch Allergy Appl Immunol. 1970;38(3):315–319. doi: 10.1159/000230284. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Gibbens J. W., Knox K. W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973 Jan;113(1):365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Zabriskie J. B., McCarty M. Group A streptococcal antigens cross-reactive with myocardium. Purification of heart-reactive antibody and isolation and characterization of the streptococcal antigen. J Exp Med. 1977 Aug 1;146(2):579–599. doi: 10.1084/jem.146.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]