Abstract
Adherence of Streptococcus mutans to smooth surfaces has been attributed to the production of sucrose-derived d-glucans. However, several studies indicate that the bacterium will adhere in the absence of sucrose. The present data confirmed that S. mutans adherence to saliva-coated hydroxyapatite beads in the absence of sucrose is described by the Langmuir equation. The nature of the sucrose-independent adherence was studied with the Persea americana agglutinin as a selective adherence inhibitor. Pretreatment of the bacterium with P. americana agglutinin caused a 10-fold reduction in adherence, and the inhibition was not reversed with the addition of sucrose. Pretreatment of S. mutans with proteases also reduced adherence, regardless of the sucrose content, whereas periodate oxidation and glucanohydrolase treatment of the bacteria reduced sucrose-mediated adherence to the levels found for sucrose-independent adherence. The P. americana agglutinin, glucanohydrolase, and pepsin pretreatment of the cells did not eliminate sucrose-induced agglutination. Scanning electron microscopy showed that short streptococcal chains were bound to saliva-coated hydroxyapatite crystals in the sucrose-independent system, whereas the presence of sucrose caused larger bacterial clumps to be found. A two-reaction model of S. mutans adherence was developed from these data. It is proposed that one reaction is attachment to the tooth pellicle which is mediated by cell-surface proteins rather than glucans or teichoic acids. The other reaction is cellular accumulation mediated by sucrose-derived d-glucans and cell surface lectins. A series of sequential adherence experiments with P. americana agglutinin as a selective inhibitor provided presumptive evidence for the validity of our model of S. mutans adherence.
Full text
PDF


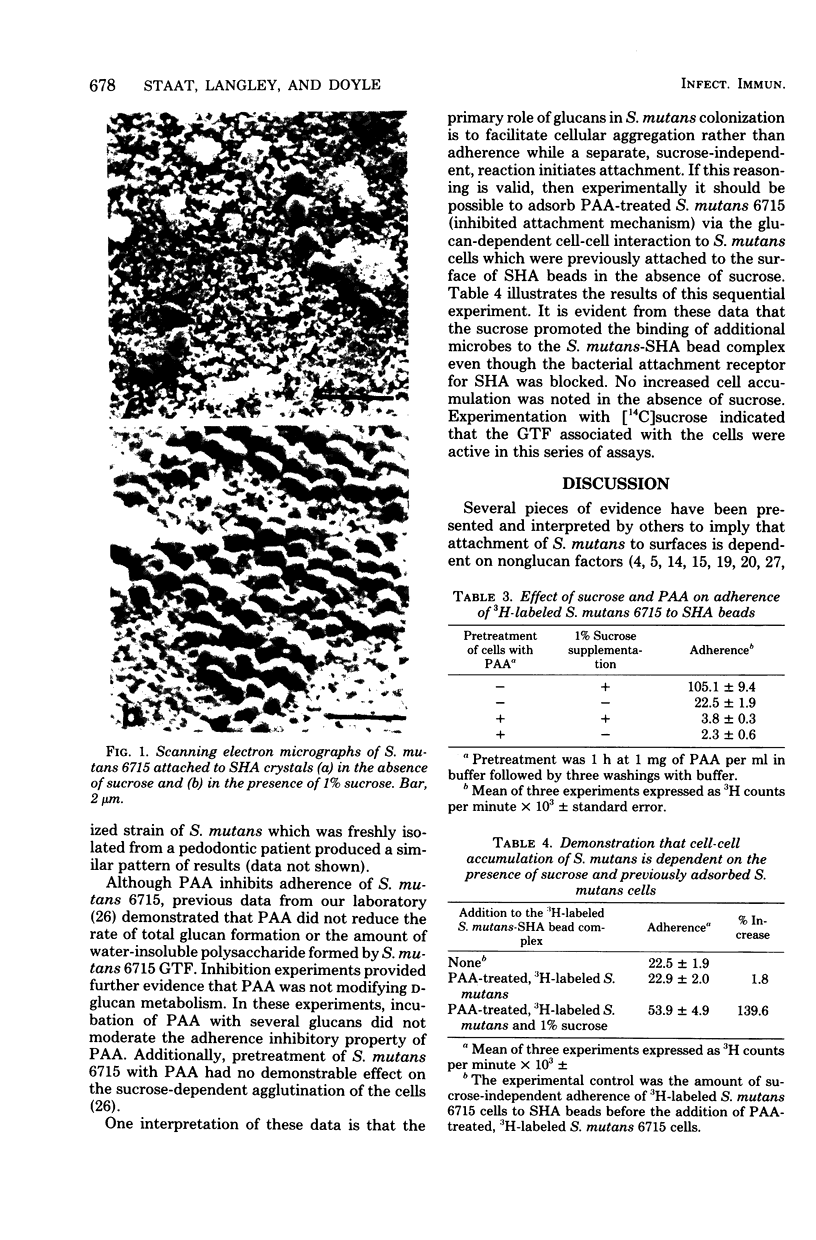



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgeau G., McBride B. C. Dextran-mediated interbacterial aggregation between dextran-synthesizing streptococci and Actinomyces viscosus. Infect Immun. 1976 Apr;13(4):1228–1234. doi: 10.1128/iai.13.4.1228-1234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets L., Vieth U. C., Hermann G. Isolation and new biological properties of Arion empiricorum lectin. Biochim Biophys Acta. 1979 Jan 4;582(1):154–163. doi: 10.1016/0304-4165(79)90298-8. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H., Ingersoll L. Molecular basis for the different sucrose-dependent adherence properties of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1977 Aug;17(2):330–337. doi: 10.1128/iai.17.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Schauer S. V. Studies on the bacterial components which bind Streptococcus sanguis and Streptococcus mutans to hydroxyapatite. Arch Oral Biol. 1975 Sep;20(9):609–615. doi: 10.1016/0003-9969(75)90082-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M. Multiple forms of dextran-binding proteins from Streptococcus mutans. Adv Exp Med Biol. 1978;107:749–759. doi: 10.1007/978-1-4684-3369-2_84. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Jefferson W., Campbell G. L. Cell membrane-binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975 May 1;141(5):990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosan B. Relationship of the cell wall composition of group H streptococci and Streptococcus sanguis to their serological properties. Infect Immun. 1976 Apr;13(4):1144–1153. doi: 10.1128/iai.13.4.1144-1153.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rölla G., Iversen O. J., Bonesvoll P. Lipoteichoic acid - the key to the adhesiveness of sucrose grown Streptococcus mutans. Adv Exp Med Biol. 1978;107:607–617. doi: 10.1007/978-1-4684-3369-2_69. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Staat R. H., Harlander S. K. Dextranases from oral bacteria: inhibition of water-insoluble glucan production and adherence to smooth surfaces by Streptococcus mutans. Infect Immun. 1975 Aug;12(2):309–317. doi: 10.1128/iai.12.2.309-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Lectins. Sci Am. 1977 Jun;236(6):108-16, 118-9. doi: 10.1038/scientificamerican0677-108. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Doyle R. J., Langley S. D., Suddick R. P. Modification of in vitro adherence of Streptococcus mutans by plant lectins. Adv Exp Med Biol. 1978;107:639–647. doi: 10.1007/978-1-4684-3369-2_72. [DOI] [PubMed] [Google Scholar]
- Wu-Yuan C. D., Tai S., Slade H. D. Properties of Streptococcus mutans grown in a synthetic medium: binding of glucosyltransferase and in vitro adherence, and binding of dextran/glucan and glycoprotein and agglutination. Infect Immun. 1979 Mar;23(3):600–608. doi: 10.1128/iai.23.3.600-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte J., Burgess R. C., Onose H. Oral implantation of human strains of Streptococcus mutans in rats fed sucrose or glucose diets. Arch Oral Biol. 1976;21(9):561–564. doi: 10.1016/0003-9969(76)90023-6. [DOI] [PubMed] [Google Scholar]
- van Houte J., Duchin S. Streptococcus mutans in the mouths of children with congenital sucrase deficiency. Arch Oral Biol. 1975 Nov;20(11):771–773. doi: 10.1016/0003-9969(75)90050-3. [DOI] [PubMed] [Google Scholar]



