Abstract
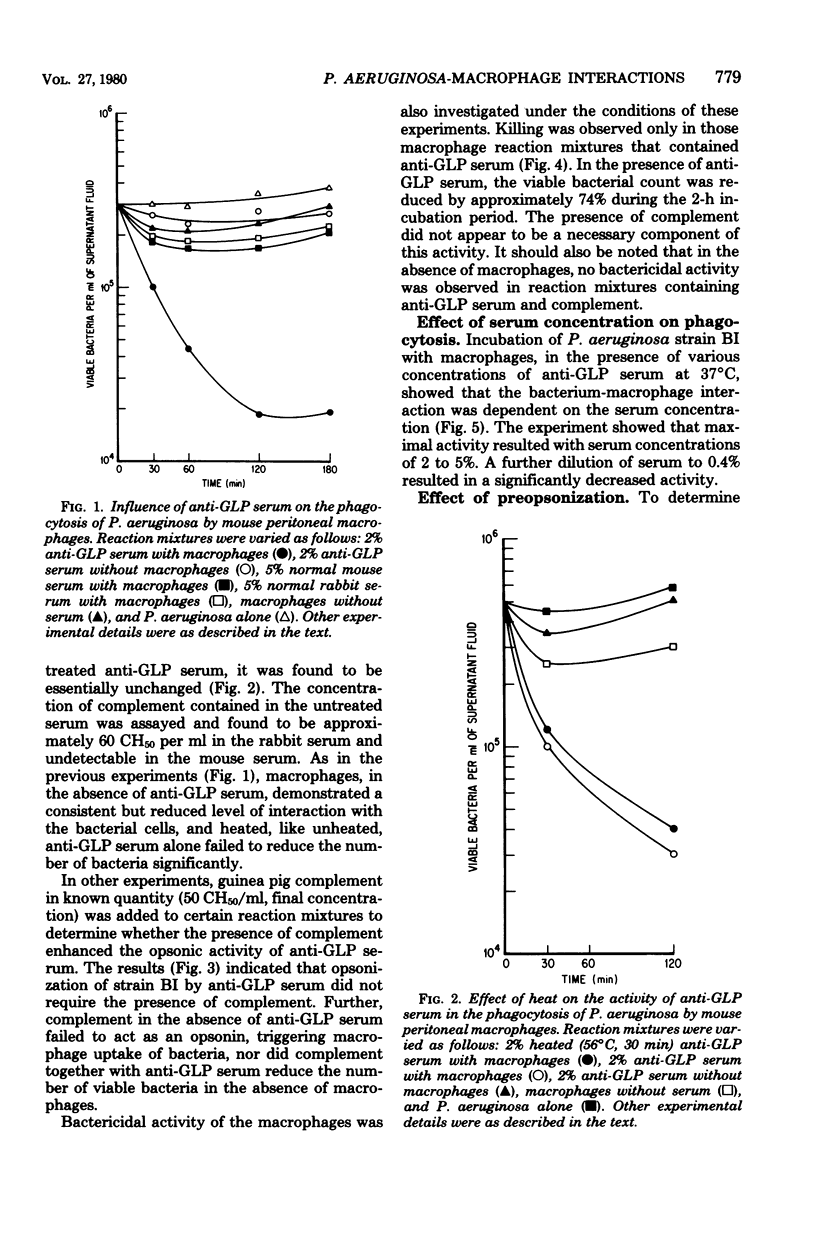
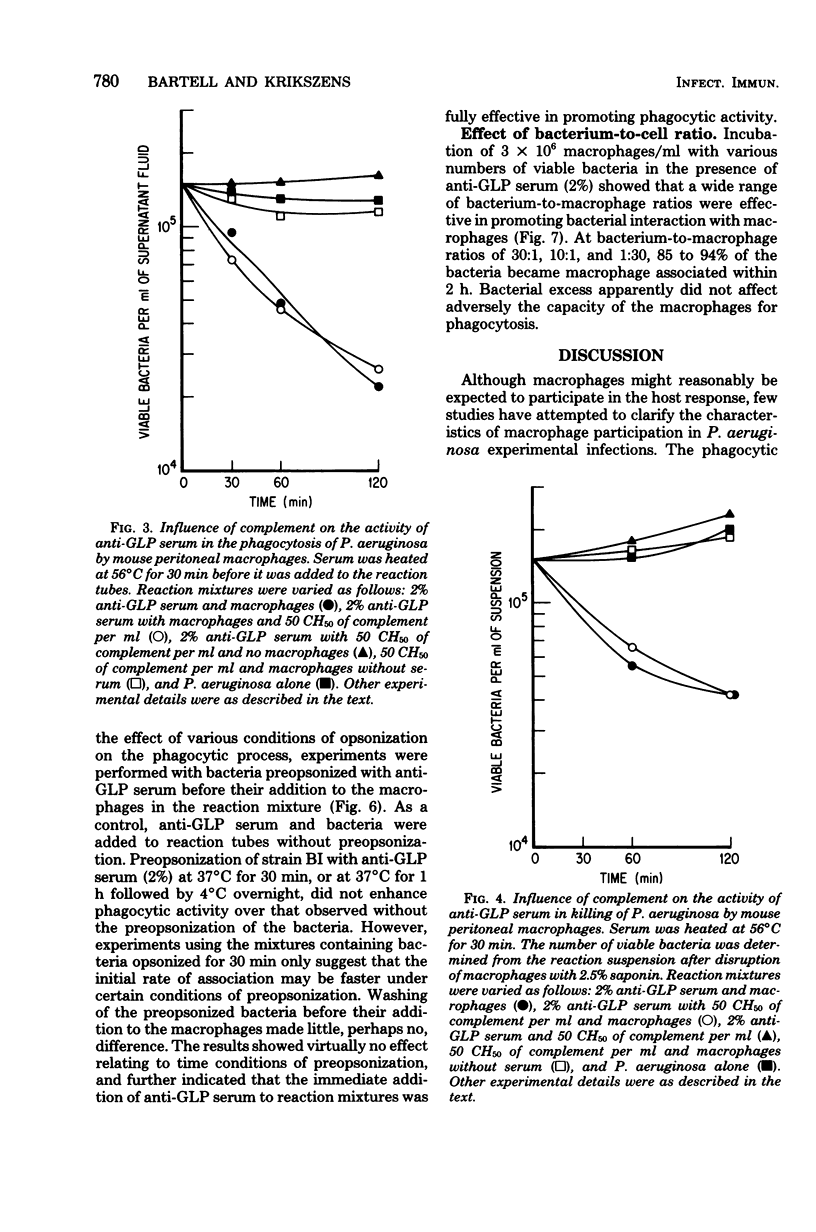
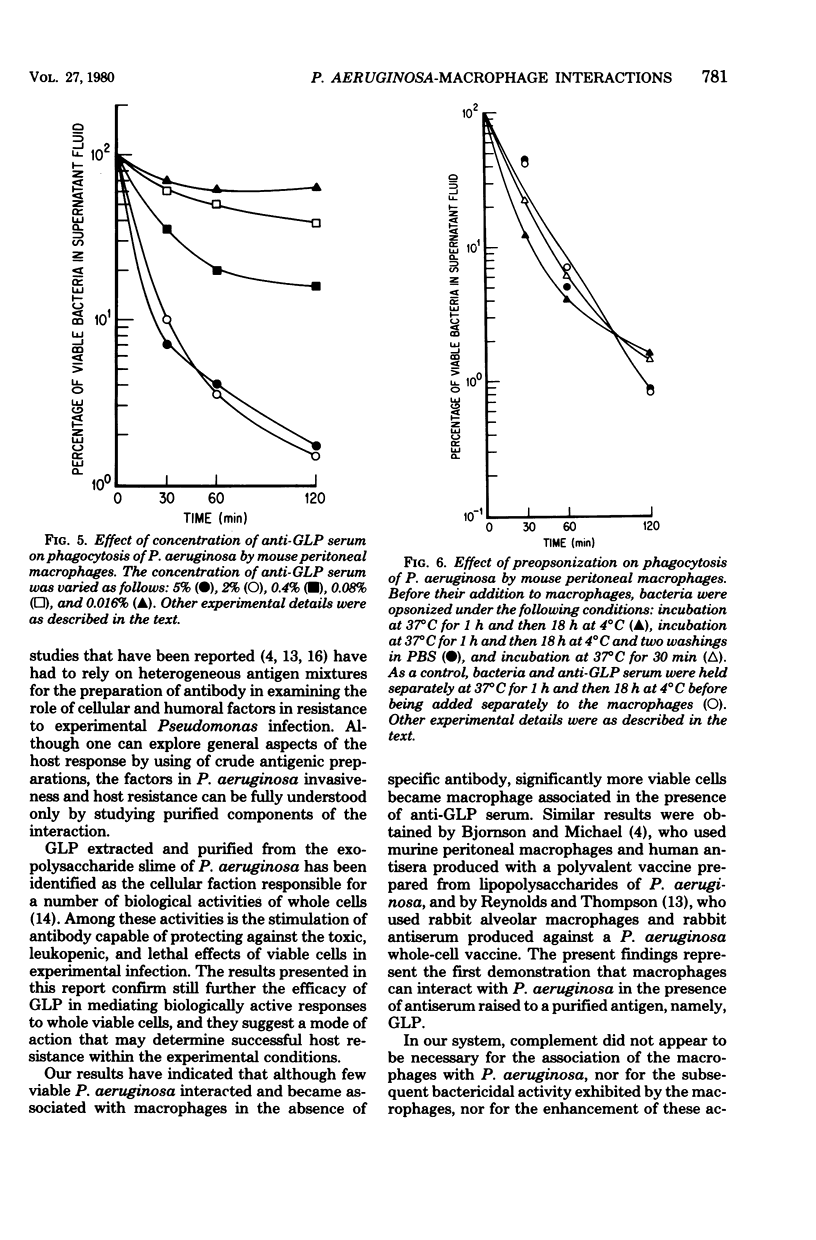
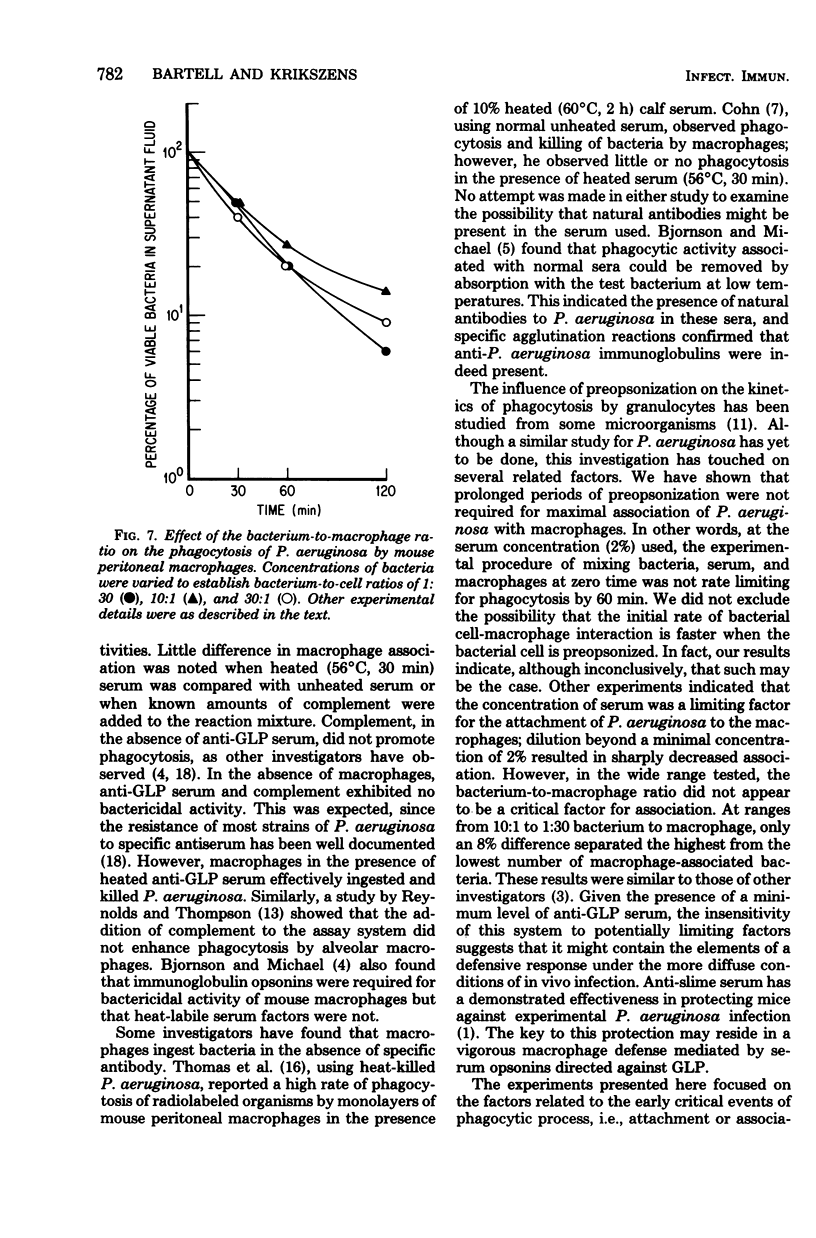
Glycolipoprotein, a purified fraction of the exopolysaccharide slime of Pseudomonas aeruginosa, was identified as responsible for a number of the biological activities of viable cells, including toxicity and immunogenicity capable of stimulating protective antibody against the lethal effects of viable cells. Antiserum against glycolipoprotein also mediated the phagocytosis and subsequent killing of viable P. aeruginosa by unstimulated mouse peritoneal macrophages. In the absence of anti-glycolipoprotein serum, macrophages did not significantly reduce the number of bacteria. The presence of complement in the experimental mixture did not affect the reduction of bacteria by the macrophage in the presence of anti-glycolipoprotein serum. The limiting effect of antiserum concentration on macrophage activity was studied, and maximal activity was found at 2%, with no further increase in activity at 5% Preopsonization of the bacteria with anti-glycolipoprotein serum had little effect on the course of phagocytosis within the experimental conditions. Variations in bacterium-to-macrophage input ratios, ranging from 30:1 to 1:30, did not affect the capacity of the macrophages for phagocytosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartell P. F., Orr T. E., Chudio B. Purification and Chemical Composition of the Protective Slime Antigen of Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. I. Interaction between immunoglobulins, heat-labile serum factors, and phagocytic cells in the killing of bacteria. Infect Immun. 1971 Oct;4(4):462–467. doi: 10.1128/iai.4.4.462-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. II. Opsonic, agglutinative, and protective capacities of immunoglobulin G anti-Pseudomonas antibodies. Infect Immun. 1972 May;5(5):775–782. doi: 10.1128/iai.5.5.775-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkstén B., Peterson P. K., Verhoef J., Quie P. G. Limiting factors in bacterial phagocytosis by human polymorphonuclear leukocytes. Acta Pathol Microbiol Scand C. 1977 Oct;85(5):345–349. doi: 10.1111/j.1699-0463.1977.tb03652.x. [DOI] [PubMed] [Google Scholar]
- Boxerbaum B., Kagumba A., Matthews L. W. Selective inhibition of phagocytic activity of rabbit alveolar macrophages by cystic fibrosis serum. Am Rev Respir Dis. 1973 Oct;108(4):777–783. doi: 10.1164/arrd.1973.108.4.777. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Shilo M. Role of cell wall structure of salmonella in the interaction with phagocytes. Infect Immun. 1970 Sep;2(3):279–285. doi: 10.1128/iai.2.3.279-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Dubbeldeman-Rempt I., van Furth R. Kinetics of phagocytosis of Staphylococcus aureus and Escherichia coli by human granulocytes. Immunology. 1979 Jun;37(2):453–465. [PMC free article] [PubMed] [Google Scholar]
- Lynn M., Sensakovic J. W., Bartell P. F. In vivo distribution of Pseudomonas aeruginosa slime glycolipoprotein: association with leukocytes. Infect Immun. 1977 Jan;15(1):109–114. doi: 10.1128/iai.15.1.109-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. II. Interaction of respiratory antibodies with Pseudomonas aeruginosa and alveolar macrophages. J Immunol. 1973 Aug;111(2):369–380. [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Glycolipoprotein from Pseudomonas aeruginosa as a protective antigen against P. aeruginosa infection in mice. Infect Immun. 1977 Nov;18(2):304–309. doi: 10.1128/iai.18.2.304-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Thomas W. R., Holt P. G., Keast D. Phagocytosis and processing of bacteria by peritoneal macrophages. J Reticuloendothel Soc. 1974 Jan;15(1):16–21. [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]


