Abstract
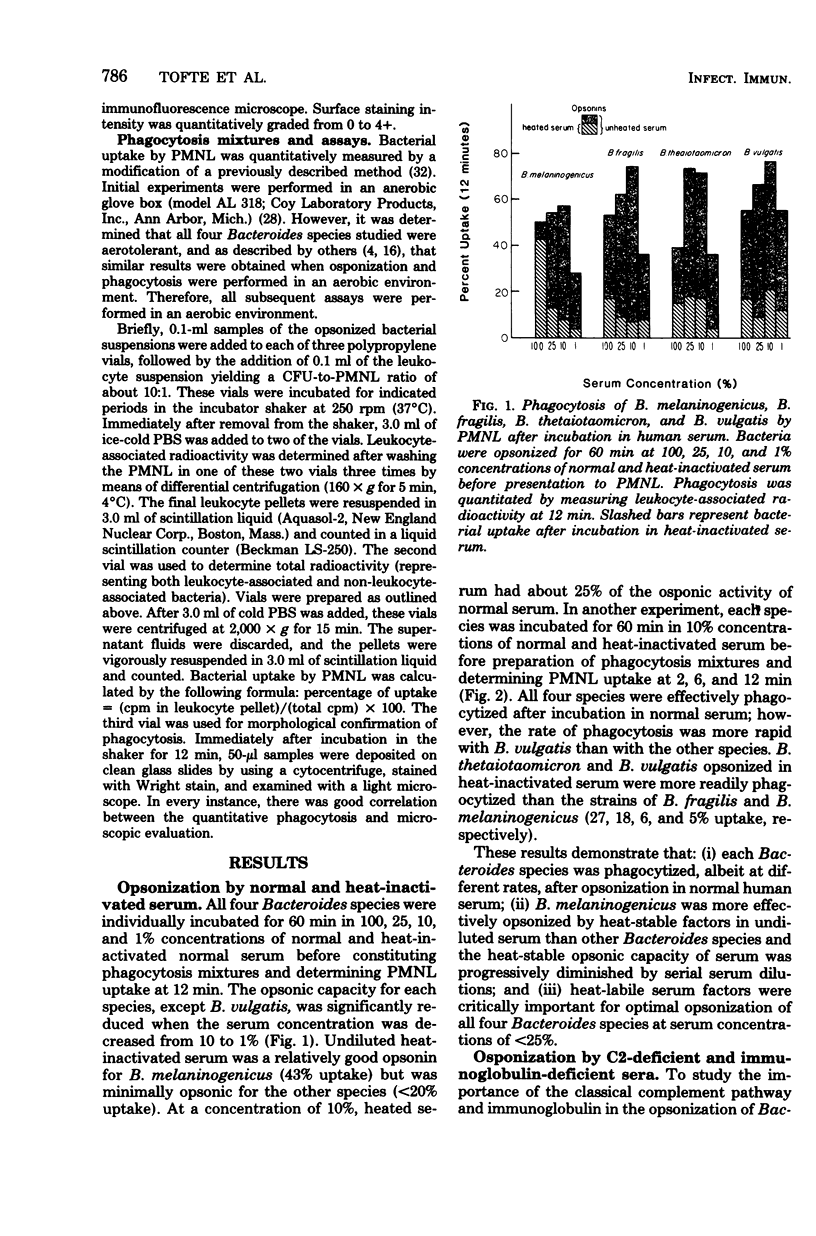
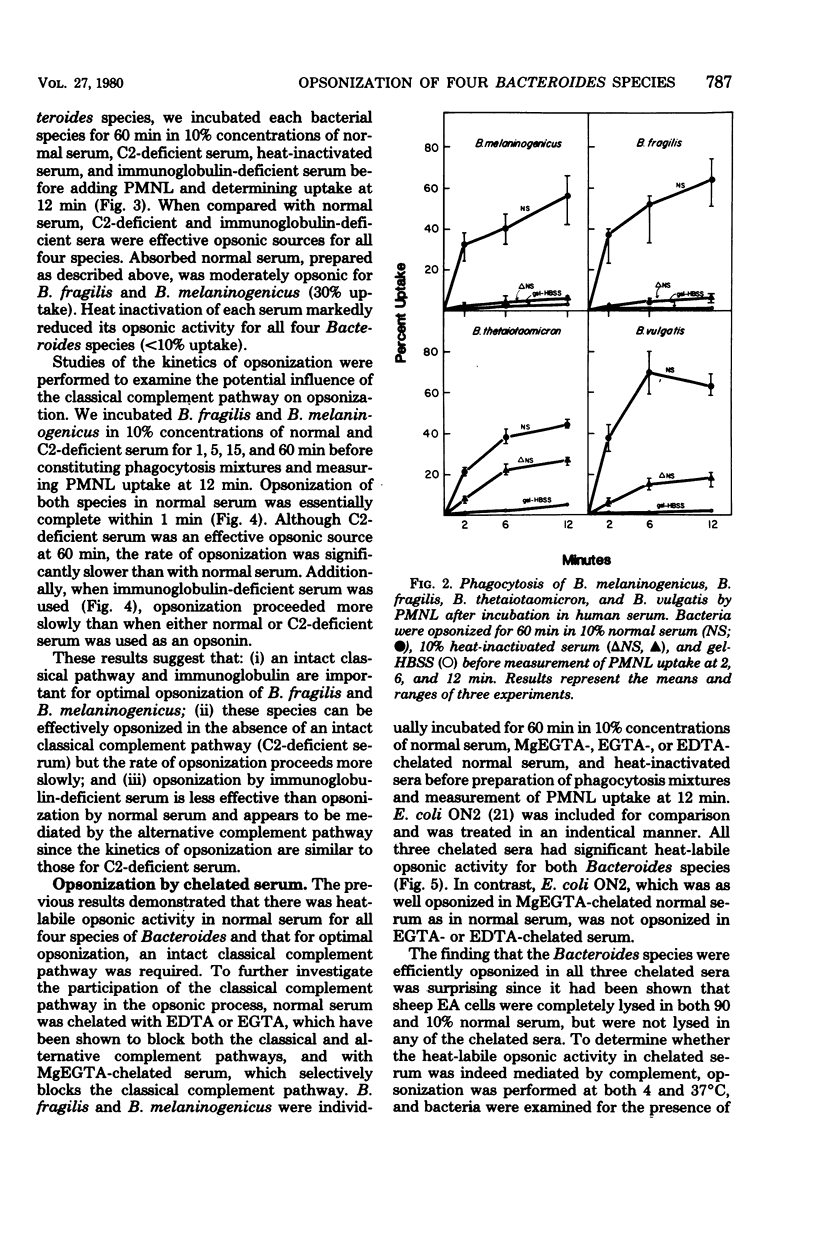
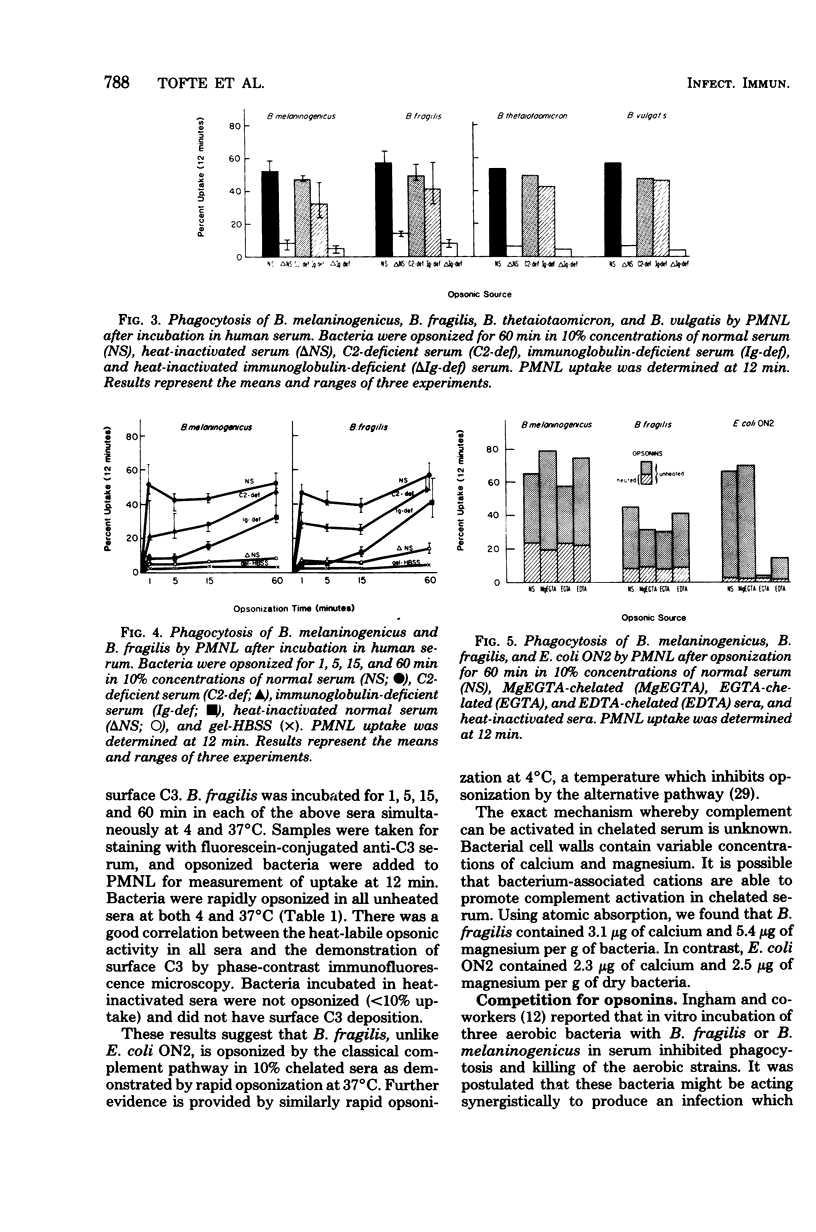
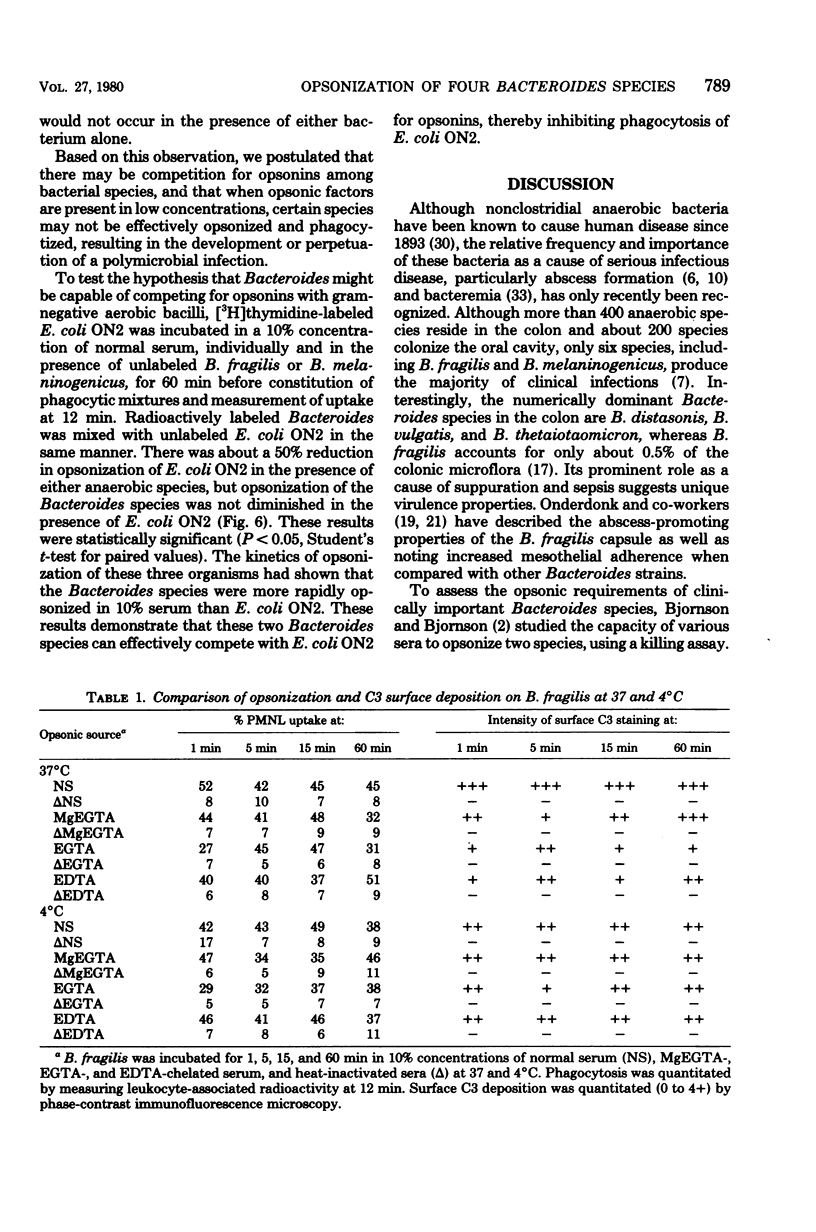
Previous investigators have suggested that opsonization of two Bacteroides species is mediated exclusively by the alternative complement pathway and requires immunoglobulins. In this study, the nature of the opsonic factors in nonimmune human serum for four species of Bacteroides was investigated by measuring uptake of [3H]thymidine-labeled bacteria by human polymorphonuclear leukocytes. Normal human serum, C2-deficient serum, immunoglobulin-deficient serum, and serum chelated with ethylene glycol-bis(β-aminoethyl ether)-N,N-tetraacetic acid (EGTA), MgEGTA, and ethylenediaminetetraacetic acid (EDTA) were used as opsonic sources. Heat inactivation of each of these sera significantly reduced its opsonic activity for all four Bacteroides species, suggesting that serum complement was essential for effective opsonization. All strains were opsonized in the absence of the classical complement pathway; however, kinetics studies revealed that opsonization proceeded at a significantly faster rate when the classical complement pathway was intact. Although two strains were opsonized in immunoglobulin-deficient sera, opsonization was less efficient and appeared to occur via the alternative complement pathway. Unexpectedly, all strains were well opsonized by the classical complement pathway in 10% serum which had been effectively chelated with EGTA or EDTA. The explanation for this finding is unknown; however, it is possible that cell wall cations of Bacteroides species may participate in the activation of complement in chelated serum, resulting in effective opsonization. It was also found that Bacteroides, when incubated with an Escherichia coli strain in normal serum, could compete for opsonins and thereby reduce phagocytosis of E. coli. It is possible that competition for opsonins among bacterial species contributes to the synergistic role these organisms share in mixed floral infections.
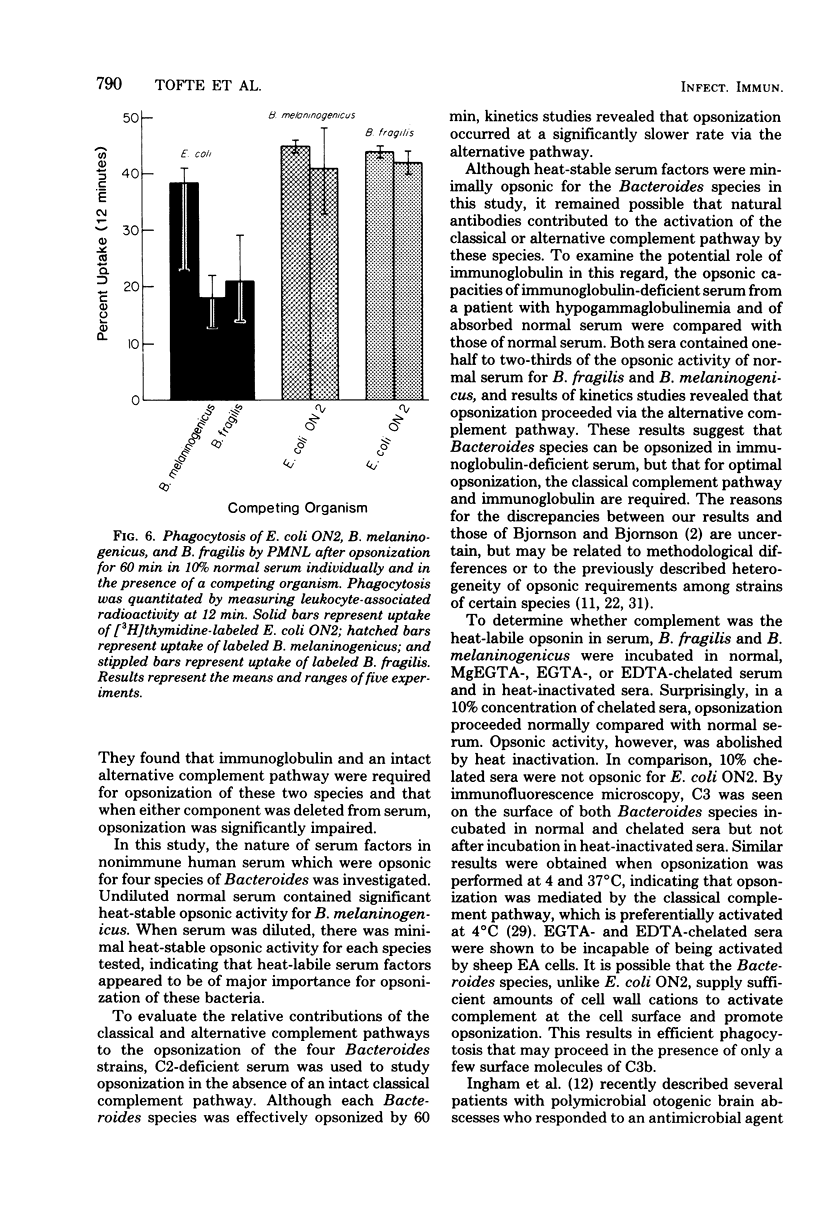
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Johnston R. B., Jr, Smith D. H. Human serum activities against Hemophilus influenzae, type b. J Clin Invest. 1972 Jan;51(1):31–38. doi: 10.1172/JCI106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Participation of immunoglobulin and the alternative complement pathway in opsonization of Bacteroides fragilis and Bacteroides thetaiotaomicron. J Infect Dis. 1978 Sep;138(3):351–358. doi: 10.1093/infdis/138.3.351. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Factors in normal human serum that promote bacterial phagocytosis. J Infect Dis. 1973 Jul;128(Suppl):182–186. doi: 10.1093/infdis/128.supplement_1.s182. [DOI] [PubMed] [Google Scholar]
- Casciato D. A., Rosenblatt J. E., Goldberg L. S., Bluestone R. In vitro interaction of Bacteroides fragilis with polymorphonuclear leukocytes and serum factors. Infect Immun. 1975 Feb;11(2):337–342. doi: 10.1128/iai.11.2.337-342.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M. Taxonomy, enzymes, and clinical relevance of anaerobic bacteria. Rev Infect Dis. 1979 Mar-Apr;1(2):248–253. doi: 10.1093/clinids/1.2.248. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Quie P. G. Influence of the alternate complement pathway in opsonization of several bacterial species. Infect Immun. 1974 Aug;10(2):402–404. doi: 10.1128/iai.10.2.402-404.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Clark R. A., Frank M. M. Kinetic analysis of chemotactic factor generation in human serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1975 Jan;3(3):334–346. doi: 10.1016/0090-1229(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Guckian J. C., Christensen W. D., Fine D. P. Evidence for quantitative variability of bacterial opsonic requirements. Infect Immun. 1978 Mar;19(3):822–826. doi: 10.1128/iai.19.3.822-826.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Jasin H. E. Human heat labile opsonins: evidence for their mediation via the alternate pathway of complement activation. J Immunol. 1972 Jul;109(1):26–31. [PubMed] [Google Scholar]
- Johnson F. R., Agnello V., Williams R. C., Jr Opsonic activity in human serum deficient in C2. J Immunol. 1972 Jul;109(1):141–145. [PubMed] [Google Scholar]
- Kim Y., Friend P. S., Dresner I. G., Yunis E. J., Michael A. F. Inherited deficiency of the second component of complement (C2) with membranoproliferative glomerulonephritis. Am J Med. 1977 May;62(5):765–771. doi: 10.1016/0002-9343(77)90881-6. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Bactericidal activity of aerobic and anaerobic polymorphonuclear neutrophils. Infect Immun. 1974 Feb;9(2):337–341. doi: 10.1128/iai.9.2.337-341.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. L., Smith J. W., Fossedal E. N., Condon R. E. Efficacy of parenteral antibiotics in the treatment of experimentally induced intraabdominal sepsis. Rev Infect Dis. 1979 Mar-Apr;1(2):302–312. doi: 10.1093/clinids/1.2.302. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Mansheim B. J., Louie T. J., Gorbach S. L., Bartlett J. G. Experimental animal models for anaerobic infections. Rev Infect Dis. 1979 Mar-Apr;1(2):291–301. doi: 10.1093/clinids/1.2.291. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Moon N. E., Kasper D. L., Bartlett J. G. Adherence of Bacteroides fragilis in vivo. Infect Immun. 1978 Mar;19(3):1083–1087. doi: 10.1128/iai.19.3.1083-1087.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Schmeling D., Lindemann M., Verhoef J., Quie P. G. Complement-mediated phagocytosis of Pseudomonas aeruginosa. J Lab Clin Med. 1978 Dec;92(6):883–894. [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Quie P. G. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978 Mar;19(3):943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polhill R. B., Jr, Pruitt K. M., Johnston R. B., Jr Kinetic assessment of alternative complement pathway activity in a hemolytic system. I. Experimental and mathematical analyses. J Immunol. 1978 Jul;121(1):363–370. [PubMed] [Google Scholar]
- Quinn P. H., Crosson F. J., Jr, Winkelstein J. A., Moxon E. R. Activation of the alternative complement pathway by Haemophilus influenzae type B. Infect Immun. 1977 Apr;16(1):400–402. doi: 10.1128/iai.16.1.400-402.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Fallon A., Finegold S. M. Comparison of methods for isolation of anaerobic bacteria from clinical specimens. Appl Microbiol. 1973 Jan;25(1):77–85. doi: 10.1128/am.25.1.77-85.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E. Isolation and identification of anaerobic bacteria. Hum Pathol. 1976 Mar;7(2):177–186. doi: 10.1016/s0046-8177(76)80021-4. [DOI] [PubMed] [Google Scholar]
- Sandberg A. L., Osler A. G. Dual pathways of complement interaction with guinea pig immunoglobulins. J Immunol. 1971 Nov;107(5):1268–1273. [PubMed] [Google Scholar]
- Verhoef J., Peterson P. K., Quie P. G. Kinetics of staphylococcal opsonization, attachment, ingestion and killing by human polymorphonuclear leukocytes: a quantitative assay using [3H]thymidine labeled bacteria. J Immunol Methods. 1977;14(3-4):303–311. doi: 10.1016/0022-1759(77)90141-7. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd Comparison of two commercially available media for detection of bacteremia. Appl Microbiol. 1971 Oct;22(4):604–607. doi: 10.1128/am.22.4.604-607.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Quie P. G. Opsonic activity of agammaglobulinemic human sera. J Immunol. 1971 Jan;106(1):51–55. [PubMed] [Google Scholar]



