Abstract
Purpose
Tools to estimate survival, such as ePrognosis (http://eprognosis.ucsf.edu/carey2.php), were developed for general, not cancer, populations. In older patients with breast cancer, accurate overall survival estimates would facilitate discussions about adjuvant therapies.
Methods
Secondary analyses were performed of data from two parallel breast cancer studies (CALGB/Alliance 49907/NCT000224102 and CALGB/Alliance 369901/NCT00068328). We included patients (n=971) who were age 70 years and older with complete baseline quality of life data (194 from 49907; 777 from 369901). Estimated versus observed all-cause two-year mortality rates were compared. ePrognosis score was calculated based on age, sex, and daily function (derived from EORTC QLQ-C30). ePrognosis scores range from 0 to 10, with higher scores indicating worse prognosis based on mortality of community-dwelling elders, and were categorized into three groups (0–2, 3–6, 7–10). Observed mortality rates were estimated using Kaplan-Meier methods.
Results
Patient mean age was 75.8 years (range 70–91) and 73% had stage I-IIA disease. Most patients were classified by ePrognosis as good prognosis (n=562, 58% 0–2) and few (n=18, 2% 7–10) poor prognosis. Two-year observed mortality rates were significantly lower than ePrognosis estimates for patients scoring 0–2 (2% vs 5%, p=0.001) and 3–6 (8% vs 12%, p=0.01). The same trend was seen with scores of 7–10 (23% vs 36%, p=0.25).
Conclusions
ePrognosis tool only modestly overestimates mortality rate in older breast cancer patients enrolled in two cooperative group studies. This tool, which estimates non-cancer mortality risk based on readily available clinical information, may inform adjuvant therapy decisions, but should be validated in non-clinical trial populations.
Keywords: Breast cancer, survival estimates, ePrognosis, elderly
INTRODUCTION
Cancer systemic adjuvant therapy prevents future distant recurrences and improves long-term survival.[1] In older patients, the balance of benefits and risks of cancer treatment is complicated by competing causes of mortality, presence of frailty, and advanced age. These factors are associated with increase treatment toxicity and limited overall and cancer-specific survival and, therefore, hinder discussions about preventive therapies and participation in clinical trials.[2,3] Data on life expectancy could support treatment decision-making.
Several extant tools are available to estimate life expectancy in older individuals and they are compiled for clinical use in ePrognosis (http://eprognosis.ucsf.edu/).[4] The ePrognosis tools were designed for older adults without severe illnesses, such as cancer, and are validated in community-dwelling, nursing-home, or hospitalized older individuals. None of these indices is validated in patients with non-metastatic cancer, who might be considering potentially curative adjuvant therapies.
We conducted a secondary analysis of data from two cancer cooperative group studies to compare short-term (two-year) ePrognosis estimated versus observed all-cause mortality rates among older women with non-metastatic breast cancer. Studies included were (1) a phase III, randomized study of adjuvant chemotherapy for breast cancer in women age 65 years and older (CALGB 49907/NCT000224102)[5] and (2) a parallel observational study with the same eligibility criteria (CALGB 369901/NCT00068328)[6]. CALGB is now a part of the Alliance for Clinical Trials in Oncology. By design, the two studies included a common core of sociodemographic and quality of life data.[7]
METHODS
This study was deemed exempt by the Duke University Hospital System Institutional Review Board.
Patients with early-stage breast cancer participating in CALGB/Alliance 49907 and 369901, who were age 70 years and older and had completed baseline quality of life questionnaires, were included in the analysis (Figure 1). Age 70 years was chosen to parallel ePrognosis. We selected two-year all-cause mortality because short-term limits in life expectancy would have a large influence on systemic therapy decisions, particularly among women with estrogen-receptor negative. Cause of death was grouped as breast cancer-related and other, which included unknown cause.
Figure 1.
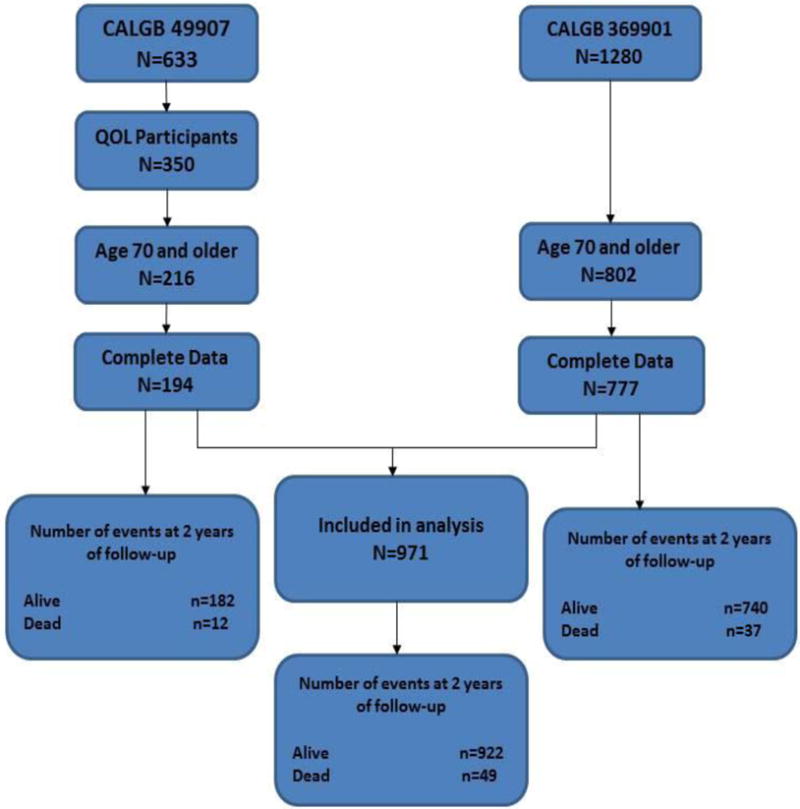
Sample derivation and number of events.
Estimations of Prognosis
The two-year ePrognosis tool[8] was developed from the Asset and Health Dynamics Among the Oldest Old (AHEAD) study of 4516 community dwelling adults, age 70 years and older, and provides mortality estimates based on answers to six function questions. We matched data items collected from the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) at baseline to the ePrognosis tool.
The ePrognosis tool assigns a score to each item, for a summary score ranging from 1–10, with zero indicating the best prognosis. We applied the scoring system to the study data as follows:
“How old is your patient?” Age was available in both studies and was scored as 0 if age 70–75, 1 if age 76–80, and 2 if age >80.
“What is your patient’s sex?” All patients were female and were score as 0.
“Does your patient need help from another person to bathe?” Defined from the question “do you need help with eating, dressing, washing yourself, or using the toilet?” on the EORTC QLQ-C30. Scored as 0 if answered “not at all”, otherwise 1 point.
“Does your patient need help from another person to shop for groceries?” Defined from the EORTC QLQ-C30: During the past week, were you limited in doing either your work or other daily activities? Scored as 0 if answered “not at all”, otherwise 2 points.
“Does your patient have difficulty walking several blocks?” Defined from the EORTC QLQ-C30: Do you have any trouble taking a long walk? Scored as 0 if answered “not at all”, otherwise 2 points.
“Does your patient have difficulty pushing or pulling a heavy object, such as an arm chair?” Defined from the EORTC QLQ-C30: Have you felt weak? Scored as 0 if answered “not at all”, otherwise 1 point.
Statistical Analysis
Observed all-cause mortality rates (and 95% confidence intervals) were calculated for both samples combined and each protocol separately from study entry to death using Kaplan-Meier methods.[9,10] All-cause mortality rates were also calculated for each stratum of the ePrognosis score categories (0–2 points, 3–6 points, and 7–10 points). Observations were censored at the date last known alive. The two-year survival Index scores from ePrognosis were used to generate an estimated survival rate for each score category. A two-sided z-test was used to compare the observed versus estimated mortality rates. Data collection and statistical analyses were conducted by the Alliance Statistical and Data Center. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC, USA) on a dataset locked on May 15, 2015.
RESULTS
Participant mean age was 76 years with range of 70–91 years. Patient characteristics, overall and by study, are presented in Table 1. Of those that died within 2 years, 55% died from causes related to breast cancer. Most women were in the good prognosis category (n=562, 58%) and 18 (2%) in the poor prognosis category.
Table 1.
Characteristics of Non-Metastatic Breast Cancer Patients ages 70+ Enrolled in Two CALGB/Alliance Protocols
| Characteristic | Total (N=971) |
49907 (N=194) |
369901 (N=777) |
p-value |
|---|---|---|---|---|
| Years of enrollment | 2001–2006 | 2004–2011 | ||
|
Median follow-up (years) range |
6.67 (0, 12.6) |
8.17 (0, 12.61) |
6.59 (0.14, 10.75) |
|
|
Age, (years) mean (SD) range 70–75 76–80 >80 |
75.8 (4.6) (70, 91) 535 (55.1%) 287 (29.6%) 149 (15.3%) |
74.6 (3.7) (70, 89) 124 (63.9%) 57 (29.4%) 13 (6.7%) |
76.1 (4.7) (70, 91) 411 (52.9%) 230 (29.6%) 136 (17.5%) |
0.0002 0.001 |
|
ECOG Performance status 0 1 2 Missing |
131 (67.5%) 59 (30.4%) 4 (2.1%) |
Not Available |
||
|
Race or ethnic group White Black or African American Other Unknown |
863 (88.9%) 88 (9.1%) 15 (1.5%) 5 (<1%) |
166 (85.6%) 23 (11.9%) 3 (1.5%) 2(1.0%) |
697 (89.7%) 65 (8.4%) 12 (1.5%) 3 (0.4%) |
0.30 |
|
Ethnicity Hispanic or Latino Non-Hispanic Unknown/Not reported |
18 (1.8%) 936 (96.4%) 18 (1.8%) |
4 (2.0%) 178 (91.8%) 12 (6.2%) |
14 (1.8%) 758 (97.4%) 6 (<1%) |
0.74 |
|
Tumor size ≤ 2 cm > 2 to ≤ 5 cm > 5 cm Missing |
560 (57.7%) 365 (37.6%) 43 (4.4%) 3 (<1%) |
76 (39.2%) 107 (55.2%) 10 (5.2%) 1 (< 1%) |
484 (62.3%) 258 (33.2%) 33 (4.2%) 2 (<1%) |
<0.0001 |
|
No. of positive lymph nodes 0 1–3 4–9 ≥ 10 Missing |
308 (31.7%) 218 (22.5%) 89 (9.2%) 52 (5.3%) 304 (31.3%) |
51 (26.3%) 104 (53.6%) 31 (16.0%) 7 (3.6%) 1 (< 1%) |
257 (33.1%) 114 (14.7%) 58 (7.5%) 45 (5.8%) 303 (39.0%) |
<0.0001 |
|
ER Positive status Negative Positive Missing |
210 (21.6%) 760 (78.3%) 1 (<1%) |
80 (41.2%) 114 (58.8%) 0 |
130 (16.7%) 646 (83.1%) 1 (<1%) |
<0.0001 |
|
HER2 status Negative Positive Unknown |
155 (79.9%) 29 (14.9%) 10 (5.2%) |
Not available |
||
|
Type of surgery Partial mastectomy/lumpectomy/excisional bx Mastectomy/NOS Missing |
589 (60.7%) 381 (39.2%) 2(<1%) |
80 (41.2%) 113 (58.2%) 1 (< 1%) |
509 (65.5%) 268 (34.4%) 1 (<1%) |
<0.0001 |
|
Axillary evaluation Sentinel-node biopsy only Axillary dissection only Sentinel-node biopsy and axillary dissection Neither sentinel-node nor axillary dissection Missing |
311 (32.0%) 208 (21.4%) 387 (40.0%) 64 (6.6%) 1 (<1%) |
0 71 (36.6%) 87 (44.8%) 35 (18.0%) 1 <1%) |
311 (40.0%) 137 (17.6%) 300 (38.6%) 29 (3.7%) 0 |
<0.0001 |
|
Adjuvant chemotherapy Chemotherapy No Chemotherapy Not reported/Unknown |
442 (45.5%) 492 (50.7%) 37 (3.8%) |
194 (100%) 0 0 |
248 (31.9%) 492 (63.3%) 37 (4.8%) |
|
|
AJCC Stage Stage I Stage IIA Stage IIB Stage IIIA Stage IIIB Stage IIIC Missing |
380 (40.2%) 308 (32.6%) 159 (16.8%) 67 (7.1%) 6 (0.6%) 25 (2.6%) 26 (2.7%) |
18 (10.7%) 67 (39.6%) 68 (40.2%) 14 (8.3%) 0 (0.0%) 2 (1.2%) 25 (12.9%) |
362 (46.6%) 241 (31.1%) 91 (11.7%) 53 (6.8%) 6 (0.8%) 23 (3.0%) 1 (<1%) |
<0.0001 |
|
Cause of Death Breast Cancer-related Cause Other Cause |
27 (55.1%) 22 (44.9%) |
7 (58.3%) 5 (41.7%) |
20 (54.1%) 17 (45.9%) |
0.796 |
Predicted and observed mortality estimates by ePrognosis are shown in Table 2 and Appendix Figure 1. Two-year observed mortality was significantly lower than estimated from ePrognosis for patients scoring 0–2 (2% vs 5%, p=0.001) and 3–6 (8% vs 12%, p=0.01) points. Although not significant, the same trend was seen for the small number with scores of 7–10 (23% vs 36%, p=0.25).
Table 2.
Predicted vs. Observed 2-year Mortality Rates in a Sample of Non-Metastatic Breast Cancer Patients ages 70 and Older
| ePrognosis Prediction | 49907 and 369901 Patients | ||||
|---|---|---|---|---|---|
| Points | Predicted Probability of Mortality | N | Number of Deaths | Observed Probability of Overall Mortality at 2 years (%, 95% CI) | p-value |
| 0–2 | 5% | 562 | 12 | 2% (1–4%) | 0.001 |
| 3–6 | 12% | 391 | 33 | 8% (6–12%) | 0.01 |
| 7–10 | 36% | 18 | 4 | 23% (9–49%) | 0.25 |
The results are similar within each study (Appendix Table 1 and Figures 2 and 3).
DISCUSSION
Older women with non-metastatic breast cancer participating in cooperative group studies have very low two-year all-cause mortality rates. The ePrognosis tool systematically, but only slightly, over-estimated mortality. This may have implications for practice, since the tool may be used by clinicians when discussing systemic treatment options that have short-term toxicity but long-term benefits in preventing recurrence.[1]
These results are consistent with studies of linked SEER-Medicare datasets showing that women age 65 years and older with early-stage breast cancer have higher non-breast cancer survival than matched women without breast cancer.[11,12] Higher socio-economic status, healthier behaviors, better healthcare access, and more routine doctor visits to treat coexisting illness are thought to explain the better non-breast cancer survival seen in older breast cancer patients.[11,13,14] Use of older datasets to derive ePrognosis estimates and lower mortality over time due to improvements in management of acute toxicities and comorbidities may also explain why ePrognosis provided an overestimate.[15] Interestingly, other online prognostic tools, such as Adjuvant! Online and PREDICT, slightly over-estimate 10-year survival.[16,17]
This study is limited by the secondary nature of the analysis, some non-correspondence between items on the tool and those available from EORTC, and use of cooperative group data. These results will need to be confirmed in broader, older breast cancer populations and community settings. However, the prospective design of the original studies, high quality of data collection, and inclusion of many community hospitals and observational data enhances the generalizability of the finding.
Despite these caveats, the results were robust across data sets and prognostic groups and suggest that tools developed in general populations provide a slight overestimate of mortality in older breast cancer patients, which may not be clinically significant. Even so, development and testing of more accurate tools to estimate mortality in older breast cancer patients could enhance shared decision-making about the risks and benefits of adjuvant therapies.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA003927, U10CA007968, U10CA032291, U10CA035279, U10CA041287, U10CA047559, U10CA047577, U10CA077597, U10CA180790, U10CA180836, U10CA180838, U10CA180857, and U10CA180867. The research was also supported by National Cancer Institute at the National Institutes of Health grants U10CA084131 and R01CA127617 to JSM. The research was also supported, in part, by National Cancer Institute at the National Institutes of Health grant R35CA197289, R01CA129769, R01CA124924, and K05CA096940 to JSM. Alliance protocol 369901 was further supported, in part, by the National Cancer Institute at the National Institutes of Health legacy grants #U10CA031946 to the Cancer and Leukemia Group B (now Alliance for Clinical Trials in Oncology) and #U10CA033601 to the CALGB Statistical Center (now Alliance Statistics and Data Center). Earlier portions of the Alliance 369901 trial were also funded in part by a grant to support patient accrual from Amgen Pharmaceuticals to the CALGB Foundation. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute at the National Institutes of Health.
Appendix
Appendix Figure 1.
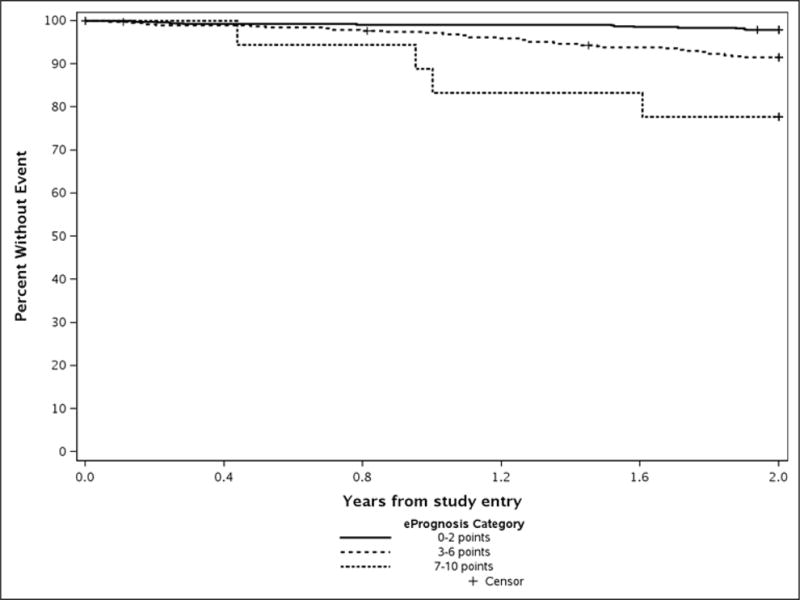
Observed 2-year Survival by ePrognosis Category
Appendix Figure 2.
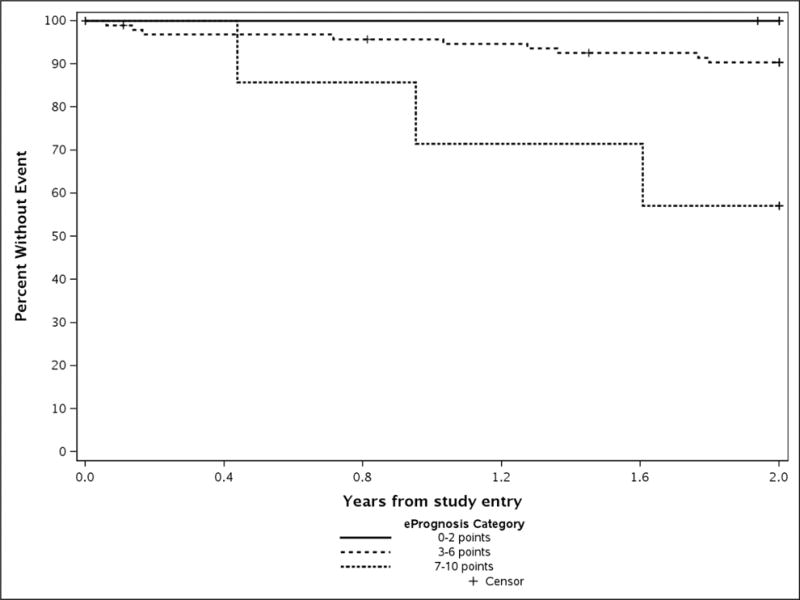
Observed 2-year Survival among CALGB/Aliance 49907 Patients by ePrognosis Category
Appendix Figure 3.
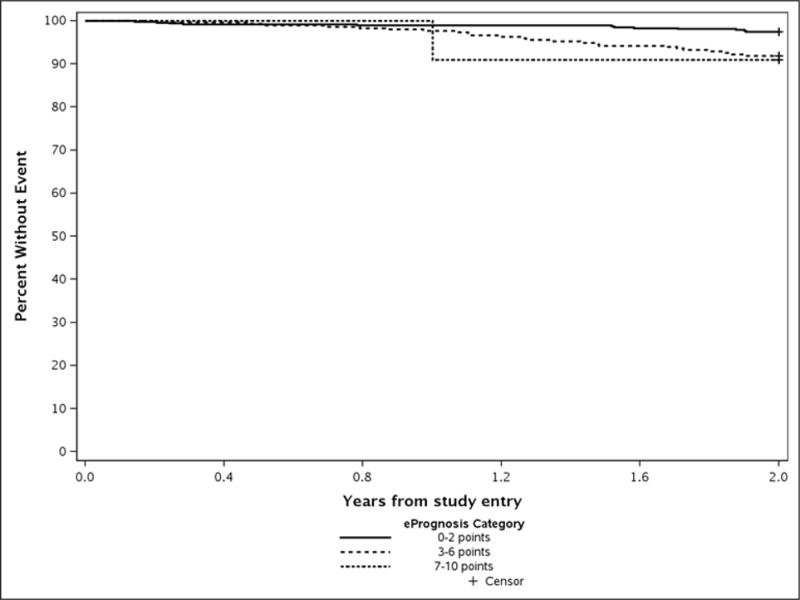
Observed 2-year Survival among CALGB/Alliance 369901 Patients by ePrognosis Category
Appendix Table 1.
Predicted vs. Observed 2-year All-Cause Mortality Rates in a Sample of Non-Metastatic Breast Cancer Patients Ages 70 years and Older by Study
| ePrognosis Prediction | 49907 Patients | 369901 Patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Points | Predicted Probability of Survival | N | Number of Deaths | Observed Probability of Overall Survival at 2 years (%, 95% CI) | p-value | N | Number of Deaths | Observed Probability of Overall Survival at 2 years (%, 95% CI) | p-value |
| 0–2 | 5% | 92 | 0 | 0% | 0.03 | 470 | 12 | 3% (1–4%) | 0.047 |
| 3–6 | 12% | 95 | 9 | 10% (5–18%) | 0.55 | 296 | 24 | 8% (6–12%) | 0.03 |
| 7–10 | 36% | 7 | 3 | 43% (26–83%) | 0.70 | 11 | 1 | 9% (1–49%) | 0.06 |
Footnotes
Conflicts of Interest:
None of the authors has potential conflicts of interest or specific financial interests relevant to the subject of their manuscript.
References
- 1.Muss HB. Adjuvant chemotherapy in older women with breast cancer: who and what? J Clin Oncol. 2014;32(19):1996–2000. doi: 10.1200/JCO.2013.54.8586. [DOI] [PubMed] [Google Scholar]
- 2.Hurria A. Management of elderly patients with cancer. J Natl Compr Canc Netw. 2013;11(5 Suppl):698–701. doi: 10.6004/jnccn.2013.0205. [DOI] [PubMed] [Google Scholar]
- 3.Kimmick G. Clinical trial accrual in older cancer patients: The most important steps are the first ones. J Geriatr Oncol. 2016;7(3):158–161. doi: 10.1016/j.jgo.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. doi:307/2/182 [pii] 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, Partridge AH, Dressler LG, Cohen HJ, Becker HP, Kartcheske PA, Wheeler JD, Perez EA, Wolff AC, Gralow JR, Burstein HJ, Mahmood AA, Magrinat G, Parker BA, Hart RD, Grenier D, Norton L, Hudis CA, Winer EP. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. doi:360/20/2055 [pii] 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandelblatt JS, Faul LA, Luta G, Makgoeng SB, Isaacs C, Taylor K, Sheppard VB, Tallarico M, Barry WT, Cohen HJ. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30(21):2609–2614. doi: 10.1200/JCO.2011.40.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Makgoeng SB, Luta G, Hurria A, Kimmick G, Isaacs C, Tallarico M, Barry WT, Pitcher B, Winer EP, Hudis C, Cohen HJ, Muss HB. A planned, prospective comparison of short-term quality of life outcomes among older patients with breast cancer treated with standard chemotherapy in a randomized clinical trial vs. an observational study: CALGB #49907 and #369901. J Geriatr Oncol. 2013;4(4):353–361. doi: 10.1016/j.jgo.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–1033. doi: 10.1111/j.1525-1497.2004.40016.x. doi:JGI40016 [pii] 10.1111/j.1525-1497.2004.40016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. Springer-Verlag; New York: 2000. [Google Scholar]
- 10.Sprinthall RC. Basic Statistical Analysis. 9th. Pearson Education; Old Tappan, NJ: 2011. [Google Scholar]
- 11.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, McCarthy EP. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29(12):1570–1577. doi: 10.1200/JCO.2010.33.0472. doi:JCO.2010.33.0472 [pii] 10.1200/JCO.2010.33.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing NonCancer-Related Health Status of US Cancer Patients: Other-Cause Survival and Comorbidity Prevalence. American Journal of Epidemiology. 2013;178(3):339–349. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RT, Yang TC, Matthews SA, Camacho F, Kern T, Mackley HB, Kimmick G, Louis C, Lengerich E, Yao N. Breast cancer screening, area deprivation, and later-stage breast cancer in Appalachia: does geography matter? Health Serv Res. 2014;49(2):546–567. doi: 10.1111/1475-6773.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 15.Mariotto AB, Wesley MN, Cronin KA, Johnson KA, Feuer EJ. Estimates of long-term survival for newly diagnosed cancer patients - A projection approach. Cancer. 2006;106(9):2039–2050. doi: 10.1002/cncr.21803. [DOI] [PubMed] [Google Scholar]
- 16.de Glas NA, van de Water W, Engelhardt EG, Bastiaannet E, de Craen AJ, Kroep JR, Putter H, Stiggelbout AM, Weijl NI, van de Velde CJ, Portielje JE, Liefers GJ. Validity of Adjuvant! Online program in older patients with breast cancer: a population-based study. Lancet Oncol. 2014;15(7):722–729. doi: 10.1016/S1470-2045(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 17.de Glas NA, Bastiaannet E, Engels CC, de Craen AJ, Putter H, van de Velde CJ, Hurria A, Liefers GJ, Portielje JE. Validity of the online PREDICT tool in older patients with breast cancer: a population-based study. Br J Cancer. 2016;114(4):395–400. doi: 10.1038/bjc.2015.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


