Abstract
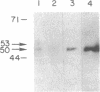
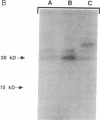
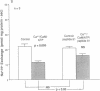
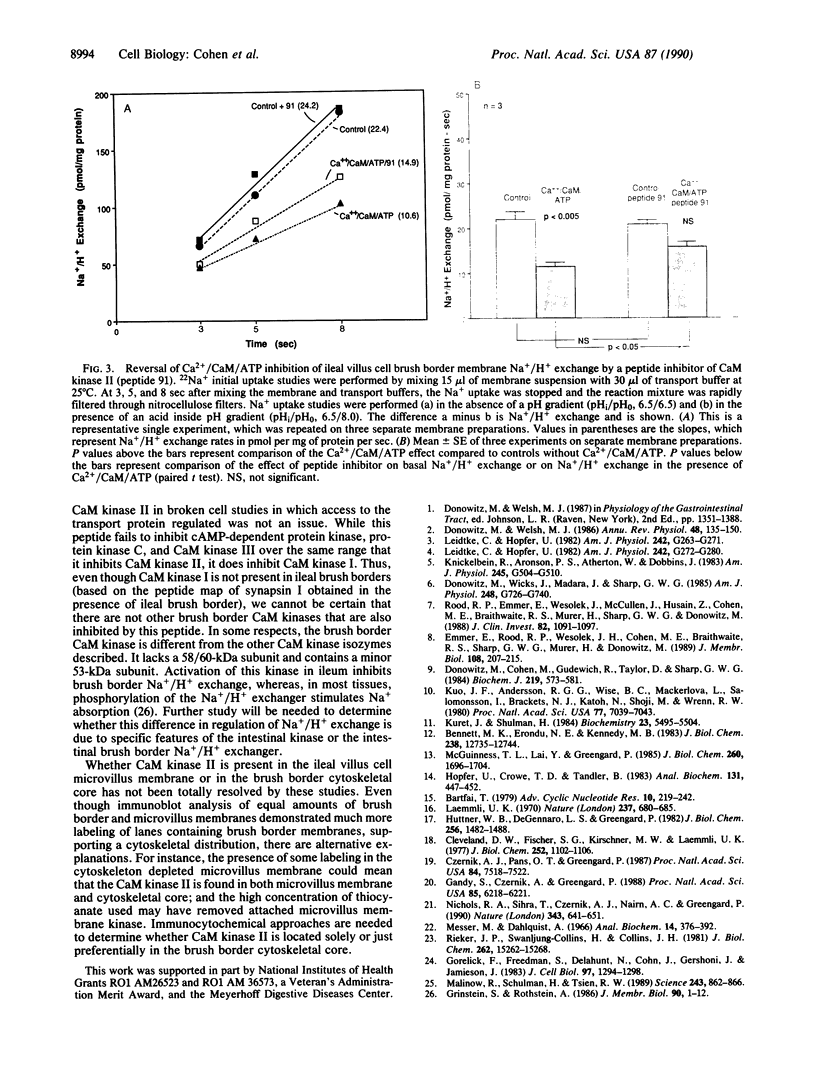
Ileal brush border membranes contain an endogenous Ca2+/calmodulin (CaM)-dependent protein kinase activity that modulates the activity of the apical membrane Na+/H+ exchanger. To further characterize this kinase, synapsin I, a substrate for Ca2+/CaM-dependent protein kinases, was added to preparations of ileal brush border membranes. In the presence of Ca2+/CaM, synapsin I was phosphorylated. Phosphopeptide mapping demonstrated that the addition of Ca2+/CaM to brush border membranes stimulated the phosphorylation of sites in synapsin I specific for Ca2+/CaM-dependent protein kinase II. Immunoblots containing brush border and microvillus membrane proteins were probed with an antibody that recognizes the 50-kDa subunit of rat brain Ca2+/CaM-dependent protein kinase II. This antibody labeled major and minor species of 50 and 53 kDa, respectively, with more labeling of the brush border than the microvillus membranes. Right-side-out ileal villus cell brush border vesicles were prepared containing CaM, ATP, and 350 nM free Ca2+. Na+/H+ exchange was inhibited by the presence of Ca2+/CaM/ATP within the vesicles. A 21-amino acid peptide inhibitor of CaM kinase II was enclosed within some vesicle preparations by freeze-thaw. The effect on Na+/H+ exchange of Ca2+/CaM/ATP was partially reversed by the inhibitor peptide. These studies demonstrate the presence of Ca2+/CaM-dependent protein kinase II in rabbit ileal villus cell brush border membranes. Based on the effect of a specific inhibitor peptide of Ca2+/CaM kinase II, it is concluded that this kinase inhibits brush border Na+/H+ exchange, which participates in the regulation of ileal Na+ absorption.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cohen M. E., Gudewich R., Taylor L., Sharp G. W. Ca2+-calmodulin-, cyclic AMP- and cyclic GMP-induced phosphorylation of proteins in purified microvillus membranes of rabbit ileum. Biochem J. 1984 Apr 15;219(2):573–581. doi: 10.1042/bj2190573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Welsh M. J. Ca2+ and cyclic AMP in regulation of intestinal Na, K, and Cl transport. Annu Rev Physiol. 1986;48:135–150. doi: 10.1146/annurev.ph.48.030186.001031. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Wicks J., Madara J. L., Sharp G. W. Studies on role of calmodulin in Ca2+ regulation of rabbit ileal Na and Cl transport. Am J Physiol. 1985 Jun;248(6 Pt 1):G726–G740. doi: 10.1152/ajpgi.1985.248.6.G726. [DOI] [PubMed] [Google Scholar]
- Emmer E., Rood R. P., Wesolek J. H., Cohen M. E., Braithwaite R. S., Sharp G. W., Murer H., Donowitz M. Role of calcium and calmodulin in the regulation of the rabbit ileal brush-border membrane Na+/H+ antiporter. J Membr Biol. 1989 Jun;108(3):207–215. doi: 10.1007/BF01871735. [DOI] [PubMed] [Google Scholar]
- Gandy S., Czernik A. J., Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick F. S., Cohn J. A., Freedman S. D., Delahunt N. G., Gershoni J. M., Jamieson J. D. Calmodulin-stimulated protein kinase activity from rat pancreas. J Cell Biol. 1983 Oct;97(4):1294–1298. doi: 10.1083/jcb.97.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Crowe T. D., Tandler B. Purification of brush border membrane by thiocyanate treatment. Anal Biochem. 1983 Jun;131(2):447–452. doi: 10.1016/0003-2697(83)90197-5. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuret J., Schulman H. Purification and characterization of a Ca2+/calmodulin-dependent protein kinase from rat brain. Biochemistry. 1984 Nov 6;23(23):5495–5504. doi: 10.1021/bi00318a018. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. II. Demonstration of Cl--OH- exchange and Cl- conductance. Am J Physiol. 1982 Mar;242(3):G272–G280. doi: 10.1152/ajpgi.1982.242.3.G272. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Nichols R. A., Sihra T. S., Czernik A. J., Nairn A. C., Greengard P. Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature. 1990 Feb 15;343(6259):647–651. doi: 10.1038/343647a0. [DOI] [PubMed] [Google Scholar]
- Rieker J. P., Swanljung-Collins H., Collins J. H. Purification and characterization of a calmodulin-dependent myosin heavy chain kinase from intestinal brush border. J Biol Chem. 1987 Nov 5;262(31):15262–15268. [PubMed] [Google Scholar]
- Rood R. P., Emmer E., Wesolek J., McCullen J., Husain Z., Cohen M. E., Braithwaite R. S., Murer H., Sharp G. W., Donowitz M. Regulation of the rabbit ileal brush-border Na+/H+ exchanger by an ATP-requiring Ca++/calmodulin-mediated process. J Clin Invest. 1988 Sep;82(3):1091–1097. doi: 10.1172/JCI113665. [DOI] [PMC free article] [PubMed] [Google Scholar]