Abstract
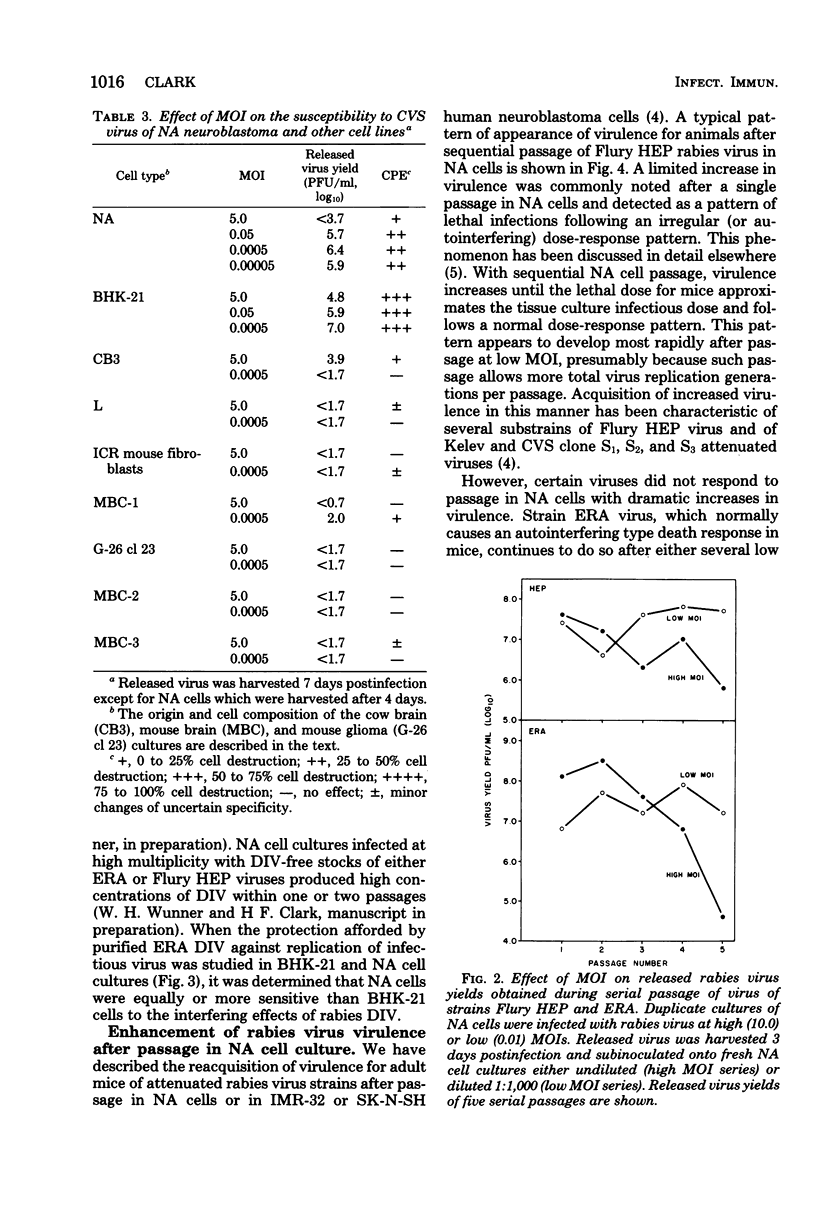
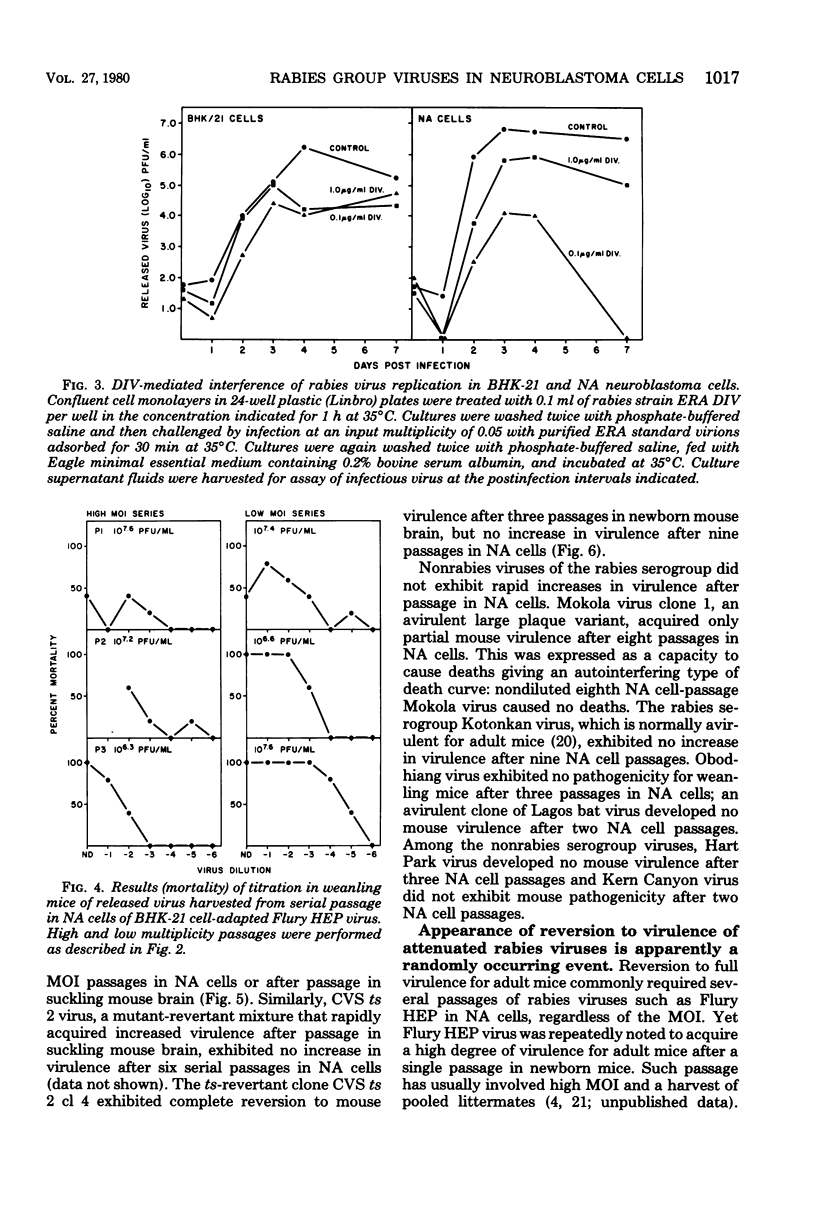
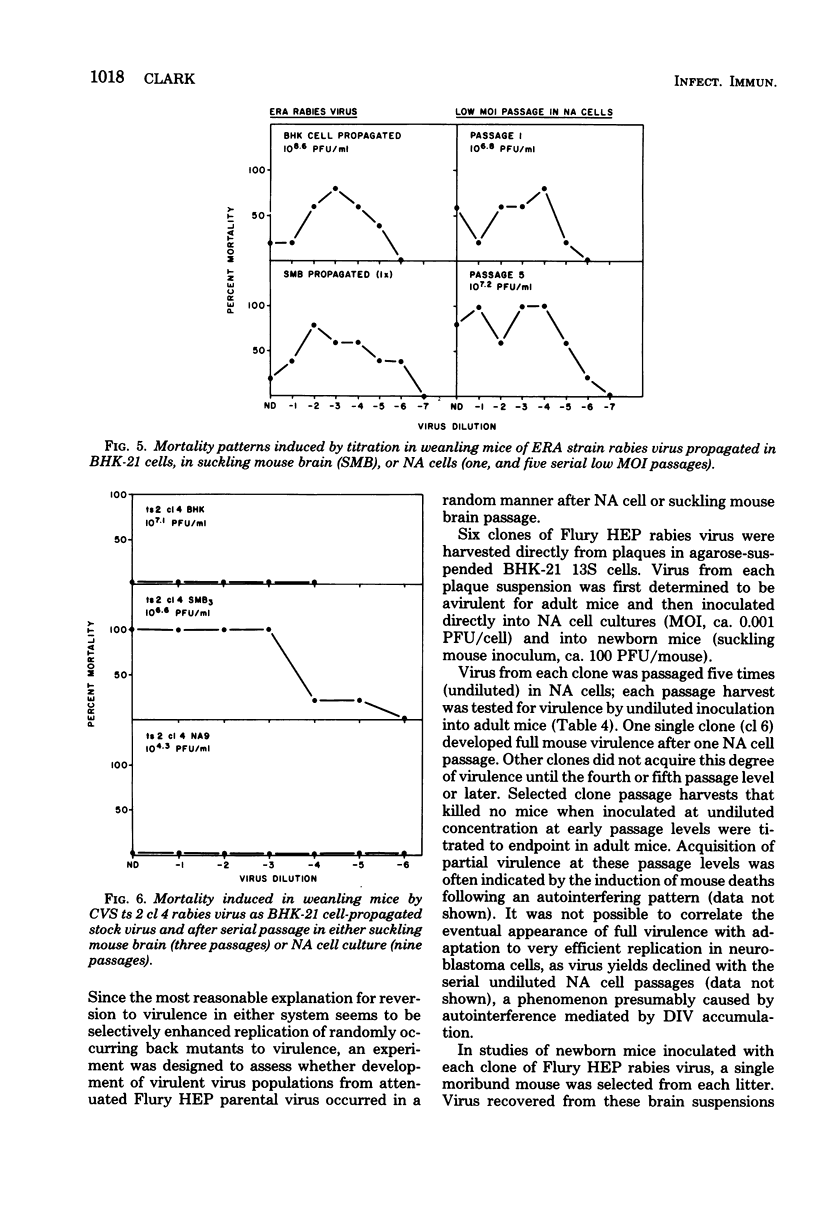
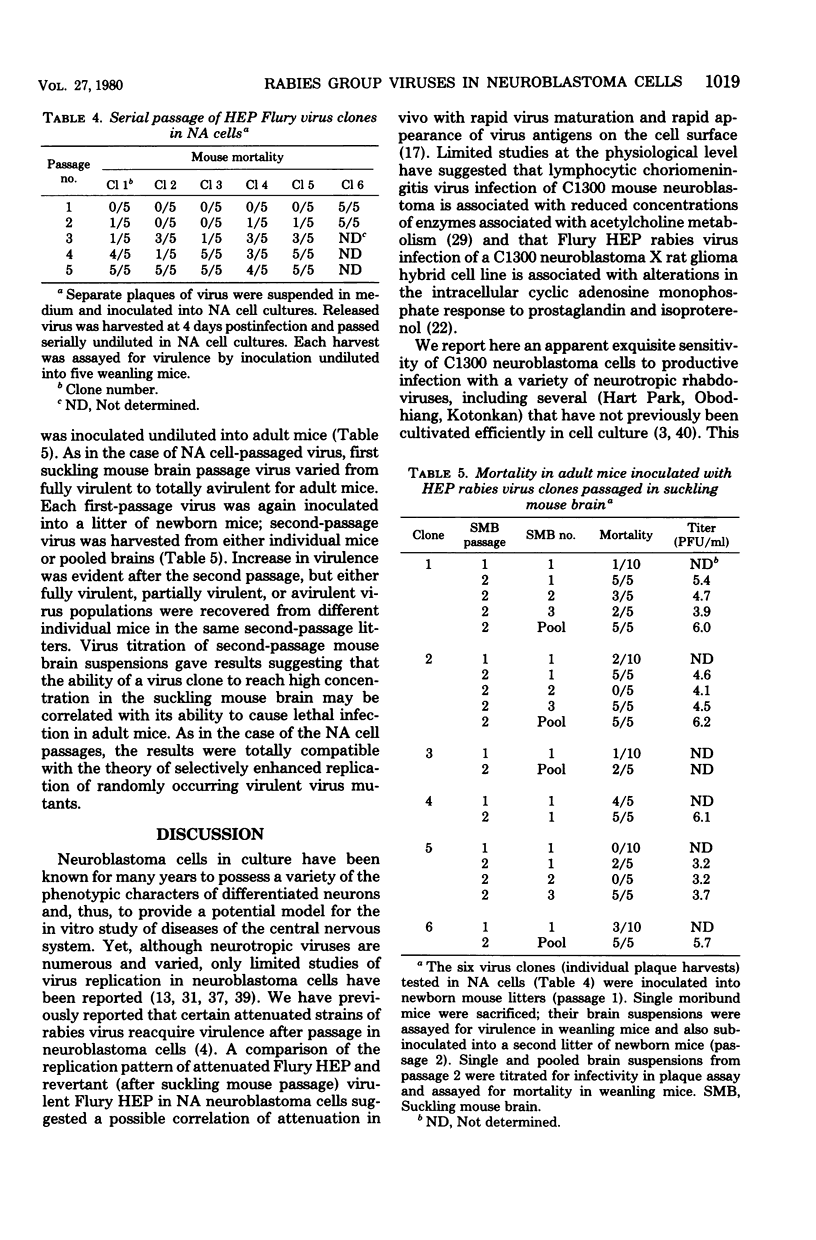
Each of several strains of fixed rabies virus was found to replicate to high titers in C1300 mouse neuroblastoma (clone NA) cells, without adaptation. Rabies serogroup Lagos bat, Mokola, and Duvenhage viruses also replicated efficiently in NA cells. Kotonkan and Obodhiang viruses replicated efficiently after adaptation, to titers not previously obtained in vitro. Infection in NA cells was frequently more cytopathic than in BHK-21 cells, allowing titration of Kotonkan and Obodhiang viruses by plaque assay. Duvenhage virus caused syncytium formation. Serial propagation of rabies viruses at a high multiplicity of infection in NA cells led to a rapid decline in virus yields; similar "autointerference" has not previously been demonstrated with rabies virus in other cell systems. Rabies virus infection in NA cells exhibited extreme sensitivity to interference by experimentally added defective interfering virions. Although several strains of attenuated rabies virus consistently reverted rapidly to virulence after propagation in NA cells, other strains of attenuated rabies and rabies serogroup viruses acquired increased virulence at a more gradual rate or not at all, suggesting that diverse characters may control virulence. When attenuated Flury HEP rabies virus was serially propagated at a low multiplicity of infection in either NA cells or suckling mouse brain, virulence appeared at a very variable rate, indicating that these systems may selectively enhance replication of randomly occurring virulent virus mutants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Clark H. F., Bishop D. H., Koprowski H. Comparison of the ribonucleic acid polymerases of two rhabdoviruses, Kern Canyon virus and vesicular stomatitis virus. J Virol. 1971 Jun;7(6):726–735. doi: 10.1128/jvi.7.6.726-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley S. M. Arbovirus infection of vertebrate and insect cell cultures, with special emphasis on Mokola, Obodhiang, and kotonkan viruses of the rabies serogroup. Ann N Y Acad Sci. 1975;266:241–250. doi: 10.1111/j.1749-6632.1975.tb35105.x. [DOI] [PubMed] [Google Scholar]
- Buckley S. M. Singh's Aedes albopictus cell cultures as helper cells for the adaptation of Obodhiang and kotonkan viruses of the rabies serogroup to some vertebrat cell cultures. Appl Microbiol. 1973 Apr;25(4):695–696. doi: 10.1128/am.25.4.695-696.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Koprowski H. Isolation of temperature-sensitive conditional lethal mutants of "fixed" rabies virus. J Virol. 1971 Mar;7(3):295–300. doi: 10.1128/jvi.7.3.295-300.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Ohtani S. Temperature-sensitive mutants of rabies virus in mice: a mutant (ts2) revertant mixture selectively pathogenic by the peripheral route of inoculation. Infect Immun. 1976 May;13(5):1418–1425. doi: 10.1128/iai.13.5.1418-1425.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F. Rabies viruses increase in virulence when propagated in neuroblastoma cell culture. Science. 1978 Mar 10;199(4333):1072–1075. doi: 10.1126/science.628831. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Wiktor T. J. Plasticity of phenotypic characters of rabies-related viruses: spontaneous variation in the plaque morphology, virulence, and temperature-sensitivity characters of serially propagated Lagos bat and Mokola viruses. J Infect Dis. 1974 Dec;130(6):608–618. doi: 10.1093/infdis/130.6.608. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Wiktor T. J. Temperature-sensitivity characteristics distinguishing substrains of fixed rabies virus: lack of correlation with plague-size markers or virulence for mice. J Infect Dis. 1972 Jun;125(6):637–646. doi: 10.1093/infdis/125.6.637. [DOI] [PubMed] [Google Scholar]
- Crick J., Brown F. In vivo interference in vesicular stomatitis virus infection. Infect Immun. 1977 Feb;15(2):354–359. doi: 10.1128/iai.15.2.354-359.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Marsden H. S., Cook M. L., Stevens J. G. Acute infection of differentiated neuroblastoma cells by latency-positive and latency-negative herpes simplex virus ts mutants. Virology. 1979 Apr 30;94(2):430–441. doi: 10.1016/0042-6822(79)90473-2. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Hughes J. V., Dille B. J., Thimmig R. L., Johnson T. C., Rabinowitz S. G., Dal Canto M. C. Neuroblastoma cell fusion by a temperature-sensitive mutant of vesicular stomatitis virus. J Virol. 1979 Jun;30(3):883–890. doi: 10.1128/jvi.30.3.883-890.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Clark H. F. Rabies virus infection in mouse neuroblastoma cells. Lab Invest. 1977 Jun;36(6):578–574. [PubMed] [Google Scholar]
- Iwasaki Y., Tsuchida N. Transmission electron microscopy surveillance of retroviruses in tissue culture cells prepared by the critical-point drying method. J Natl Cancer Inst. 1978 Aug;61(2):431–436. [PubMed] [Google Scholar]
- KOPROWSKI H. Biological modification of rabies virus as a result of its adaptation to chicks and developing chick embryos. Bull World Health Organ. 1954;10(5):709–724. [PMC free article] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S., Tanabe K. Characterization of rabies viruses recovered from persistently infected BHK cells. Virology. 1975 Oct;67(2):520–533. doi: 10.1016/0042-6822(75)90452-3. [DOI] [PubMed] [Google Scholar]
- Kemp G. E., Lee V. H., Moore D. L., Shope R. E., Causey O. R., Murphy F. A. Kotonkan, a new rhabdovirus related to Mokola virus of the rabies serogroup. Am J Epidemiol. 1973 Jul;98(1):43–49. doi: 10.1093/oxfordjournals.aje.a121531. [DOI] [PubMed] [Google Scholar]
- Koschel K., Halbach M. Rabies virus infection selectively impairs membrane receptor functions in neuronal model cells. J Gen Virol. 1979 Mar;42(3):627–632. doi: 10.1099/0022-1317-42-3-627. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S. ELECTRON MICROSCOPE STUDIES OF RABIES VIRUS IN MOUSE BRAIN. J Cell Biol. 1963 Dec;19:565–591. doi: 10.1083/jcb.19.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris F. A. Expression and extinction of glial properties in glioma X neuroblastoma cell hybrids. Exp Cell Res. 1978 Jul;114(2):269–276. doi: 10.1016/0014-4827(78)90483-4. [DOI] [PubMed] [Google Scholar]
- McMorris F. A., Ruddle F. H. Expression of neuronal phenotypes in neuroblastoma cell hybrids. Dev Biol. 1974 Aug;39(2):226–246. doi: 10.1016/0012-1606(74)90237-1. [DOI] [PubMed] [Google Scholar]
- Meredith C. D., Prossouw A. P., Koch H. van P. An unusual case of human rabies thought to be of chiropteran origin. S Afr Med J. 1971 Jul 17;45(28):767–769. [PubMed] [Google Scholar]
- Murphy F. A., Coleman P. H., Whitfield S. G. Electron microscopic observations of Flanders virus. Virology. 1966 Oct;30(2):314–317. doi: 10.1016/0042-6822(66)90107-3. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Holmstoen J., Welsh R. M., Jr Alterations of acetylcholine enzymes in neuroblastoma cells persistently infected with lymphocytic choriomeningitis virus. J Cell Physiol. 1977 Jun;91(3):459–472. doi: 10.1002/jcp.1040910316. [DOI] [PubMed] [Google Scholar]
- Rabinowitz S. G., Dal Canto M. C., Johnson T. C. Infection of the central nervous system produced by mixtures of defective-interfering particles and wild-type vesicular stomatitis virus in mice. J Infect Dis. 1977 Jul;136(1):59–74. doi: 10.1093/infdis/136.1.59. [DOI] [PubMed] [Google Scholar]
- Rice M., Holland L., Wagner E. K. Viral nucleic acid synthesis in HSV infected neural cells. Arch Virol. 1979;59(4):345–355. doi: 10.1007/BF01317474. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Wiktor T. J. Reproducible plaquing system for rabies, lymphocytic choriomeningitis,k and other ribonucleic acid viruses in BHK-21-13S agarose suspensions. J Virol. 1967 Dec;1(6):1224–1226. doi: 10.1128/jvi.1.6.1224-1226.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shope R. E., Murphy F. A., Harrison A. K., Causey O. R., Kemp G. E., Simpson D. I., Moore D. L. Two African viruses serologically and morphologically related to rabies virus. J Virol. 1970 Nov;6(5):690–692. doi: 10.1128/jvi.6.5.690-692.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Tignor G. H., Emmons R. W., Woodie J. D. Isolation of field rabies virus strains in CER and murine neuroblastoma cell cultures. Intervirology. 1978;9(6):359–361. doi: 10.1159/000148958. [DOI] [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Stability of neurotropic mouse hepatitis virus (JHM strain) during chronic infection of neuroblastoma cells. Arch Virol. 1978;57(1):53–61. doi: 10.1007/BF01315637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlne A., Lycke E. Herpes simplex virus infection of in vitro cultured neuronal cells (mouse neuroblastoma C 1300 cells). J Gen Virol. 1978 May;39(2):321–332. doi: 10.1099/0022-1317-39-2-321. [DOI] [PubMed] [Google Scholar]
- WHITNEY E. FLANDERS STRAIN, AN ARBOVIRUS NEWLY ISOLATED FROM MOSQUITOES AND BIRDS OF NEW YORK STATE. Am J Trop Med Hyg. 1964 Jan;13:123–131. doi: 10.4269/ajtmh.1964.13.123. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN H. M. The nature of gliomas as revealed by animal experimentation. Am J Pathol. 1955 Jan-Feb;31(1):1–29. [PMC free article] [PubMed] [Google Scholar]



