Abstract.
Cerebral malaria (CM) is a common cause of death and disability among children in sub-Saharan Africa. Many prior studies of neuropsychiatric morbidity have been limited by a cross-sectional design or a short duration of follow-up. Most have included subjects who may have presented with coma due to a disease process other than CM. No studies have assessed the relationship between magnetic resonance imaging (MRI) findings and long-term outcomes. The Cognitive Outcomes and Psychiatric symptoms of retinopathy-positive CM (COPS) cohort is the first large (N = 221) prospectively recruited cohort of stringently defined cases of CM and hospital-based, age-matched, non-CM controls in whom cognitive and psychiatric outcomes are assessed with standardized measures semi-annually for up to 5 years. We report baseline characteristics of the cohort and outcomes at 1 month. At enrollment, CM cases were more likely to come from families with fewer socioeconomic resources and to have health characteristics that increase risk for malaria. In children younger than 5 years, cases were delayed in motor, language, and social development by approximately 6 months, compared with controls. More significant delays occurred in those with MRI abnormalities at the 1-month follow-up visit. There were no differences between cases and controls in inhibitory self-control, nor in cognitive function in children ≥ 5 years of age. The latter finding may be related to the smaller sample size, case-control imbalance in socioeconomic status, or the use of cognitive and behavioral assessments that are less culturally appropriate to this population. Continued follow-up will help determine predictors of long-term outcomes.
INTRODUCTION
Malaria due to mosquito-borne transmission of the Plasmodium falciparum parasite is one of the leading causes of illness, death, and neurodisability in malaria-endemic areas.1 More than 40% of the world’s population is at risk, although most transmission occurs in sub-Saharan Africa (SSA).1,2 Cerebral malaria (CM) is the most severe complication of falciparum infection and is associated with an estimated case fatality rate of 15%.3 Approximately half of children admitted to hospital with malaria have signs of neurological involvement.4 Approximately 85% of CM cases in SSA occur in children less than 5 years of age.1
CM is clinically defined by P. falciparum parasitemia and coma with no other evident etiology for coma.5 Overt neurologic sequelae observed at the time of discharge include cortical blindness, gross motor deficits, ataxia, language regression, epilepsy, or behavioral abnormalities in up to 20% of patients.3,6–11 Prognostic factors for neurologic sequelae include preadmission seizures, seizures during admission, higher maximum temperature, male gender, profound coma, and neurologic deficit upon discharge.3,12 Although neurologic defects in some children resolve by 6 months from the time of discharge,6,7 persisting cognitive impairment has been reported after initial neurologic recovery.3,6
It is important to quantify the incidence of long-term neurocognitive sequelae and behavioral changes in child CM survivors and to identify children at risk of suffering long-term sequelae so that appropriate resources can be targeted to prevention and rehabilitation. The most accurate estimates of children requiring additional services after recovery will come from prospective studies of large, well-characterized cohorts with serial evaluations over a sufficient period to ascertain clinically relevant outcomes. Previous studies have been limited by cross-sectional designs, unreported rates of patient retention after initial illness, sample sizes < 100, follow-up of < 1 year, samples that are heterogeneous with respect to the underlying disease, and assessments that were discontinued when neurologic examination had normalized.3,6,8–10,12–14 Although a majority of CM cases occur in children less than 5 years of age, most studies have evaluated children older than 4 years at the time of diagnosis. There are no studies examining the association of initial and subsequent radiologic evidence of CM-related brain injury to outcomes. Finally, most previous studies have used a clinical definition of CM5 that allows other etiologies of coma to be included. In an autopsy study, 23% of children with clinically defined CM died of other causes (Reye’s syndrome, ruptured arteriovenous malformation [AVM], hepatic necrosis, severe anemia, pneumonia, and meningitis).15 Although outcomes of usual care for retinopathy-negative and positive CM children may not differ,16 it is reasonable to hypothesize that predictors of outcomes and opportunities for acute intervention may differ, based on differences in underlying pathological processes.
To more fully characterize the long-term cognitive and behavioral impact of CM, we designed a prospective case-control study of children with incident, retinopathy-positive CM that included semi-annual neurobehavioral assessments and serial magnetic resonance imaging (MRI) evaluations over a 5-year period. We report here the baseline characteristics of the cohort, the developmental, cognitive, and behavioral outcomes at 1 month postillness and the relationship of these characteristics and outcomes to initial neuroimaging. Longer-term outcomes and predictors will be described in subsequent reports.
METHODS
Design: prospective, longitudinal, case-control study.
Participants.
Cases were drawn from a cohort of survivors of retinopathy-positive CM who were 6 months of age or older at the time of presentation. They had been admitted to the Pediatric Research Ward at the Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi, during the peak malaria seasons (January–June) of 2012–2014. QECH is the major, public referral and research hospital in the southern region of Malawi where malaria is endemic. Survivors were included if they 1) met a generally accepted clinical definition of CM (a Blantyre Coma Scale of ≤ 2, P. falciparum parasitemia, and no other evident etiology for coma), 2) lived within 1.5 hours’ drive from QECH, and 3) had evidence of malarial retinopathy. Malarial retinopathy was established by a direct and indirect, dilated ophthalmic examination by an experienced ophthalmologist.15
Controls were children admitted to the general pediatrics ward at QECH for nonneurologic conditions such as dehydration, pneumonia, or other acute infection. They were age-matched one to one to CM survivors within 6 months of age at time of recruitment. Additional controls were recruited if a control patient was lost to follow-up within the first year of enrollment. All recruited participants were included in the analyses.
All participants consented for human immunodeficiency virus (HIV) testing with appropriate caregiver precounseling before recruitment into the cohort. Status with respect to HIV infection was determined with the use of two rapid tests: Uni-Gold Recombigen HIV-1/2 (Trinity Biotech, Jamestown, NY) and Determine HIV-1/2 (Alere, Waltham, MA).17 Discrepancies were resolved with a third rapid test, CapillusR (Trinity Biotech).
Procedures.
Enrollment.
Participants were enrolled and consented before hospital discharge. Caregivers agreed to attend a baseline assessment after 1 month of recovery from the initial illness, with follow-up visits scheduled every 6 months thereafter for 5 years. We obtained a clinical history from caregivers and from the medical record including information about CM severity (e.g., duration of fever, length of coma, and frequency of seizures, acute MRI results), significant past medical illnesses, previous hospitalizations, seizures, malaria episodes, and developmental or neurologic disability before admission. To assess for preadmission seizures, we used the Epilepsy Screening Questionnaire, which has been used in similar populations for the detection of epilepsy with a 70–73% sensitivity and 92–99% specificity.3 A previous history suggestive of febrile seizures was not a criterion for exclusion. To assess for preadmission developmental or neurologic disability, we used the Ten Questions screen, which has been shown to be valid in children as young as 2 years in similar populations with 85% sensitivity. Both screening instruments have been used in previous studies at QECH3 and were translated and back translated into the local language before their utilization.
Participant follow-up.
For the baseline and all subsequent assessments, caregivers were contacted by phone or in person during routine field visits the week before their scheduled baseline assessment. All participants received reimbursement for travel expenses as well as a small breakfast before the start of the assessment session. If families did not attend their scheduled visit, they were contacted up to three times by phone or in person. They were considered lost to follow-up if they did not attend an assessment after three reminders. Individuals known to have died or have moved from the catchment area, or whose family declined further study involvement were also considered lost to follow-up.
We obtained information on weight, height, mid-upper arm circumference, family living situation, socioeconomic status, and family structure at the baseline visit. We also performed developmental, cognitive, and behavioral and brain MRI studies, as described below. Three Malawian study nurses fluent in the local language completed the individual assessments in a quiet room. Caregivers were encouraged to observe all assessment sessions. Study nurses were trained to perform each assessment by a UK-trained child psychologist before the start of the study. The psychologist held training review sessions on each assessment on a 4- to 8-week revolving basis during the 2012 and 2013 season. The COPS manager also received training and continued subsequent bimonthly training sessions. Each nurse was observed every other month and given feedback to ensure visit consistency and assessment characteristics over time. Assessments were scored before participant departure and results were discussed with caregivers. All caregiver questions were answered at the start and conclusion of assessments. Caregivers were also asked a series of qualitative questions about their concerns about their child including any concerns specific to the child’s health, behavior, and development. Families were also asked about any acute stressors that could be impacting the child’s behavior or performance on the day’s assessments. All data were entered into a secure, password-protected REDCap database.
Development.
For children under 5 years of age at the baseline visit, we assessed development using the raw number of items passed on the Gross Motor, Fine Motor, Language, and Social subscales of the Malawi Developmental Assessment Tool (MDAT). The MDAT is a culturally appropriate developmental screening tool with 97% sensitivity and 82% specificity in identifying neurodisability in a similar, Malawian population.18 Each of the 34 items on the four subscales is scored on a pass/fail basis. We classified a child as developmentally delayed if they fell 2 standard deviations (SDs) below the mean of same-aged Malawian children on two or more MDAT subscales.
Cognitive function.
For children 5 years and older, we assessed cognitive function using translated and back-translated versions of the Kaufman Assessment Battery for Children, Second Editions (KABC II), preschool, and school-age versions.19,20 This battery includes tests that measure problem solving, memory, and visual spatial abilities. It has been used in multiple studies of cognitive function in studies of CM and HIV in SSA children.6–10,12–14,21 Its underlying factor structure has been confirmed in these settings. Because there are no KABC-II normative data for Malawian children, we calculated Sequential, Learning, and Delayed Recall summary scores by summing raw scores for the subtests and summary scales that are common to the preschool and school-age versions.
Behavior.
For children 2 years and older recruited during the 2013–2014 season, we assessed behavior using caregiver responses to the Behavioral Rating Inventory of Executive Function (BRIEF) (preschool and school-age versions), a standardized behavior rating scale that assesses executive function.22 It has demonstrated reliability, validity, and clinical utility across a range of conditions including individuals with learning disabilities, traumatic brain injuries, attention disorders, and other neurologic, psychiatric, and medical conditions. There are no local normative data for the BRIEF. We estimated an Inhibitory Self Control Index (ISCI) by summing raw scores of the Emotional Control and Inhibit subscales, to maintain consistency between the ISCI of the BRIEF-preschool version and the Emotional Regulation Index of the BRIEF school-age version. These two subscales have been shown to constitute a Behavioral Adjustment factor along with Externalizing Behavior subscales of the Child Behavior Checklist (CBCL) in Ugandan children.23
Magnetic resonance imaging.
MRI scans were performed on a 0.35 T Signa Ovation Excite MRI scanner (General Electric). The CM survivors had scans during the acute illness and at the 1-month, 12-month, and 60-month follow-up visits. All control participants had a baseline MRI obtained at the 1-month visit; additional scans were obtained if clinically indicated by a change in neurologic status. A Malawian radiologist (Sam Kampondeni) provided a clinical interpretation at the time of each scan, and these results were reviewed with the caregiver at the following visit. MRIs were classified as “abnormal” if there was any mention in the clinical report of an intracranial abnormality other than sinusitis. The radiologist was aware of case-control status but was blind to results of the cognitive and behavioral assessments.
Statistical analyses.
We used t tests and χ2 analyses to compare demographics and health history among enrolled participants who did and did not return for baseline assessments at 1 month, to assess bias due to attrition. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented for selected comparisons. We also compared health history, anthropometric measures, family living situation, socioeconomic status, and family structure between those cases and controls who attended baseline assessments at 1 month, to see if there were baseline differences between groups that might confound interpretation of any observed differences in developmental, cognitive, and behavioral status.
We compared the groups’ developmental, cognitive, and behavioral status by performing univariate (BRIEF) or multivariate (MDAT and KABC-II) analyses of covariance on raw test scores. (Preliminary analyses showed no difference in the pattern of results using MDAT raw scores versus age-corrected z scores, so results for raw scores are presented here). We used SAS 9.3 PROC GLM to calculate Wilks’ Lambda to determine overall effects on the MDAT and KABC-II subtests, entering covariates into the model first and examining Type III sums of squares to determine unique effects of each variable, given other effects in the model. Cases missing one or more covariates were excluded.
Age in months, gender, and family resources served as covariates. Preliminary analyses showed that cases and controls differed in socioeconomic status, reflected in differences in parental education and quality of housing. These family attributes were also associated with some outcome measures. To adjust for these confounding factors while preserving degrees of freedom in the analysis, we created a single index of “family resources” by summing indicators of parental education and housing quality on which the cases and controls differed (Table 1). Similar indicators of socioeconomic status have been used in prior studies6,7,24 and have been predictive of cognitive performance.25 We did not consider parental employment, since 88% of fathers were employed, some mothers may be able to afford not to work for pay, and employment categories in this setting (farming versus vending versus wage worker) are not unambiguous indicators of household resources.
Table 1.
Demographic characteristics
| Demographics and health status | Cases (N = 104) | Controls (N = 117) | P value |
|---|---|---|---|
| Physical characteristics | |||
| Age (months) | 50.3 (104) | 50.7 (117) | NS |
| Sex (% male) | 47.1 (104) | 53.9 (117) | NS |
| Height (cm) | 105.3 (78) | 106.3 (98) | NS |
| Weight (kg) | 15.0 (84) | 15.8 (98) | NS |
| Mid-upper arm circumference (cm) | 15.1 (87) | 15.4 (101) | NS |
| Abnormal MRI at 1 month (%) | 34.5 (87) | 17.3 (81) | 0.01 |
| Preadmission health problems | |||
| HIV+ (%) | 15.4 (104) | 7.1 (98) | 0.07 |
| Seizures (%) | 9.7 (103) | 4.3 (117) | NS |
| Malaria episode (%) | 29.9 (97) | 14.8 (108) | 0.01 |
| Other serious illness (%) | 45.4 (97) | 35.2 (108) | NS |
| Developmental problems (%) | 11.0 (100) | 13.3 (113) | NS |
| Family environment | |||
| Mother with < standard 5 education (%) | 43.4 (99) | 24.1 (108) | 0.003 |
| Father with < standard 5 education (%) | 29.7 (91) | 11.8 (102) | < 0.001 |
| Child is attending school (%) | 26.5 (102) | 31.9 (116) | NS |
| Mother working (%) | 64.4 (101) | 61.7 (107 | NS |
| Father working (%) | 91.7 (96) | 87.5 (104) | NS |
| One or both parents work as farmers (%) | 43.9 (98) | 22.0 (100) | 0.001 |
| Rudimentary housing characteristics | |||
| No electricity (%) | 86.5 (104) | 62.4 (117) | < 0.0001 |
| Well or surface water source (%) | 51.0 (104) | 33.3 (114) | 0.01 |
| Rudimentary or dirt floor (%) | 81.7 (104) | 66.7 (114) | 0.01 |
| Natural (thatch) roof (%) | 49.5 (103) | 27.4 (113) | 0.0008 |
| Pit latrine (%) | 98.1 (103) | 92.0 (112) | 0.04 |
HIV = human immunodeficiency virus; MRI = magnetic resonance imaging; NS = nonsignificant. For patient characteristic variables, numbers in parentheses reflect the number in the sample with nonmissing information for that analysis. Baseline MRI and anthropometric measures were not performed consistently at the 1-month visit until the 2013 season. Basic literacy and numeracy are achieved by most Malawians who complete the Standard 5 level of primary schooling.
We used χ2 analyses to compare prevalence of developmental delay (as determined by the MDAT) and how often caregivers reported the new onset of problems with growth, development, or behavior at the baseline visit.
Ethics approval.
The study was approved by the University of Malawi College of Medicine Research Ethics Committee and by the appropriate institutional review boards of Michigan State University, the University of Minnesota, and the University of Rochester.
RESULTS
Enrollment and retention.
During the study period, we enrolled 117 of 124 (95%) eligible CM survivors and 141 age-matched controls. Of the 258 participants, 221 (86%, 104 cases, 117 controls) returned for the baseline assessment. Of those who did not return, one died, eight were withdrawn from the study by caregivers, 24 moved out of the area, and four did not return for unknown reasons. Those who returned were more likely to have had a prior serious illness (OR = 3.9, CI = 1.4–10.4) or a prior episode of malaria (OR = 4.5, CI = 1.04–19.5). They did not differ from participants who did not return in age at enrollment, HIV status, or preadmission history of seizures or developmental problems. This report focuses on the 104 cases and 117 controls who returned for the 1-month follow-up visit.
Case-control differences: demographics and health status.
Table 1 shows preadmission health and socioeconomic indicators and MRI results at the 1-month visit. The groups did not differ in preadmission health history or anthropometrics, except that cases tended to be more likely to be HIV+ (P = 0.07, OR = 2.4, CI = 0.93–6.0) and were more likely to have had an episode of malaria before the index illness (P = 0.01, OR = 2.4, CI = 1.2–4.9). They did not differ in rates of school attendance or caregiver employment. Families of cases were more likely to have fewer family resources (P < 0.001) and to have at least one caregiver working in farming (P < 0.001, OR = 2.8, CI = 1.4–5.1). Cases were more likely to have an abnormal clinical reading of their MRI (P = 0.01, OR = 2.5, CI = 1.2–5.2). The majority (78%) of abnormalities noted were focal or diffuse atrophy or gliosis. Other findings included increased periventricular T2 signal (N = 3) and stroke (N = 3).
Case-control differences: neurobehavioral outcomes.
MDAT.
In the 144 participants younger than 5 years in whom we had complete data, cases scored lower overall on the MDAT (P = 0.001) and across all four domains individually (all P < 0.03). There was a significant effect for age overall and across all four domains (all P < 0.0001), with older children performing better. There was no effect of family resources overall or on any domain score (all P > 0.10). Twelve (16%) cases were developmentally delayed in two or more domains, compared with none of the controls (P < 0.0001, OR is undefined) (Table 2).
Table 2.
Neurobehavioral outcomes
| Neurobehavioral outcomes |
Cases |
Controls |
P value |
|---|---|---|---|
| Development (age < 60 months) | (N = 70) | (N = 74) | |
| MDAT gross motor (items passed) | 22.5 (21.2–23.8) | 26.0 (24.7–27.2) | 0.0002 |
| Fine motor (items passed) | 22.8 (21.5–24.2) | 26.0 (24.8–27.4) | 0.0009 |
| Language (items passed) | 21.4 (20.0–22.7) | 23.6 (22.3–24.9) | 0.02 |
| Social (items passed) | 24.1 (22.7–25.5) | 26.3 (25.0–27.7) | 0.02 |
| Developmentally delayed (%) | 16.0 | 0.0 | < 0.0001 |
| Cognitive function (age ≥ 60 months) | (N = 30) | (N = 27) | |
| KABC-II sequential processing (raw score) | 18.5 (17.4–19.7) | 19.5 (18.5–20.6) | NS |
| Learning (raw score) | 79.6 (71.0–88.2) | 90.9 (82.8–99.0) | NS |
| Delayed recall (raw score) | 26.4 (22.3–30.5) | 29.0 (25.1–32.9) | NS |
| Behavior (age ≥ 24 months) | (N = 60) | (N = 61) | |
| BRIEF inhibition/self-control (raw score) | 33.0 (31.5–34.6) | 31.0 (29.4–32.6) | NS |
| Caregiver reporting new concerns since discharge | |||
| Behavior (%) | 10.8 (102) | 2.6 (114) | 0.02 |
| Growth and development (%) | 7.8 (103) | 1.7 (115) | 0.03 |
BRIEF = Behavioral Rating Inventory of Executive Function; MDAT = Malawi Developmental Assessment Tool; MRI = magnetic resonance imaging. MDAT, KABC-II, and BRIEF results are shown as adjusted least squares means with 95% confidence limits in parentheses. BRIEF was administered only for the 2013–2014 season.
KABC-II.
Among the 57 participants who were 5 years or older and for whom data were complete, KABC-II scores did not differ between cases and controls in the overall model (P > 0.15) or on the three subtests individually, although there was a trend for controls to perform better than cases on learning (P = 0.08). For all three KABC-II measures, the 95% CIs for the adjusted mean difference between cases and controls were large and exceeded the unadjusted SD for each measure, pooled across groups. Younger age (all P < 0.0001) and fewer family resources (all P < 0.02) were associated with poorer performance overall and on the three subtests individually. To determine whether case-control differences in family resources confounded our ability to observe differences attributable to CM, we reran the analyses, excluding the family resources variable from the model. No group differences were observed (P = 0.55).
Behavioral Rating Inventory of Executive Function.
Caregivers of cases and of girls tended to report more problems with inhibition and emotional control on the BRIEF than caregivers of controls or of boys, but the differences were not significant. Younger age was associated with more problems overall (P < 0.0001). There was no effect of family resources (P > 0.20).
Caregiver report.
When interviewed, caregivers of cases more often reported new concerns about growth and development (7.8% versus 1.7%, P = 0.03, OR = 4.8, CI = 0.99–2.0) and behavior (10.8% versus 2.6%, P = 0.02, OR = 4.5, CI = 1.2–16.5) than did caregivers of controls. New-onset behavior problems involved aggression (N = 5), willfulness (N = 3), emotional dysregulation (N = 3), jerks and twitches (N = 2), dependence (N = 1), poor memory (N = 1), and hyperactivity (N = 1).
HIV status.
Because cases were slightly more likely to be HIV+, the MDAT, KABC-II, and BRIEF analyses were repeated after excluding HIV+ subjects. The pattern of significance in the results was unchanged.
Relationship of assessment results to MRI findings.
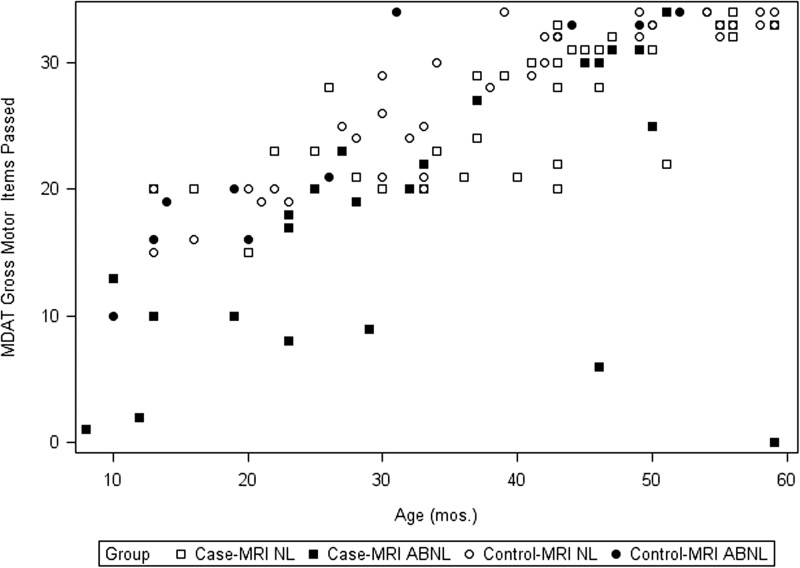
We reran the above analyses, adding MRI findings as a factor, in the subgroup of 81 cases and 87 controls from the 2013 and 2014 seasons who had had an MRI at the 1-month, postdischarge visit. An abnormal MRI was associated with lower performance on the MDAT overall (P < 0.05, N = 93), and on all four MDAT domains individually (all P < 0.03). The overall effect of CM and age remained significant (P = 0.04, P < 0.0001, respectively). Figure 1 shows the distribution of the sum of items passed in the MDAT Gross-Motor domain for both groups as a function of age and MRI status. The pattern of lower scores in participants with abnormal MRI was similar for the other three domains (Supplemental Figures 1–3). On the KABC II, there was a trend overall (P = 0.052) for lower KABC-II scores in those with abnormal MRIs. There was no effect of MRI on behavior as assessed by the BRIEF (P > 0.10, N = 112) (Supplemental Figures 4–7).
Figure 1.
MDAT Gross Motor (n items passed) by age (in months) for cases (squares) and controls (circles) with MRI read clinically as normal (open) or abnormal (filled).
DISCUSSION
In this prospective study of a rigorously defined and well-characterized cohort of pediatric survivors of CM, cases under the age of 5 years were more likely than age-matched hospital controls to show delays in development 1 month after the index illness. There were no differences in cognitive function in children 5 years of age or over or in inhibitory self-control among children 2 years of age or over. Cases also were more likely to come from families with fewer socioeconomic resources and had individual health characteristics that are risk factors for malaria, HIV infection, and poor developmental, cognitive, and behavioral outcomes that are independent of any effect of CM. Each of these observations is discussed in detail below.
Development.
Cases scored 2–3 points lower on average than controls on the MDAT, a screening task adapted specifically to assess development in SSA children. This corresponds roughly to a 6-month delay among the 1- to 4-year age groups, using normative data from a large cohort of healthy Malawian children.18 MDAT scores in this cohort were not associated with level of family resources. Cases also were more likely to have abnormal MRIs and children with abnormal MRIs were more likely to be delayed. These observations support the idea that some younger children suffer postacute, brain-based effects of CM and that the differences observed in this cohort do not simply reflect the lower family resources and more rural lifestyle that put children at risk both for CM and for developmental delay in SSA. These observations are also consistent with previous studies that suggest that development is delayed after CM in children under the age of 5 years and that there is a subset of CM survivors who are more severely affected.3,6,13
Cognitive function.
Case-control differences in cognitive function (as measured by the KABC II) were less apparent among children 5 years or older. MRI abnormalities also were less clearly associated with KABC II performance than they were with performance on the MDAT.
The age-based discrepancy in our results (case-control differences in younger children with the MDAT, but not in older children with the KABC-II) has a number of possible explanations. Younger brains may be more susceptible to the effects of CM, although little is known about the impact of age at the time of exposure.8 The sample size of older children (N = 57) was smaller, reflecting the earlier typical age of onset of CM. Thus, we may have had insufficient power to detect a clinically significant effect. To our knowledge, the minimally clinically important difference (MCID) for KABC-II measures in this setting has not been described, making it difficult to perform clinically meaningful power calculations. Nevertheless, we note that the 95% CIs for case-control differences in KABC-II adjusted mean values were large (> 1 SD of unadjusted scores). If the true MCID in this setting is ≤ 1 SD, then it is reasonable to suppose that we failed to detect a true, clinically meaningful difference between cases and controls because of low power. On the other hand, two prior studies of larger (N > 100) Ugandan cohorts also failed to find case-control differences at 3, 6, and 24 months postillness in the frequency of impairment or in raw scores, using these KABC-II subtests.6,7,24 In one of these cohorts, subsequent analyses did show case-control differences at multiple time points on a summary measure that combined one of these KABC-II measures with other measures of attention and learning.26
This pattern of results raises concerns that these specific KABC-II subtests may not be sufficiently sensitive to true, CM-related cognitive deficits in school-aged children. If so, the age-based discrepancies in our results and elsewhere in the literature could reflect differences in the cultural-appropriateness of KABC-II, compared with the MDAT. The MDAT was developed and validated within Malawi, whereas the KABC-II is a translated, Western measure. On the other hand, the KABC-II was sensitive to the expected effects of age and family resources in this sample and has shown evidence of construct validity in Ugandan children.19 Given these uncertainties, further studies are needed of the criterion and predictive validity of the KABC-II and other cognitive measures in SSA school-aged children, using a range of extratest criteria that are meaningful within each culture.
Behavior.
There were no differences in inhibitory self-control between cases and controls when measured by the BRIEF. However, caregiver interviews revealed numerous new behavioral concerns including externalizing behaviors that were more common among cases than controls. This discrepancy may reflect the relative heterogeneity of new behavioral problems in CM survivors that are not adequately assessed by the inhibitory self-control subscale of the BRIEF. It may also reflect limitations in cross-cultural validity of the BRIEF, a translated version of a measure of emotional control that was developed and validated in Western children (although see Familiar and others23). This raises concerns that the scope of assessment of new onset behavior problems may be limited by the lack of culturally valid methods for diagnosing a broad range of behavior and psychiatric disorders for children living in SSA.
Strengths of this study include the prospective assessment of developmental, cognitive, and behavioral outcomes in a population of well-characterized pediatric CM survivors and the opportunity to assess outcomes at sequential time points over a 5-year follow-up period. The detailed clinical data during the index event and serial MRI imaging may provide information on clinical characteristics that predict brain injury, the evolution of recovery, and the long-term neurocognitive deficits in survivors. The inclusion of malarial retinopathy in the diagnostic criteria also improved the classification of cases, as children more likely to have nonmalarial causes of coma were excluded. Few studies have followed children longitudinally and therefore have not accounted for other illnesses or events that may result in neurocognitive deficits including recurrent CM, other neurologic illness, poorly controlled epilepsy, malnutrition, or other familial stressors. Given our close follow-up, we hope to be able to address in this cohort how these various factors combine to affect outcomes. Finally, this is the first study to include MRI, an important independent indicator of short-term CM morbidity and possibly a strong predictor of long-term outcome. Long-term follow-up of this cohort should be able to address this important issue.
Limitations include the relatively small sample of older children, although this subsample will increase as the cohort ages. Our sample is representative of children with CM who receive care in urban, tertiary care settings within SSA, but it may not be representative of the entire population. The effect of this ascertainment bias is unclear, since CM children who do not come to the hospital could have less severe disease (and thus are not thought sick enough to warrant hospital care) or more severe disease (and die before reaching the hospital). The use of hospital-based controls was intended to enhance our ability to identify the specific impact of CM, independent of other factors associated with acute illness, including the impact on school attendance and the ability to learn. At the same time, it may underestimate the effects of CM if control children suffered cognitive problems as a result of their hospitalization. Controls also came from better-educated families with more economic resources than the cases. Other studies have attempted to mitigate this confound by using community controls drawn from a more similar socioeconomic background.6,7,24 However, their findings are necessarily confounded by the effects of having an illness that required hospitalization, above and beyond the effects of CM. Thus, each choice of controls has its unique limitations that have to be accounted for in interpreting results.
Finally, case-control socioeconomic differences were associated with school-aged children’s cognitive function, but not with development in the younger children. One possible explanation for this difference is that KABC-II tests are more like school activities than MDAT tasks. Parental education and other family resources may be more influential in fostering school-based skills than early developmental tasks. For example, when they are young, poorer rural children may have ample opportunities to develop motor, language, and social skills while helping parents and older siblings on the farm, but may have less time and support for schooling when they get older. These observations have implications for developing outreach and interventions for this population. For example, the lower maternal education levels in cases may limit a mother’s health literacy or ability to access relevant medical information about early signs and symptoms of CM. This lack of medical knowledge may, in turn, correlate with a delay to care which correlates with a more severe acute illness. More resourced families may also be more likely to seek hospital care for nonmalarial illness, whereas underresourced families may be at a higher risk of acquiring severe malaria.
This prospective, longitudinal cohort will allow for further investigation into the natural progression of neurocognitive sequelae in children affected by CM. Little is known about the specific impact of CM when compared with severe malaria or other common neurologic infections. The use of adapted neurocognitive assessment combined with the routine collection of caregiver reports could allow for expansion of our understanding of the unique features of CM. This could also enhance our ability to design and validate more culturally appropriate tools to assess cognitive, behavior, and development in Malawi. Ultimately, we hope to use these observations to develop targeted prevention strategies and scalable behavioral interventions to mitigate the neurocognitive impact of a disease that affects a large number of the world’s most vulnerable children.
Supplementary Material
Acknowledgments:
We are extremely grateful to Clara Antonio, for initial training on MDAT, KABC-II, and BRIEF; and Chimwemwe Kalengo and Edith Kafoteka for performing the assessments.
Note: Supplemental figures appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization , 2016. World Malaria Report 2016. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Idro R, Marsh K, John CC, Newton CR, 2010. Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 68: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE, 2010. Blantyre malaria project epilepsy study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 9: 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley J, Crawley J, Fegan G, Bauni E, Peshu N, 2007. Burden, features, and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA 297: 2232–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization , 2014. Severe malaria. Trop Med Int Health 19: 7–131. [DOI] [PubMed] [Google Scholar]
- 6.Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, John CC, 2007. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 119: e360–e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, Wu B, Boivin MJ, 2008. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 122: e92–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holding PA, Stevenson J, Peshu N, Marsh K, 1999. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg 93: 529–534. [DOI] [PubMed] [Google Scholar]
- 9.Kihara M, Carter JA, Holding PA, Vargha-Khadem F, Scott RC, Idro R, Fegan GW, de Haan M, Neville BG, Newton CR, 2009. Impaired everyday memory associated with encephalopathy of severe malaria: the role of seizures and hippocampal damage. Malar J 8: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boivin MJ, Vokhiwa M, Sikorskii A, Magen JG, Beare NA, 2014. Cerebral malaria retinopathy predictors of persisting neurocognitive outcomes in Malawian children. Pediatr Infect Dis J 33: 821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Idro R, Kakooza-Mwesige A, Balyejjussa S, Mirembe G, Mugasha C, Tugumisirize J, Byarugaba J, 2010. Severe neurological sequelae and behaviour problems after cerebral malaria in Ugandan children. BMC Res Notes 3: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idro R, Carter JA, Fegan G, Neville BG, Newton CR, 2006. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 91: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, Shapiro E, John CC, 2014. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis 59: 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, John CC, 2016. Neurocognitive domains affected by cerebral malaria and severe malarial anemia in children. Learn Individ Differ 46: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME, 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. [DOI] [PubMed] [Google Scholar]
- 16.Postels DG, Taylor TE, Molyneux M, Mannor K, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Birbeck GL, 2012. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology 79: 1268–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, Taylor TE, 2015. Brain swelling and death in children with cerebral malaria. N Engl J Med 372: 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladstone M, Lancaster GA, Umar E, Nyirenda M, Kayira E, van den Broek NR, Smyth RL, 2010. The Malawi developmental assessment tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med 7: e1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bangirana P, Seggane-Musisi Allebeck P, Giordani B, John CC, Opoka OR, Byarugaba J, Ehnvall A, Boivin MJ, 2009. A preliminary examination of the construct validity of the KABC-II in Ugandan children with a history of cerebral malaria. Afr Health Sci 9: 186–192. [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman AS, 2004. KABC-II: Kaufman Assessment Battery for Children. Bloomington, MN: NCS Pearson. [Google Scholar]
- 21.Boivin MJ, Gladstone MJ, Vokhiwa M, Birbeck GL, Magen JG, Page C, Semrud-Clikeman M, Kauye F, Taylor TE, 2011. Developmental outcomes in Malawian children with retinopathy-positive cerebral malaria. Trop Med Int Health 16: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gioia GA, Isquith PK, Guy SC, Kenworthy L, 2000. Behavior Rating Inventory of Executive Function: BRIEF. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- 23.Familiar I, Ruisenor-Escudero H, Giordani B, Bangirana P, Nakasujja N, Opoka R, Boivin M, 2015. Use of the behavior rating inventory of executive function and child behavior checklist in Ugandan children with HIV or a history of severe malaria. J Dev Behav Pediatr 36: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bangirana P, Musisi S, Boivin MJ, Ehnvall A, John CC, Bergemann TL, Allebeck P, 2011. Malaria with neurological involvement in Ugandan children: effect on cognitive ability, academic achievement and behaviour. Malar J 10: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, Boivin MJ, 2009. Socioeconomic predictors of cognition in Ugandan children: implications for community interventions. PLoS One 4: e7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergemann TL, Bangirana P, Boivin MJ, Connett JE, Giordani BJ, John CC, 2012. Statistical approaches to assess the effects of disease on neurocognitive function over time. J Biom Biostat (Suppl 7): 7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.