Abstract
In order to determine the comparative efficacy of injectable and intranasal vaccines to stimulate Bordetella bronchiseptica (Bb)-reactive anamnestic antibodies, a trial was conducted using 144 adult household dogs of various breeds and ages, which had been previously administered intranasal Bb vaccine approximately 12 months before enrollment. Dogs were randomized into 2 groups and blood, nasal swabs, and pharyngeal swabs were collected prior to the administration of single component Bb vaccines intranasally or parenterally. Ten to 14 days later all dogs were resampled to measure changes in systemic and local antibody to Bb. There were no differences in the changes in Bb-reactive serum IgG and nasal IgA between the groups, whereas intranasally vaccinated dogs had significantly higher Bb-reactive serum IgA. These data indicate that both of the current generation of intranasal (modified-live) and injectable (acellular) Bb vaccines can stimulate anamnestic local and systemic antibody responses in previously vaccinated, Bb-seropositive adult household dogs.
Résumé
Efficacité comparative des vaccins intranasaux et injectables pour stimuler les réponses des anticorps anamnestiques réagissant à Bordetella bronchiseptica chez les chiens domestiques. Afin de déterminer l’efficacité comparative des vaccins injectables et intranasaux pour stimuler les anticorps anamnestiques réagissant à Bordetella bronchiseptica (Bb), un essai a été réalisé à l’aide de 144 chiens domestiques adultes de diverses races et d’âges différents, auxquels l’on avait déjà administré le vaccin Bb intranasal environ 12 mois avant le recrutement. Les chiens ont été assignés au hasard à deux groupes et des échantillons sanguins, et écouvillons nasaux et pharyngés ont été prélevés avant l’administration de vaccins Bb à composant unique soit par voie intranasale ou parentérale. Dix à 14 jours plus tard, on a prélevé de nouveaux échantillons pour tous les chiens afin de mesurer les changements dans les anticorps systémiques et locaux pour Bb. Il n’y avait aucune différence au niveau des changements pour l’IgG sérique et l’IgA nasal réactif à Bb entre les groupes, tandis que les chiens vaccinés par voie intranasale présentaient un niveau significativement supérieur d’IgA sériques réactives à Bb. Ces données indiquent que les deux générations actuelles de vaccins Bb intranasal (vivant modifié) et injectable (acellulaire) peuvent stimuler les réponses locale et systémique des anticorps Bb chez les chiens adultes domestiques antérieurement vaccinés.
(Traduit par Isabelle Vallières)
Introduction
Bordetella bronchiseptica (Bb) is a Gram-negative bacterium that is one of about 12 pathogens that have been causally associated with the canine infectious respiratory disease complex (CIRDC) (1). Various parenteral and mucosal vaccines against Bb are available and have frequently been used in veterinary practices for more than 30 y (2). Throughout this period there has been controversy about the relative efficacy of these vaccines in stimulating primary protective immune responses and in their comparative utility as “booster shots” (2).
Environmental co-factors, such as natural exposure to Bb, that could provide a “boosting” effect for iatrogenically primed immune responses likely significantly contribute to vaccine efficacy and duration of immunity (DOI) in client-owned dogs (3,4). The involvement of these cofactors, including dose and frequency of Bb exposure in settings such as boarding kennels and grooming operations, is virtually impossible to model in a laboratory setting, requiring the use of household dogs to best gauge the Gestalt of immunity to Bb and other pathogens. For various reasons, perhaps most notably logistical difficulties related to owner participation and compliance, there are few studies that have sequentially examined immune responses to Bb vaccines in real-world dogs (5). Neither are there many recent data concerning the carriage of Bb in clinically normal household dogs, that could affect responses to vaccination and DOI (6,7). The purpose of this study was to extend extant laboratory findings related to the immunogenicity of Bb vaccines by comparing the anamnestic systemic and mucosal antibody responses induced by the current generation of injectable or intranasal single component Bb vaccines in adult household dogs presenting for their annual “booster shots.”
Materials and methods
Study population and experimental design
Clinically normal client-owned household dogs of various ages and breeds (Table 1) with a documented history of intranasal vaccination for Bb approximately 1 y before enrollment (a common and often recommended interval between vaccinations for Bb) (2) were subjects, and had written owner consent. Owners were questioned regarding their dogs’ lifestyle as related to potential exposures to other dogs. Patients were randomized into 2 groups using a computerized random number generator. Dogs in 1 group received a single injectable Bb vaccine; those in the other received a single intranasal vaccine. When there were 2 or more dogs in a household, all dogs received the same treatment. Venous blood (for serum), nasal swabs (sterile polyester tipped), and pharyngeal swabs were collected on day 0 prior to vaccination and again 10 to 14 d later. Individual swabbing was performed in both nares, left first, and then in the deep pharynx (including tonsil whenever possible). All sampling was done away from owners, and fractious dogs were mildly sedated, if necessary. Only pharyngeal swabbing was done in dogs with stenotic nares (i.e., too small to insert swab). Nasal swabs were placed in 1 mL, and pharyngeal swabs in 2 mL, of transport medium and frozen at −20°C, then −80°C prior to analysis.
Table 1.
Dog breeds categorized by treatment group and breed size
| Dog breeds | Treatment group | Breeds | Treatment group | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Injectable Bb vaccine | Intranasal Bb vaccine | Total | Injectable Bb vaccine | Intranasal Bb vaccine | Total | ||
| Large breed dogs | Small breed dogs | ||||||
| Alaskan malamute | 0 | 1 | 1 | Bichon frisé | 0 | 3 | 3 |
| American foxhound | 1 | 0 | 1 | Boston terrier | 0 | 1 | 1 |
| Boxer mix | 1 | 1 | 2 | Cairn terrier | 1 | 0 | 1 |
| Bullmastiff | 0 | 1 | 1 | Chihuahua | 1 | 1 | 2 |
| Dalmatian | 0 | 1 | 1 | Chihuahua mix | 1 | 0 | 1 |
| Doberman | 0 | 1 | 1 | Dachshund | 1 | 3 | 4 |
| Flat-coated retriever | 1 | 0 | 1 | Dachshund mix | 0 | 2 | 2 |
| Fox terrier (wire-haired) | 2 | 0 | 2 | Jack Russell terrier | 1 | 1 | 2 |
| German shepherd | 7 | 1 | 8 | Maltese | 0 | 1 | 1 |
| German shepherd mix | 0 | 2 | 2 | Maltese mix | 1 | 1 | 2 |
| Great Dane mix | 1 | 0 | 1 | Miniature dachshund | 1 | 0 | 1 |
| Greyhound | 0 | 1 | 1 | Miniature schnauzer | 3 | 0 | 3 |
| German shorthaired pointer | 0 | 1 | 1 | Papillion | 1 | 0 | 1 |
| Hound mix | 1 | 0 | 1 | Pekingese | 1 | 0 | 1 |
| Husky | 0 | 1 | 1 | Pomeranian | 1 | 0 | 1 |
| Husky mix | 0 | 1 | 1 | Pomeranian mix | 0 | 1 | 1 |
| Labrador | 3 | 8 | 11 | Poodle mix | 0 | 1 | 1 |
| Labrador mix | 2 | 0 | 2 | Pug | 0 | 1 | 1 |
| Old English sheepdog | 0 | 1 | 1 | Scottish terrier | 1 | 0 | 1 |
| Rhodesian ridgeback | 2 | 0 | 2 | Shih tzu | 3 | 4 | 7 |
| Schnauzer | 0 | 2 | 2 | Terrier mix | 3 | 1 | 4 |
| Weimaraner | 1 | 0 | 1 | Toy poodle | 1 | 2 | 3 |
| Total | 24 | 23 | 47 | West Highland terrier | 1 | 2 | 3 |
| West Highland terrier mix | 0 | 1 | 1 | ||||
| Medium breed dogs | Welsh corgi | 2 | 0 | 2 | |||
| Australian shepherd | 1 | 0 | 1 | Welsh corgi mix | 1 | 0 | 1 |
| Australian shepherd mix | 1 | 0 | 1 | Yorkshire terrier | 2 | 1 | 3 |
| Bassett hound | 0 | 2 | 2 | Yorkshire terrier mix | 1 | 0 | 1 |
| Beagle | 2 | 2 | 4 | Total | 28 | 27 | 55 |
| Beagle mix | 1 | 1 | 2 | ||||
| Border collie | 0 | 1 | 1 | No classification | |||
| Brittany spaniel | 0 | 1 | 1 | Mix | 14 | 5 | 19 |
| Cocker spaniel | 1 | 1 | 2 | ||||
| Collie | 0 | 1 | 1 | ||||
| French bulldog | 1 | 0 | 1 | ||||
| King Charles spaniel | 1 | 0 | 1 | ||||
| Pitbull | 1 | 1 | 2 | ||||
| Pitbull Mix | 1 | 0 | 1 | ||||
| Pointer Mix | 0 | 1 | 1 | ||||
| Shar Pei | 1 | 0 | 1 | ||||
| Shetland sheepdog | 0 | 1 | 1 | ||||
| Total | 11 | 12 | 23 | ||||
Vaccines
Single component injectable (Bronchicine; Zoetis, Parsippany, New Jersey, USA) and intranasal (Vanguard B; Zoetis) Bb vaccines were obtained from a distributor.
Quantitation of Bb-reactive antibodies
Enzyme-linked immunosorbent assays (ELISAs) to measure IgG and IgA reactive with Bb were performed as previously described (8) using Bb-antibody positive and negative sera and saliva as controls.
Polymerase chain reaction (PCR)
A real time PCR for Bb (and other respiratory pathogens; RealPCR test code 2524; Idexx Reference Laboratories, Calgary, Alberta) was performed on deep pharyngeal swabs.
Statistical analysis
Statistical analyses were performed using a commercial software package (SPSS Statistics 23.0; IBM, Markham, Ontario). Changes in Bb-reactive serum IgG, serum IgA, and nasal IgA between the enrollment/vaccination visit and the follow-up visit were the 3 outcome variables examined. Non-parametric Mann-Whitney U-tests were used to determine the differences between treatment groups (9). Baseline data collected from the primary visit were also used to examine the secondary hypothesis that activities which increase the likelihood of interaction with other dogs increase the chance of natural exposure to Bb, and subsequently provide a “boosting” effect for iatrogenically primed immune responses. A score was created for each dog by categorizing the potential risk of natural Bb exposure based on activities (boarding, grooming, etc.) which could increase interaction with other dogs in the 12 mo prior to the onset of the study (Table 2). These categories were then summed for each dog in order to create a total risk score. The risk for natural Bb exposure was considered greater for dogs with a higher total score for these parameters. The relationships between baseline IgG or IgA and the total risk score for potential natural Bb exposure were examined using a Spearman’s correlation (10).
Table 2.
Categorization of potential risk factors for being naturally exposed to Bordetella bronchiseptica
| Potential Bordetella exposure variables | Bordetella exposure risk category |
|---|---|
| Boarded (previous 12 months) | |
| a. None | 0 |
| b. 1 time | 1 |
| c. 1 to 2 times | 2 |
| d. 2 times | 3 |
| e. 2 to 3 times | 4 |
| f. 3 times | 5 |
| g. 3 to 4 times | 6 |
| h. > 4 times | 7 |
| i. Other | 8 |
| Groomed (last 12 months) | |
| a. None | 0 |
| b. Monthly | 6 |
| c. Every other month | 5 |
| d. 4 to 5 times per year | 4 |
| e. 2 to 3 times per year | 3 |
| f. 1 time per year | 2 |
| g. Other (stated regularly but not specified) | 1 |
| Other factors in last 12 months for risk of exposure | |
| a. None | 0 |
| b. Dog park | 1 |
| c. Travel with family | 1 |
| d. Interact with other dogs | 1 |
| e. Fostering other dogs (75 dogs in a home) | 2 |
Results
A total of 144 dogs were enrolled between September 4, 2014 and October 28, 2015. Seventy-seven dogs were randomly assigned to the injectable Bb vaccine group and 67 to the intra-nasal Bb vaccine group. Before initiation of the study, historical data revealed that there were 22 dogs in the injectable Bb vaccine group which were vaccinated for Bb between January and November of 2013, and 55 dogs vaccinated between January and October of 2014. In the intranasal Bb vaccine group, there were 16 dogs vaccinated between July and December of 2013, 50 dogs vaccinated between January and October of 2014, and 1 dog in May of 2012. There was no statistical difference in the previous vaccination dates between the 2 treatment groups (P = 0.87).
There were also no statistical differences between breed classification (large, medium, small) between the 2 treatment groups (Table 1, P = 0.24). The dogs in the injectable Bb vaccine group and the intranasal Bb vaccine group were also not different from each other for any of the other potential confounding variables explored (Bb exposure variables, Tables 2, 3 and baseline Bb-reactive antibodies, Table 4).
Table 3.
Counts and associated P-values for potential Bordetella bronchiseptica exposure variables
| Bordetella exposure variables | Treatment group | P-value | |
|---|---|---|---|
|
| |||
| Injectable Bb vaccine Number of dogs | Intranasal Bb vaccine Number of dogs | ||
| Boarded (previous 12 months) | 0.98 | ||
| a. None | 21 | 17 | |
| b. 1 time | 4 | 6 | |
| c. 1 to 2 times | 1 | 1 | |
| d. 2 times | 21 | 17 | |
| e. 2 to 3 times | 10 | 12 | |
| f. 3 times | 2 | 3 | |
| g. 3 to 4 times | 5 | 2 | |
| h. > 4 times | 7 | 5 | |
| i. Other | 6 | 4 | |
| Total | 77 | 67 | |
| Groomed (previous 12 months) | 0.87 | ||
| a. None | 47 | 40 | |
| b. Monthly | 6 | 5 | |
| c. Every other month | 8 | 9 | |
| d. 4 to 5 times per year | 4 | 7 | |
| e. 2 to 3 times per year | 6 | 4 | |
| f. 1 time per year | 0 | 0 | |
| g. Other (regularly) | 6 | 2 | |
| Total | 77 | 67 | |
| Other exposure factors (previous 12 months) | 0.64 | ||
| a. None | 67 | 60 | |
| b. Dog park | 2 | 1 | |
| c. Travel with family | 4 | 3 | |
| d. Interact with other dogs | 0 | 1 | |
| e. Fostering other dogs | 4 | 2 | |
| Total | 77 | 67 | |
| Potential exposure (1 month prior to sample) | 0.40 | ||
| a. None | 55 | 52 | |
| b. Boarded | 5 | 1 | |
| c. Groomed | 7 | 11 | |
| d. Foster dogs | 3 | 1 | |
| e. Other (travel, dog day care, dog show, extended family dog interaction) | 7 | 2 | |
| Total | 77 | 67 | |
| Potential exposure (between 2 test periods) | 0.84 | ||
| a. None | 61 | 54 | |
| b. Boarded | 8 | 7 | |
| c. Groomed | 2 | 5 | |
| d. Foster dogs | 3 | 0 | |
| e. Other (travel, dog day care, dog show, extended family dog interaction) | 3 | 1 | |
| Total | 77 | 67 | |
Table 4.
Descriptive statistics and associated P-values for dog gender, age, baseline Bordetella bronchiseptica (Bb)-specific serum IgG, baseline Bb-specific serum IgA and baseline Bb-specific nasal IgA
| Variable | Treatment group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Injectable Bb vaccine | Intranasal Bb vaccine | ||||||
|
|
|
||||||
| Number of dogs | Female | Male | Number of dogs | Female | Male | P-value | |
| Gender (male/female) | 77 | 44 | 33 | 67 | 40 | 27 | 0.8 |
|
| |||||||
| Number of dogs | Median | Range | Number of dogs | Median | Range | P-value | |
|
| |||||||
| Age (years) | 77 | 6.5 | 1 to 15 | 67 | 6 | 1 to 13 | 0.85 |
| Baseline Bb-specific serum IgG | 77 | 72.1 | 5.5 to 111.1 | 67 | 75.1 | −3.5 to 128.9 | 0.62 |
| Baseline Bb-specific serum IgA | 77 | 67 | 0 to 169 | 67 | 63.5 | 0 to 173 | 0.99 |
| Baseline Bb-specific nasal IgA | 62 | 66 | 3 to 116 | 58 | 73 | 0 to 137 | 0.41 |
In a subset of dogs the Bb-reactive serum IgG (n = 9 dogs), serum IgA (n = 12 dogs) and nasal IgA (n = 30 dogs) decreased between the enrollment/vaccination visit and follow-up visits. Therefore, when calculating the change in these parameters from baseline a negative value was obtained. Since, it is biologically less probable to have these parameters decrease in the 10- to 14-day period between the initial vaccination and follow-up visit there was uncertainty as to the best way to manage these data. To ensure that either including or excluding dogs that had lower serum IgG, IgA, and/or nasal IgA after vaccination did not bias the outcomes, the data were analyzed using 5 approaches; dogs that had a lower serum IgG on the post-vaccination visit excluded, dogs that had a lower serum IgA on the post-vaccination visit excluded, dogs that had a lower nasal IgA on the post-vaccination visit excluded, dogs with a lower serum IgG, IgA, or nasal IgA on the post-vaccination visit excluded and no dogs excluded. Interpretation of the analyses for both the explanatory variables or primary outcomes of interest did not change regardless of the dataset used; therefore, for brevity only the analysis for all of the enrolled dogs is presented.
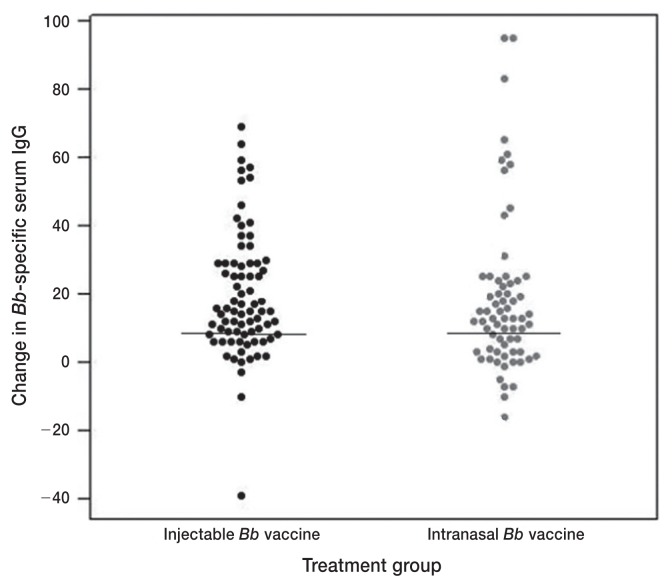
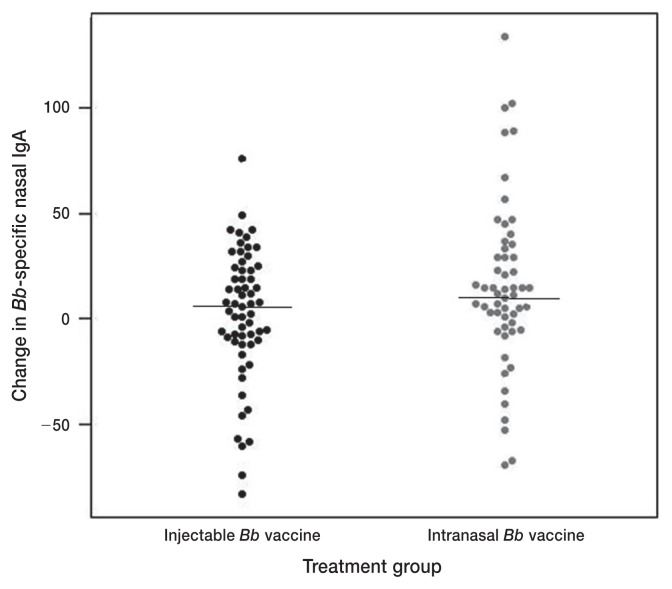
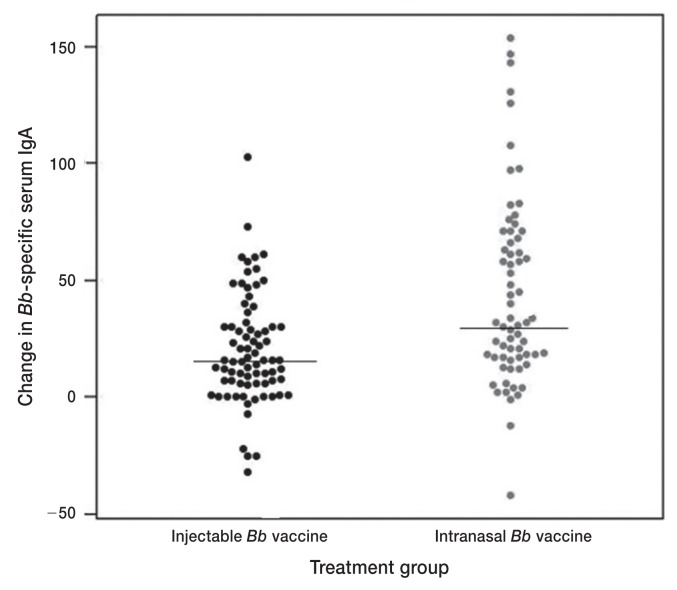
The changes in Bb-reactive serum IgG and nasal IgA were not significantly different between the 2 vaccination types (Table 5; Figures 1, 2); whereas the change in Bb-reactive serum IgA was significantly higher in the intranasally vaccinated (median = 32, range −42 to 154) versus the injected dogs (median 16, range −32 to 103) (Table 5; Figure 3) (P = 0.007).
Table 5.
Descriptive statistics and associated P-values for the 3 outcomes of interest; change in Bordetella bronchiseptica (Bb)-specific serum IgG, change in Bb-specific serum IgA, and change in Bb-specific nasal IgA between initial and final sampling
| Variable | Treatment group | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Injectable Bb vaccine | Intranasal Bb vaccine | ||||||
|
|
|
||||||
| Number of dogs | Median | Range | Number of dogs | Median | Range | P-value | |
| Change in Bb-specific serum IgG | 77 | 15 | −39 to 69 | 67 | 13 | −16 to 95 | 0.31 |
| Change in Bb-specific serum IgA | 77 | 16 | −32 to 103 | 67 | 32 | −42 to 154 | 0.007 |
| Change in Bb-specific nasal IgA | 60 | 6.5 | −83 to 76 | 55 | 12 | 69 to 134 | 0.23 |
Figure 1.
Beeswarm plot of change in Bordetella bronchiseptica-reactive serum IgG concentrations between initial and final sampling. Solid lines depict the median values for each treatment group.
Figure 2.
Beeswarm plot of change in Bordetella bronchiseptica-reactive IgA in nasal secretions between initial and final sampling. Solid lines depict the median values for each treatment group.
Figure 3.
Beeswarm plot of change in Bordetella bronchiseptica-reactive serum IgA concentrations between initial and final sampling. Solid lines depict the median values for each treatment group.
There was no statistical association between the calculated total lifestyle risk score for potential natural Bb exposure and baseline IgG (P = 0.12) or IgA (P = 0.93).
Pharyngeal swabs from 4/101 dogs from 2014 (3 injectable-vaccinated/1 intranasally vaccinated) were positive for Bb DNA. Because of the low prevalence and variable baseline and post-vaccination responses, the pharyngeal swab data were not further analyzed.
Discussion
The results of this study extend our previous investigations related to the immunogenicity of the current generation of parenteral vaccines for Bb in dogs, an acellular nonadjuvanted filtrate preparation similar to the vaccines used against Bordetella pertussis (Bp) in humans (5,10). To our knowledge, this is the first study to compare the ability of injectable and intranasal vaccines to stimulate anamnestic mucosal and systemic antibody responses in a large cohort of previously intranasally vaccinated, variably Bb seropositive adult household dogs.
To the extent that there were no significant differences in the change in Bb-reactive IgG responses in serum between groups that received the injectable versus intranasal vaccines, these results are in contrast to a previous study that documented significantly higher systemic antibody responses, that developed more rapidly in parenterally vaccinated Bb-seropositive adult laboratory beagles compared to a similar group of intranasally vaccinated dogs (8). That study examined a whole cell alum-adjuvanted bacterin; therefore, it is perhaps not surprising that the latter vaccine was apparently more immunogenic since it contained orders of magnitude more of the protein antigen (5,8,10). As well, the whole cell bacterin contained more and different pathogens associated molecular patterns (PAMPs) or “danger signals” that likely had adjuvant activity (5,10,11,12) in addition to the inclusion of aluminum hydroxide.
In human medicine, until about the early 1990’s, various whole cell Bp vaccines were used to successfully control whooping cough in vaccinated populations (10). However, these vaccines were associated with an approximately 50% incidence of adverse reactions, most often local inflammatory reactions and/or transient malaise and pyrexia (10). It is most likely that the PAMPs in the whole cell vaccines were responsible for not only adjuvant effects but also for inducing the inflammatory responses that constituted the majority of the adverse reactions (10,12). These adverse reactions were a main instigator in the development of less reactive acellular vaccines containing both less PAMPs and less potential antigens (10). The adverse reaction rate to the previously widely used whole cell Bb vaccines in dogs was apparently considerably less than with the Bp vaccines according to available, primarily anecdotal data; however, there is a dearth of reliable prevalence data on adverse reactions to Bb vaccines in dogs. Nevertheless, a similar desire to reduce perceived reaction rates was a major reason for the development of the current acellular Bb vaccine. Therefore, a safety-driven “parallel-evolution” has occurred in human and veterinary medicine related to vaccines for relevant Bordetella spp., resulting in less reactive acellular vaccines. However, as part of that process it is important to acknowledge the expected “trade-off’ between overall immunogenicity and less reactogenicity; it is generally not biologically possible to have both (10,12,13).
It was of interest that in some dogs, Bb-reactive antibodies in the serum and nasal secretions apparently decreased 10 to 14 d after vaccination. In the case of the nasal secretions, this could simply be a sampling “artifact” related to the volume of nasal secretion collected on the swabs prior to placement into a standard amount of transport medium; there is a high degree of variation in collection of these samples. This factor is difficult to control, especially when collecting from client-owned dogs, with variably sized, often small, nares. This constraint alludes to the logistical difficulty of conducting minimally invasive studies in household dogs, and warrants the use of the immunological surrogate, serum IgA, as an indicator of a relevant mucosal response (14). In the case of serum antibodies this possible dilution effect is not relevant, as a standard dilution of serum is tested in the ELISAs. A decrease in antibody 10 to 14 d after antigen exposure in serum or nasal secretions could be an indicator of a variable lack of response to the vaccine in an already antibody positive animal, in combination with a decay in antibody related to the expected half-life of those proteins in plasma and on mucosal surfaces, and/or immune complexing of antibody with vaccinal antigens (15). Alternatively, an apparent decrease in antibody measured in the ELISAs which used a whole cell preparation of Bb as antigen, could be due to the failure to accurately measure responses to immunodominant Bb antigens or different IgG subtype antibody responses in different dogs (13,16,17). In other words, after boosting with vaccine some dogs may respond preferentially to particular antigens versus others, which could then be detected in an apparent overall decrease in reaction with the standardized constellation of antigens in the whole cell preparation used as the ELISA antigen.
The site of production and immunological relevance of canine serum IgA has been controversial (18,19). The finding that most adult dogs in this study that received intranasal vaccine had increases in Bb-reactive IgA in the serum post-vaccination is consistent with the concept, based primarily on studies in the gut, that mucosal production following antigen exposure is the source of the dimeric IgA in the serum (20–22). This concept is supported by our recent finding of substantial increases in serum IgA subsequent to the administration of a single dose of oral or intranasal Bb, to young Bb-seronegative beagle puppies (14). Perhaps more controversial is the ability of parenteral immunization to boost measurable IgA on non-diseased mucosal surfaces, and there are few comparative data that address this issue. Although the numbers of nasal swab samples were decreased compared to serum, the finding that there were no significant differences in mucosal Bb-reactive IgA in the groups of dogs that received injectable or intranasal vaccines is consistent with the concept that parenteral administration of vaccine can result in increases in antigen-specific IgA in nasal secretions in mucosally-primed individuals (23). More relevant to Bb, it has been demonstrated that parenteral vaccination of previously naturally exposed human adolescents with acellular Bp (DTaP) stimulated not only anamnestic Bp-specific IgG responses, but IgA as well (24). This was not the case following primary parenteral immunization with the DTaP vaccine indicating that mucosal priming was required to affect the latter response (24). The practical implication from these observations is that priming puppies with intranasal vaccine followed by parenteral delivery in an initial series achieves boosting of both systemic and mucosal antibody responses (25,26).
It was suggested 40 y ago that natural transmission of Bb from clinically affected, convalescent, or asymptomatic carrier dogs could contribute to the duration of immunity to Bb, and that this effect could vary with a dog’s “lifestyle” (3). We were unable to associate the lifestyle co-factor of potential exposure to other dogs with baseline antibody responses to Bb in this cohort of dogs. This may be due to limitations of the data such as recall bias of owners, inadvertently missing or misclassifying key exposure risk(s), or an inability to quantify the exposure risk appropriately; for example, a lack of knowledge related to the “circulation” of Bb in subsets of the canine population. Data concerning the carriage of Bb by asymptomatic dogs are somewhat conflicting. Early studies based on culture reported no isolation of Bb in 2 populations of laboratory beagles [n = 467 (27); n = 25 (28)], and 3/50 (6.0%) asymptomatic dogs entering, or housed in a university veterinary clinic (29) were Bb culture positive. In recent studies using potentially more sensitive PCR, 2/22 (9.1%) normal dogs presenting at veterinary clinics (7) and 98/503 (19.5%) asymptomatic dogs presenting at shelters were positive for Bb DNA (30). Unfortunately, the immune status to Bb was not known/reported in any of those studies. Based on the low prevalence of Bb positive dogs (4%) in this population of routinely vaccinated dogs, carriage of Bb could not be implicated as an important co-factor in the response to Bb vaccines in this study. Nevertheless, exposure to Bb from acutely infected individuals, carrier animals, or fomites is undoubtedly an important, if difficult to measure co-factor in the response to Bb vaccines (4), which begs further investigation.
In conclusion, these data demonstrate that both intranasal and parenteral administration of current vaccines for Bb to previously intranasally vaccinated variably Bb seropositive adult household dogs can stimulate anamnestic systemic and mucosal antibody responses that have been associated with disease sparing in Bordetella infections (10,25). Therefore, these data suggest that either vaccine could be considered for use as a booster in animals with immunological memory established by previous immunization (25) and/or natural exposure.
Acknowledgments
This work was supported in part by an unrestricted grant from Zoetis to study the clinical immunity to Bb vaccines, and by the principal author’s discretionary funds to study clinical immunology. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Priestnall SL, Mitchell JA, Walker CA, Erles K, Brownlie J. New and emerging pathogens in canine infectious respiratory disease. Vet Pathol. 2014;51:492–504. doi: 10.1177/0300985813511130. [DOI] [PubMed] [Google Scholar]
- 2.Ellis JA. How well do vaccines for Bordetella bronchiseptica work in dogs? A critical review of the literature 1997–2014. Vet J. 2015;204:5–16. doi: 10.1016/j.tvjl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Bemis DA, Carmichael LE, Appel MJG. Naturally occurring respiratory disease in a kennel caused by Bordetella bronchiseptica. Cornell Vet. 1977;67:282–293. [PubMed] [Google Scholar]
- 4.Lavine JS, King AA, Bjørnstad ON. Natural immune boosting in pertussis dynamics and the potential for long-term vaccine failure. Proc Natl Acad Sci (USA) 2011;108:7259–7264. doi: 10.1073/pnas.1014394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis JA, Rhodes C, Lacoste S, Krakowka S. Antibodies to Bordetella bronchiseptica in vaccinated and infected dogs. Can Vet J. 2014;55:1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Ellis JA, Anseeuw E, Gow S, et al. Seroepidemiology of respiratory (group 2) canine coronavirus, canine parainfluenza virus and Bordetella bronchiseptica infections in urban dogs in a humane shelter and in rural dogs in small communities. Can Vet J. 2011;52:861–868. [PMC free article] [PubMed] [Google Scholar]
- 7.Joffe DJ, Lelewski R, Weese JS, et al. Factors associated with the development of Canine Infectious Respiratory Disease Complex (CIRDC) in dogs in 5 Canadian small animal practices. Can Vet J. 2016;57:46–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis JA, Krakowka GS, Dayton AD, Konoby C. Comparative efficacy of an injectable vaccine and an intranasal vaccine in stimulating Bordetella bronchiseptica-reactive antibody responses in seropositive dogs. J Am Vet Med Assoc. 2002;220:43–48. doi: 10.2460/javma.2002.220.43. [DOI] [PubMed] [Google Scholar]
- 9.Dohoo I, Martin W, Henrik S. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island: VER; 2009. [Google Scholar]
- 10.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benn CS, Netea MG, Selin LK, Aaby P. A small jab — A big difference: Nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:43–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 13.Vaughan K, Seymour E, Peters B, Sette A. Substantial gaps in knowledge of Bordetella pertussis antibody and T cell epitopes relevant for natural immunity and vaccine efficacy. Hum Immunol. 2014;75:440–451. doi: 10.1016/j.humimm.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis JA, Gow SP, Waldner CL, et al. Comparative efficacy of intra-nasal and oral vaccines against Bordetella bronchiseptica in dogs. Vet J. 2016;212:71–77. doi: 10.1016/j.tvjl.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Dixon FJ, Talmage DW, Maurer PH, Deichmille M. The half-life of homologous gamma globulin (antibody) in several species. J Exptl Med. 1952;96:313–318. doi: 10.1084/jem.96.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hijnen M, He Q, Schepp R, et al. Antibody responses to defined regions of the Boredetella pertussis virulence factor pertactin. Scan J Infect Dis. 2008;40:94–104. doi: 10.1080/00365540701642138. [DOI] [PubMed] [Google Scholar]
- 17.Leininger E, Bowen S, Renauld-Mongenie G, et al. Immunodominant domains present on the Bordetella pertussis vaccine component filamentous hemagglutinin. J Infect Dis. 1997;175:1423–1431. doi: 10.1086/516475. [DOI] [PubMed] [Google Scholar]
- 18.Davis RB, Jayappa H, Abdelmagid OY, Armstrong R, Sweeney D, Lehr C. Comparison of the mucosal immune response in dogs vaccinated with either an intranasal avirulent live culture or a subcutaneous antigen extract vaccine of Bordetella bronchiseptica. Vet Ther. 2007;8:32–40. [PubMed] [Google Scholar]
- 19.Larson LJ, Thiel BE, Sharp P, Schultz RD. A comparative study of protective immunity provided by oral, intranasal and parenteral canine Bordetella bronchiseptica vaccines. Int J Appl Res Vet Med. 2013;11:153–160. [Google Scholar]
- 20.Vaerman JP, Heremans JE. Origin and molecular size of immunoglobuliin-A in the mesenteric lymph of the dog. Immunol. 1970;18:27–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Batt RM, Barnes A, Rutgers HC, Carter SD. Relative IgA deficiency and small intestinal bacterial overgrowth in German shepherd dogs. Res Vet Sci. 1991;50:106–111. doi: 10.1016/0034-5288(91)90062-s. [DOI] [PubMed] [Google Scholar]
- 22.Olsson M, Frankowiack M, Tengvall K, et al. The dog as a genetic model for immunoglobulin A (IgA) deficiency: Identification of several breeds with low serum IgA concentrations. Vet Immunol Immunopathol. 2014;160:255–259. doi: 10.1016/j.vetimm.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Brown TA, Murphy BR, Radl J, Haaijman JJ, Mestecky J. Subclass distribution and molecular form of immunoglobulin A hemagglutinin antibodies in sera and nasal secretions after experimental secondary infection with influenza A virus in humans. J Clin Microbiol. 1985;22:259–264. doi: 10.1128/jcm.22.2.259-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thuan L, Cherry JD, Chang ST, et al. Immune responses and antibody decay afer immunization of adolescents and adults with an acellular pertussis vaccine: The APERT study. J Infect Dis. 2004;190:535–544. doi: 10.1086/422035. [DOI] [PubMed] [Google Scholar]
- 25.Ellis JA, Haines DM, West KH, et al. Effect of vaccination on experimental infection with Bordetella bronchiseptica dogs. J Am Vet Med Assoc. 2001;218:367–375. doi: 10.2460/javma.2001.218.367. [DOI] [PubMed] [Google Scholar]
- 26.Feunou PF, Kammoun H, Debrie A-S, Locht C. Heterologous prime-boost immunization with live attenuated B. pertussis BPZE1 followed by acellular pertussis vaccine in mice. Vaccine. 2014;32:4281–4288. doi: 10.1016/j.vaccine.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Brennan PC, Simkins RC. Throat flora of a closed colony of beagles. Proc Soc Exptl Biol Med. 1970;134:566–570. doi: 10.3181/00379727-134-34836. [DOI] [PubMed] [Google Scholar]
- 28.Clapper WE, Meade GH. Normal flora of the nose, throat, and lower intestine of dogs. J Bacteriol. 1956;85:399–420. doi: 10.1128/jb.85.3.643-648.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bailie WE, Stowe EC, Schmitt AM. Aerobic bacterial flora of oral and nasal fluids of canines with reference to bacteria associated with bites. J Clin Microbiol. 1978;7:223–231. doi: 10.1128/jcm.7.2.223-231.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavan R, Knesl O. Prevalence of canine infectious respiratory pathogens in asymptomatic dogs presented at US animal shelters. J Small Anim Pract. 2015;56:572–576. doi: 10.1111/jsap.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]